Abstract
Poorly formed tumor blood vessels lead to regions of microenvironmental stress due to depletion of oxygen and glucose and accumulation of waste products (acidosis). These conditions contribute to tumor progression and correlate with poor patient prognosis. Here we show that the microenvironmental stresses found in the solid tumor are able to inhibit the canonical Wnt/β-catenin signaling pathway. However, tumor cells harboring common β-catenin pathway mutations, such as loss of adenomatous polyposis coli, are insensitive to this novel hypoxic effect. The underlying mechanism responsible is hypoxia-induced endoplasmic reticulum (ER) stress that inhibits normal Wnt protein processing and secretion. ER stress causes dissociation between GRP78/BiP and Wnt, an interaction essential for its correct posttranslational processing. Microenvironmental stress can therefore block autocrine and paracrine signaling of the Wnt/β-catenin pathway and negatively affect tumor growth. This study provides a general paradigm relating oxygen status to ER function and growth factor signaling.
Tumor hypoxia is found in regions that are distant from the supporting tumor vasculature (6). These microenvironments can also suffer from low levels of nutrients (hypoglycemia) and high levels of waste products (acidosis). The net result is a stressful environment that can adversely effect tumor cell proliferation and survival and select for aggressive clonogens (12). Cells respond to these stresses through the induction of both HIF-1-dependent and HIF-1-independent mechanisms (8). Recent data from model tumors has identified how the endoplasmic reticulum (ER) can be stressed by severe hypoxia and how an incomplete response to this stress can inhibit tumor cell growth and survival (5, 19, 30). One potential mechanism by which ER stress could inhibit tumor formation would be through the inhibition of secreted growth factors, blocking autocrine and paracrine signaling of many molecules promoting proliferation and survival.
Wnt genes encode a family of secreted cysteine-rich glycoproteins that plays a significant role in development and homeostasis (21). Wnt proteins can bind to the Frizzled transmembrane receptors and activate different intracellular signaling pathways, such as the “canonical pathway.” Here, the key element is stabilization and nuclear translocation of β-catenin which then dimerizes with T-cell-specific transcription factor/lymphoid enhancer-binding factor 1 (TCF/LEF) transcription factors to transactivate target genes. In the absence of Wnt ligand, β-catenin is phosphorylated by a cytosolic multiprotein complex, which includes adenomatous polyposis coli (APC), axin, and glycogen synthase kinase 3 (GSK-3) and is thus targeted for degradation by the proteasome. Loss of APC, or even mutation of the phosphorylation sites in β-catenin itself, leads to a constitutively stable and active β-catenin (11, 29). In colorectal cancer, more than 90% of tumor samples have canonical Wnt pathway activating mutations. However, it is extremely rare to find mutations that directly lead to unrestricted expression of the Wnt genes.
Colorectal cancers, and even precancerous adenomas, have been reported to be hypoxic (14); therefore, we hypothesized that hypoxia might impact the Wnt/β-catenin signaling pathway. In the present study we report that hypoxia has a profound effect on Wnt-stabilized β-catenin protein but not on β-catenin that is stabilized by common tumor-derived mutations in APC or β-catenin. Further analysis revealed that hypoxia alone or combined with other tumor stresses, such as acidosis or glucose deprivation, is able to inhibit Wnt processing and secretion. In an effort to identify the mechanism underlying this phenomenon, we found that hypoxia interferes with the normal interaction of Wnt growth factors with the ER chaperone GRP78. This interaction is necessary for proper processing of the protein. Incompletely processed Wnt protein is targeted for proteasomal degradation. These findings have biologic significance because mutant β-catenin can confer a survival advantage relative to Wnt3a in cells treated with hypoxia in vitro and also yield increased xenograft tumor growth in vivo.
MATERIALS AND METHODS
Cells, cell culture, and reagents.
HEK293, RKO, L, SW480, and SW48 cells were cultured in Dulbecco modified Eagle medium with 10% fetal bovine serum. RCH-ACV cells were cultured in RPMI with 10% fetal bovine serum, and mouse ES cells were as described previously (28). Mouse L cells transfected with PGK-Wnt3a or empty vector were kindly provided by Roel Nusse (Stanford University). Wnt conditioned media was generated as described previously (32). For moderate hypoxia, cells were treated in a variable-oxygen In Vivo2 humidified hypoxia workstation (Ruskinn Technologies, Bridgend, United Kingdom). Severe hypoxia was generated in an anaerobic workstation gassed with 5% CO2, 5% H2, and 95% N2 containing a palladium catalyst (Sheldon Co., Cornelius, OR). Transient and stable transfection were performed by using Lipofectamine (Invitrogen, Carlsbad, CA). MG-132, tunicamycin, and 2-bromopalmitate were purchased from Sigma-Aldrich (St. Louis, MO). Dithiothreitol (DTT) was purchased from Invitrogen.
Plasmids, small interfering RNAs (siRNAs), and sequence analysis.
The pRK5SK-ΔGSK-β-catenin, PGK-Wnt3a, and Super8XTOPFlash in pTA-Luc vector were kindly provided by Roel Nusse (Stanford University). In mutant β-catenin (ΔGSK-β-catenin), four serine/threonine residues involved in GSK-3 phosphorylation have been changed into alanine as previously described (3). The creation of RKO cells stably transfected with a shHIF-1α plasmid has been previously described (26). Protein sequences were aligned by using CLUSTAL X program with default parameters (http://bips.u-strasbg.fr/fr/Documentation/ClustalX/). The GenBank accession numbers of the sequences used were as follows: human Wnt3a (NP149122), mouse Wnt3a (NP033548), zebra fish Wnt3a (AAT38336), and Drosophila melanogaster Wingless (M17230).
Reverse transcription-PCR (RT-PCR) analysis.
Total RNA was isolated by using TRIzol (Invitrogen). One microgram of total RNA was used for cDNA synthesis using SuperScript II reverse transcriptase (Invitrogen). In PCRs, the following primers were used: β-catenin (forward), 5′-TACCATTCCATTGTTTGTGCAG-3′; β-catenin (reverse), 5′-TGAAGAGAGAGCTGGTCAGCTC-3′; nip3L (forward), 5′-ATGTCGTCCCACCTAGTCGA-3′; nip3L (reverse), 5′- CTTCAGAAATTCTGCGGAGAAAATACCCCC-3′; 18S (forward), 5′-CCATCCAATCGGTAGTAGCG-3′; and 18S (reverse), 5′-GTAACCCGTTGAACCCCATT-3′.
Western blotting.
In brief, treated cells were harvested in radioimmunoprecipitation assay (RIPA) buffer, lysates were sonicated and cleared by centrifugation, and the protein concentrations were quantitated. Portions (25 to 50 μg) were electrophoresed on a reducing Tris-Tricine gel and electroblotted onto polyvinylidene difluoride membrane. The antibodies used were mouse anti-β-catenin (E5; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-GSK3β (BD Biosciences Pharmingen, San Diego, CA), mouse anti-HIF-1α (BD Biosciences Pharmingen), mouse anti-VHL (BD Biosciences Pharmingen), mouse anti-α-tubulin (Research Diagnostics, Flanders NJ), rabbit anti-ATF3 (sc-188; Santa Cruz Biotechnology), rabbit anti-human Wnt3a (ab28472; Abcam, Cambridge, MA), rat anti-mouse Wnt3a (R&D Systems, Minneapolis, MN), mouse anti-human Wnt16 (BD Biosciences Pharmingen), mouse anti-p53 (DO-1; Santa Cruz Biotechnology), rabbit anti-GRP78/BiP (Abcam), goat anti-GRP78/BiP (N-20; Santa Cruz Biotechnology), and mouse anti-β-actin (Abcam). Primary antibodies were detected with species-specific AP-secondary antibodies (1:3,000; Vector Labs) and visualized with ECF (Amersham) on a Storm 860 phosphorimager (Molecular Devices).
Cell fractionation.
Cells were washed and scraped on ice into TBS (10 mM Tris-HCl [pH 7.5], 140 mM NaCl, 2 mM DTT, protease inhibitors). The cells were then homogenized with 30 strokes in a Dounce homogenizer, and the nuclei were removed at 2,000 × g for 30 min (to pellet unlysed cells and nuclei). The lysates were spun at 100,000 × g for 60 min at 4°C, and the supernatant (cytosolic fraction) was analyzed by Western blotting.
