Abstract
Human AP-endonuclease (APE1/Ref-1), a central enzyme involved in the repair of oxidative base damage and DNA strand breaks, has a second activity as a transcriptional regulator that binds to several trans-acting factors. APE1 overexpression is often observed in tumor cells and confers resistance to various anticancer drugs; its downregulation sensitizes tumor cells to such agents. Because the involvement of APE1 in repairing the DNA damage induced by many of these drugs is unlikely, drug resistance may be linked to APE1's transcriptional regulatory function. Here, we show that APE1, preferably in the acetylated form, stably interacts with Y-box-binding protein 1 (YB-1) and enhances its binding to the Y-box element, leading to the activation of the multidrug resistance gene MDR1. The enhanced MDR1 level due to the ectopic expression of wild-type APE1 but not of its nonacetylable mutant underscores the importance of APE1's acetylation in its coactivator function. APE1 downregulation sensitizes MDR1-overexpressing tumor cells to cisplatin or doxorubicin, showing APE1's critical role in YB-1-mediated gene expression and, thus, drug resistance in tumor cells. A systematic increase in both APE1 and MDR1 expression was observed in non-small-cell lung cancer tissue samples. Thus, our study has established the novel role of the acetylation-mediated transcriptional regulatory function of APE1, making it a potential target for the drug sensitization of tumor cells.
The mammalian AP-endonuclease APE1 is a ubiquitous and remarkably multifunctional protein. It plays a central role in the base excision repair (BER) pathway for repairing damaged bases and DNA single-strand breaks induced by reactive oxygen species and alkylating agents and also repairing AP sites that are generated spontaneously or after the excision of oxidized and alkylated bases by DNA glycosylases (12, 40). APE1 was also shown to incise DNA 5′ to oxidatively damaged bases including 5,6-dihydrothymidine and alpha-2′-deoxyadenosine in the nucleotide incision repair pathway (11, 21). The deletion of the N-terminal 61 amino acid residues, which are dispensable for the AP-endonuclease activity, affected its incision activity as well (21, 28). Moreover, the ability of APE1 to incise DNA 5′ to a bulky exocyclic adduct such as p-benozoquinone has also been reported (22). Besides its repair function, mammalian APE1 has two unique and apparently distinct transcriptional regulatory activities. It was independently identified as a reductive activator of c-Jun in vitro and named Ref-1 (62). Subsequently, several other transcription factors (including p53, NF-κB, hypoxia-inducible factor 1-α, PAX5, and PAX8) were also shown to be activated by APE1, presumably via the same redox process (13, 31, 55). A third and distinct function of APE1 as a trans-acting factor was discovered when APE1 was identified as being one of the regulatory proteins that binds to the negative Ca2+ response elements (nCaRE-A and -B) in the Ca2+-dependent downregulation of the parathyroid hormone gene (48) and subsequently in the human renin gene (17). We later showed that human APE1 is acetylated at Lys6 and Lys7 by the histone acetyltransferase p300, both in vivo and in vitro, and that acetylation enhances APE1's binding to nCaRE-B, leading to the repression of the parathyroid hormone promoter (2). Our follow-up studies indicated that a small but significant fraction of APE1 in HeLa cells and mouse liver is present in the acetylated form (14, 56). Recently, we have shown that the early growth response protein (Egr-1)-mediated activation of phosphoinositol phosphatase and tensin homologue (PTEN) is dependent on APE1 acetylation (14).
Although APE1 heterozygous mice are viable and appear to be normal, APE1 nullizygous mice show early embryonic lethality (37, 39, 63). We recently showed that APE1 inactivation induced apoptosis in mouse embryo fibroblasts conditionally nullizygous for endogenous APE1, an effect that could be prevented by the ectopic expression of human APE1 (27). Using a complementation assay, we also showed that both the repair activity and acetylation-mediated transcriptional regulatory functions of APE1 are required to prevent the apoptosis of APE1-null mouse embryo fibroblasts. The unexpected essentiality of APE1's regulatory function suggests that APE1 is a coregulator of many critical genes.
Resistance to many common anticancer drugs often occurs due to the enhanced expression of ATP-binding cassette (ABC) transporter proteins (6, 20). Among the ABC transporters, P glycoprotein/MDR1, the product of the multidrug resistance gene MDR1, mediates resistance to a wide variety of anticancer agents. Tumor cells frequently develop drug resistance after initial chemotherapy due to the elevated level of MDR1. MDR1 expression is often upregulated due to promoter activation after drug treatment (8, 36). The Y box (inverted CCAAT box) was identified as being one of the key cis elements in the MDR1 promoter that binds both Y-box-binding protein 1 (YB-1) and NF-Y (33, 45, 46). YB-1 is ubiquitously expressed in human tissues, and it is overexpressed in many human cancer cell lines that are resistant to anticancer agents (35, 45). YB-1 is normally localized in the cytoplasm; however, treatment with anticancer drugs, hypothermia, and UV light induces its rapid translocation to the nucleus, where its binding to the Y box leads to the activation of the MDR1 gene (35, 57). Immunostaining analyses of various human cancers including breast cancer and osteosarcoma further supported these observations and showed that the elevated nuclear level of YB-1 is closely associated with the acquisition of MDR1-mediated multidrug resistance (1, 18, 43). It is thus evident that YB-1 plays a central role in the enhanced drug resistance of tumor cells by activating the MDR1 gene.
APE1 is often overexpressed in tumor cells, and its altered level or intracellular distribution has been found in various cancers (13, 34, 51, 64). We recently showed that wild-type (WT) but not mutant p53 is a negative regulator of APE1 expression in tumor cells (66). A high level of APE1 expression is also associated with tumor cell resistance to chemo- and radiotherapy and poor prognoses (4, 15, 51), and APE1 downregulation induces apoptosis and also sensitizes cancer cells to chemotherapy (27, 52, 60). Resistance to many anticancer drugs that induce cytotoxic lesions could be due to the enhanced repair of such lesions. Because APE1 is involved primarily in the DNA BER pathway responsible for the removal of small base adducts, the enhanced resistance of tumor cells to drugs such as cisplatin or etoposide, which induce lesions that are not repaired via the BER or nucleotide incision repair pathway, could not be explained by a causal relationship between APE1 overexpression and drug resistance. We addressed this paradox by analyzing APE1-interacting proteins in vivo. We have shown in this study that APE1 stably interacts with YB-1. Acetylated APE1 (AcAPE1) shows enhanced YB-1 binding and activates MDR1 gene expression. The activation of MDR1 expression by the ectopic expression of WT APE1, but not with its nonacetylable mutant, underscores the importance of this modification in APE1's ability to act as a coactivator. We also demonstrate that the downregulation of APE1 sensitizes MDR1-overexpressing tumor cells to anticancer drugs such as doxorubicin and cisplatin, which suggests a molecular basis by which APE1's transcriptional function and YB-1 could be linked to induce the drug resistance of tumor cells. This is further supported by our observations showing significant correlation between APE1 and MDR1 levels in many lung cancer cells.
MATERIALS AND METHODS
Cell culture and transfection.
The human colon carcinoma line HCT116 (with WT p53, a gift from B. Vogelstein, Johns Hopkins University) was cultured in McCoy's 5A modified medium. Chlorambucil-resistant human ovarian carcinoma A2780/100 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and maintained with 50 μM chlorambucil (25). Colchicine-resistant MCF-7MDR1 cells (a gift from M. Gottesman, NCI) were cultured in Dulbecco's modified Eagle's medium and maintained in 60 ng/ml colchicine (9). All cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Antibodies and plasmids.
A synthetic peptide CDGKETKAADPPAENS (45) corresponding to amino acid residues 299 to 313 of human YB-1 was used as a hapten to raise polyclonal antibody in rabbits (ProteinTech Company Group, Chicago, IL). An AcAPE1-specific rabbit antibody was generated using the hapten (MPKRGAcKKGAVAED-C) corresponding to residues 1 to 13 of human APE1 with acetylated Lys6 (boldface) (ProteinTech Company Group, Chicago, IL). Antibodies were purified by peptide immunoaffinity chromatography. Anti-rabbit human APE1 (50) and anti-mouse human APE1 (catalog number Sc-2104; Santa Cruz Biotechnology), mouse monoclonal APE1 antibody (catalog number NB100-116; Novus Biologicals), and mouse monoclonal MDR1 antibody (MAB 448; Chemicon) were also used. The cDNAs encoding APE1, K6R/K7R APE1, or NΔ33APE1 were cloned into mammalian expression plasmid pFLAG-CMV 5.1 (Sigma). Both WT and mutated Y-box-containing MDR1 promoter-luciferase plasmids were described previously (47). The plasmids containing full-length glutathione S-transferase (GST)-YB-1 cDNA and five GST-YB-1 deletion mutants (GST-YB-1 Δ1 to GST-YB-1 Δ5) were also used previously (26). The additional deletion constructs Δ6 to Δ9 were generated by PCR amplification with BamHI/XhoI restriction sites and cloned into pGEX-4T. Their authenticity was confirmed prior to use by the direct sequencing of the coding regions.
Identification of APE1-associated proteins and coimmunoprecipitation.
HCT116 or A2780/100 cells were transfected with expression plasmids for FLAG-tagged APE1 or YB-1, and 48 h after transfection, cells were lysed, and the extracts were immunoprecipitated with anti-FLAG M2 antibody-conjugated agarose beads (Sigma) as described previously (3).
Expression and purification of WT and deletion mutants of YB-1.
A recombinant baculovirus, a kind gift from G. W. Teebor (New York University School of Medicine), was used to purify His-tagged YB-1 (38). GST-tagged WT and deletion mutants of YB-1 were purified using glutathione-agarose affinity beads (Sigma) according to the manufacturer's protocol.
Far-Western analysis.
Purified proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (12% polyacrylamide gel) and transferred onto nitrocellulose membranes for far-Western analysis as described previously (61).
Luciferase assay.
