Abstract
The nuclear receptor Ad4BP/SF-1 is essential for development of the adrenal cortex and the gonads, which derive from a common adrenogonadal primordium. The adrenal cortex subsequently forms morphologically distinct compartments: the inner (fetal) and outer (definitive or adult) zones. Despite considerable effort, the mechanisms that mediate the differential development of the adrenal and gonadal primordia and the fetal and adult adrenal cortices remain incompletely understood. We previously identified a fetal adrenal-specific enhancer (FAdE) in the Ad4BP/SF-1 locus that directs transgene expression to the fetal adrenal cortex and demonstrated that this enhancer is autoregulated by Ad4BP/SF-1. We now combine the FAdE with the Cre/loxP system to trace cell lineages in which the FAdE was active at some stage in development. These lineage-tracing studies establish definitively that the adult cortex derives from precursor cells in the fetal cortex in which the FAdE was activated before the organization into two distinct zones. The potential of these fetal adrenocortical cells to enter the pathway that eventuates in cells of the adult cortex disappeared by embryonic day 14.5. Thus, these studies demonstrate a direct link between the fetal and adult cortices involving a transition that must occur before a specific stage of development.
The two major steroidogenic organs are the adrenal cortex and the gonads (ovaries or testes). The adrenal cortex produces mineralocorticoids and glucocorticoids, corticosteroid hormones which are essential to maintain fluid and electrolyte balance, to modulate intermediary metabolism, and to downregulate inflammatory processes, while the gonads synthesize estrogens and androgens, sex steroids which mediate female and male sex differentiation, respectively. Many of the same steroidogenic enzymes catalyze essential reactions in both the adrenal and gonadal steroidogenic pathways. Moreover, steroidogenesis in both organs is regulated by trophic hormones controlled by the hypothalamus-pituitary endocrine axes (27). Finally, studies that examined the expression of the nuclear receptor Ad4BP/SF-1 (NR5A1) identified a common pool of Ad4BP/SF-1-positive precursor cells that contributed to both the adrenal cortex and the gonads, designated the adrenogonadal primordium (AGP) (5, 9). Despite considerable effort, the mechanisms that establish the AGP and direct its subsequent separation into two distinct steroidogenic organs remain poorly understood.
As the adrenal gland emerges rostral to the kidney, the fetal adrenal cortex contains two distinct cell types: small, tightly packed outer cells termed the adult/definitive zone and larger, irregularly aligned inner cells known as the fetal zone. The fetal zone is particularly prominent in the adrenal glands of humans and other primates, whereas there has been some debate regarding its existence in the rodent adrenal (26). Recently, we identified a fetal adrenal-specific enhancer (FAdE) of the Ad4BP/SF-1 gene, which allowed us to demonstrate a transient fetal zone in mice (40). From the later fetal stages to puberty, cells of the adult zone increased in number, whereas those of the fetal zone decreased in number and accumulated at the boundary of the cortex and medulla. Thereafter, the fetal zone, originally termed the X zone in histological studies with mice, gradually disappeared. Thus, the transition from the fetal to the adult adrenal gland possibly involves a similar process in most mammalian species. However, the precise developmental relation of the fetal and adult zones is poorly understood.
Since tissue-specific enhancers are key determinants of tissue-specific gene expression, study of enhancer function should provide important insights into the mechanisms underlying tissue development. Recently, different enhancers that selectively drive the expression of Ad4BP/SF-1 to the ventromedial hypothalamic nucleus (29), the fetal adrenal cortex (40), or pituitary gonadotropes (30) were identified, and a number of tissue-specific upstream regulators of the Ad4BP/SF-1 gene were defined. Interestingly, the FAdE, which directs expression to the fetal adrenal gland, includes elements that bind homeobox-containing proteins, which initiate Ad4BP/SF-1 expression, and binding sites for Ad4BP/SF-1 itself, which maintains the expression of Ad4BP/SF-1 in an autoregulatory loop (40). In the present study, a transgene (Tg) in which the FAdE-directed expression of Cre recombinase was used to trace the fate of the fetal adrenocortical cells. These studies demonstrate that cells of the adult adrenal cortex derive from precursors in which the FAdE was transiently active, providing direct evidence for the common origin of the fetal and adult zones of the adrenal cortex.
MATERIALS AND METHODS
DNA preparation for the generation of Tg mice.
To place the expression of Cre recombinase under the control of the FAdE, FAdE-Ad4BP-IRES-Cre was constructed as follows. The internal ribosome entry site (IRES) fragment (SmaI-NcoI) prepared from pIRES2-enhanced green fluorescent protein (EGFP) (Clontech) and Cre coding sequences (XhoI-HindIII fragment) from pBS185 (Gibco) were ligated, and the resulting fragment was cloned into pBluescript SK (SmaI-HindIII sites) (Stratagene). An Ad4BP/SF-1 Tg cassette containing the FAdE and 5.8 kb of the 5′ flanking region (40) was inserted upstream of IRES-Cre to generate FAdE-Ad4BP-IRES-Cre. To provide tamoxifen-induced Cre activity, FAdE-Ad4BP-Cre-ERT2 was constructed by cloning an EcoRI-NotI fragment from pGS-Cre-ERT2 (10) into SplI (blunt-ended)-NotI sites of the Ad4BP/SF-1 Tg cassette to generate Cre-ERT2. The FAdE-Hsp68-EGFP construct was described previously (40).
Generation of Tg mice and tamoxifen treatment.
Tg mice were generated as described previously (7). The recombinant plasmids FAdE-Ad4BP-IRES-Cre, FAdE-Ad4BP-Cre-ERT2, and FAdE-Hsp68-EGFP were digested with NotI-SalI, NotI-SalI, and XhoI, respectively, and the linearized DNAs were used for microinjection. Founder mice were identified by PCR with primers for lacZ (5′-GCCGAAATCCCGAATCTCTATC-3′ and 5′-GATTCATTCCCCAGCGACCAG-3′), EGFP (5′-GAGCTGGACGGCGACGTAAAC-3′ and 5′-CACCTTGATGCCGTTCTTCTGC-3′), and Cre (5′-TGCCACGACCAAGTGACAGCA-3′ and 5′-CCGGCAAAACAGGTAGTTATTC-3′). To test the expression and activity of Cre or Cre-ERT2, the Tg mouse lines harboring Cre and Cre-ERT2 were crossed with the ROSA26R Cre reporter allele (31). Tamoxifen (T5648; 10 mg/ml; Sigma) dissolved in corn oil-ethanol (9:1) was injected into pregnant females (4 mg/mouse intraperitoneally) at the indicated time points. Mice were maintained and experimental protocols performed in accord with the guidelines of Institutional Animal Care and Use Committee of National Institute for Basic Biology.
LacZ staining, immunohistochemistry, and in situ hybridization.