Thiol modification blocking assay (treatment with NEM).
Cells were cultured for the indicated times and then lysed in RIPA buffer (1% Triton X-100, 150 mM NaCl, 20 mM HEPES [pH 7.5], 10% glycerol, 1 mM EDTA, 100 mM NaF, 17.5 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 4 μg of aprotinin/ml, 2 μg of pepstatin A/ml) supplemented with fresh 20 mM N-ethylmaleimide (NEM; Sigma-Aldrich). Cell lysates (30 μg) were analyzed on an 8% sodium dodecyl sulfate-polyacrylamide gel under reducing (125 mM DTT) and nonreducing (0 mM DTT) conditions.
Reporter assays.
L cells expressing the Super8XtopFlash luciferase reporter were plated and 24 h later were cultured in Wnt3a or control L conditioned medium (CM). At 48 h after plating, cells were lysed, and reporter activity was measured by using a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA) and a luciferase reporter assay kit (Roche, Indianapolis, IN). The data were normalized for total protein concentration. Values are reported as mean ± the standard deviation for three independent experiments.
Mouse VEGF ELISA.
The quantity of mouse vascular endothelial growth factor (VEGF) in L Wnt3a conditioned media was determined by solid phase enzyme-linked immunosorbent assay (ELISA) using a Quantikine mouse VEGF immunoassay kit, according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Coimmunoprecipitation.
Cells were harvested in RIPA buffer with or without 20 mM NEM. A total of 30 μl of protein A/G Plus-agarose beads (Santa Cruz Biotechnology) was rinsed with phosphate-buffered saline (PBS) and incubated with 2 μg of rabbit anti-GRP78/BiP (Abcam) for 1 h. The beads were then incubated with 200 μg of protein lysates at 4°C overnight. After incubation, the beads were washed four times with ice-cold lysis buffer and then boiled in Tricine sample buffer with or without DTT and analyzed by Western blotting.
Immunocytochemistry and fluorescence microscopy.
Cells were grown on glass chamber slides (Nalge Nunc, Naperville, IL), or frozen tumor sections were analyzed. Tumor-bearing animals were given 60 mg of pimonidozole (Chemicon)/kg intraperitoneally 3 h prior to harvest. Slides were fixed in 4% paraformaldehyde and blocked in PBS-Tween-milk (0.2% Tween 20 and 5% nonfat dry milk in PBS) overnight at 4°C. Primary antibodies were as described above and were visualized with anti-mouse Alexa 594- or anti-rat Alexa 488-conjugated secondary antibody (Molecular Probes) as indicated. Slides were mounted in Vectashield (Vector Laboratories, Burlingame, CA). Cells were visualized on a Nikon Eclipse E800 microscope, with a Spot RT Slider charge-coupled device digital camera using standard fluorescein isothiocyanate and Texas Red filter blocks (Diagnostic Instruments, Sterling Heights, MI). ImageJ software (National Institutes of Health, Bethesda, MD) was used for image analysis and quantification.
Cell survival assays.
For clonogenic assays, 300 to 500 cells were plated in 60-mm dishes and 6 h later placed in anoxia, followed by a return to normoxia. Ten days later, cells were fixed in 96% ethanol and stained with crystal violet, and colonies (>50 cells) were scored. Trypan blue exclusion assay was performed with cells that were plated in 60-mm dishes (106 cells/dish) and treated. All cells were harvested, and resuspended in PBS. An equal volume of cell suspension was mixed with 0.4% trypan blue solution (Sigma-Aldrich), The number of blue cells determined on a hemacytometer, and the percentage of total cells was reported. Assays were performed in triplicate.
Xenograft tumor growth in nude mice.
A total of 5 × 106 cells were implanted subcutaneously into each flank of 8-week-old female athymic nu/nu mice (Charles River Laboratories, Wilmington, MA). Tumor sizes in two dimensions were measured with calipers, and volumes were calculated by using the formula: α2 × β × 0.52, where α is the smaller dimension and β is the larger dimension. A total of 8 to 10 tumors were measured for each line in duplicate experiments. Mice were euthanized as defined by Stanford animal care guidelines.
Statistical analysis.
All data were presented as means ± the standard error of the mean. Statistical analysis was performed with Microsoft Excel software. Differences between individual groups were analyzed by using the Student t test. P values of <0.05 were considered statistically significant.
RESULTS
Hypoxia downregulates stabilized β-catenin.
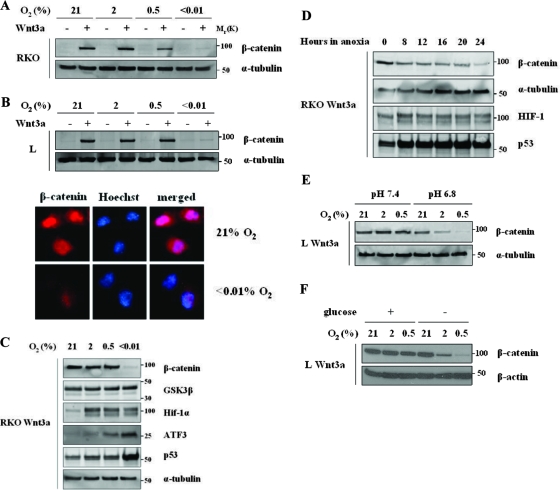
To investigate the possibility that hypoxia might impact the Wnt signaling pathway, we studied RKO cells derived from a human colorectal tumor with an intact β-catenin regulatory pathway. We generated RKO Wnt3a-expressing, or vector cells, cultured them in different oxygen concentrations, and analyzed lysates to detect β-catenin protein levels. Interestingly, β-catenin levels were significantly decreased in severe hypoxia (<0.01%) (Fig. 1A).
FIG. 1.
Hypoxia inhibits β-catenin stabilization by Wnt3a. (A) Hypoxia downregulates β-catenin protein levels in RKO colorectal tumor cells. A PGK-Wnt3a vector was used to create RKO cell line stably expressing mouse Wnt3a. RKO cells expressing Wnt3a or empty vector were cultured for 24 h in the indicated oxygen concentrations. Total cell lysates (30 μg) were analyzed by Western blotting and probed for β-catenin and α-tubulin. (B) Hypoxia downregulates β-catenin protein levels in murine L cells. L cells expressing Wnt3a or empty vector were cultured for 24 h in the indicated oxygen concentrations. Total cell lysates (30 μg) were analyzed by Western blotting and probed for β-catenin and α-tubulin (top panel). Subcellular localization of β-catenin in L cells is also shown (bottom panel). Cells expressing Wnt3a were cultured in chamber slides in normoxia or anoxia for 24 h. Immunofluorescence was performed for β-catenin, nuclei were visualized by Hoechst staining. Identical exposures were used for the different conditions. (C) Hypoxia downregulates wild-type β-catenin protein levels but does not affect GSK3β levels. RKO Wnt3a cells were cultured for 24 h in the indicated oxygen concentrations. Total cell lysates (30 μg) were analyzed by Western blotting and probed for β-catenin, GSK3β, Hif-1α, ATF3, p53, and α-tubulin. (D) Time course analysis of β-catenin protein levels in Wnt3a-expressing RKO cells cultured in severe hypoxia. Cells were cultured for the indicated time and then harvested for protein. Western blots were probed for β-catenin, α-tubulin, HIF-1α, and p53. (E) Low pH synergizes with hypoxia to inhibit β-catenin stabilization. L Wnt3a cells were cultured for 24 h in the indicated oxygen concentrations in media without bicarbonate with HEPES buffer (pH 7.4) or with PIPES buffer (pH 6.8). Total lysates (30 μg) were analyzed by Western blotting and probed for β-catenin and α-tubulin. (F) Glucose deprivation synergizes with hypoxia to inhibit β-catenin stabilization. L Wnt3a cells were cultured for 24 h in regular media or media without glucose, in the indicated oxygen concentrations. Total lysates were analyzed as described in panel A.