HCT116 cells were cotransfected with MDR1-promoter-reporter (bp −136 to +121) and expression plasmids for WT APE1 or its K6R/K7R mutant. Forty hours after transfection, cells were washed and lysed with reporter lysis buffer (Promega), and the luciferase activity of the extracts was measured in a luminometer (AutoLumant LB 953; Berthold) using the luciferase assay kit (Promega) according to the manufacturer's protocol.
Electrophoretic mobility shift assays (EMSAs).
The 5′ 32P-labeled oligonucleotide contained the WT (5′-GGTGAGGCTGATTGGCTGGGCAGGA-3′) or mutated Y box (5′-GGTGAGGCTGATCAACTGGGCAGGA-3′) (underlined WT and mutated Y-box sequences were annealed with appropriate complementary stands to generate the duplex oligonucleotides). Each oligonucleotide (50 fmol) was then incubated with 10 ng of YB-1 or various amounts of APE1 or nuclear extracts (NEs) (3 μg) for 20 min at 25°C in a buffer containing 40 mM HEPES-KOH (pH 7.5), 50 mM KCl, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 10% glycerol, and 1 μg of poly(dI-dC) (Sigma). After electrophoresis in nondenaturing 5% polyacrylamide gels in Tris-borate buffer at 4°C, the gels were dried and exposed to a PhosphorImager apparatus (Molecular Dynamics), and the radioactivity was analyzed with IMAGEQUANT software.
Purification of AcAPE1.
Recombinant APE1 (0.5 mg) was acetylated with the p300 histone acetyltransferase domain (5 μg), and the acetylated protein was purified as previously described (2). The identity of AcAPE1 was confirmed by sequencing and Western analysis with AcAPE1-specific antibody.
AP-endonuclease assay.
A 43-mer oligonucleotide containing the AP-site analog tetrahydrofuran was 5′ end labeled with [γ-32P]ATP using T4 polynucleotide kinase. After annealing to the complementary strand, the duplex oligonucleotide (500 nM) was incubated with recombinant APE1 (0.05 nM) together with or without YB-1 at 37°C for 10 min, during which the reaction rate was linear in a 20-μl reaction mixture. The standard AP-endonuclease assay was performed as described previously (7).
DNA affinity precipitation.
A 5′ biotin-labeled 24-mer duplex oligonucleotide (50 pmol) containing the WT or mutant Y-box sequence of the MDR1 promoter was incubated with 100 μg of Dynabead M-280 streptavidin (Dynal AS, Oslo, Norway) for 30 min at 25°C. The biotinylated oligonucleotides bound to the paramagnetic beads were retrieved using a magnetic particle concentrator (Dynal AS) and washed twice with 1× B&W buffer (10 mM Tris-HCl [pH 7.5], 0.5 mM EDTA, 250 mM NaCl). NEs (100 μg) from HCT116 cells pretreated with or without cisplatin (40 μg/ml) were added to the beads and incubated at 4°C for 30 min. After washing the beads twice with the B&W buffer to remove the unbound proteins, the bound proteins were recovered by boiling in 1× Laemmli buffer, separated by 10% SDS-PAGE, and then transferred onto a nylon membrane for immunoblotting.
APE1 downregulation by siRNA.
For the downregulation of APE1, 80 nM each of the ON-Target Plus APE1 short interfering RNAs (siRNAs) (catalog number LU-010237, human APEX1; Dharmacon) was used according to the manufacturer's protocol. Total RNA was isolated using an RNeasy Mini kit (Qiagen) according to the manufacturer's protocol, and the APE1 mRNA level was quantitated by real-time reverse transcription (RT)-PCR analysis. Cell extracts were prepared at 48 h after transfection, and the APE1 polypeptide level was assessed by immunoblotting.
ChIP assay.
A QuickChIP chromatin immunoprecipitation (ChIP) kit (Imgenex) was used according to the manufacturer's protocol. Briefly, 5 × 106 A2780/100 or HCT116 cells in 10-cm dishes were first treated with or without cisplatin (40 μg/ml) or trichostatin A (TSA) (100 ng/ml) for 6 h and then incubated with the culture medium containing 1% formaldehyde at room temperature for 10 min to allow the reversible cross-linking of proteins to DNA. After stopping the cross-linking reaction with glycine, the cells washed with phosphate-buffered saline were resuspended in 1 ml of SDS lysis buffer and kept on ice for 10 min. After fragmenting the chromatin DNA to an average length of 0.5 to 3 kb by sonication, the lysates were centrifuged (12,000 × g for 10 min), and the supernatants were diluted in dilution buffer. The diluted chromatin was precleared with DNA-protein G-agarose, which was pretreated with salmon sperm DNA. For immunoprecipitation, the treated chromatin solution was incubated at 4°C with 5 μg of APE-1 antibody (Novus Biologicals). After reversing the DNA-protein cross-links in the immunocomplexes, the MDR1 promoter sequence in the DNA containing the Y-box element (positions −82 to −73) was quantitated by real-time PCR using an ABI 7000 thermal cycler. The primers and probe sequences used were 5′ primer 5′-CCAGCCAATCAGCCTC-3′, 3′ primer 5′-CCCGGCTGTTCCT-3′), and TaqMan probe CCGGGAGCAGTCATCTGT. For qualitative comparison, the DNA was also amplified by standard PCR to generate a 359-bp region (positions −254 to +105) of the human MDR1 promoter using the following primers: 5′-ATAAGTTGAAATGTCCCCAATGAT-3′ and 5′-AGACGTGAAATTTTGGAAGAAGAT-3′ as 5′ and 3′ primers, respectively.
Nuclear colocalization of APE1 and YB-1.
HCT116 cells grown on coverslips were washed with phosphate-buffered saline and fixed in acetone-methanol (1:1) for 10 min after air drying for further processing for immunocytochemical analysis as described previously (3).
RNA isolation and RT-PCR analysis of tumor samples.
Cryopreserved non-small-cell lung cancer (NSCLC) and proximal normal lung tissues were obtained from the Sealy Center for Cancer Cell Biology tissue bank. Total RNA was extracted after homogenizing tissues in Trizol followed by purification using an RNeasy Mini kit (Qiagen) according to the manufacturer's protocol. For quantitative real-time RT-PCR, one-step RT-PCR was performed with 100 ng of cellular RNA using a TaqMan one-step RT-PCR master mixture reagent kit (catalog number P/N 4309196) in the Cancer Cell Biology real-time RT-PCR core facility. The mRNA levels of APE1 and MDR1 with 18S rRNA as an internal control were measured using an ABI Prism 7000 thermal cycler. The relative expression of each mRNA was calculated by the ΔCT method (cycle numbers obtained by subtracting the threshold cycle [CT] value of 18S mRNA from the CT value of the target mRNA).
Statistical analyses.
The correlation between APE1 and MDR1 mRNA expression levels was determined by Pearson relative correlation coefficient analysis using Sigma Plot software.
Clonogenic survival assay.
Log-phase cultures of A2780/100 or MCF-7MDR1 cells were treated with APE1 siRNA (80 nM) or a control siRNA for 48 h. Cells were trypsinized and plated onto 60-mm dishes at a density of ∼300 cells/plate. Twelve hours after plating, A2780/100 cells were treated with cisplatin (0 to 200 μM), and MCF-7MDR1 cells were treated with doxorubicin (0 to 300 nM). After allowing the cells to grow for 10 days, the colonies were counted after staining with crystal violet for calculating the survival fraction (25).
RESULTS
Identification of YB-1 as an APE1-interacting protein.
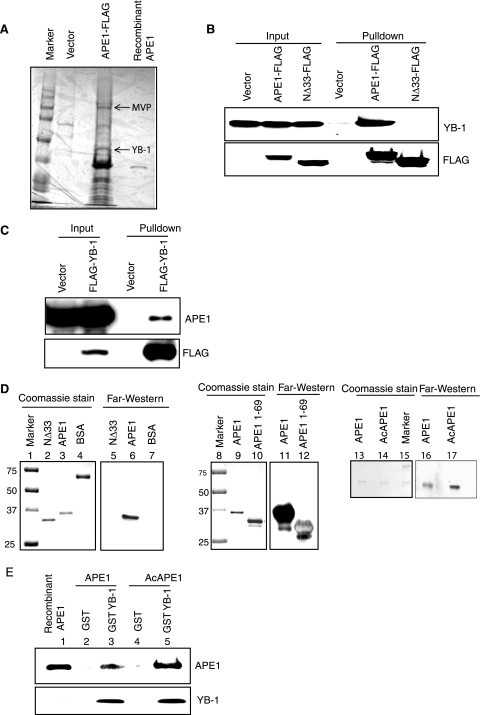
Given its multiple functions, it is not surprising that many interacting partners of APE1 have already been identified (29). To examine the role of APE1 in inducing drug resistance in tumor cells, we decided to characterize APE1's interactome by affinity analysis in the HCT116 human colon carcinoma line. FLAG-tagged APE1 was immunoprecipitated from HCT116 cell extracts using FLAG antibody beads, and bound proteins in the immunocomplex were eluted with the FLAG peptide. Subsequent matrix-assisted laser desorption ionization-time of flight analysis of proteins separated by SDS-PAGE identified several APE1-interacting partners, among which YB-1 and major vault protein (MVP) were prominent (Fig. 1A). Similar screening with a control FLAG vector confirmed that the APE1-YB-1 or APE1-MVP interaction was specific (Fig. 1A). Furthermore, a similar screening in human embryonic kidney (HEK-293) cells also identified YB-1 and MVP as interacting partners of APE1, indicating that these interactions are not cell type specific (data not shown). We then carried out a detailed analysis of the APE1-YB-1 interaction.
FIG. 1.