LacZ activity in fetal tissues was examined as described previously (7). After being stained, the tissue samples were fixed with 4% paraformaldehyde in phosphate-buffered saline (PFA-PBS) for 6 h at 4°C, embedded in optimal-cutting-temperature compound, and sectioned at a 16- to 18-μm thickness. For immunohistochemical analyses of LacZ, samples were fixed for 2 to 3 h in PFA-PBS at 4°C and then embedded in paraffin. Five-micrometer sections were incubated with rabbit anti-Ad4BP antiserum (1/2,000) (24), rat monoclonal antibody for Ad4BP/SF-1 (30), guinea pig polyclonal antibody for Dax-1 (25), or rabbit anti-β-galactosidase (provided by K. Mihara and M. Sakaguchi [Kyushu University]). For double immunofluorescence analyses, the sections were deparaffinized, rehydrated, and boiled in a microwave oven for 10 min in 10 mM citrate (pH 2.0) for antigen retrieval. After being washed with PBS three times for 5 min, the sections were blocked with 2% skim milk in PBS for 30 min and incubated with anti-β-galactosidase (LacZ) antibody overnight at 4°C. After being washed, the sections were incubated with Alexa 488 goat anti-rabbit antibody (Invitrogen) for 1 to 2 h. After being washed with PBS, the sections were blocked again with 2% skim milk in PBS and incubated with the rat monoclonal antibody for Ad4BP/SF-1 or antibody for Dax-1 in 2% skim milk. After being washed with PBS, the sections were incubated with Cy3-conjugated goat anti-rat or Cy3-conjugated donkey anti-guinea pig (Jackson ImmunoResearch, West Grove, PA) and washed with PBS.
For whole-mount immunohistochemistry, embryos were fixed with 4% PFA for 30 min at 4°C and washed with 0.1% Triton X-100 in PBS (PBTX) three times for 10 min. The fixed embryos were dehydrated using an ethanol-PBTX series and kept in absolute ethanol for 30 min at room temperature. Following rehydration with graded ethanol-PBTX washes, embryos were incubated in blocking solution (5% bovine serum albumin with 1% heat-inactivated sheep serum in PBTX) for 1 h at room temperature and thereafter with rabbit polyclonal anti-Ad4BP/SF-1 antibody in blocking solution overnight at 4°C. After being washed with PBTX four times for 1 h, the embryos were incubated with Alexa 488-anti-rabbit immunoglobulin G for 4 h and washed with PBTX four times for 1 h. Images were collected on an Olympus SZX-16 microscope and processed using Adobe Photoshop. Whole-mount in situ hybridization was performed as described previously (37) with a digoxigenin-labeled probe (Roche Molecular Biochemicals, Germany) for Ad4BP/SF-1 (accession no. AB000490, 192 to 2407).
For in situ hybridization and LacZ immunohistochemistry analyses in sections of mouse embryos, samples were fixed with 0.5% PFA-PBS for 2 to 3 h at 4°C and then embedded in optimal-cutting-temperature compound. Transverse sections cut to a 10-μm thickness were processed for in situ hybridization with digoxigenin-labeled probes. The probes were 3β-hydroxysteroid dehydrogenase (3β-Hsd; accession no. NM_008293, 512 to 1523), cholesterol side chain cleavage enzyme (Cyp11a1; accession no. NM_019779, 132 to 691), and 21-hydroxylase (Cyp21; accession no. NM_009995, 469 to 1554). Prehybridization and hybridization were done in buffer containing 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 0.1% Tween 20, 1 mg/ml yeast (Saccharomyces cerevisiae) RNA, 0.1 mg/ml heparin, 5 mM EDTA, and 50% formamide at 65°C overnight. After being washed with 1× SSC and 0.1× SSC (both twice at 65°C for 30 min), samples were incubated with antidigoxigenin antibody (1/2,000; Roche) in PBS with 10% sheep serum and 2% bovine serum albumin and washed three times for 30 min with 100 mM maleic acid, 150 mM NaCl, 0.1% Tween 20 (pH 7.5). The alkaline phosphatase activity was detected by nitro blue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate). The samples then were processed for immunohistochemistry. Briefly, samples were incubated with 0.2 M glycine (pH 2.0) for 30 min at room temperature, washed with PBS, and fixed with 4% PFA for 30 min at room temperature. After being washed twice with PBS for 5 min, the slides were blocked with 2% skim milk in PBS for 30 min at room temperature, incubated with rabbit anti-β-galactosidase, and detected using Alexa 488-goat anti-rabbit antibody as described above. To detect the expression of Cre, in situ hybridization probed with an EcoRI-XhoI fragment of pGS-Cre-ERT2 was performed. Transverse sections cut to a 10-μm thickness from the embryonic day 14.5 (E14.5) adrenals of FAdE-Ad4BP-IRES-Cre mice (line 1) were used.
Cell proliferation study.
To label dividing cells, bromodeoxyuridine (BrdU) (B-5002; Sigma) at 50 mg/kg of body weight was injected intraperitoneally to pregnant mice. One hour after injection, the pregnant females were sacrificed, and the fetuses were removed, fixed for 2 h in 4% PFA-PBS at 4°C, and embedded into paraffin with standard procedures. The adrenal glands were sectioned transversely at a 5-μm thickness; immunohistochemical analyses were performed on every 10th serial section (a total of six or seven sections, spanning roughly half of the adrenal gland). Sections to be analyzed were deparaffinized, rehydrated, and boiled in a microwave oven for 10 min in 10 mM citrate (pH 2.0) for antigen retrieval. After being washed with PBS three times for 5 min, the sections were blocked with 2% skim milk in PBS for 30 min and incubated overnight with mouse monoclonal anti-BrdU (B8434, 1:500; Sigma) and rabbit anti-β-galactosidase antibodies. After being washed, the sections were incubated with Alexa 488-conjugated goat anti-mouse antibody (Invitrogen) for 1 to 2 h and then with Cy3-conjugated goat anti-rabbit antibody (Jackson ImmunoResearch), washed with PBS three times, and mounted for fluorescence imaging. Data are expressed as percentages of LacZ-positive cells that were also positive for BrdU.
Luciferase assay.
HEK293T cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (HyClone Laboratories, Inc.) and penicillin-streptomycin (Invitrogen) at 37°C in 5% CO2. Cells were plated at 105 cells/well in 24-well plates 24 h before transfection and were transfected in triplicate with the relevant combination of plasmids by use of FuGENE 6 (Roche, Basel, Switzerland) according to the manufacturer's protocol. Combinations of total plasmids were 400 ng/well, this including the linearized luciferase reporter plasmid (100 ng), CMX-SF-1 (18) or pcDNA-Dax-1 as indicated below (see Fig. 6C), and cytomegalovirus-β-galactosidase (1 ng) as a control for transfection efficiency. The cells were harvested 48 h after transfection, and activities in cell lysates were determined using the luciferase reporter assay system (1501; Promega) and the β-galactosidase activity kit (T1007; ABI). The luciferase values were normalized to the β-galactosidase activity. The reporter plasmid was constructed by inserting the FAdE fragment (40) together with the minimal promoter of thymidine kinase of herpes simplex virus into the KpnI-HindIII sites of pGL3-Basic (Promega). To construct pcDNA-Dax-1, the mouse Dax-1 cDNA was derived from pSP65 (15) and inserted into HindIII-NotI sites of pcDNA3.1 (Invitrogen).
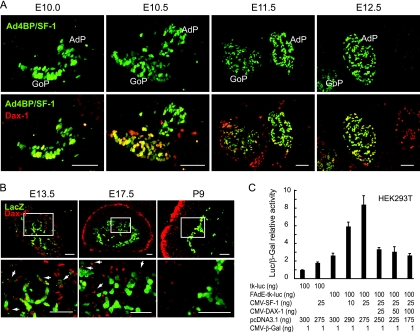
FIG. 6.