An identical effect of hypoxia on β-catenin was seen in mouse L cells, which are commonly used to study Wnts (32) (Fig. 1B, top panel). Immunofluorescence analysis of these cells also showed that stabilized β-catenin can be detected in the cytoplasm and the nucleus, but only in normoxic conditions (Fig. 1B, bottom panel). Further analysis in RKO cells showed that although hypoxia destabilized β-catenin, p53, HIF-1α, and ATF3 were all stabilized under the same conditions, implying a specific negative regulation of β-catenin by hypoxia and not just toxicity in these culture conditions (Fig. 1C). A more detailed time course analysis of RKO Wnt3a-expressing cells showed that after as little as 8 h hypoxia β-catenin levels have already started to decrease (Fig. 1D).
We then examined the effect of other tumor environment stresses on β-catenin. We examined the effect of either mild acidosis or glucose deprivation in combination with hypoxia on β-catenin in L Wnt3a cells. To achieve this, cells were cultured in media with either HEPES buffer (pH 7.4) or PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] buffer (pH 6.8). Interestingly, low pH (6.8), in combination with very mild hypoxia, could cause a significant decrease in β-catenin levels (Fig. 1D). Likewise, glucose deprivation in combination with mild hypoxia also led to a significant decrease in β-catenin levels (Fig. 1E).
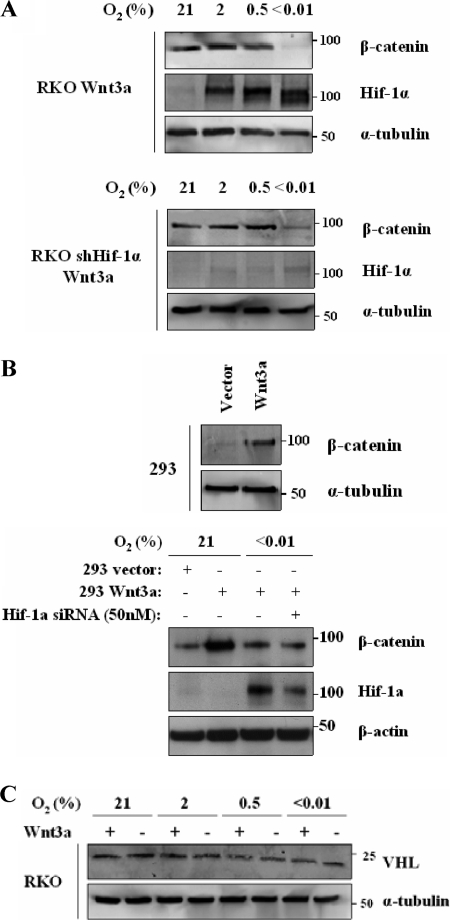
The response to mild hypoxia and recent publications have raised the possibility that HIF-1 might be involved in this effect (15). We therefore directly tested this possibility using RKO cells expressing an shRNA against HIF-1α (26). In these cells without HIF-1α, β-catenin was still downregulated in hypoxia, suggesting that this phenomenon is HIF-1 independent (Fig. 2A). To further confirm HIF-1 independence, we generated 293 cells expressing Wnt3a (Fig. 2B, top panel). Using these cells, we examined β-catenin stability after treatment with siRNA to HIF-1α (Fig. 2B, bottom panel). Here again, downregulation of HIF1 did not affect β-catenin levels. In addition, a recent study has shown that Wnt3a is able to upregulate VHL in 293T cells (10); however, we observed that Wnt3a expression had no effect on VHL levels in RKO cells (Fig. 2C).
FIG. 2.
Hypoxic downregulation of β-catenin is Hif-1 independent. (A) Anoxic downregulation of β-catenin is HIF-1 independent in RKO cells. RKO cells stably expressing Wnt3a and RKO cells expressing Wnt3a and an shRNA against HIF-1α were cultured for 24 h in the indicated oxygen concentrations. Cell lysates (30 μg) were analyzed by Western blotting for β-catenin, HIF-1α and α-tubulin. (B) Hypoxic downregulation of β-catenin is HIF-1-independent in HEK 293 cells. HEK 293 cells stably expressing Wnt3a were generated (top panel). Protein was harvested and fractionated, and cytoplasmic fraction analyzed by Western blotting for β-catenin. Cells stably expressing Wnt3a were transiently transfected with HIF-1α siRNA (50 nM), cultured in normoxia for 48 h, and then cultured in severe hypoxia for an additional 24 h. Cell lysates (30 μg) were analyzed by Western blotting for β-catenin, HIF-1α and β-actin (bottom panel). (C) Neither Wnt3a nor hypoxia affects VHL expression in RKO cells. RKO stably expressing Wnt3a and control vector cells were cultured for 24 h in the indicated oxygen concentrations. Western blots were probed for VHL and α-tubulin.
Hypoxia destabilizes β-catenin through endogenous degradation machinery.
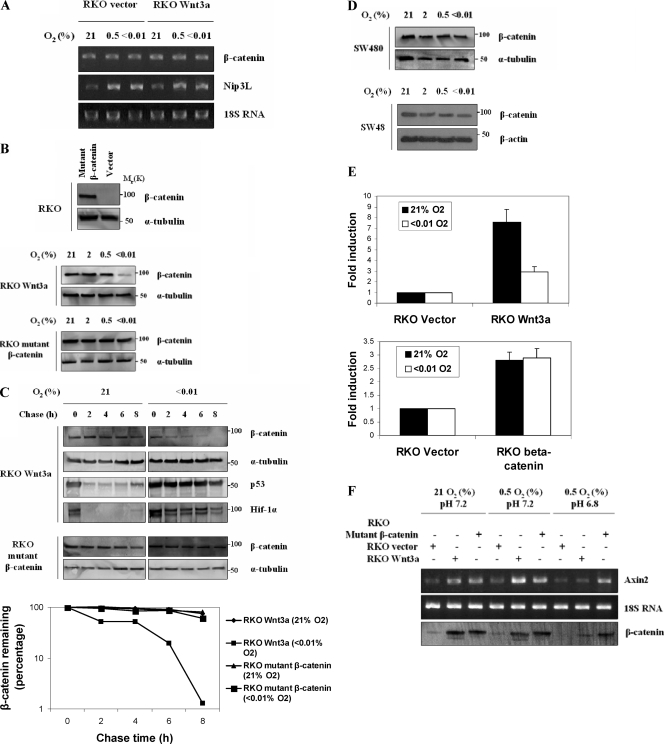
Because we did not see downregulation of β-catenin mRNA in response to severe hypoxia (Fig. 3A), we examined its posttranscriptional regulation for a mechanism responsible for its downregulation. We first generated an RKO cell line expressing a nonphosphorylatable form of β-catenin that is resistant to proteolytic degradation (Fig. 3B, top panel). These cells have high levels of β-catenin in the absence of Wnt stabilization that we found to be resistant to treatment with hypoxia (Fig. 3B). This result suggested that hypoxia was acting to destabilize β-catenin through the action of the endogenous degradation machinery, even in cells expressing Wnt3a. We confirmed this hypothesis with the following two experiments. First, we measured the half-life of β-catenin in normoxic or hypoxic Wnt3a cells treated with cycloheximide. Cells expressing the mutant β-catenin showed an extended half-life irrespective of oxygen concentrations (Fig. 3C).
FIG. 3.