Stable interaction between YB-1 and APE1. (A) Identification of YB-1 as an APE1-associated protein. Extracts of HCT116 cells transfected with FLAG-tagged APE1 or empty vector were immunoprecipitated with FLAG antibody, and bound proteins were eluted with FLAG peptide, resolved by SDS-PAGE, and visualized with Coomassie stain. YB-1 and MVP (marked by arrow) were identified by matrix-assisted laser desorption ionization-time of flight analysis. (B, top) Extracts of HCT116 cells transfected with empty vector or WT APE1-FLAG or NΔ33APE1-FLAG were immunoprecipitated with FLAG antibody for Western analysis with YB-1 antibody. (Bottom) Western analysis of the same blot with FLAG antibody. (C) Western analysis of the FLAG-YB-1 immunoprecipitate with APE1 antibody (top) or with FLAG antibody (bottom). (D) Far-Western analysis with YB-1 antibody using the WT or NΔ33APE1 (left), the GST-tagged N-terminal 69 amino acid residues of APE1 (middle), and unmodified APE1 or AcAPE1 (right). Bovine serum albumin (BSA) was used as a control. (E) GST-tagged YB-1 was incubated separately with unmodified APE1 (lane 3) or in vitro-acetylated APE1 (lane 5). Bound proteins were eluted and immunoblotted with APE1 antibody. (Bottom) Western analysis of the same blot with YB-1 antibody.
In vivo and in vitro association of APE1 and YB-1 and the mapping of their interaction domains.
The preliminary identification of YB-1 as a stable interacting partner of APE1 was further confirmed by coimmunoprecipitation analysis using C-terminal FLAG-tagged full-length or N-terminal 33-amino-acid-deleted APE1 (NΔ33APE1). Western analysis of the FLAG immunoprecipitate showed that YB-1 was present in the immunoprecipitate of full-length APE1 but not NΔ33APE1 (Fig. 1B, top). The presence of comparable levels of the FLAG fusion polypeptide in the starting extracts and immunoprecipitates was confirmed by Western analysis with FLAG antibody (Fig. 1B, bottom). Quantitative immunoblots of the immunoprecipitate in parallel with the input cell extracts showed that the proportion of APE1 or YB-1 associated in vivo is low, corresponding to <10% of YB-1 and <6% of APE1 (see Fig. S1A in the supplemental material). The binding of YB-1 to full-length APE1 but not the NΔ33APE1 deletion mutant indicated that the interaction domain includes the N-terminal region. To further confirm the stable interaction between APE1 and YB-1, we performed the reciprocal analysis to show the presence of APE1 in the FLAG-YB-1 immunocomplex (Fig. 1C, top). To test whether APE1 interacts directly with YB-1, we carried out far-Western analysis using recombinant YB-1 and APE1. The strong interaction of YB-1 with full-length APE1 but not NΔ33APE1 (Fig. 1D, left, lanes 5 and 6) indicated that APE1 directly interacts with YB-1 via its N-terminal domain. Moreover, the interaction of YB-1 with the GST-tagged N-terminal 69 amino acid residues of APE1 (Fig. 1D, middle, lanes 11 and 12) showed that the N-terminal region of APE1 is necessary and sufficient for the interaction with YB-1. Our previous studies showed that the addition of APE1 acetylated at Lys6 and Lys7 strongly stimulated the binding of NEs to nCaRE-B (2). To test whether APE1 acetylation enhances its interaction with YB-1, we carried out far-Western analysis with recombinant APE1 or AcAPE1. It is evident that AcAPE1 binds more strongly to YB-1 than the unacetylated protein when used at the same concentration (Fig. 1D, right, lanes 16 and 17).
We then used GST-tagged YB-1 to further examine whether acetylation affects APE1's interaction with YB-1. We prepared a GST-YB-1 immunoaffinity column and separately incubated it with acetylated or unmodified APE1, eluted the bound proteins with reduced glutathione, and then immunoblotted them with APE1 antibodies. The elution profile revealed that AcAPE1 preferentially binds to YB-1 compared to the unmodified APE1 (Fig. 1E, lanes 3 and 5), thus confirming that acetylation enhances APE1's binding to YB-1.
APE1 interacts with a long C-terminal stretch of YB-1.
We next characterized YB-1's interaction interface by using nested deletion mutants. Far-Western analysis showed that APE1 interacts with a long stretch of the C-terminal region of YB-1 (see Fig. S1B and S1C in the supplemental material).
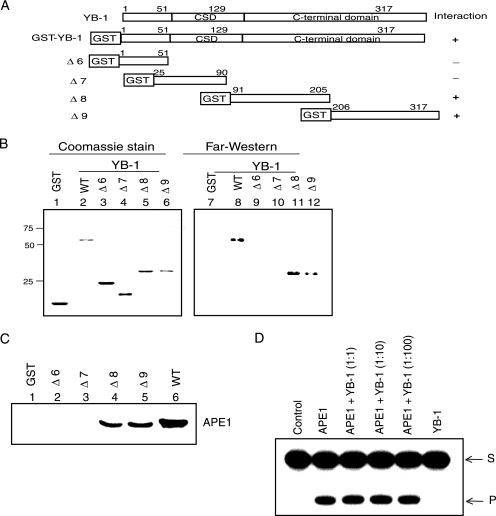
For a more precise characterization of the APE1-interacting domain, we tested additional deletion mutants of YB-1 (Fig. 2A). It is evident from Fig. 2B that APE1 has multiple interaction sites in YB-1, containing residues 91 to 205 (Δ8) and 206 to 317 (Δ9). To further confirm this, we performed GST pull-down assays with various deletion mutants of GST-YB-1. Figure 2C shows a robust interaction of GST-YB-1 (residues 91 to 205) and GST-YB-1 (residues 206 to 317) with APE1. Together, these results confirmed that a long C-terminal stretch of YB-1 is involved in interaction with APE1, similar to that observed previously for interactions with Smad3 (23).
FIG. 2.
Mapping of the APE1-interacting region in YB-1 by far-Western and GST pull-down analyses. (A) Schematic representation of the GST-YB1 deletion mutants. CSD, cold shock domain. (B) Far-Western analysis. (Right) APE1 antibody was used to test different GST-YB-1 deletion mutants. (Left) Coomassie staining of YB-1 deletion mutants. (C) GST pull-down assay. Recombinant APE1 was incubated with glutathione-agarose affinity beads containing different GST-YB-1 deletion mutants (Δ6 to Δ9, as indicated), and the eluted proteins were immunoblotted with APE1 antibody. (D) Effect of YB-1 on APE1's AP-endonuclease activity. APE1 (50 pM) was incubated for 10 min with the tetrahydrofuran-containing 32P-labeled duplex in the presence of increasing amounts of YB-1 (0.05 to 5 nM), and the reaction products were analyzed in a 20% urea-polyacrylamide gel as described previously (7). S, substrate; P, product.
APE1 stimulates YB-1 binding to the Y-box-containing oligonucleotide.
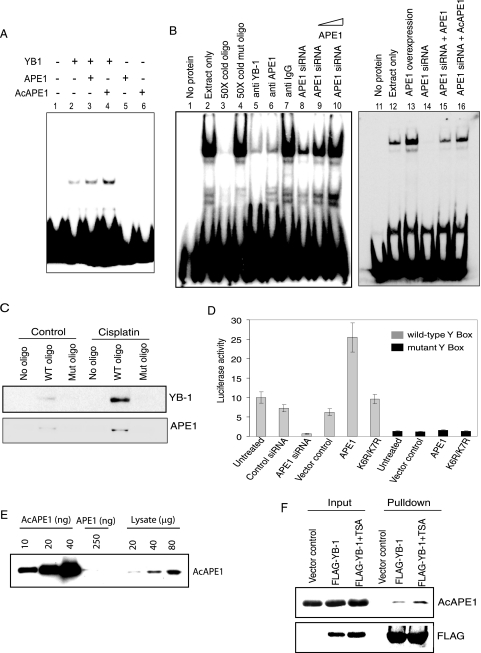
The stable interaction of YB-1 with human NTH1, a DNA glycosylase specific for the repair of oxidized bases, was previously reported, which was also shown to enhance NTH1's activity as a result of this interaction (38). In contrast, we observed no difference in the AP-endonuclease activity of APE1 in the presence or absence of YB-1 (Fig. 2D). Because APE1 was shown to modulate the binding of several transcription factors to their cognate cis elements (13), we examined the effect of APE1 on YB-1 binding to the Y box in the MDR1 promoter by EMSA. APE1 stimulated the Y-box-binding activity of YB-1 in vitro (Fig. 3A). More importantly, AcAPE1 stimulated YB-1 binding more strongly than did the unacetylated APE1 protein at the same concentration (Fig. 3A, lanes 3 and 4). APE1 or AcAPE1 alone had no affinity for the Y-box oligonucleotide under the same experimental conditions (Fig. 3A, lanes 5 and 6). To show that APE1 enhances YB-1's cis element binding in vivo, we investigated the effect of the downregulation of APE1 on YB-1-binding activity in HCT116 cells. A strong reduction in the level of the Y-box-bound complex was observed with the NE of cells in which the APE1 level was downregulated with siRNA (Fig. 3B, lanes 8 and 14). The possibility that this was a direct effect of APE1 was indicated by the observation that the level of the complex was restored when recombinant APE1 or AcAPE1 was added exogenously to the NE (Fig. 3B, left, lanes 9 and 10, and right, lanes 15 and 16). Furthermore, a strong enhancement of binding with the HCT116 extracts was observed after the ectopic expression of APE1 (Fig. 3B, right, lane 13). These findings indicate that both YB-1 and APE1 are necessary for the binding to the Y box and that APE1 is limiting in vivo for the formation of the Y-box complex for which AcAPE1 is preferred over APE1 (Fig. 3B, right). Although the preincubation of the NE with either YB-1 (Fig. 3B, lane 5)- or APE1 (Fig. 3B, lane 6)-specific antibody did not show the formation of a supershifted complex, a significant reduction in the amount of the complex was observed when antibodies to YB-1 or APE1 were added to the NE. The finding that the inhibition of complex formation was specific was confirmed by using nonspecific immunoglobulin G (IgG) as a control (Fig. 3B, lane 7).