Expression of Ad4BP/SF-1 and Dax-1 in the developing gonads and adrenal glands. (A) Differential expression of Dax-1 in the developing gonads and adrenal glands. Cross sections of the urogenital region were prepared from the Ad4BP-LacZ-FAdE Tg fetuses at E10.0, E10.5, E11.5, and E12.5 and used for immunofluorescence analysis for Ad4BP/SF-1 (green) and Dax-1 (red). Expression of Ad4BP/SF-1 is shown at the top, while superimposed images of Dax-1 and Ad4BP/SF-1 immunostaining are shown at the bottom. GoP, gonadal primordium. Bars = 50 μm. (B) Expression of LacZ and Dax-1 in the adrenal glands of the Ad4BP-LacZ-FAdE Tg mice at E13.5, E17.5, and P9. Sections of the adrenal gland were subjected to immunofluorescent detection of LacZ (green) and Dax-1 (red). The areas enclosed by the rectangles are enlarged at the bottom. Arrows indicate cells expressing Dax-1 which have decreased expression of LacZ. Bars = 50 μm. (C) Stimulation of FAdE activity by Ad4BP/SF-1 and inhibitory effect of Dax-1. The mouse Ad4BP/SF-1 and Dax-1 expression constructs were cotransfected into HEK293T cells with reporter tk-luciferase (tk-luc) plasmids that either lacked or contained the FAdE as indicated. The relative luciferase activities were normalized with β-galactoside (β-Gal) activity and are shown as means ± standard deviations from triplicate transfections. CMV, cytomegalovirus promoter.
RESULTS
Downregulation of FAdE activity during fetal adrenal development.
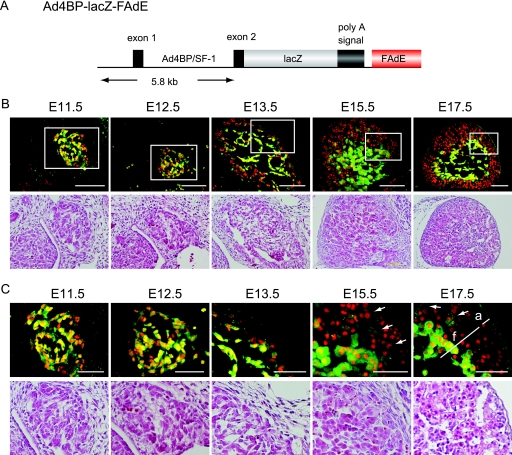
We previously studied the activity of the FAdE during adrenal development in mice harboring lacZ reporter Tgs (40). At E10.5, LacZ expression was observed in the anterior urogenital region (the presumptive adrenal primordium [AdP]), coincident with its separation into the adrenal and gonadal primordia (40). Expression persisted throughout the AdP until E12.5 to E13.5; by E17.5, expression was restricted to the inner part of the developing adrenal cortex (fetal zone). Eventually, these cells became the postnatal X zone and then disappeared with the onset of sex steroid production. These observations strongly suggested that the FAdE is active in the fetal but not in the adult adrenal cortex.
In the present study, we used immunohistochemistry to compare the expressions of Tg LacZ and endogenous Ad4BP/SF-1 in the fetal adrenal gland. The Ad4BP-LacZ-FAdE Tg directs the expression of the lacZ reporter to cells in which the FAdE is transcriptionally active and thus provides a dynamic indicator of enhancer function at different stages of development. The histological appearance of the developing adrenal gland at different stages at lower and higher magnifications is shown at the bottoms of Fig. 1B and C. At early stages (i.e., E11.5 and E12.5), LacZ was expressed in all Ad4BP/SF-1-immunoreactive cells, although the LacZ signal in a few cells appeared to be weaker. Cells that expressed neither LacZ nor Ad4BP/SF-1 were also present; these cells may have represented chromaffin cell precursors that were to have populated the adrenal medulla. By E13.5, the putative chromaffin cells (negative for both Ad4BP/SF-1 and LacZ) had migrated medially to form the adrenal medulla. While the expression of Ad4BP/SF-1 persisted throughout the cortex, LacZ expression was clearly diminished in cells in the outer region of the cortex but persisted in cells of the inner cortex. This differential expression of LacZ became more evident at E15.5: many outer cells gave no signal for LacZ, whereas most inner cells still expressed LacZ. Interestingly, some of the cells between the outer and inner cell layers expressed LacZ only weakly (Fig. 1C). By E17.5, the tightly packed outer adult and larger inner fetal zones were morphologically evident. The LacZ staining was undetectable in the cells of the outer adult zone, while LacZ expression varied within the fetal zone; some cells in the fetal zone expressed LacZ, and others were unstained. The different levels of LacZ expression in cells forming the adult and fetal zones suggest that the FAdE is regulated differentially in the two zones; however, the temporal decrease in LacZ expression in cells of the outer (adult) zone raised the possibility that cells in which the FAdE was transiently activated might also contribute to the adult zone.
FIG. 1.
Region-specific downregulation of LacZ expression in the developing fetal adrenal cortices in Ad4BP-LacZ-FAdE Tg mice. (A) Schematic representation of the Ad4BP-LacZ-FAdE Tg construct (40). A fragment that contains 5.8 kb upstream from the initiation methionine in the second exon of Ad4BP/SF-1 was used as the basal promoter. The FAdE was placed downstream of LacZ coding sequences and a poly(A) signal. (B) Lower-power views of sections of Ad4BP-LacZ-FAdE Tg fetuses at the indicated developmental stages stained with hematoxylin and eosin to reveal adrenal morphology (bottom) or with specific antibodies for LacZ (green) and rat monoclonal antibody for Ad4BP/SF-1 (red). Superimposed images of LacZ and Ad4BP/SF-1 immunolocalization are shown. (C) Higher-power views of hematoxylin-and-eosin staining (bottom) or immunostaining (top) for LacZ and Ad4BP/SF-1. The areas enclosed by the rectangles in the immunohistochemical studies shown in panel B are enlarged. The arrows in the E15.5 and E17.5 sections indicate Ad4BP/SF-1-positive cells displaying weak LacZ staining. Fetal (f) and adult (a) zones are indicated in the E17.5 section. Bars = 100 μm (B) and 50 μm (C).
Lineage tracing of the fetal adrenal cortex by use of FAdE function.