Hypoxic downregulation of β-catenin requires canonical degradation machinery. (A) Hypoxia does not affect β-catenin mRNA expression. RKO Wnt3a-expressing and control vector cells were analyzed by RT-PCR to assess β-catenin mRNA expression. Cells were cultured for 24 h in the indicated oxygen concentrations. Total RNA was isolated, and RT-PCR was used to quantitate mRNA for β-catenin or the hypoxia-responsive gene BNip3L. (B) Hypoxia downregulates wild-type but not mutant β-catenin protein levels. A pRK5-SK-ΔGSK-β-catenin vector was used to stably transfect RKO cells (top panel). RKO Wnt3a cells (middle panel) and RKO mutant (stabilized) β-catenin (bottom panel) were cultured and analyzed as described in Fig. 1A. (C) Hypoxia decreases half-life of wild-type β-catenin but not mutant β-catenin. A cycloheximide decay assay was performed with RKO Wnt3a cells. Cells were cultured in normoxia or severe hypoxia for 14 h, and then cycloheximide (50 μg/ml) was added. The cells were chased for the indicated time points, and total lysates were analyzed by Western blotting. Blots were probed for β-catenin, p53, HIF-1α, and α-tubulin. Quantification of the results for β-catenin is presented. The graph is the average of three independent experiments ± the standard error of the mean. (D) Hypoxia does not affect wild-type β-catenin protein levels in APC mutant cells or in mutant β-catenin cells. SW480 cells were cultured for 24 h in the indicated oxygen concentrations, and cytosolic fractions were analyzed by Western blotting for β-catenin and α-tubulin (top panel). SW48 cells were cultured for 24 h in the indicated oxygen concentrations and cytosolic fractions were analyzed (bottom panel). (E) Transcriptional activity of wild-type β-catenin but not mutant β-catenin is inhibited by hypoxia. RKO cells overexpressing Wnt3a or mutant β-catenin were transiently transfected with SuperTopflash luciferase reporter and after 24 h were cultured in severe hypoxia for an additional 12 h. The reporter activity in cells overexpressing Wnt3a (top panel) and mutant β-catenin (bottom panel) is expressed relative to the activity of control empty vector cells. (F) Hypoxia combined with acidosis inhibit Wnt-induced transcriptional activity of endogenous Axin2, a common Wnt target. RKO cells overexpressing Wnt3a, mutant β-catenin, or control vector cells were cultured for 8 h in 0.5% hypoxia at normal (7.2) or low (6.8) pH. RNA from these cells was analyzed by RT-PCR using primers specific for human Axin2. Western analysis of β-catenin from the same cells is also shown. Note that hypoxia combined with low pH destabilizes wild-type but not mutant β-catenin and inhibits Wnt3a-induced transcriptional activity of Axin2.
The second analysis of hypoxia-mediated β-catenin downregulation was to test its stability in colorectal tumor cells SW480 and SW48. SW480 cells have wild-type β-catenin, but the protein is stabilized due to APC truncation, while SW48 cells have wild-type APC, but a stable, mutant β-catenin (S33Y). As with the mutant β-catenin in the RKO cells, hypoxia did not affect β-catenin protein levels when SW480 or SW48 cytoplasmic fractions were analyzed (Fig. 3D). The resistance of mutant β-catenin to hypoxic downregulation was also evident in reporter assays, in which extreme hypoxia reduced wild-type β-catenin transcriptional activity but not that of the mutant version (Fig. 3E). Also, combination of mild hypoxia and acidosis downregulated wild-type, but not mutant, β-catenin and led to a significant decrease in the levels of endogenous Axin2 mRNA, a common Wnt target (Fig. 3F). These data support the premise that the mechanism of hypoxic downregulation of β-catenin requires the normal cellular degradation machinery, and tumor-derived mutations rendered cells resistant to this effect.
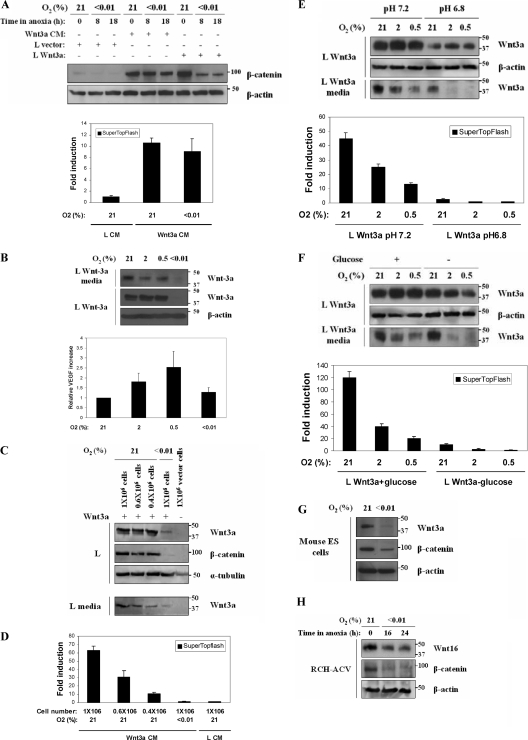
These observations led us to hypothesize that hypoxia might have an impact on β-catenin through an upstream effect at the level of the Wnt growth factor. We next cultured parental L cells in Wnt3a CM and L cells expressing Wnt3a in normoxia and hypoxia and saw that extreme hypoxia could significantly reduce β-catenin levels only in Wnt3a-expressing cells (Fig. 4A, top panel). Moreover, hypoxia did not have a significant effect on TCF-mediated transcription in L reporter cells cultured in Wnt CM (Fig. 4A, bottom panel).
FIG. 4.
Hypoxia downregulates β-catenin through a mechanism that involves the inhibition of Wnt secretion. (A) Wild-type β-catenin stabilization by Wnt3a CM is not affected by hypoxia. L cells overexpressing Wnt3a, control empty vector cells, and L cells cultured in Wnt3a CM were maintained in normoxia or hypoxia for the indicated times. Total lysates were analyzed by Western blotting for β-catenin and β-actin. The activity of β-catenin was also analyzed in L cells by a reporter assay. Wnt3a CM and control L CM were added to SuperTopflash luciferase L cells and were cultured in normoxia or hypoxia for 24 h. Luciferase activity is expressed relative to control media. (B) Hypoxia downregulates intracellular and secreted Wnt3a protein levels. L Wnt3a cells were incubated in the indicated oxygen concentrations for 24 h. Total lysates (30 μg) and culture media were analyzed by Western blotting for Wnt3a and β-actin (top panel). Hypoxia increases VEGF expression in mouse L cells. VEGF quantity in the culture media was determined by solid-phase ELISA (bottom panel). Values are normalized for cell number. (C) Hypoxia downregulates intracellular, secreted levels and activity of Wnt3a protein. The indicated numbers of L Wnt3a cells were incubated in normoxia or anoxia for 24 h. Western blots of lysates were probed for Wnt3a, β-catenin, and α-tubulin. Equal volumes of culture media (30 μl) were also analyzed for secreted Wnt3a. (D) Hypoxia inhibits TCF reporter-luciferase activity in media from cells shown in Fig. 4C. (E) Acidosis synergizes with hypoxia to downregulate intracellular and secreted levels of Wnt3a protein. L Wnt3a cells were cultured in the indicated oxygen concentrations for 24 h in either HEPES buffer (pH 7.4) or PIPES buffer (pH 6.8). Total lysates and culture media from the cells were analyzed by Western blotting for Wnt3a (top panel). At the bottom panel, a reporter assay is shown, using L SuperTopflash reporter cells treated with conditioned media made by the L cells described in top panel. (F) Glucose deprivation synergizes with hypoxia to downregulate intracellular and secreted levels of Wnt3a protein. L Wnt3a cells were cultured in media with or without glucose in the indicated oxygen concentrations. Western blots of lysates and media were probed for Wnt3a (top panel). At the bottom panel, a reporter assay is shown, using L SuperTopflash reporter cells treated with conditioned media made by the L cells described in top panel. (G) Hypoxia downregulates expression of endogenous Wnt3a in ES cells. Murine ES cells were cultured under control or hypoxic conditions for 24 h, and then lysates were blotted for Wnt3a, β-catenin, and β-actin. (H) Hypoxia downregulates expression of endogenous Wnt16. RCH-ACV cells were cultured in normoxia or hypoxia for 16 and 24 h, and lysates were probed for Wnt16, β-catenin, and β-actin.
Hypoxia blocks Wnt protein expression and secretion.
These data suggested that hypoxia might directly modulate the level of Wnt3a secreted into the media and/or the activity of the secreted growth factor. Therefore, we cultured L Wnt3a-expressing cells in different oxygen concentrations for 24 h and observed a profound decrease in intracellular Wnt3a in cells cultured in severe hypoxia (Fig. 4B, top panel), which correlated to a significant reduction in the levels of secreted Wnt3a (Fig. 4B, top panel). In contrast, endogenous VEGF protein was still secreted when measured in the same media (Fig. 4B, bottom panel). To quantitate the secreted Wnt3a, we analyzed the intracellular and secreted Wnt3a levels in cultures containing different cell numbers. These results show a reduction of at least 90% in Wnt3a protein secretion into the media in hypoxia, which was again coupled to a failure of β-catenin stabilization (Fig. 4C). Moreover, media from these cells were analyzed for Wnt3a activity by adding it to reporter L cells and showed only 3% of the activity of media from the normoxic culture (Fig. 4D). Collectively, our data show that severe hypoxia causes a profound decrease in intracellular and secreted Wnt3a protein, which blocks β-catenin from being stabilized. We were not able to detect any Wnt3a protein or activity in the media from the RKO cells (data not shown), possibly due to inefficient secretion commonly observed in a large number of cell lines (7).