FIG. 3.
APE1 enhances YB-1 binding to the Y-box sequence. (A) EMSA of YB-1 (10 ng) with or without added APE1 (20 ng) or AcAPE1 (20 ng) using 32P-labeled duplex oligonucleotides containing the WT Y-box sequence. Lanes 5 and 6, APE1 or AcAPE1 alone. (B) APE1 enhances binding of HCT116 NE to 5′ 32P-labeled Y-box-containing oligonucleotide (oligo). Lane 1, free probe. Lanes 2 to 16, EMSA with NE (3 μg) alone or in the presence of 50-fold unlabeled WT or mutant oligonucleotide (lanes 2 to 4); NE preincubated with anti-YB-1, anti-APE1, and control rabbit IgG (lanes 5 to 7); NE from APE1 siRNA-treated cells alone or supplemented with 100 ng or 500 ng recombinant APE1 (lanes 8 to 10); and NE from cells expressing ectopic APE1 (lane 13) or APE1 siRNA-treated cells alone (right, lane 14) or supplemented with 500 ng of APE1 (lane 15) or recombinant AcAPE1 (lane 16). (C) Western analysis of eluted proteins bound to 5′ biotin-labeled WT or mutant Y-box-containing oligonucleotide with YB-1 (top) or APE1 (bottom) antibody. (D) HCT116 cells were cotransfected with a MDR1 promoter-driven luciferase reporter plasmid containing either the WT (gray) or mutant (black) Y box and expression plasmids for APE1 (WT or the K6R/K7R mutant) or equivalent amounts of empty vector. Where indicated, cells were transfected with APE1-specific siRNA or control siRNA (80 nM) 24 h before transfection with the reporter plasmid. Luciferase activity was normalized with coexpressed β-galactosidase. The bar graph shows the averages ± standard deviations for three independent experiments performed in duplicate. (E) Western analysis of recombinant AcAPE1 and HCT116 cell extracts with AcAPE1-specific antibody. (F) HCT116 cells were transfected with FLAG-YB-1 and treated 24 h later with TSA (100 ng/ml) for 12 h. Immunoprecipitates of the cell extracts with FLAG antibody were analyzed by Western blotting with AcAPE1 (top) or FLAG antibody (bottom).
To examine the ability of APE1 to form a ternary complex with YB-1 and DNA, we used an alternative approach, namely, DNA affinity precipitation of cell extracts. A biotin-labeled 25-mer duplex oligonucleotide containing the Y-box sequence of the MDR1 gene was incubated with Dynabead M-280 streptavidin at room temperature. The biotin/streptavidin-conjugated beads were then incubated with NEs of HCT116 cells pretreated with or without cisplatin. As a control, we incubated NEs with a biotinylated oligonucleotide containing a mutated Y-box sequence. The protein-oligonucleotide complexes were washed with high-salt buffer to remove unbound proteins, followed by the recovery of the bound proteins. Western analysis with APE1 or YB-1 antibody showed the presence of YB-1 as well as APE1 in the bound complex with the WT Y-box oligonucleotide but not with the mutant oligonucleotide (Fig. 3C). Moreover, an increased binding of both YB-1 and APE1 to the Y-box sequence was observed in cells treated with cisplatin (Fig. 3C). These results indicate that both YB-1 and APE1 could be present in the Y-box-bound complex.
The APE1 level modulates Y-box-dependent MDR1 promoter activity.
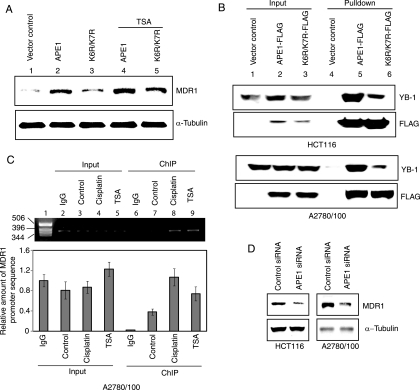
Because YB-1's binding to the Y box in the MDR1 promoter is a major contributor to the transcriptional activation of MDR1 (46), we used a luciferase reporter assay to examine the effect of APE1 on YB-1-mediated MDR1 promoter activity. HCT116 cells were transiently transfected with a luciferase reporter fused to the 258-bp promoter region of the MDR1 gene containing the WT or mutant Y-box sequence. A significant (about sixfold) decrease in luciferase activity in the extracts of APE1 siRNA-transfected cells compared to those from control siRNA-treated cells (Fig. 3D) indicated a strong dependence of MDR1 promoter activity on the APE1 level. This was further supported by the observation that ectopic WT APE1 enhanced luciferase expression by more than threefold. Because APE1 acetylation stimulates YB-1's DNA binding, we asked whether APE1 acetylation modulates MDR1 expression by measuring MDR1 promoter activity after the expression of the nonacetylable K6R/K7R APE1 mutant. The overexpression of the mutant, unlike that of the WT protein, did not enhance luciferase activity (Fig. 3D). The activation of the MDR1 promoter thus appears to be dependent on APE1 acetylation. The finding that this modulating effect of APE1 occurs via the Y box was confirmed by using a mutant Y-box-containing reporter that showed no significant effect of an overexpression of WT or mutant APE1 (Fig. 3D). To confirm the presence of endogenous AcAPE1, we generated and then affinity purified the AcAPE1 antibody. Western analysis showed that this antibody could detect 10 ng recombinant AcAPE1 and showed no cross-reactivity even with 250 ng unmodified APE1, confirming its high specificity for AcAPE1 (Fig. 3E). The presence of AcAPE1 in the HCT116 cell extract (Fig. 3E) provided unequivocal evidence for the presence of endogenous AcAPE1 under normal conditions. We used this antibody to confirm the presence of AcAPE1 in the FLAG-YB-1 immunoprecipitate (Fig. 3F). Furthermore, treatment with TSA, a histone deacetylase inhibitor, enhanced the level of AcAPE1 in the YB-1 immunocomplex (Fig. 3F).
Stress-induced nuclear translocation of YB-1 and APE1 enhances APE1/YB-1 complex formation.
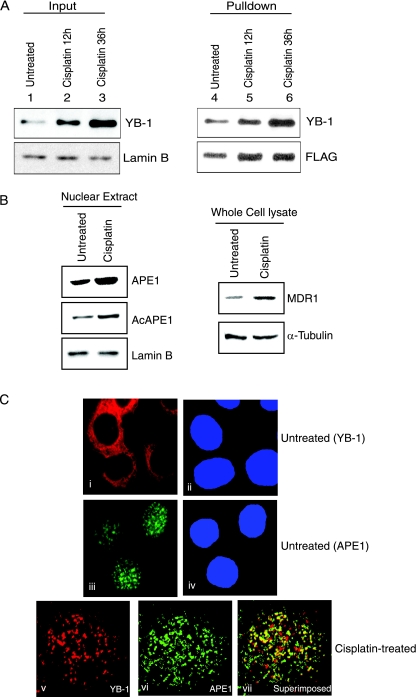
As cisplatin activates MDR1 expression, presumably via an enhanced binding of YB-1 to the MDR1 promoter (26, 45), we examined the effect of cisplatin on the interaction between APE1 and YB-1 in APE1-FLAG-expressing HCT116 cells. After treating the cells with cisplatin, we isolated FLAG immunoprecipitate from the NE, which showed an approximately twofold-higher level of YB-1 in the APE1 immunocomplex than in the untreated control (Fig. 4A, top right and bottom right). We also observed an approximately twofold increase in the nuclear levels of both ectopic (Fig. 4A, lower right) and endogenous (Fig. 4B, left) APE1 or AcAPE1 after cisplatin treatment. Furthermore, consistent with previously reported observations (45), we observed a 2.5-fold increase in the MDR1 polypeptide level at 12 h after cisplatin treatment (Fig. 4B, top right) concomitantly with enhanced nuclear localization of YB-1 (Fig. 4A, top left). Although cisplatin treatment enhanced the nuclear localization of both YB-1 and APE1, the ectopic overexpression of APE1 alone did not change YB-1's nuclear localization (see Fig. S1D in the supplemental material). These results strongly suggest that the nuclear translocation of both YB-1 and APE1 after genotoxic stress leads to an increased association. The enhanced association between APE1 and YB-1 after genotoxic stress was further confirmed by confocal microscopy. Immunostaining with YB-1 (red) and APE1 (green) antibodies showed that before stress, YB-1 was mostly cytosolic, while APE1 was mostly nuclear (Fig. 4Ci and ii). However, after cisplatin treatment, YB-1 was translocated to the nucleus (Fig.4Cv), and a superimposition of the images showed a significant overlapping of YB-1 and APE1 staining in the subnuclear region, as indicated by yellow speckles (Fig.4Cvii).
FIG. 4.
Enhanced interaction between APE1 and YB-1 after cisplatin treatment. (A) HCT116 cells transfected with APE1-FLAG were treated with 40 μg/ml cisplatin for 12 h (lanes 2 and 5) and 36 h (lanes 3 and 6). NEs were immunoprecipitated with FLAG antibody beads, followed by Western analysis using YB-1 antibody (top right) or FLAG antibody (bottom right). (Left) Western analysis for YB-1 in input nuclear extract (lamin B as the loading control). (B) Western analysis of APE1, AcAPE1, and MDR1 in nuclear (left) and whole-cell (right) extracts after cisplatin treatment for 12 h. Lamin B (bottom left) or α-tubulin (bottom right) was used as a loading control. (C) Colocalization of APE1 and YB-1 after cisplatin treatment. HCT116 cells were immunostained with rabbit YB-1 (i and v) and mouse APE1 antibody (iii and vi) followed by goat anti-rabbit Texas Red-labeled and goat anti-mouse fluorescein isothiocyanate-labeled secondary antibody. vii, superimposition of images v and vi. DAPI (4′,6′-diamidino-2-phenylindole) was used for nuclear staining (ii and iv).