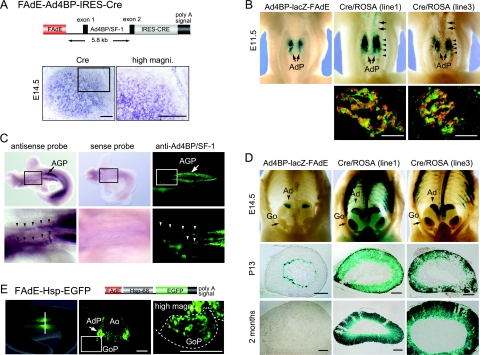
In order to trace cell lineages that at least transiently activated the FAdE, we generated Tg mice in which the expression of Cre recombinase was driven by the FAdE and the basal promoter of Ad4BP/SF-1 (FAdE-Ad4BP-IRES-Cre) (Fig. 2A). Four Cre-expressing Tg lines were generated. To confirm that Cre expression in FAdE-Ad4BP-IRES-Cre Tg mice corresponds to LacZ expression in Ad4BP-LacZ-FAdE Tg mice (Fig. 1), we performed in situ hybridization using adrenal sections from FAdE-Ad4BP-IRES-Cre mice (line 1) at E14.5. As expected, Cre mRNA in the Tg was distributed similar to LacZ in Ad4BP-LacZ-FAdE mice (Fig. 2A). The Cre-expressing Tg lines were mated with ROSA26R reporter mice (31). Since the cells that express the FAdE will permanently activate the Cre/ROSA Tg, the fate of cells that activated the FAdE can be traced thereafter by examining LacZ staining. When the whole abdominal staining patterns of the mice at E11.5 (lines 1 and 3 in Fig. 2B) were compared with those of an Ad4BP-LacZ-FAdE Tg mouse (Fig. 2B, left), LacZ was comparably expressed in the AdP (Fig. 2B). Dual labeling for LacZ and endogenous Ad4BP/SF-1 expression in the AdP revealed that LacZ staining overlapped with that of Ad4BP/SF-1 (Fig. 2B, bottom). This overlapping staining was quite similar to that for the Ad4BP-LacZ-FAdE Tg mouse shown in Fig. 1B, showing that the Cre activity is spatially and temporally controlled by the FAdE and that the cells in which the FAdE was activated can be traced thereafter.
FIG. 2.
Cell lineage tracing using FAdE activity. (A) Schematic representation of the FAdE-Ad4BP-IRES-Cre Tg and expression of Cre. IRES-Cre was inserted downstream of the 5.8-kb Ad4BP/SF-1 basal promoter and the FAdE. In situ hybridization was performed with Ad4BP-LacZ-FAdE Tg fetuses (line 1) at E14.5. Cre mRNA was detected in the inner part of cortical layer of the adrenal at this stage. The area enclosed by the rectangle in the middle panel is enlarged as the right panel. Bars = 100 μm. (B) Detection of LacZ expression as a surrogate marker of Cre activity driven by the FAdE. The FAdE-Ad4BP-IRES-Cre Tg mice (lines 1 and 3) were mated with ROSA26R mice (31), and LacZ activity in the Cre/ROSA Tg fetuses (lines 1 and 3) at E11.5 was examined. An Ad4BP-LacZ-FAdE Tg fetus is shown for comparison. The LacZ staining localized to the AdP of the control mouse (arrows). While strong staining in the two Cre/ROSA Tg mice was again seen for the AdP (arrows), staining was also detected anterior to the AdP and within the gonadal primordium (arrowheads). Sections of the AdP of Cre/ROSA Tg fetuses were immunostained with the antibodies to LacZ (green) and Ad4BP/SF-1 (red). Superimposed images are shown. Bars = 50 μm. (C) Detection of endogenous expression of Ad4BP/SF-1 in the region anterior to the AdP. In situ hybridization using RNA antisense probe (left) and sense probe (middle), and immunohistochemistry with antibody for Ad4BP/SF-1 (right), were performed with wild-type fetuses at E10.5. Strong signals of Ad4BP/SF-1 were detected in the AGP (arrows). The bottom panels show enlarged views of the areas enclosed by rectangles in the top panels. As indicated (arrowheads), both Ad4BP/SF-1 transcripts and protein were expressed in the region anterior to the AGP. In situ hybridization with the Ad4BP/SF-1 sense probe showed no specific signal. The dark caudal staining in the top left panel corresponds to signal in the AGP but is seen from a dorsal view because the sample is rotated. (D) Cell lineage tracing of the fetal adrenal cortex via Cre-mediated activation of LacZ expression. LacZ expression in Cre/ROSA Tg mice (lines 1 and 3) was examined at E14.5, P13, and 2 months after birth. For comparison, age-matched Ad4BP-LacZ-FAdE mice were examined. Since the fetal adrenal (X) zone disappears at puberty in males (8, 40), male mice at P13 (prepubertal) and 2 months after birth were used. The LacZ signal in the Ad4BP-LacZ-FAdE Tg mice remains at the inner part of the adrenal cortex at P13 but disappears at 2 months after birth; in contrast, LacZ expression in the Cre/ROSA Tg mice persisted throughout the adrenal cortex at all stages examined. Go, gonad. Bars = 200 μm. (E) Schematic presentation of the FAdE-Hsp-EGFP Tg construct. EGFP was placed downstream of the basal promoter of Hsp68 and FAdE as indicated. A representative fluorescence image of the FAdE-Hsp-EGFP Tg fetus (E11.0) is shown. A cross section (middle) of the Tg fetus at the indicated region in the left panel (vertical bar) reveals that EGFP is expressed strongly in the AdP and weakly in a part of the gonadal primordium (GoP). The area enclosed by the rectangle in the middle panel is enlarged as the right panel. The region of the gonadal primordium is enclosed by a dotted line. Ao; aorta; magni., magnification. Bars = 100 μm.
In addition to being observed for the AdP, weaker LacZ staining was observed for the developing gonad (Fig. 2B, lines 1 and 3) in the region anterior to the AdP (Fig. 2B, lines 1 and 3). This contrasts with what was found by analyses of Ad4BP-LacZ-FAdE Tg mouse fetuses, where the region anterior to the AdP at E11.5 was not stained. Anticipating that Ad4BP/SF-1 might be expressed in this anterior region at an earlier stage, we investigated the endogenous expression of Ad4BP/SF-1 at E10.5. Whole-mount in situ hybridization and immunohistochemical assays (Fig. 2C, left and right, respectively) revealed that Ad4BP/SF-1 expression is confined to a narrow strip of cells in the anterior region. Thus, this low expression of Ad4BP/SF-1 was not sufficient to direct detectable LacZ expression in Ad4BP-LacZ-FAdE Tg mice but apparently was sufficient to provide Cre-mediated activation of the Cre/ROSA Tg.
We next examined LacZ expression in Cre/ROSA Tg male mice (lines 1 and 3) at E14.5, postnatal day 13 (P13), and 2 months after birth (Fig. 2D). For comparison, we examined LacZ expression in age-matched samples prepared from Ad4BP-LacZ-FAdE Tg mice. While LacZ was expressed in the adrenal gland of Ad4BP-LacZ-FAdE Tg fetuses at E14.5, staining also was evident in the anterior part of the gonad and in the thoracic region (Fig. 2D). At P13, LacZ staining persisted at the inner part of the adrenal cortex (corresponding to the X zone) in Ad4BP-LacZ-FAdE Tg adrenal glands; this staining disappeared by 2 months after birth. By contrast, in lineage-mapping experiments with the Cre/ROSA Tg mice, LacZ clearly was detected not only in the fetal adrenal cortex but also throughout the adult cortex. Collectively, these results indicate that the adult adrenal cortex derives from cells in which the FAdE was transiently activated during development.
As noted above, some gonadal cells at E11.5 and the anterior region of the gonad at E14.5 in the Cre/ROSA Tg mice expressed LacZ (Fig. 2B and D). Since this construct contained the basal promoter of Ad4BP/SF-1, which can induce gene expression in the developing gonad (36, 40), it was unclear whether the basal promoter or the FAdE determined this gonadal LacZ expression. We therefore examined a separate Tg (FAdE-Hsp68-EGFP) in which the basal promoter of Hsp68 replaced that of Ad4BP/SF-1 (Fig. 2E). Consistent with analyses of Cre/ROSA Tg mice, cells in the AdP in these mice strongly expressed EGFP (Fig. 2E); again, however, a few gonadal cells also weakly expressed EGFP. These findings indicate that some cells that have activated the FAdE are present in the early developing gonad and that the Ad4BP/SF-1 basal promoter is not essential for this expression.