In an effort to explore the mechanism behind the hypoxic downregulation of β-catenin in combination with other microenvironmental stresses, we also tested Wnt3a-expressing L cells in low-pH and glucose deprivation conditions. Both intracellular and secreted Wnt3a levels were substantially reduced even in mild hypoxia (Fig. 4E and F). Furthermore, media taken from these cultures also had very little activity when placed on L TOPFlash reporter cells (Fig. 4E and F).
We also tested hypoxia for its effect on the endogenous Wnt3a protein. There are no reported colorectal cell lines that express Wnt3a, so we tested murine embryonic stem (ES) cells. Wnt3a protein is detectable in control ES cell extracts, and it is reduced in cells treated with severe hypoxia (Fig. 4G). We see the biologic significance of this reduction as a loss of β-catenin stability in hypoxic ES cells (Fig. 4G). In addition, we examined the expression of Wnt16 in the ALL cell line RCH-ACV. These cells have been reported to have elevated Wnt16 due to the activity of the fusion protein E2A-PBX that is generated in the transformation of these cells (24). Endogenous Wnt16 and β-catenin are similarly reduced in these cells treated with hypoxia (Fig. 4H).
ER stress blocks Wnt secretion.
Wnt proteins are highly modified posttranslationally. Wnt proteins have many conserved cysteines necessary for proper peptide folding, they also require N glycosylation and acylation for their full biologic activity (25, 33, 36) (Fig. 5). Hypoxia may interfere with the machinery responsible for the posttranslational modifications and therefore reduce the efficiency of Wnt secretion.
FIG. 5.
Multiple species alignment of Wnt3a. Human (hWnt3a) mouse (mWnt3a), zebra fish (zWnt3a) Wnt3a, and Wingless (D. melanogaster) protein sequences were aligned. Asterisks denote identical amino acids, while double dots represent similar amino acids. The Wnt3a N-glycosylation sites are indicated by “#.” Conserved cysteines are shown in boldface. The Wnt3a cysteine that is palmitoylated is indicated by “@.”
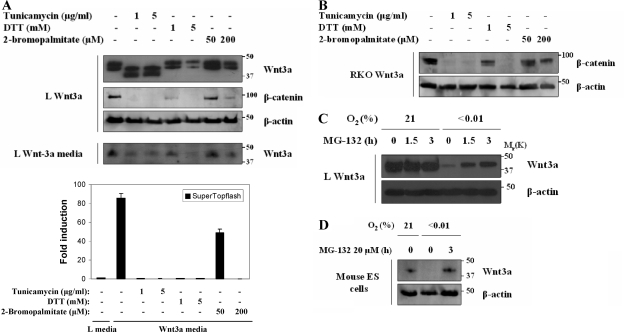
We examined the impact of chemically blocking each of the three major forms of modification on Wnt3a expression using tunicamycin to inhibit N glycosylation, DTT to inhibit disulfide bond formation, and 2-bromopalmitate to inhibit palmitolylation. Wnt3a-expressing cells were exposed to the indicated concentrations of inhibitors, and then we determined the intracellular Wnt3a, the secreted Wnt3a, and the biologic activity of the secreted Wnt3a (using reporter cells). Although all three drugs could inhibit the amount and activity of the Wnt3a in the media (Fig. 6A), only the treatment with DTT showed a significant decrease in the amount of intracellular Wnt3a (Fig. 6A and B). Hypoxia has been shown to generate ER stress (34) through the accumulation of malfolded proteins. Proteins with incorrect disulfide bonds accumulate in the ER until they undergo retrograde transport to the cytoplasm for proteasomal degradation (13). If this were the case for Wnt3a, then treatment of cells with a proteasome inhibitor would result in an accumulation of Wnt3a only in hypoxic cultures. This is indeed what we determined for L cells treated with hypoxia and MG-132 (Fig. 6C) and for murine ES cells expressing endogenous Wnt3a (Fig. 6D).
FIG. 6.
ER stress blocks Wnt3a expression and activity. (A) L Wnt3a cells were treated with the indicated amounts of tunicamycin, DTT, or 2-bromopalmitate for 24 h. Cell lysates were analyzed by Western blotting for Wnt3a, β-catenin, and β-actin. Culture media from these cells were analyzed for secreted Wnt3a. Media from these cells were collected and used to treat a culture of L SuperTopflash luciferase cells. All media showed significant reduction in TCF-mediated transcription. (B) RKO Wnt3a cells were treated with tunicamycin, DTT, or bromopalmitate as described in panel A. Lysates were probed for β-catenin and β-actin. (C) Inhibition of the proteasome leads to the accumulation of intracellular Wnt3a in hypoxia. L Wnt3a cells were cultured in normoxia or anoxia for 18 h and then treated with MG-132 for 1.5 and 3 h. Levels of Wnt3a and β-actin proteins were analyzed by Western blotting. (D) Murine ES cells were treated with hypoxia for 18 h, followed by proteasome inhibitor for 3 h. Endogenous Wnt3a is degraded by the proteasome in hypoxia.
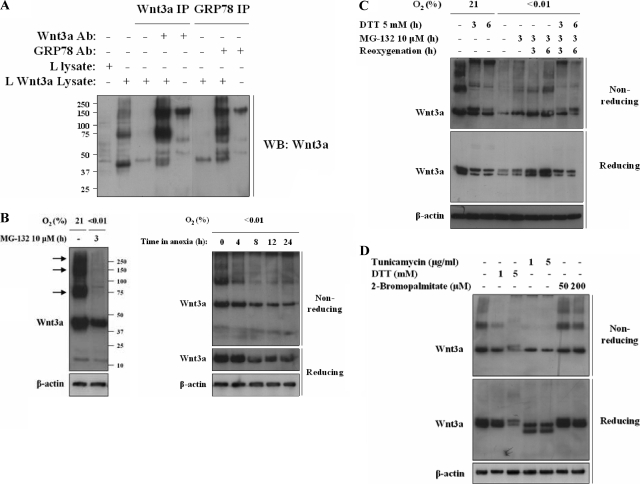
To investigate the folding intermediates involved in proper disulfide bond formation, we examined Wnt3a electrophoretic migration under denaturing, but nonreducing conditions. Enzymes of the protein disulfide isomerase (PDI) family (9) can form metastable disulfide-based intermediates with their cargo proteins during disulfide bond formation (31). Extraction of cellular proteins with buffer containing NEM blocks the oxidation of any existing disulfide bonds and allows examination of these intermediates. Under nonstressed conditions Wnt3a forms large, slowly migrating folding intermediates (Fig. 7B, left panel). The specificity of these intermediates was demonstrated by immunoprecipitation using an anti-Wnt3a antibody (Fig. 7A). Large Wnt folding intermediates have been reported in nonreducing conditions (7). These bands could represent Wnt3a covalently attached to members of the PDI family of proteins (9) and are completely lost in cells treated with 24 h of hypoxia (Fig. 7B, left panel). A time course of extracts made from cells treated with hypoxia shows that these intermediates are lost within 4 h (Fig. 7B, right panel). Treatment of these extracts with DTT in vitro caused all of the slowly migrating bands collapse to a single monomeric Wnt3a band (Fig. 7B, C, and D).
FIG. 7.
Hypoxia causes loss of Wnt3a folding intermediates. (A) Immunoprecipitation of GRP78 and Wnt3a in Wnt3a-expressing cells captures folding intermediates. Wnt3a folding intermediates immunoprecipitate with Wnt3a antibody in control (normoxic) conditions (lane 4). Immunoprecipitation of GRP78 captures Wnt3a folding intermediates (lane 7). Precipitates run in nonreducing conditions. An ∼150-kDa band is detected when either Wnt3a or GRP78 antibodies alone are analyzed in nonreducing conditions (lanes 5 and 8, respectively). (B) Wnt3a expressing L cells were treated with control or 24 h hypoxia and 3 h proteasome inhibitor, and lysates were collected in buffer containing NEM. Proteins were run in nonreducing conditions to visualize folding intermediates and probed for Wnt3a and β-actin (left panel). The time course of extracts of Wnt3a-expressing cells treated with hypoxia and run in nonreducing and reducing conditions was determined. Blots were probed for Wnt3a and β-actin as indicated (right panel). Note the loss of slower-migrating forms of Wnt3a within 4 h of hypoxia. (C) Treatment of Wnt3a-expressing L cells with DTT, hypoxia, and reoxygenation and proteasome inhibitor as indicated. Lysates were run in nonreducing or reducing gels as indicated. Note the loss of slower-migrating forms of Wnt3a with treatment by DTT or hypoxia and the recovery of bands within 3 h of reoxygenation. (D) Treatment of Wnt3a-expressing cells with tunicamycin, DTT, or 2-bromopalmitate as indicated. Lysates were run in nonreducing and reducing gels. Note the loss of slower-migrating forms of Wnt3a after treatment with either DTT or tunicamycin but not after treatment with 2-bromopalmitate.