APE1 acetylation enhances MDR1 expression.
The functional consequence of enhanced YB-1 binding to AcAPE1 was assessed by measuring the endogenous MDR1 polypeptide level. We quantitated the MDR1 level in the A2780/100 cell line, a cisplatin-resistant ovarian cancer cell line (25), after the overexpression of the WT versus the K6R/K7R APE1 mutant. The overexpression of WT APE1, but not the K6R/K7R mutant, caused a significant (about threefold) increase in the MDR1 protein level. These data support the scenario that APE1 acetylation enhances its binding to YB-1 in order to activate MDR1 expression. Furthermore, treatment with TSA caused a moderate increase in the level of MDR1 when WT APE1 was overexpressed (Fig. 5A, top), which further supports this model. We then compared the amount of YB-1 associated with the WT to that associated with the K6R/K7R APE1 mutant. Extracts of HCT116 and A2780/100 cells overexpressing the FLAG-tagged WT or K6R/K7R APE1 mutant were immunoprecipitated with FLAG antibody. Western analysis with YB-1 antibody showed a significantly smaller amount of YB-1 (about threefold) (Fig. 5B, top) complexed with K6R/K7R APE1, with comparable amounts of WT versus mutant K6R/K7R APE1 (Fig. 5B, bottom). This result further supports the contribution of APE1 acetylation to its binding to YB-1.
FIG. 5.
APE1 acetylation-dependent enhancement of MDR1 expression. (A) A2780/100 cells were transfected with expression plasmids for WT (lanes 2 and 4) or K6R/K7R (lanes 3 and 5) APE1. After 24 h, one set of cells was treated with 100 ng/ml TSA (lanes 4 and 5) for 12 h, and the MDR1 level was analyzed by Western blotting with MDR1 antibody. (B, top) Extracts of HCT116 and A2780/100 cells transfected with FLAG-tagged WT APE1 or K6R/K7R APE1 were immunoprecipitated with FLAG antibody beads and analyzed by Western blotting using YB-1 antibody. (Bottom) Western analysis of the same blot with FLAG antibody. (C) ChIP assay for in vivo association of APE1 with the MDR1 promoter. (Top) PCR analysis of immunoprecipitated MDR1 promoter sequence. Lane 1, DNA molecular weight marker; lanes 2 to 5, MDR1 promoter sequence in control IgG, untreated control, and cisplatin- and TSA-treated input chromatin, respectively; lanes 7 to 9, immunoprecipitated MDR1 promoter sequence with APE1 antibody from control and cisplatin- and TSA-treated cell extracts, respectively; lane 6, immunoprecipitated MDR1 promoter with control IgG. (Bottom) Quantitation of immunoprecipitated MDR1 promoter DNA by real-time PCR analysis as described in Materials and Methods. (D) HCT116 and A2780/100 cells were transfected with 80 nM APE1 siRNA oligonucleotide. The MDR1 level (top) was measured by Western analysis at 48 h after transfection (α-tubulin was used as the loading control).
We then utilized ChIP assays to demonstrate the presence of APE1 in the Y-box-binding complex on the MDR1 promoter in vivo. After treating A2780/100 cells with cisplatin or TSA for 6 h, ChIP analysis was carried out with the APE1 antibody, as described in Materials and Methods. The amount of immunoprecipitated MDR1 promoter DNA was quantitated by real-time PCR analysis with primers that amplified an MDR1 promoter sequence spanning the Y-box element. Figure 5C (bottom) shows a significant enrichment (∼15-fold) of this sequence in the immunocomplex with APE1 antibody (lane 7) compared to the control immunocomplex with IgG, indicating that APE1 is associated with the MDR1 promoter in vivo in A2780/100 cells. We performed appropriate control experiments to validate our results. Thus, no significant enrichment of the MDR1 promoter sequence was observed by the standard PCR assay when control IgG was used for immunoprecipitation (Fig. 5C, top, lane 6). Similarly, no significant enrichment of the MDR1 promoter sequence was observed in chromatin extracts from either cell line when the formaldehyde cross-linking step was omitted (data not shown). Treatment with cisplatin caused a 2.8-fold increase (Fig. 5C, bottom) in the amount of MDR1 promoter sequence in the immunocomplex, indicating the enhanced binding of the APE1/YB-1 complex to the MDR1 promoter after cisplatin treatment (Fig. 5C, lane 8). TSA treatment also significantly enhanced (twofold) promoter occupancy of APE1 in the MDR1 promoter, further confirming that APE1 acetylation enhances YB-1 binding to the Y box in the MDR1 promoter in vivo (Fig. 5C, bottom). To demonstrate that endogenous APE1 modulates MDR1 expression, we downregulated APE1 by siRNA, which caused a significant decrease (about threefold) in the MDR1 level in both HCT116 and A2780/100 cells (Fig. 5D).
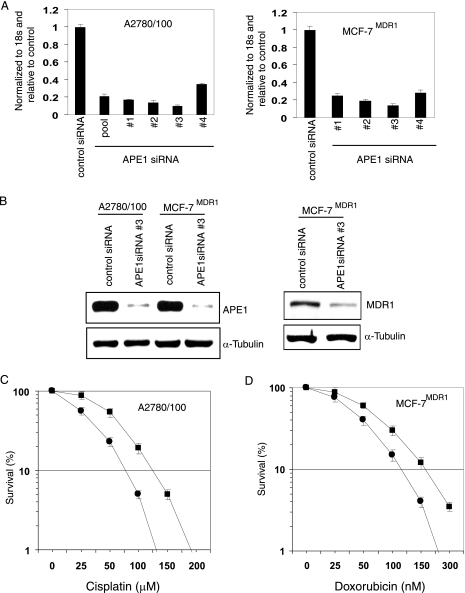
APE1 downregulation increases drug sensitivity of tumor cells.
The results described so far suggest that APE1 stably interacts with YB-1 and that the APE1/YB-1 complex modulates MDR1 expression. This raises the possibility that APE1 could be targeted for MDR1 downregulation, leading to an enhanced drug sensitivity of tumor cells. We used A2780/100 cells and the doxorubicin-resistant breast cancer line MCF-7MDR1, which express high levels of MDR1 (9, 25). Figure 6A shows that four different APE1 siRNA oligonucleotides were effective in decreasing the APE1 mRNA level in both A2780/100 and MCF-7MDR1 cells. With oligonucleotide 3, we observed a >80% reduction in the APE1 protein level (Fig. 6B, left) in both cell lines, with a concomitant decrease in the MDR1 level (Fig. 5D and 6B, right). APE1-downregulated A2780/100 cells were more sensitive to cisplatin than the control cells, with a twofold reduction in the 50% inhibitory concentration (Fig. 6C). Similarly, APE1 downregulation caused a 1.5-fold decrease in the 50% inhibitory concentration for doxorubicin with MCF-7MDR1 cells (Fig. 6D). These results confirm that APE1 could be an indirect target for increasing the therapeutic index of various drugs in MDR1-overexpressing tumor cells.
FIG. 6.
APE1 downregulation sensitizes drug-resistant cells. (A) Real-time RT-PCR analysis of APE1 mRNA from A2780/100 (left) and MCF-7MDR1 (right) cells at 48 h after transfection with 80 nM of APE1-specific or control duplex siRNAs. (B, left) Western analysis of cell extracts with APE1 (top) or α-tubulin (bottom) antibody. (Right) Western analysis of MDR1 levels in MCF-7 MDR1 cells at 48 h after transfection with 80 nM APE1 siRNA oligonucleotide. (C and D) Survival assay of A2780/100 and MCF-7MDR1 cells transfected with either control (▪) or APE1 (•) siRNA; 48 h after transfection, ∼300 cells were plated into 60-mm dishes and treated with increasing concentrations of cisplatin (0 to 200 μM) or doxorubicin (0 to 300 nM). Cells were maintained for 10 days with or without drug treatment, and the colonies were counted using crystal violet. One hundred percent corresponds to the colony numbers in the control without drug treatment. Other details are given in Materials and Methods.
Correlation of MDR1 and APE1 levels in NSCLC tissues.
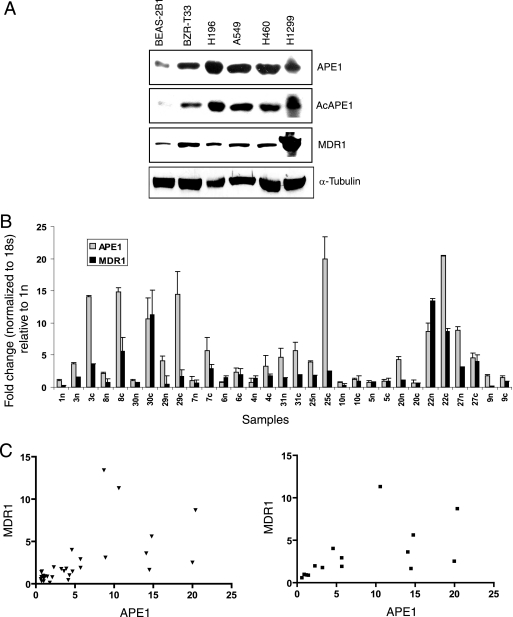
Several studies suggested that elevated MDR1 protein levels are responsible for the drug resistance of NSCLC, which could be correlated with the tumor response to chemotherapy (19, 53, 58). Because of the altered level and subcellular localization of APE1 in non-small-cell lung carcinomas (49, 65) and the availability of the lung tumor samples from our institutional tumor bank (Sealy Center for Cancer Cell Biology Core), we examined possible correlation between APE1 and MDR1 levels in both NSCLC cell lines and tumor samples. Western analysis using four human NSCLC cell lines, namely, A549, H196, H460, H1229, and BZR-T33, an H-ras-transfected lung epithelial BEAS-2B1 cell line, we observed that all of these tumor cell lines have APE1 and MDR1 proteins at a considerably higher level than the normal lung epithelial BEAS-2B1 cell line, which is immortalized after transfection with simian virus 40 T antigen (Fig. 7A).