Temporal window in which FAdE-active cells can contribute to the adult adrenal cortex.
These observations demonstrated that fetal adrenal cells that activated the FAdE contributed to the adult adrenal cortex. To extend this observation, we generated Tg mice with Cre-ERT2 under the control of the FAdE (FAdE-Ad4BP-Cre-ERT2 in Fig. 3A). The Cre-ERT2 fusion protein is activated only in the presence of tamoxifen, such that Cre-mediated recombination should occur only when the FAdE stimulates the expression of Cre-ERT2 and tamoxifen activates the fusion protein. Three Tg lines (lines 4, 5, and 7) were mated with ROSA26R Cre reporter mice, tamoxifen was injected intraperitoneally into the pregnant mothers at 10.5 days postcoitum, and lacZ expression in the Cre-ERT2/ROSA fetuses was investigated at E14.5. Although the LacZ activity of Ad4BP-LacZ-FAdE Tg mice disappeared from the outer adrenocortical region by E14.5, the cells in this layer in all three Cre-ERT2/ROSA Tg mice retained LacZ expression (Fig. 3B); this finding argues that cells in the outer adult zone activated the FAdE only transiently.
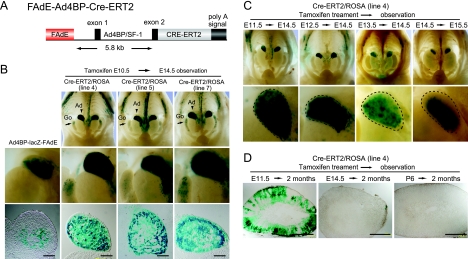
FIG. 3.
Restricted window in which the FAdE-active fetal adrenocortical cells can develop into adult adrenal cortex. (A) Schematic representation of the FAdE-Ad4BP-Cre-ERT2 Tg construct. Cre-ERT2 is placed downstream of the FAdE and the 5.8-kb promoter fragment of Ad4BP/SF-1. (B) Monitoring Cre-ERT2 activity through LacZ activity. The FAdE-Ad4BP-Cre-ERT2 Tg mice (lines 4, 5, and 7) were mated with ROSA26R mice, pregnant mice were treated with tamoxifen at E10.5, and the lacZ activity of the Cre-ERT2/ROSA Tg fetuses was examined at E14.5. For comparison, the LacZ expression for an Ad4BP-LacZ-FAdE Tg fetus at E14.5 is shown (left). Whole-abdomen views of the urogenital region are shown at the top (the control for the whole-abdomen view is shown in Fig. 2D), while enlarged views and sections of the adrenal gland are shown at the middle and bottom panels, respectively. LacZ is expressed throughout the adrenal cortices in fetuses from the three Cre-ERT2/ROSA Tg lines but only in the inner part of the cortex of the Ad4BP-LacZ-FAdE Tg fetus. Ad, adrenal gland; Go, gonad. Bars = 100 μm. (C) Restricted potential of the FAdE-active fetal adrenocortical cells to develop into the adult adrenal cortex. The Cre-ERT2/ROSA Tg fetuses (line 4) were treated with tamoxifen, thereby activating Cre-ERT2 at E11.5, E12.5, E13.5, or E14.5, and then subjected to LacZ staining at E14.5 or E15.5 as indicated. Whole-abdomen views are shown at the top, while enlarged views of the adrenal gland are shown at the bottom. The fetal adrenal glands are indicated by dotted lines. (D) Cell lineage tracing of the FAdE-active fetal adrenocortical cells in adult mice. The Cre-ERT2/ROSA Tg fetuses were treated with tamoxifen at E11.5, E14.5, or P6, and then LacZ expression in the adrenal glands of males was examined 2 months after birth. LacZ-positive cells were observed only in the mouse treated with tamoxifen at E11.5. Bars = 500 μm.
We next determined at which stage the fetal adrenal cells that activated the FAdE could contribute to the adult zone, activating Cre-ERT2 with tamoxifen at different time points of pregnancy and then analyzing LacZ expression at E14.5. When tamoxifen was injected at E11.5, LacZ was expressed throughout the adrenal cortex at E14.5 (Fig. 3C). When tamoxifen was administered at E12.5 or E13.5, there was a progressive decrease in LacZ activity in cells in the outer adult layer at E14.5. Finally, when tamoxifen was injected at E14.5, the adrenal expression of LacZ at E15.5 (Fig. 3C) was generally comparable to what was shown by the staining of Ad4BP-LacZ-FAdE (Fig. 3B).
This potential of the FAdE-active fetal adrenal cells to contribute to the adult (definitive) cortex was further examined by use of adult Cre-ERT2/ROSA mice. Following tamoxifen administration at E11.5, E14.5, or P6, mice were examined at 2 months after birth. As shown in Fig. 3D, most but not all adult cortical cells in mice treated at E11.5 expressed LacZ. However, if tamoxifen treatment was delayed until E14.5 (Fig. 3D, middle) or P6 (Fig. 3D, right), cells that retained LacZ expression were not present in the adult adrenal glands.
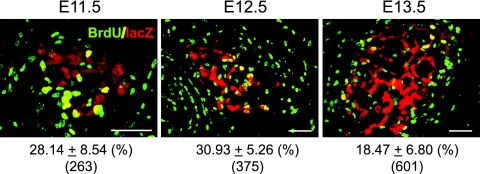
These observations suggested that FAdE-active cells proliferate rapidly at early stages of adrenal development and then gradually lose proliferative activity. In fact, a previous study examining wild-type adrenal glands at E15.5 and later stages showed that the outer cells were actively proliferating, while proliferation was significantly lower in the inner layer (28). Therefore, we used BrdU incorporation to examine whether these FAdE-active cells actively proliferate at earlier stages. As shown in Fig. 4, the LacZ-positive cells incorporated BrdU at all times examined (E11.5, E12.5, and E13.5), but the proliferation rate decreased as development progressed.
FIG. 4.
Proliferation of FAdE-positive cells at early stages of adrenal development. Cell proliferation in Ad4BP-LacZ-FAdE Tg mice was examined using BrdU incorporation at E11.5, E12.5, and E13.5. Shown are sections stained with anti-BrdU (green) and anti-LacZ (red) antibodies. Data are expressed as percentages of LacZ-positive cells that were also positive for BrdU. Means of proliferating and total cell numbers are shown below along with standard deviations. Bars = 50 μm.
Collectively, these data demonstrate that the cells in the outer (adult) adrenal cortex derive from precursors in which the FAdE was active during a specific stage of development (i.e., E11.5). By E14.5, however, tamoxifen administration to Cre-ERT2/ROSA mice did not lead to the incorporation of LacZ-expressing cells in the adult cortex. Thus, the potential ability of precursor cells that activate the FAdE to contribute to the adult adrenal cortex largely disappears by E14.5, even though the FAdE itself is still active in the fetal zone.
Expression of adrenal cortical cell markers in LacZ-positive cells.