In order to better understand what these large intermediates represented, we analyzed their formation in cells treated with DTT, hypoxia, and reoxygenation (Fig. 7C). We find that these intermediates are lost within 3 h in cells treated with DTT (Fig. 7C). Furthermore, they are formed again within 3 h in cells that are reoxygenated. However, cell reoxygenation in the presence of DTT cannot reform these intermediates. Similarly, we find loss of the intermediates in cells treated with tunicamycin but not in bromopalmitate-treated cells (Fig. 7D). These findings suggest that glycosylation is necessary for correct disulfide bond formation but that lipidation is not.
Wnt interaction with ER chaperone GRP78 is lost in hypoxia.
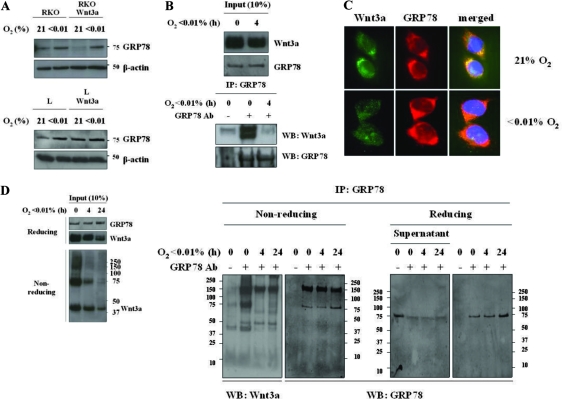
One of the major facilitators of ER function is the molecular chaperone GRP78/BiP, a member of the Hsp70 family of heat shock proteins. A proportion of overexpressed Wnt1 was reported to bind to GRP78/BiP (16), although the exact reason for this interaction was not defined. Furthermore, GRP78 is induced by hypoxia in a number of cell lines (8, 17). We found a significant induction of GRP78 in RKO cells and a slight induction in L cells by hypoxia, independent of Wnt3a expression (Fig. 8A). To determine a functional interaction between GRP78 and Wnt3a, we immunoprecipitated GRP78 and probed the precipitates for Wnt3a. We find that there is a robust interaction between the two proteins in control conditions, but this interaction is lost within 4 h of hypoxia (Fig. 8B).
FIG. 8.
Hypoxia causes loss of interaction between ER chaperone GRP78 and Wnt3a. (A) Hypoxia upregulates GRP78 protein levels. Wnt3a-expressing L and RKO cells or parental cells were grown in normoxia or hypoxia for 24 h. Lysates were probed for ER chaperone GRP78. (B) Immunoprecipitate of GRP78 captures Wnt3a in control extracts but not in extracts from hypoxic cells. Note that the input material shows no reduction in total amount of Wnt3a. (C) Immunolocalization of both GRP78 and Wnt3a in L Wnt3a cells exposed to control or 24 h of hypoxia. Wnt3a staining is greatly reduced in hypoxia, while GRP78 staining continues to identify punctuate cytoplasmic structures. (D) Immunoprecipitation of GRP78 in Wnt3a-expressing cells captures folding intermediates. An aliquot of input material is shown in the left panel. Precipitates were run in reducing and nonreducing conditions. Wnt3a folding intermediates immunoprecipitate with GRP78 in control conditions but not after 4 h of hypoxia.
Although GRP78 does not covalently attach to client proteins, it can form higher-order complexes. We next tested the GRP immunoprecipitates in nonreducing conditions in order to see whether the GRP78-Wnt3a interactions were with the monomeric Wnt3a protein or with the Wnt3a in the larger intermediates. Figure 7A and 8D show that by running the GRP78 precipitates under nonreducing conditions the Wnt3a that precipitates with GRP78 contains the slower-migrating forms. All of the interactions with Wnt3a are lost within 4 h of treatment with hypoxia. Lastly, immunostaining of Wnt3a-expressing L cells cultured in normoxia showed that Wnt3a and GRP78 colocalized in cytoplasmic structures resembling ER (Fig. 8C). However, in L Wnt3a cells cultured in hypoxia, Wnt3a staining intensity was reduced, and its pattern was much more diffuse (Fig. 8C). These results support the model that GRP78 facilitates the formation of correct disulfide bonds within Wnt3a under control conditions. This interaction is lost under hypoxia, and therefore the Wnt3a protein is not able to fold properly, targeting it for proteasomal degradation.
Mutant β-catenin is more effective than Wnt3a at protecting cells against hypoxic stress in vitro and offers model tumors a growth advantage in vivo.
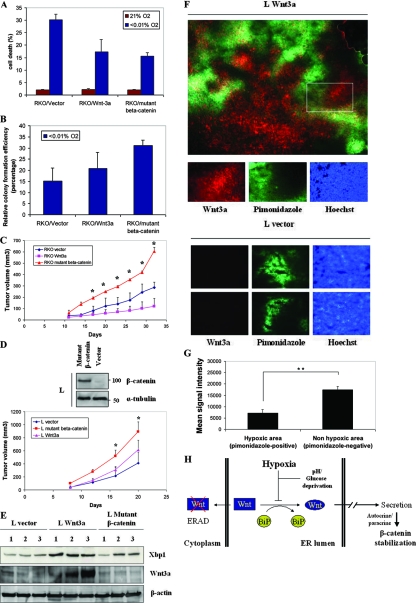
To explore the cellular significance of the hypoxic downregulation of β-catenin, we performed survival assays using RKO tumor cells, Wnt3a RKO and mutant β-catenin RKO cells. In an acute survival assay using trypan blue exclusion, cells expressing the Wnt3a, or mutant β-catenin showed increased survival in severe hypoxic conditions. Although all cells were >95% viable at 24 h of treatment, there was significant toxicity by 48 h (Fig. 9A). These cells were also tested by clonogenic assay in which cells were cultured in normoxia or severe hypoxia for 48 h, and then they were moved to normoxic conditions for colony formation. The mutant β-catenin provided greater survival than Wnt3a in hypoxia, a finding consistent with the finding that this protein is resistant to hypoxic downregulation (Fig. 9B).
FIG. 9.