FIG. 7.
MDR1 expression is correlated with APE1 levels in normal lung and NSCLC tissues. (A) Western analysis of APE1, AcAPE1, and MDR1 levels in cell extracts of various lung cancer cell lines. α-Tubulin was used as the loading control. (B) Real-time RT-PCR analysis of APE1 and MDR1 mRNA expression levels in 15 NSCLC specimens and proximal normal lung tissues as described in Materials and Methods. The level of APE1 mRNA was expressed relative to the level in normal lung tissue of patient 1n,which was normalized to 1. n, normal lung tissues; c, NSCLC tissues. The bar graph shows the averages ± standard deviations for three independent experiments performed in duplicates. (C) Correlation between APE1 and MDR1 expression levels in both normal and NSCLC samples together (left) (r = 0.581; P = 0.0005) and in NSCLC samples (right) (r = 0.558; P = 0.03).
To further validate this observation, we then analyzed NSCLC and proximal normal lung tissues of 15 patients for APE1 and MDR1 mRNA levels using real-time RT-PCR. Both APE1 and MDR1 are frequently expressed in both normal and cancer tissues (Fig. 7B). Although the levels of APE1 and MDR1 varied among the samples, a significant correlation (r = 0.581; P = 0.0005) between APE1 and MDR1 mRNA levels was observed in both normal and cancer tissue samples (Fig. 7C, left). Pairwise comparisons between cancer and normal tissues from the same patients showed that 9 out of 15 (60%) patient cancer tissues had elevated APE1 mRNA levels (Fig. 7B); 3 patients had no significant difference between normal and cancer tissues, while 3 had higher APE1 mRNA levels in normal tissues. An elevated expression level of MDR1 was observed in six out of nine (66%) NSCLC samples that overexpressed APE1. Importantly, a significant correlation (r = 0.558; P = 0.0305) between APE1 and MDR1 mRNA levels was also observed in NSCLC tissue samples (Fig. 7C, right). These results further confirm the strong correlation between APE1 and MDR1 levels in vivo.
DISCUSSION
Several studies have shown that APE1 downregulation sensitized tumor cells to various genotoxic agents via the induction of apoptosis (16, 52, 60). Whether such enhanced sensitivity is due solely to the loss of APE1's DNA repair activity or is also due to the loss of its transcriptional regulatory function or both is unknown. Because APE1 plays a central role in the repair of endogenous DNA damage and small base adducts induced by alkylating agents via the BER pathway, it is not surprising that APE1 overexpression is associated with tumor cell resistance to alkylating drugs. However, the downregulation of APE1 was shown to sensitize tumor cells to etoposide or sensory neuronal cells to cisplatin (32, 60), which cannot be easily explained by the repair function of APE1 because these drugs induce DNA double-strand breaks or DNA intrastrand cross-links that are repaired via the APE1-independent nonhomologous-end-joining or nucleotide excision repair pathways (5, 10). Thus, the higher sensitivity to cisplatin and doxorubicin that we observed in APE1-downregulated A2780/100 and MCF-7MDR1 cells cannot be explained by the loss of APE1's DNA repair function; rather, it is likely to result from the deficiency in APE1's regulatory activity. This study provides the first evidence for the involvement of APE1's transcriptional regulatory function in MDR1 expression and the resulting drug resistance, a mechanism that could provide a molecular basis for the sensitization of tumor cells to doxorubicin or cisplatin via APE1 downregulation. For the first time, we have thus documented nonrepair functions of APE1 in affecting the sensitivity of tumor cells to genotoxic agents. We have also unraveled the molecular mechanism by which APE1 acetylation controls YB-1-mediated MDR1 expression, again suggesting that such an acetylation-mediated transactivation function of APE1 could be used as a novel therapeutic target.
We have shown that 33 N-terminal amino acid residues of APE1 including the acetyl acceptor Lys6 and Lys7 are necessary for its interaction with YB-1. Our previous studies indicated that this N-terminal region of APE1, which is dispensable for APE1's endonuclease activity, is intrinsically disordered (29, 41). It appears likely that acetylation-mediated conformational changes in APE1's N-terminal domain modulate its interaction with partner proteins. Thus, the acetylation of APE1 enhances its binding to YB-1, leading to the activation of the Y-box-dependent MDR1 promoter, while the lack of acetylation in the Lys6 and Lys7 mutants significantly decreased binding (Fig. 1E and 5B).
Our observations that the addition of APE1 did not alter the electrophoretic mobility of the Y-box-bound complex (Fig. 3B) and that the APE1-stimulated YB-1-DNA complex could not be supershifted by affinity-purified APE1 antibody suggest that either APE1's binding to YB-1 masked its epitope or the ternary complex of APE1, YB-1, and DNA is too unstable to withstand the gel electrophoresis conditions. However, we resolved this issue by using a DNA affinity precipitation assay and showed that both APE1 and YB-1 remain bound to Y-box-containing DNA in a ternary complex. Thus, such a complex may not survive during EMSA. A very similar situation was observed with high-mobility-group protein 1 (HMG1), which directly interacts with p53 and stimulates its cis element binding (30), while its addition did not affect the mobility of the p53-bound complex in EMSA, and HMG1 antibody did not cause a supershift of the complex (30). Interestingly, the preincubation of the nuclear extract with YB-1 antibody resulted in a significant decrease in its DNA-binding activity, suggesting that antibody binding may mask the DNA-binding domain of YB-1 and thus reduce its binding to target DNA. Consistent with this, one previous study using the same YB-1 antibody (raised against the same peptide residues, residues 299 to 313, as those used in our study) showed a strong reduction in YB-1's binding to the gamma interferon response element in the human α2-collagen promoter in EMSA (24). Because the downregulation of APE1 significantly reduces the formation of the Y-box-bound complex, it is possible that the addition of APE1 antibody titrates out the free APE1 and prevents YB-1's binding. Whether APE1 could alter YB-1's conformation, thus facilitating its binding to target DNA by enhancing its loading or preventing a dissociation from the Y box, remains to be determined.
The exact mechanism by which the acetylation of APE1 activates MDR1 expression is not clear. While Cys65 was proposed to be the redox-active residue in APE1 for the activation of several transcription factors (59), the Cys65Ser and Cys138Ser mutants behaved like WT APE1 in modulating YB-1-mediated MDR1 promoter activity (data not shown). This suggests that APE1's redox activity is not involved in MDR1 activation. Both APE1 and YB-1 interact with p300 (23), best known as a scaffold protein that facilitates transcriptional complex assembly and also involved in chromatin remodeling via its intrinsic histone acetyltransferase activity (44). p300 acetylates APE1 at Lys6 (or Lys7), thus enhancing its binding affinity with YB-1. Thus, p300-mediated APE1 acetylation in concert with other proteins may be the key to the incorporation of APE1 in the MDR1 promoter. Using ChIP assays, we have provided direct evidence that APE1 is associated with the Y-box-binding complex on the MDR1 promoter in vivo and that TSA treatment enhanced (presumably via increased APE1 acetylation) MDR1 promoter occupancy. It is thus likely that the AcAPE1/YB-1/p300 complex is recruited to the MDR1 promoter, where p300 hyperacetylates the promoter-bound histones and unfolds the chromatin structure to promote transcription. A similar mechanism has been proposed for the NF-Y-mediated activation of the MDR1 promoter by recruiting the coactivator P/CAF, a p300/CBP-associated factor with histone acetyltransferase activity, to the MDR1 promoter (33).
Several studies support YB-1's role as a major transcription factor for MDR1 expression and consequent drug resistance. For example, an early study showed that the stable transfection of a YB-1 antisense expression plasmid caused a twofold decrease in MDR1 gene expression in KB cells (45). Furthermore, human cancer cell lines overexpressing YB-1 are resistant to cisplatin, while decreasing their YB-1 level sensitized them to UV and DNA-interacting drugs (46). The targeted disruption of one allele of YB-1 in mouse embryonic stem cells enhanced their sensitivity to cisplatin and mitomycin C (54). Nevertheless, we cannot rule out that the APE1-mediated activation of MDR1 expression involves not only YB-1 but also NF-Y. In support of this possibility, a previous study showed that the redox function of APE1 is involved in modulating NF-Y binding (42).
We have provided evidence for the systematic elevation of APE1 and MDR1 levels in NSCLC specimens, which underscores the physiological relevance of our study. Pairwise comparisons between normal and cancer tissues indicated that elevated levels of APE1 in NSCLC are associated with enhanced MDR1 expression. Consistent with this, one recent study demonstrated that the APE1 protein level in the nuclear and cytoplasmic fractions of NSCLC tissues is significantly higher than those in the nontumor region (65). We thus postulate that the APE1 level could be used as a predictive marker for multidrug resistance in NSCLC. At the same time, the nonlinear correlation of APE1 and MDR1 levels in both normal and cancer tissues is consistent with the possibility that MDR1 expression is regulated by multiple factors, including APE1.
In summary, we have shown that APE1 and its acetylation play an important role in YB-1-mediated MDR1 expression, leading to drug resistance, raising the possibility that the acetylation-dependent transcriptional regulatory function of APE1 could be a key parameter in drug resistance. Thus, the attenuation of the AcAPE1 level by a small molecular inhibitor(s) is a potential approach for reversing drug resistance. Our studies also demonstrate that posttranslational modifications provide a subtle means for channeling multifunctional proteins such as APE1 to diverse functions. Whether this is a general mechanism remains to be established.
Supplementary Material
Acknowledgments
We thank George W. Teebor, New York University School of Medicine, for the YB-1 expression plasmid; Michael Gottesman, NCI, for the MCF-7MDR1 cell line; and Lori Bernstein, Texas A&M University System Health Science Center, College Station, TX, for the FLAG-YB-1 construct. We thank Peter B. Ernst, University of Virginia, for statistical analysis. We also thank D. Konkel for critically reading and editing the manuscript and Wanda Smith for secretarial assistance.