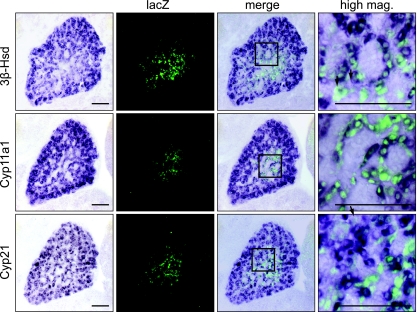
According to a prominent model of adrenal development, cells of the adult adrenal cortex in mice develop from a pool of stem cells immediately adjacent to the capsule (16, 21-23, 39). While not incompatible with this model, our data argue that cells of the adult cortex arise from cells in which the FAdE is active transiently, which then undergo a transition to the “adult” phenotype and extinguish FAdE maintenance. To explore further the properties of these putative LacZ-positive transitional precursors, we compared the adrenal expression of LacZ in Ad4BP-LacZ-FAdE Tg mice with that of three steroidogenic enzymes: 3β-Hsd, cholesterol Cyp11a1, and Cyp21. At E13.5, E15.5, and E17.5, all three markers were detected in nearly all cells of the adrenal cortex (see Fig. S1 in the supplemental material). In double-labeled sections at E14.5, most of the LacZ-positive cells also expressed the three steroidogenic enzymes; the few cells that expressed LacZ but not the steroidogenic enzymes (Fig. 5, right) may not yet have fully acquired the steroidogenic phenotype. By E17.5, all LacZ-positive cells also expressed 3β-Hsd (see Fig. S1B in the supplemental material). Taken together, these data show that LacZ-positive cells also express several steroidogenic enzymes in a manner indistinguishable from that of other Ad4BP/SF-1-positive cells, such that a distinct population of cells cannot be defined by the differential expression of the steroidogenic program.
FIG. 5.
Expression of steroidogenic enzymes in the developing adrenal glands. Three steroidogenic enzymes, 3β-Hsd, Cyp11a1, and Cyp21, were analyzed by combined in situ hybridization (blue) and immunofluorescence for LacZ (green). Cross sections of the adrenal gland were prepared from the Ad4BP-LacZ-FAdE Tg fetuses at E14.5. Expression of each marker is shown at the left as indicated, with the merged picture shown at the right. The areas enclosed by the rectangles are enlarged at the far right. Arrows indicate cells that express LacZ but not the steroidogenic markers. Bars = 100 μm.
In a previous study, the definitive adrenal cortex failed to differentiate fully in Wnt4 knockout (KO) mice, implicating Wnt4 in the development of the adult adrenal cortex (6). We therefore examined the effect of a Wnt4 KO on LacZ expression in the developing adrenal glands of Cre/ROSA Tg mice at E14.5 and E18.5. As shown in Fig. S2 in the supplemental material, the absence of Wnt4 affected neither the distribution of LacZ-positive cells nor the overall structure of the adrenal gland.
Expression of Dax-1 during adrenal development.
As described above, some gonadal cells in Cre/ROSA Tg mice expressed LacZ, indicating that cells that had activated the FAdE persisted in the fetal gonad. In contrast, no gonadal cells expressed LacZ in Ad4BP-LacZ-FAdE Tg mice. This difference suggests that these cells silence the FAdE after they localize to the gonad. Considering that the FAdE remains active in the AdP, FAdE function apparently is regulated differentially in these two cell types. We previously defined two steps that regulate the FAdE function: activation by a Hox/Pbx/Prep homeobox complex and autoregulatory maintenance by the gene product, Ad4BP/SF-1 (40). Since the FAdE function is at least transiently activated in some gonadal cells, it is plausible that the maintenance of FAdE function is suppressed in these gonadal cells. Dax-1 has been shown to suppress the transcriptional activity of Ad4BP/SF-1 through direct heterodimerization (4, 11, 32); we therefore examined Dax-1 expression during early adrenal and gonadal development.
As shown in Fig. 6A, Ad4BP/SF-1 was expressed in the common adrenogonadal precursor at E10.0. By E11.5, these cell populations separated into two distinct cell populations: the adrenal and gonadal primordia. At E10.0, Dax-1 expression was detected in a few cells in the region that were to have become the gonad. This gonadal expression became stronger and expanded to most cells of the gonadal primordium by E10.5; in contrast, Dax-1 in the AdP was not expressed at E10.0 and was only weakly expressed at E10.5. By E11.5 and E12.5, Dax-1 clearly was expressed in the AdP.
We next compared the expressions of Dax-1 and LacZ driven by FAdE in the adrenal glands of Ad4BP-LacZ-FAdE Tg fetuses at E13.5, E17.5, and P9. At this time point, Dax-1 is expressed strongly in the outer adult zone, where LacZ expression is no longer detected. Considering the differing expressions of LacZ and Dax-1, it seems plausible that a new cellular zone had developed between the LacZ-positive fetal and Dax-1-positive adult zones. Dax-1 is expressed weakly in some cells of this new zone but clearly at levels below that seen for the adult zone. Interestingly, when the boundary between the fetal- and new-zone cells was examined carefully, we noted cells expressing Dax-1 that appeared to have a relatively decreased expression of LacZ (Fig. 6B). Eventually (P9), the remaining LacZ-positive cells in the adrenal X zone contained no Dax-1 (Fig. 6B, right). This inverse relationship suggests that Dax-1 suppresses the FAdE function and that the new zone consists of cells in the process of changing from a fetal- to an adult-zone program. However, our functional characterization of steroidogenic enzyme expression in the developing adrenal cortex (Fig. 5) did not reveal a distinct population with respect to differentiated function.
To test whether Dax-1 is involved in the regulation of the FAdE function through the suppression of Ad4BP/SF-1, we performed a transient transfection assay with HEK293T cells (Fig. 6C). As expected, increasing amounts of the Ad4BP/SF-1 expression construct increased the luciferase activity directed by the FAdE, and this Ad4BP/SF-1 activity was almost completely suppressed by Dax-1. Thus, these data reveal that Dax-1 can repress the FAdE function mediated by Ad4BP/SF-1 in cultured cells.
DISCUSSION
Establishment of adrenal and gonadal primordial cells.
The AdP is first identified as a thickening of cells of the coelomic epithelium in the medial region of the primitive urogenital ridge (21). Thereafter, these precursors appear to migrate mediodorsally and eventually accumulate adjacent to the anterior pole of the mesonephros. The gonadal primordium similarly arises from a thickening of the coelomic epithelial cells. Although it is unclear whether the epithelial compartments for the gonadal and adrenal primordia overlap or are completely different, immunohistochemical detection of Ad4BP/SF-1 strongly suggests that the two primordia initially comprise a single cell group (the AGP) before becoming separate (5).
Even though the two primordia are highly related functionally and likely arise from a common precursor, they soon begin to develop into distinct cell lineages. This assumption was supported by our previous (40) and present observations that the FAdE is activated in the AdP but inactivated in the gonadal primordium even before complete separation has occurred. Therefore, considering that Ad4BP/SF-1 is expressed in the gonadal primordium, another yet-to-be-identified enhancer presumably directs its expression in gonadal cells. Because both primordia emerge from the common AGP, it is plausible that cells localized at the boundary of separation may accidentally migrate into the gonadal primordium even though they activate the FAdE. In fact, our cell lineage-tracing studies with FAdE-driven Cre Tg mice are consistent with this model, demonstrating that some cells in which the FAdE once was activated nonetheless localized in the anterior part of the gonad. This model is consistent with the presence of “adrenal-like” cells in the testes (34). Although it is still unclear whether the “adrenal-like” cells are the cells identified by our cell lineage-tracing study, these observations strongly suggest that the process of separation of the two primordial pools potentially generates cells whose fate does not correspond with their localization.