Stabilization of β-catenin by mutation provides an in vitro and vivo growth advantage compared to Wnt3a-mediated stabilization. (A) Stabilization of β-catenin diminishes cell death levels under severe hypoxia. RKO Wnt3a cells and RKO β-catenin and control cells with empty vector were cultured in normoxia and anoxia for 48 h. Cells were stained with trypan blue, and the viable percentage was calculated in triplicate experiments. (B) Mutant β-catenin increases colony formation efficiency under hypoxia. A total of 300 RKO Wnt3a, RKO β-catenin, and RKO control cells with empty vector were plated, cultured in anoxia for 48 h, and then moved into normoxia for 12 days. Colonies were stained with crystal violet and counted in triplicate experiments. (C) Mutant β-catenin increases RKO xenograft tumor growth. RKO empty vector, RKO Wnt3a, and RKO mutant β-catenin cells (5 × 106) were injected subcutaneously into the flanks of nude mice. Tumor volumes were calculated every 4 days with calipers. The average of eight tumors from each cell line in triplicate experiments was determined. Statistical analysis showed that from day 17 onward there is a significant growth advantage (*, P < 0.05) in tumors expressing mutant β-catenin versus Wnt3a. (D) Mutant β-catenin increases L xenograft tumor growth. A pRK5-SK-ΔGSK-β-catenin vector was used to stably transfect L cells (top panel). L Wnt3a and L mutant β-catenin cells (5 × 106) were injected subcutaneously into the flanks of nude mice. Tumor volumes were calculated every 4 days (bottom panel). The average of eight tumors from each cell line in triplicate experiment was determined (*, P < 0.05). (E) ER stress conditions were detected in L Wnt3a xenografts. Lysates from L Wnt3a, L mutant β-catenin, and L vector tumors (30 μg) were probed for the spliced form of Xbp1 protein, Wnt3a, and β-actin. (F) Wnt3a expression is significantly reduced in hypoxic regions of tumors. L Wnt3a cells were grown as tumors and treated with pimonidazole prior to sacrifice. Sections were stained for hypoxic regions (pimonidazole positive) and Wnt3a and overlaid to determine colocalization (top panel). Pictures with Wnt3a (red), pimonidazole (green), and Hoechst (blue) staining, which are shown at a higher magnification, represent the rectangular area shown at the top panel. Tumor sections from L vector mouse xenografts are shown as a negative control for Wnt3a staining (bottom panel). (G) Quantification of Wnt3a signal in hypoxic and nonhypoxic regions in L Wnt3a mouse xenografts. The intensity of Wnt3a staining was analyzed in pimonidazole-positive and pimonidazole-negative tumor regions. The graph represents the quantification of the staining intensity from three different section fields from each of three different tumors. **, P < 0.01. (H) Model for the hypoxic inhibition of Wnt3a secretion. Severe hypoxia inhibits the formation of disulfide bonds in Wnt by disrupting the interaction with GRP78. As a result, Wnt is retained in the ER, targeted for degradation by the proteasome, and unable to stabilize β-catenin.
We next studied the effect of Wnt3a and mutant β-catenin on tumor growth in vivo where all of the stresses of the tumor microenvironment exist. We injected parental RKO, Wnt3a- and mutant β-catenin-expressing cells for xenograft formation in nude mice. Expression of stabilized mutant β-catenin increased tumor growth relative to the control vector cells, while expression of Wnt3a caused decreased growth (Fig. 9C). A possible explanation for this result would be that expression of Wnt3a promotes chronic ER stress, which negatively affects cell growth in vivo (30). We also found a similar effect in the L cells expressing mutant β-catenin (Fig. 9D, top panel) versus Wnt3a, grown as xenografts (Fig. 9D, bottom panel). L-Wnt3a tumors showed elevated levels of Xbp1 splicing, a finding consistent with increased ER stress (Fig. 9E).
Finally, we examined sections of the tumors grown from the Wnt3a expressing L cells for both expression of Wnt3a and hypoxia (using the marker drug pimonidozole). In the top panel of Fig. 9F, we show a representative whole section of the tumor stained for both hypoxic regions (pimonidozole positive) and well-oxygenated regions (pimonidozole negative). While not a perfect inverse relationship exists, there is reduced expression of Wnt3a staining in hypoxic regions (green), with robust Wnt3a expression in the pimonidozole-negative sections. L vector tumors have been used as negative controls for Wnt3a staining (Fig. 9F, bottom panel). Quantification of Wnt3a expression in hypoxic and nonhypoxic tumor regions is shown in Fig. 9G.
DISCUSSION
Severe hypoxia has been shown to block tumor cell proliferation and eventually leads to cellular death (27). However, tumor hypoxia can also predict for a more aggressive clinical outcome. To reconcile these two observations, hypoxia has been proposed to act as a selective pressure that kills the sensitive clonogens and leaves only the most resistant. Harsh tumor microenvironmental conditions, such as hypoxia, present a significant selective force on the tumor favoring an increase in the invasive potential of the tumor (1). In model tumors, hypoxia has been shown to select for cells that have lost p53 expression or gained bcl2 expression (12). Here we show that tumor cells with an activated β-catenin pathway have increased survival in severe hypoxia, and they grow faster in model tumors (Fig. 9). Although the survival advantage of activating β-catenin in tumors has been presented in the literature (37), the ability of hypoxia to selectively block this signal has not been recognized. These results might also provide a possible explanation for why colorectal tumors have frequent activating mutations in APC or β-catenin, while it is not common to find activating or overexpression mutations in any of the Wnt genes. However, other possibilities exist to explain this discrepancy, such as noncanonical signaling that can be activated from secreted Wnt ligands.
Synthesis, modification, and secretion of Wnt proteins are not well understood processes. Wnt proteins are very hydrophobic molecules that are secreted inefficiently by many cell lines and tend to associate with the cell membrane and the extracellular matrix. Several studies using Wingless, the Wnt1 ortholog of Drosophila, and also mammalian Wnts, such as Wnt1 and Wnt3a, have revealed a variety of posttranslational modifications of these proteins, including disulfide bond formation, N glycosylation, and acylation by palmitate and the monounsaturated fatty acid palmitoleic (33, 36). Although more information regarding the Wnt posttranslational modifications accumulates, little is known about the proteins that are directly or indirectly involved in these modifications, especially in the ER. Increasing evidence suggests that porcupine, an ER protein and member of the membrane-bound O-acyltransferase family, is responsible for lipid modification of Wnts affecting their transport and secretion. However, the role of these modifications and the components involved in the ER processing of Wnts are still largely unknown. Likewise, there is a lack of information regarding the transport from ER and secretion of Wnts. Recently, the discovery of the transmembrane protein Wntless (Wls)/Eveness interrupted (Evi)/Mom-3 has added exciting new perspectives to this issue (2, 4).
Wnt proteins have by definition a large number of highly conserved cysteine residues. The conservation of both number and spacing of Wnt cysteines suggests that these residues are necessary for the proper formation of intramolecular disulfide bonds. The importance of these cysteine residues has been demonstrated by the inability of different Wnt1 cysteine mutants to induce transformation of C57MG cells (22). Molecular oxygen is necessary for the proper formation of disulfide bonds of proteins processed in the ER (35). This process of oxidative folding is performed through the function of specific foldases, such as PDI, which gets its oxidizing equivalents through FADH2 dependent donor Ero1 (23). PDI and Ero1 are both hypoxia-inducible and are required for growth in severe hypoxia. With the use of the sulfhydryl group-alkylating reagent NEM and electrophoretic analysis under denaturing, but nonreducing conditions we observed the existence of slow-migrating Wnt3a folding intermediates. These intermediates are due to transient disulfide bonds, since DTT is able to disrupt them. Severe hypoxia has a profound effect on the formation of these intermediates within 4 h, while the total amount of Wnt3a protein is not significantly reduced. This suggests that hypoxia first inhibits the normal protein folding leading to the formation of aggregates of the malfolded protein and then their eventual destruction.
The formation of disulfide bonds is just one element of the posttranslational modifications that must occur to the Wnt proteins before they can be secreted in the active form (20). It is not clear whether there is an order to the modifications, or whether they are modified simultaneously. For example, while blocking glycosylation can inhibit some of the folding intermediates it does not cause the protein to be degraded (Fig. 6A). Conversely, blocking palmitoylation does not inhibit disulfide bond formation, it does reduce Wnt3a activity, as has been reported (Fig. 6A) (25). The mechanisms responsible for quality control within the ER are just now being identified (18). Multiple lines of evidence suggest that the ER contains several molecular chaperones able to recognize distinct features on nascent proteins. These chaperones also prevent the formation of protein aggregates due to the interaction of hydrophobic patches within the unfolded proteins. Loss of interaction between nascent Wnts and GRP78 could lead to severe interruption of normal protein folding. The malfolded Wnt proteins would form aggregates resulting in more ER stress and degradation by ERAD. Hypoxia could have a similar effect upon other secreted growth factors, especially those with a large number of disulfide bonds. Blocking secretion of additional growth factors could also modify proliferation and survival ion the tumor.
In conclusion, we have shown that hypoxia alone or in combination with other tumor stresses can downregulate wild-type, but not mutant, β-catenin. The mechanism of this phenomenon involves inhibition of Wnt protein secretion due to ER stress. We favor a model in which GRP78 is necessary for proper folding of Wnt3a, and under conditions of ER stress, that there is a competition for GRP78 to fold both Wnt and all of the other proteins within the ER (Fig. 9H). Cells with APC or β-catenin mutations would have a growth advantage in vivo by bypassing this hypoxic downregulation of Wnt.