This work was supported by American Heart Association grants 0565008Y (to K.K.B.), R01 ES08457, R01 CA53791, and P50 ES66076 (to S.M.).
Footnotes
Published ahead of print on 22 September 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bargou, R. C., K. Jurchott, C. Wagener, S. Bergmann, S. Metzner, K. Bommert, M. Y. Mapara, K. J. Winzer, M. Dietel, B. Dorken, and H. D. Royer. 1997. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat. Med. 3447-450. [DOI] [PubMed] [Google Scholar]
- 2.Bhakat, K. K., T. Izumi, S. H. Yang, T. K. Hazra, and S. Mitra. 2003. Role of acetylated human AP-endonuclease (APE1/Ref-1) in regulation of the parathyroid hormone gene. EMBO J. 226299-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakat, K. K., S. K. Mokkapati, I. Boldogh, T. K. Hazra, and S. Mitra. 2006. Acetylation of human 8-oxoguanine-DNA glycosylase by p300 and its role in 8-oxoguanine repair in vivo. Mol. Cell. Biol. 261654-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobola, M. S., L. S. Finn, R. G. Ellenbogen, J. R. Geyer, M. S. Berger, J. M. Braga, E. H. Meade, M. E. Gross, and J. R. Silber. 2005. Apurinic/apyrimidinic endonuclease activity is associated with response to radiation and chemotherapy in medulloblastoma and primitive neuroectodermal tumors. Clin. Cancer Res. 117405-7414. [DOI] [PubMed] [Google Scholar]
- 5.Boeckman, H. J., K. S. Trego, and J. J. Turchi. 2005. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol. Cancer Res. 3277-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borst, P., and R. O. Elferink. 2002. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 71537-592. [DOI] [PubMed] [Google Scholar]
- 7.Chattopadhyay, R., L. Wiederhold, B. Szczesny, I. Boldogh, T. K. Hazra, T. Izumi, and S. Mitra. 2006. Identification and characterization of mitochondrial abasic (AP)-endonuclease in mammalian cells. Nucleic Acids Res. 342067-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhary, P. M., and I. B. Roninson. 1993. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J. Natl. Cancer Inst. 85632-639. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, R., S. Currier, O. Kaplan, E. Lovelace, V. Boulay, M. M. Gottesman, and R. B. Dickson. 1992. Effect of P-glycoprotein expression on sensitivity to hormones in MCF-7 human breast cancer cells. J. Natl. Cancer Inst. 841506-1512. [DOI] [PubMed] [Google Scholar]
- 10.Damia, G., L. Imperatori, M. Stefanini, and M. D'Incalci. 1996. Sensitivity of CHO mutant cell lines with specific defects in nucleotide excision repair to different anti-cancer agents. Int. J. Cancer 66779-783. [DOI] [PubMed] [Google Scholar]
- 11.Daviet, S., S. Couve-Privat, L. Gros, K. Shinozuka, H. Ide, M. Saparbaev, and A. A. Ishchenko. 2007. Major oxidative products of cytosine are substrates for the nucleotide incision repair pathway. DNA Repair (Amsterdam) 68-18. [DOI] [PubMed] [Google Scholar]
- 12.Demple, B., and L. Harrison. 1994. Repair of oxidative damage to DNA: enzymology and biology. Annu. Rev. Biochem. 63915-948. [DOI] [PubMed] [Google Scholar]
- 13.Evans, A. R., M. Limp-Foster, and M. R. Kelley. 2000. Going APE over ref-1. Mutat. Res. 46183-108. [DOI] [PubMed] [Google Scholar]
- 14.Fantini, D., C. Vascotto, M. Deganuto, N. Bivi, S. Gustincich, G. Marcon, F. Quadrifoglio, G. Damante, K. K. Bhakat, S. Mitra, and G. Tell. 2008. APE1/Ref-1 regulates PTEN expression mediated by Egr-1. Free Radic. Res. 4220-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freitas, S., D. H. Moore, H. Michael, and M. R. Kelley. 2003. Studies of apurinic/apyrimidinic endonuclease/ref-1 expression in epithelial ovarian cancer: correlations with tumor progression and platinum resistance. Clin. Cancer Res. 94689-4694. [PubMed] [Google Scholar]
- 16.Fritz, G., S. Grosch, M. Tomicic, and B. Kaina. 2003. APE/Ref-1 and the mammalian response to genotoxic stress. Toxicology 19367-78. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs, S., J. Philippe, P. Corvol, and F. Pinet. 2003. Implication of Ref-1 in the repression of renin gene transcription by intracellular calcium. J. Hypertens. 21327-335. [DOI] [PubMed] [Google Scholar]
- 18.Fujita, T., K. Ito, H. Izumi, M. Kimura, M. Sano, H. Nakagomi, K. Maeno, Y. Hama, K. Shingu, S. Tsuchiya, K. Kohno, and M. Fujimori. 2005. Increased nuclear localization of transcription factor Y-box binding protein 1 accompanied by up-regulation of P-glycoprotein in breast cancer pretreated with paclitaxel. Clin. Cancer Res. 118837-8844. [DOI] [PubMed] [Google Scholar]
- 19.Gao, Z., Z. Gao, J. Z. Fields, and B. M. Boman. 1998. Co-transfection of MDR1 and MRP antisense RNAs abolishes the drug resistance in multidrug-resistant human lung cancer cells. Anticancer Res. 183073-3076. [PubMed] [Google Scholar]
- 20.Gottesman, M. M., T. Fojo, and S. E. Bates. 2002. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 248-58. [DOI] [PubMed] [Google Scholar]
- 21.Gros, L., A. A. Ishchenko, H. Ide, R. H. Elder, and M. K. Saparbaev. 2004. The major human AP endonuclease (Ape1) is involved in the nucleotide incision repair pathway. Nucleic Acids Res. 3273-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hang, B., A. Chenna, H. Fraenkel-Conrat, and B. Singer. 1996. An unusual mechanism for the major human apurinic/apyrimidinic (AP) endonuclease involving 5′ cleavage of DNA containing a benzene-derived exocyclic adduct in the absence of an AP site. Proc. Natl. Acad. Sci. USA 9313737-13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higashi, K., Y. Inagaki, K. Fujimori, A. Nakao, H. Kaneko, and I. Nakatsuka. 2003. Interferon-gamma interferes with transforming growth factor-beta signaling through direct interaction of YB-1 with Smad3. J. Biol. Chem. 27843470-43479. [DOI] [PubMed] [Google Scholar]
- 24.Higashi, K., Y. Inagaki, N. Suzuki, S. Mitsui, A. Mauviel, H. Kaneko, and I. Nakatsuka. 2003. Y-box-binding protein YB-1 mediates transcriptional repression of human alpha 2(I) collagen gene expression by interferon-gamma. J. Biol. Chem. 2785156-5162. [DOI] [PubMed] [Google Scholar]
- 25.Horton, J. K., G. Roy, J. T. Piper, B. Van Houten, Y. C. Awasthi, S. Mitra, M. A. Alaoui-Jamali, I. Boldogh, and S. S. Singhal. 1999. Characterization of a chlorambucil-resistant human ovarian carcinoma cell line overexpressing glutathione S-transferase mu. Biochem. Pharmacol. 58693-702. [DOI] [PubMed] [Google Scholar]
- 26.Ise, T., G. Nagatani, T. Imamura, K. Kato, H. Takano, M. Nomoto, H. Izumi, H. Ohmori, T. Okamoto, T. Ohga, T. Uchiumi, M. Kuwano, and K. Kohno. 1999. Transcription factor Y-box binding protein 1 binds preferentially to cisplatin-modified DNA and interacts with proliferating cell nuclear antigen. Cancer Res. 59342-346. [PubMed] [Google Scholar]
- 27.Izumi, T., D. B. Brown, C. V. Naidu, K. K. Bhakat, M. A. Macinnes, H. Saito, D. J. Chen, and S. Mitra. 2005. Two essential but distinct functions of the mammalian abasic endonuclease. Proc. Natl. Acad. Sci. USA 1025739-5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi, T., and S. Mitra. 1998. Deletion analysis of human AP-endonuclease: minimum sequence required for the endonuclease activity. Carcinogenesis 19525-527. [DOI] [PubMed] [Google Scholar]
- 29.Izumi, T., L. R. Wiederhold, G. Roy, R. Roy, A. Jaiswal, K. K. Bhakat, S. Mitra, and T. K. Hazra. 2003. Mammalian DNA base excision repair proteins: their interactions and role in repair of oxidative DNA damage. Toxicology 19343-65. [DOI] [PubMed] [Google Scholar]
- 30.Jayaraman, L., N. C. Moorthy, K. G. Murthy, J. L. Manley, M. Bustin, and C. Prives. 1998. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 12462-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayaraman, L., K. G. Murthy, C. Zhu, T. Curran, S. Xanthoudakis, and C. Prives. 1997. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 11558-570. [DOI] [PubMed] [Google Scholar]
- 32.Jiang, Y., C. Guo, M. R. Vasko, and M. R. Kelley. 2008. Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons. Cancer Res. 686425-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin, S., and K. W. Scotto. 1998. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol. Cell. Biol. 184377-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley, M. R., L. Cheng, R. Foster, R. Tritt, J. Jiang, J. Broshears, and M. Koch. 2001. Elevated and altered expression of the multifunctional DNA base excision repair and redox enzyme Ape1/ref-1 in prostate cancer. Clin. Cancer Res. 7824-830. [PubMed] [Google Scholar]