Regarding the mechanism(s) that mediates the separation of the two primordia, it is notable that potential fetal adrenocortical cells are localized at the anterior pole of the developing gonads in Wnt4 KO fetuses (6, 12). This observation suggests that Wnt4 contributes either to the migration of the proliferating coelomic epithelial cells or to the separation of the AGP into the adrenal and gonadal primordia. Alternatively, Wnt4 may be essential for the downregulation of the FAdE function in the gonadal cells. Data shown in Fig. S2 in the supplemental material suggest that the location of the LacZ-positive cells in our lineage-tracing studies is not altered in Wnt4 KO mice.
Possible function of Dax-1 to compensate for incomplete separation of the adrenal and gonadal primordia.
FAdE function is maintained via autoregulatory effects of the gene product, Ad4BP/SF-1, on its cognate binding sites in the FAdE (40). Therefore, a sufficient level of Ad4BP/SF-1 seems to be critical for the persistent activation of the FAdE. In fact, fetuses heterozygous for Ad4BP/SF-1 KO have marked adrenal hypoplasia at early stages of adrenal development, although compensatory “catch-up” growth can occur subsequently (2). Similarly, an adrenal defect was observed for M33 KO mice, which express Ad4BP/SF-1 at approximately 50% of the wild-type level (14). Both observations indicate that adrenal development is highly sensitive to the level of Ad4BP/SF-1 expression. By contrast, gonadal development in mice is only minimally affected by haploinsufficiency for Ad4BP/SF-1. In contrast, loss-of-function mutations of Ad4BP/SF-1 in humans preferentially impair testis development and sex differentiation while preserving apparently normal adrenal function (20).
Given that Dax-1 suppresses the transcriptional activity of Ad4BP/SF-1 (11, 19, 32), it seemed that the quantitatively differential expression of Dax-1 potentially may affect the maintenance phase of FAdE function. Interestingly, when they become separate entities at E10.5 to E11.5, we found that Dax-1 is differentially expressed in the gonadal primordium (higher expression) and the AdP (lower expression). The lower expression of Dax-1 in the AdP potentially permits the FAdE to be maintained, while the higher Dax-1 expression in the gonadal primordium potentially inactivates the FAdE. Therefore, if cells that were destined to become adrenocortical cells migrated ectopically into the gonadal primordium, the FAdE function would be arrested. As a consequence, the ectopic cells may regulate Ad4BP/SF-1 expression via an alternate enhancer(s). According to this model, an important function of Dax-1 is to mediate the functional separation of adrenal and the gonadal primordia by differential effects on the FAdE.
In addition to the suppression by Dax-1, it was reported recently that Cited2 and Wt1 are required for the initial development of the fetal adrenal cortex through the regulation of Ad4BP/SF-1 gene expression (35), although a direct role of these factors via the FAdE has not been shown. Together with our findings, the suppression and activation of the FAdE function would correlate closely with the alternative differentiation to the gonads or adrenal cortex.
The adult adrenal cortex is derived from early stages of the fetal adrenal zone.
The fetal adrenal cortices of many mammalian species consist of adult/definitive and fetal zones whose origins remain poorly understood. Morphological studies have led to three different models. First, the adult zone may derive from the coelomic epithelia after cells that form the fetal zone have migrated (33); alternatively, the fetal and adult zones may derive simultaneously from discrete populations of coelomic epithelial cells (3). Both of these models postulate that the cells comprising the fetal and adult zones are already determined in their cell fates when they develop from the coelomic epithelia. In contrast, the third model postulates that both zones differentiate from a single progenitor population that subsequently differentiates into distinct fetal and adult zones (13).
The cell lineage studies described here strongly support the third model by showing that the adult zone derives from an early stage of the AdP in which the FAdE is transiently activated. Surprisingly, the capacity of these precursors to contribute to the adult zone largely disappears by E14.5, even before a distinct fetal zone has formed. Indeed, the AdP of Ad4BP-LacZ-FAdE Tg mice at E11.5 to E12.5 variably contains some cells that weakly express LacZ, which presumably are in the process of extinguishing the FAdE. By E13.5, these cells preferentially localize in a thin layer at the surface of the cortex.
Mechanism of transition from fetal- to adult-zone cells.
An important question is what induces the apparent cell fate transition from fetal to adult cells. Interestingly, investigation of Ad4BP-LacZ-FAdE Tg mice revealed the presence of a distinct population of cells between the LacZ-positive cells of the fetal zone and the LacZ-negative adult-zone cells. Although these newly defined cells were mostly characterized as “LacZ negative,” they differ morphologically from the adult-zone cells and rather resemble fetal-zone cells. In fact, adrenocortical markers such as Cyp11B2 and Pref-1 are preferentially expressed in the adult zone but not in this newly defined zone (6). Of note is that some of the intermediate cells were characterized by a weak but detectable expression of LacZ. Although speculative, this zone may consist of cells transitioning from the fetal to adult zone and changing in terms of both shape and localization. Importantly, we propose that this transition simultaneously involves a change in enhancer usage in the Ad4BP/SF-1 locus from the FAdE to an adult adrenal enhancer which remains to be identified.
Considering that the cells of the two zones apparently differ in their enhancer usage, it is interesting that Dax-1 is highly expressed in the adult zone and, moreover, is expressed in some of the transitional cells showing a weaker LacZ signal. As discussed above, Dax-1 may be a key factor that regulates the transition from fetal to adult adrenocortical cells. Consistent with this model, the fetal zones of Dax-1 KO mice persisted even 10 weeks after birth (38).
In the present study, we used the FAdE for lineage tracing in a Tg mouse model. These studies definitively established that the fetal adrenal cells give rise to the adult adrenal cells and that the capacity for this transition is lost by E14.5. Our studies further showed rare mislocalization of cells from the AdP into the developing gonad. We propose that preferential Dax-1 expression in cells of the gonadal field corrects this mislocalization by suppressing FAdE function, causing these ectopic cells to change their cell fate to that of gonadal cells. Similarly, we propose that Dax-1 mediates the transition of cells from the fetal to adult adrenocortical program. This cellular transition may be regulated by growth factors expressed in the developing adult zone, such as Wnt4 (1, 6, 17). In fact, the adult-zone cells in Wnt4 KO fetuses fail to differentiate fully, implicating Wnt4 in development of the adult adrenal cortex (6). However, our findings for the Wnt4 KO mice (see Fig. S2 in the supplemental material) argue against an obligatory role for Wnt4 in the cell transition that we have defined. In order to define the mechanisms underlying the adrenal development, a key goal of future experiments will be to link the functions of these and other growth factors with the enhancer usage/selection of master regulatory genes such as Ad4BP/SF-1.