Acknowledgments
We are grateful to Roel Nusse, Michael Cleary, and Albert Koong for providing various reagents and cell lines; Costas Koumenis for the NEM protocol; and Sophia Chernikova for technical help with the mouse ES cells.
This study was supported by funding from the NCI (N.C.D.).
Footnotes
Published ahead of print on 29 September 2008.
REFERENCES
- 1.Anderson, A. R., A. M. Weaver, P. T. Cummings, and V. Quaranta. 2006. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell 127905-915. [DOI] [PubMed] [Google Scholar]
- 2.Banziger, C., D. Soldini, C. Schutt, P. Zipperlen, G. Hausmann, and K. Basler. 2006. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125509-522. [DOI] [PubMed] [Google Scholar]
- 3.Barth, A. I., D. B. Stewart, and W. J. Nelson. 1999. T-cell factor-activated transcription is not sufficient to induce anchorage-independent growth of epithelial cells expressing mutant beta-catenin. Proc. Natl. Acad. Sci. USA 964947-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartscherer, K., N. Pelte, D. Ingelfinger, and M. Boutros. 2006. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125523-533. [DOI] [PubMed] [Google Scholar]
- 5.Bi, M., C. Naczki, M. Koritzinsky, D. Fels, J. Blais, N. Hu, H. Harding, I. Novoa, M. Varia, J. Raleigh, D. Scheuner, R. J. Kaufman, J. Bell, D. Ron, B. G. Wouters, and C. Koumenis. 2005. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 243470-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, J. M., and A. J. Giaccia. 1998. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 581408-1416. [PubMed] [Google Scholar]
- 7.Burrus, L. W., and A. P. McMahon. 1995. Biochemical analysis of murine Wnt proteins reveals both shared and distinct properties. Exp. Cell Res. 220363-373. [DOI] [PubMed] [Google Scholar]
- 8.Denko, N. C., L. A. Fontana, K. M. Hudson, P. D. Sutphin, S. Raychaudhuri, R. Altman, and A. J. Giaccia. 2003. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene 225907-5914. [DOI] [PubMed] [Google Scholar]
- 9.Ellgaard, L., and L. W. Ruddock. 2005. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 628-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giles, R. H., M. P. Lolkema, C. M. Snijckers, M. Belderbos, P. van der Groep, D. A. Mans, M. van Beest, M. van Noort, R. Goldschmeding, P. J. van Diest, H. Clevers, and E. E. Voest. 2006. Interplay between VHL/HIF1α and Wnt/β-catenin pathways during colorectal tumorigenesis. Oncogene 253065-3070. [DOI] [PubMed] [Google Scholar]
- 11.Giles, R. H., J. H. van Es, and H. Clevers. 2003. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 16531-24. [DOI] [PubMed] [Google Scholar]
- 12.Graeber, T. G., C. Osmanian, T. Jacks, D. E. Housman, C. J. Koch, S. W. Lowe, and A. J. Giaccia. 1996. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 37988-91. [DOI] [PubMed] [Google Scholar]
- 13.Haynes, C. M., E. A. Titus, and A. A. Cooper. 2004. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell 15767-776. [DOI] [PubMed] [Google Scholar]
- 14.Jiang, Y. A., L. F. Fan, C. Q. Jiang, Y. Y. Zhang, H. S. Luo, Z. J. Tang, D. Xia, and M. Wang. 2003. Expression and significance of PTEN, hypoxia-inducible factor-1 alpha in colorectal adenoma and adenocarcinoma. World J. Gastroenterol. 9491-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaidi, A., A. C. Williams, and C. Paraskeva. 2007. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat. Cell Biol. 9210-217. [DOI] [PubMed] [Google Scholar]
- 16.Kitajewski, J., J. O. Mason, and H. E. Varmus. 1992. Interaction of Wnt-1 proteins with the binding protein BiP. Mol. Cell. Biol. 12784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koong, A. C., E. A. Auger, E. Y. Chen, and A. J. Giaccia. 1994. The regulation of GRP78 and messenger RNA levels by hypoxia is modulated by protein kinase C activators and inhibitors. Radiat Res. 138S60-S63. [PubMed] [Google Scholar]
- 18.Kostova, Z., Y. C. Tsai, and A. M. Weissman. 2007. Ubiquitin ligases, critical mediators of endoplasmic reticulum-associated degradation. Semin. Cell Dev. Biol. 18770-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koumenis, C., and B. G. Wouters. 2006. “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol. Cancer Res. 4423-436. [DOI] [PubMed] [Google Scholar]
- 20.Kurayoshi, M., H. Yamamoto, S. Izumi, and A. Kikuchi. 2007. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem. J. 402515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan, C. Y., and R. Nusse. 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20781-810. [DOI] [PubMed] [Google Scholar]
- 22.Mason, J. O., J. Kitajewski, and H. E. Varmus. 1992. Mutational analysis of mouse Wnt-1 identifies two temperature-sensitive alleles and attributes of Wnt-1 protein essential for transformation of a mammary cell line. Mol. Biol. Cell 3521-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May, D., A. Itin, O. Gal, H. Kalinski, E. Feinstein, and E. Keshet. 2005. Ero1-L alpha plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene 241011-1020. [DOI] [PubMed] [Google Scholar]
- 24.Mazieres, J., L. You, B. He, Z. Xu, A. Y. Lee, I. Mikami, F. McCormick, and D. M. Jablons. 2005. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene 245396-5400. [DOI] [PubMed] [Google Scholar]
- 25.Mikels, A. J., and R. Nusse. 2006. Wnts as ligands: processing, secretion, and reception. Oncogene 257461-7468. [DOI] [PubMed] [Google Scholar]
- 26.Papandreou, I., R. A. Cairns, L. Fontana, A. L. Lim, and N. C. Denko. 2006. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3187-197. [DOI] [PubMed] [Google Scholar]
- 27.Papandreou, I., C. Krishna, F. Kaper, D. Cai, A. J. Giaccia, and N. C. Denko. 2005. Anoxia is necessary for tumor cell toxicity caused by a low-oxygen environment. Cancer Res. 653171-3178. [DOI] [PubMed] [Google Scholar]
- 28.Pedram, M., C. N. Sprung, Q. Gao, A. W. Lo, G. E. Reynolds, and J. P. Murnane. 2006. Telomere position effect and silencing of transgenes near telomeres in the mouse. Mol. Cell. Biol. 261865-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 141837-1851. [PubMed] [Google Scholar]
- 30.Romero-Ramirez, L., H. Cao, D. Nelson, E. Hammond, A. H. Lee, H. Yoshida, K. Mori, L. H. Glimcher, N. C. Denko, A. J. Giaccia, Q. T. Le, and A. C. Koong. 2004. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 645943-5947. [DOI] [PubMed] [Google Scholar]
- 31.Schwaller, M., B. Wilkinson, and H. F. Gilbert. 2003. Reduction-reoxidation cycles contribute to catalysis of disulfide isomerization by protein-disulfide isomerase. J. Biol. Chem. 2787154-7159. [DOI] [PubMed] [Google Scholar]
- 32.Shibamoto, S., K. Higano, R. Takada, F. Ito, M. Takeichi, and S. Takada. 1998. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells 3659-670. [DOI] [PubMed] [Google Scholar]
- 33.Takada, R., Y. Satomi, T. Kurata, N. Ueno, S. Norioka, H. Kondoh, T. Takao, and S. Takada. 2006. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell 11791-801. [DOI] [PubMed] [Google Scholar]
- 34.Tu, B. P., S. C. Ho-Schleyer, K. J. Travers, and J. S. Weissman. 2000. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science 2901571-1574. [DOI] [PubMed] [Google Scholar]
- 35.Tu, B. P., and J. S. Weissman. 2002. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell 10983-994. [DOI] [PubMed] [Google Scholar]
- 36.Willert, K., J. D. Brown, E. Danenberg, A. W. Duncan, I. L. Weissman, T. Reya, J. R. Yates III, and R. Nusse. 2003. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423448-452. [DOI] [PubMed] [Google Scholar]
- 37.Woodward, W. A., M. S. Chen, F. Behbod, M. P. Alfaro, T. A. Buchholz, and J. M. Rosen. 2007. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc. Natl. Acad. Sci. USA 104618-623. [DOI] [PMC free article] [PubMed] [Google Scholar]