- 35.Kohno, K., H. Izumi, T. Uchiumi, M. Ashizuka, and M. Kuwano. 2003. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays 25691-698. [DOI] [PubMed] [Google Scholar]
- 36.Kohno, K., S. Sato, H. Takano, K. Matsuo, and M. Kuwano. 1989. The direct activation of human multidrug resistance gene (MDR1) by anticancer agents. Biochem. Biophys. Res. Commun. 1651415-1421. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig, D. L., M. A. MacInnes, Y. Takiguchi, P. E. Purtymun, M. Henrie, M. Flannery, J. Meneses, R. A. Pedersen, and D. J. Chen. 1998. A murine AP-endonuclease gene-targeted deficiency with post-implantation embryonic progression and ionizing radiation sensitivity. Mutat. Res. 40917-29. [DOI] [PubMed] [Google Scholar]
- 38.Marenstein, D. R., M. T. Ocampo, M. K. Chan, A. Altamirano, A. K. Basu, R. J. Boorstein, R. P. Cunningham, and G. W. Teebor. 2001. Stimulation of human endonuclease III by Y box-binding protein 1 (DNA-binding protein B). Interaction between a base excision repair enzyme and a transcription factor. J. Biol. Chem. 27621242-21249. [DOI] [PubMed] [Google Scholar]
- 39.Meira, L. B., D. L. Cheo, R. E. Hammer, D. K. Burns, A. Reis, and E. C. Friedberg. 1997. Genetic interaction between HAP1/REF-1 and p53. Nat. Genet. 17145. [DOI] [PubMed] [Google Scholar]
- 40.Mitra, S., T. Izumi, I. Boldogh, K. K. Bhakat, J. W. Hill, and T. K. Hazra. 2002. Choreography of oxidative damage repair in mammalian genomes. Free Radic. Biol. Med. 3315-28. [DOI] [PubMed] [Google Scholar]
- 41.Mol, C. D., T. Izumi, S. Mitra, and J. A. Tainer. 2000. DNA-bound structures and mutants reveal abasic DNA binding by APE1 and DNA repair coordination. Nature 403451-456. [DOI] [PubMed] [Google Scholar]
- 42.Nakshatri, H., P. Bhat-Nakshatri, and R. A. Currie. 1996. Subunit association and DNA binding activity of the heterotrimeric transcription factor NF-Y is regulated by cellular redox. J. Biol. Chem. 27128784-28791. [DOI] [PubMed] [Google Scholar]
- 43.Oda, Y., A. Sakamoto, N. Shinohara, T. Ohga, T. Uchiumi, K. Kohno, M. Tsuneyoshi, M. Kuwano, and Y. Iwamoto. 1998. Nuclear expression of YB-1 protein correlates with P-glycoprotein expression in human osteosarcoma. Clin. Cancer Res. 42273-2277. [PubMed] [Google Scholar]
- 44.Ogryzko, V. V., R. L. Schiltz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87953-959. [DOI] [PubMed] [Google Scholar]
- 45.Ohga, T., K. Koike, M. Ono, Y. Makino, Y. Itagaki, M. Tanimoto, M. Kuwano, and K. Kohno. 1996. Role of the human Y box-binding protein YB-1 in cellular sensitivity to the DNA-damaging agents cisplatin, mitomycin C, and ultraviolet light. Cancer Res. 564224-4228. [PubMed] [Google Scholar]
- 46.Ohga, T., T. Uchiumi, Y. Makino, K. Koike, M. Wada, M. Kuwano, and K. Kohno. 1998. Direct involvement of the Y-box binding protein YB-1 in genotoxic stress-induced activation of the human multidrug resistance 1 gene. J. Biol. Chem. 2735997-6000. [DOI] [PubMed] [Google Scholar]
- 47.Okamoto, T., H. Izumi, T. Imamura, H. Takano, T. Ise, T. Uchiumi, M. Kuwano, and K. Kohno. 2000. Direct interaction of p53 with the Y-box binding protein, YB-1: a mechanism for regulation of human gene expression. Oncogene 196194-6202. [DOI] [PubMed] [Google Scholar]
- 48.Okazaki, T., U. Chung, T. Nishishita, S. Ebisu, S. Usuda, S. Mishiro, S. Xanthoudakis, T. Igarashi, and E. Ogata. 1994. A redox factor protein, ref1, is involved in negative gene regulation by extracellular calcium. J. Biol. Chem. 26927855-27862. [PubMed] [Google Scholar]
- 49.Puglisi, F., G. Aprile, A. M. Minisini, F. Barbone, P. Cataldi, G. Tell, M. R. Kelley, G. Damante, C. A. Beltrami, and C. Di Loreto. 2001. Prognostic significance of Ape1/ref-1 subcellular localization in non-small cell lung carcinomas. Anticancer Res. 214041-4049. [PubMed] [Google Scholar]
- 50.Ramana, C. V., I. Boldogh, T. Izumi, and S. Mitra. 1998. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc. Natl. Acad. Sci. USA 955061-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson, K. A., H. A. Bullock, Y. Xu, R. Tritt, E. Zimmerman, T. M. Ulbright, R. S. Foster, L. H. Einhorn, and M. R. Kelley. 2001. Altered expression of Ape1/ref-1 in germ cell tumors and overexpression in NT2 cells confers resistance to bleomycin and radiation. Cancer Res. 612220-2225. [PubMed] [Google Scholar]
- 52.Robertson, K. A., D. P. Hill, Y. Xu, L. Liu, S. Van Epps, D. M. Hockenbery, J. R. Park, T. M. Wilson, and M. R. Kelley. 1997. Down-regulation of apurinic/apyrimidinic endonuclease expression is associated with the induction of apoptosis in differentiating myeloid leukemia cells. Cell Growth Differ. 8443-449. [PubMed] [Google Scholar]
- 53.Roy, S., E. Kenny, S. Kennedy, A. Larkin, J. Ballot, M. Perez De Villarreal, J. Crown, and L. O'Driscoll. 2007. MDR1/P-glycoprotein and MRP-1 mRNA and protein expression in non-small cell lung cancer. Anticancer Res. 271325-1330. [PubMed] [Google Scholar]
- 54.Shibahara, K., T. Uchiumi, T. Fukuda, S. Kura, Y. Tominaga, Y. Maehara, K. Kohno, Y. Nakabeppu, T. Tsuzuki, and M. Kuwano. 2004. Targeted disruption of one allele of the Y-box binding protein-1 (YB-1) gene in mouse embryonic stem cells and increased sensitivity to cisplatin and mitomycin C. Cancer Sci. 95348-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu, N., K. Sugimoto, J. Tang, T. Nishi, I. Sato, M. Hiramoto, S. Aizawa, M. Hatakeyama, R. Ohba, H. Hatori, T. Yoshikawa, F. Suzuki, A. Oomori, H. Tanaka, H. Kawaguchi, H. Watanabe, and H. Handa. 2000. High-performance affinity beads for identifying drug receptors. Nat. Biotechnol. 18877-881. [DOI] [PubMed] [Google Scholar]
- 56.Szczesny, B., K. K. Bhakat, S. Mitra, and I. Boldogh. 2004. Age-dependent modulation of DNA repair enzymes by covalent modification and subcellular distribution. Mech. Ageing Dev. 125755-765. [DOI] [PubMed] [Google Scholar]
- 57.Uchiumi, T., K. Kohno, H. Tanimura, K. Matsuo, S. Sato, Y. Uchida, and M. Kuwano. 1993. Enhanced expression of the human multidrug resistance 1 gene in response to UV light irradiation. Cell Growth Differ. 4147-157. [PubMed] [Google Scholar]
- 58.Volm, M., R. Koomagi, J. Mattern, and T. Efferth. 2002. Protein expression profiles indicative for drug resistance of non-small cell lung cancer. Br. J. Cancer 87251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker, L. J., C. N. Robson, E. Black, D. Gillespie, and I. D. Hickson. 1993. Identification of residues in the human DNA repair enzyme HAP1 (Ref-1) that are essential for redox regulation of Jun DNA binding. Mol. Cell. Biol. 135370-5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, D., M. Luo, and M. R. Kelley. 2004. Human apurinic endonuclease 1 (APE1) expression and prognostic significance in osteosarcoma: enhanced sensitivity of osteosarcoma to DNA damaging agents using silencing RNA APE1 expression inhibition. Mol. Cancer Ther. 3679-686. [PubMed] [Google Scholar]
- 61.Wiederhold, L., J. B. Leppard, P. Kedar, F. Karimi-Busheri, A. Rasouli-Nia, M. Weinfeld, A. E. Tomkinson, T. Izumi, R. Prasad, S. H. Wilson, S. Mitra, and T. K. Hazra. 2004. AP endonuclease-independent DNA base excision repair in human cells. Mol. Cell 15209-220. [DOI] [PubMed] [Google Scholar]
- 62.Xanthoudakis, S., G. Miao, F. Wang, Y. C. Pan, and T. Curran. 1992. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 113323-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xanthoudakis, S., R. J. Smeyne, J. D. Wallace, and T. Curran. 1996. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc. Natl. Acad. Sci. USA 938919-8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu, Y., D. H. Moore, J. Broshears, L. Liu, T. M. Wilson, and M. R. Kelley. 1997. The apurinic/apyrimidinic endonuclease (APE/ref-1) DNA repair enzyme is elevated in premalignant and malignant cervical cancer. Anticancer Res. 173713-3719. [PubMed] [Google Scholar]
- 65.Yoo, D. G., Y. J. Song, E. J. Cho, S. K. Lee, J. B. Park, J. H. Yu, S. P. Lim, J. M. Kim, and B. H. Jeon. 2008. Alteration of APE1/ref-1 expression in non-small cell lung cancer: the implications of impaired extracellular superoxide dismutase and catalase antioxidant systems. Lung Cancer 60277-284. [DOI] [PubMed] [Google Scholar]
- 66.Zaky, A., C. Busso, T. Izumi, R. Chattopadhyay, A. Bassiouny, S. Mitra, and K. K. Bhakat. 2008. Regulation of the human AP-endonuclease (APE1/Ref-1) expression by the tumor suppressor p53 in response to DNA damage. Nucleic Acids Res. 361555-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.