Supplementary Material
Acknowledgments
We thank K. Mihara and M. Sakaguchi (Kyushu University) for providing the anti-β-galactosidase antibody, H. Okano (Keio University) for providing nestin-Hsp68-EGFP, T. Imai and P. Chambon for providing pGS-Cre-ERT2, Isao Matsuo (Kumamoto University) for ROSA26R mice, S. Oka for technical assistance, Etoh Tomoo for helpful suggestions on the generation of the Tg mice, and A. L. Reuter for helpful discussions on the manuscript.
This work was supported in part by grants-in-aid for scientific research on priority areas and a grant-in-aid for scientific research (B) from the Ministry of Education, Culture, Sports Science, and Technology of Japan (to K.-I.M.) and by NIH grants DK54480 and HD046743 (to K.L.P.).
Footnotes
Published ahead of print on 22 September 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bitgood, M. J., and A. P. McMahon. 1995. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 172126-138. [DOI] [PubMed] [Google Scholar]
- 2.Bland, M. L., R. C. Fowkes, and H. A. Ingraham. 2004. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol. Endocrinol. 18941-952. [DOI] [PubMed] [Google Scholar]
- 3.Crowder, R. 1957. The development of the adrenal gland in man, with special reference to origin and ultimate location of cell types and evidence in favor of the “cell migration” theory. Contemp. Embryol. 251195-209. [Google Scholar]
- 4.Fan, W., T. Yanase, Y. Wu, H. Kawate, M. Saitoh, K. Oba, M. Nomura, T. Okabe, K. Goto, J. Yanagisawa, S. Kato, R. Takayanagi, and H. Nawata. 2004. Protein kinase A potentiates adrenal 4 binding protein/steroidogenic factor 1 transactivation by reintegrating the subcellular dynamic interactions of the nuclear receptor with its cofactors, general control nonderepressed-5/transformation/transcription domain-associated protein, and suppressor, dosage-sensitive sex reversal-1: a laser confocal imaging study in living KGN cells. Mol. Endocrinol. 18127-141. [DOI] [PubMed] [Google Scholar]
- 5.Hatano, O., A. Takakusu, M. Nomura, and K. Morohashi. 1996. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1663-671. [DOI] [PubMed] [Google Scholar]
- 6.Heikkila, M., H. Peltoketo, J. Leppaluoto, M. Ilves, O. Vuolteenaho, and S. Vainio. 2002. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology 1434358-4365. [DOI] [PubMed] [Google Scholar]
- 7.Hogan, B., R. Beddington, F. Constantini, and E. Lacy. 1994. Manipulating the mouse embryo: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 8.Holmes, P. V., and A. D. Dickson. 1971. X-zone degeneration in the adrenal glands of adult and immature female mice. J. Anat. 108159-168. [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda, Y., W. H. Shen, H. A. Ingraham, and K. L. Parker. 1994. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 8654-662. [DOI] [PubMed] [Google Scholar]
- 10.Indra, A. K., X. Warot, J. Brocard, J. M. Bornert, J. H. Xiao, P. Chambon, and D. Metzger. 1999. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 274324-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito, M., R. Yu, and J. L. Jameson. 1997. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol. Cell. Biol. 171476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeays-Ward, K., C. Hoyle, J. Brennan, M. Dandonneau, G. Alldus, B. Capel, and A. Swain. 2003. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 1303663-3670. [DOI] [PubMed] [Google Scholar]
- 13.Jirasek, J. 1980. Human fetal endocrines. Martinus Nijhoff, London, United Kingdom.
- 14.Katoh-Fukui, Y., A. Owaki, Y. Toyama, M. Kusaka, Y. Shinohara, M. Maekawa, K. Toshimori, and K. Morohashi. 2005. Mouse Polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood 1061612-1620. [DOI] [PubMed] [Google Scholar]
- 15.Kawabe, K., T. Shikayama, H. Tsuboi, S. Oka, K. Oba, T. Yanase, H. Nawata, and K. Morohashi. 1999. Dax-1 as one of the target genes of Ad4BP/SF-1. Mol. Endocrinol. 131267-1284. [DOI] [PubMed] [Google Scholar]
- 16.Kim, A. C., and G. D. Hammer. 2007. Adrenocortical cells with stem/progenitor cell properties: recent advances. Mol. Cell. Endocrinol. 265-26610-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lako, M., T. Strachan, P. Bullen, D. I. Wilson, S. C. Robson, and S. Lindsay. 1998. Isolation, characterisation and embryonic expression of WNT11, a gene which maps to 11q13.5 and has possible roles in the development of skeleton, kidney and lung. Gene 219101-110. [DOI] [PubMed] [Google Scholar]
- 18.Lala, D. S., D. A. Rice, and K. L. Parker. 1992. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol. Endocrinol. 61249-1258. [DOI] [PubMed] [Google Scholar]
- 19.Lalli, E., and P. Sassone-Corsi. 2003. DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol. Endocrinol. 171445-1453. [DOI] [PubMed] [Google Scholar]
- 20.Lin, L., P. Philibert, B. Ferraz-de-Souza, D. Kelberman, T. Homfray, A. Albanese, V. Molini, N. J. Sebire, S. Einaudi, G. S. Conway, I. A. Hughes, J. L. Jameson, C. Sultan, M. T. Dattani, and J. C. Achermann. 2007. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J. Clin. Endocrinol. Metab. 92991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mesiano, S., and R. B. Jaffe. 1997. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 18378-403. [DOI] [PubMed] [Google Scholar]
- 22.Mitani, F., K. Mukai, H. Miyamoto, M. Suematsu, and Y. Ishimura. 1999. Development of functional zonation in the rat adrenal cortex. Endocrinology 1403342-3353. [DOI] [PubMed] [Google Scholar]
- 23.Morley, S. D., I. Viard, B. C. Chung, Y. Ikeda, K. L. Parker, and J. J. Mullins. 1996. Variegated expression of a mouse steroid 21-hydroxylase/beta-galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol. Endocrinol. 10585-598. [DOI] [PubMed] [Google Scholar]
- 24.Morohashi, K., H. Iida, M. Nomura, O. Hatano, S. Honda, T. Tsukiyama, O. Niwa, T. Hara, A. Takakusu, Y. Shibata, et al. 1994. Functional difference between Ad4BP and ELP, and their distributions in steroidogenic tissues. Mol. Endocrinol. 8643-653. [DOI] [PubMed] [Google Scholar]
- 25.Mukai, T., M. Kusaka, K. Kawabe, K. Goto, H. Nawata, K. Fujieda, and K. Morohashi. 2002. Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells 7717-729. [DOI] [PubMed] [Google Scholar]
- 26.Nussdorfer, G. G. 1986. The fetal adrenal cortex. Int. Rev. Cytol. 98211-249. [PubMed] [Google Scholar]
- 27.Payne, A. H., and D. B. Hales. 2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25947-970. [DOI] [PubMed] [Google Scholar]
- 28.Schulte, D. M., I. Shapiro, M. Reincke, and F. Beuschlein. 2007. Expression and spatio-temporal distribution of differentiation and proliferation markers during mouse adrenal development. Gene Expr. Patterns 772-81. [DOI] [PubMed] [Google Scholar]
- 29.Shima, Y., M. Zubair, S. Ishihara, Y. Shinohara, S. Oka, S. Kimura, S. Okamoto, Y. Minokoshi, S. Suita, and K. Morohashi. 2005. Ventromedial hypothalamic nucleus-specific enhancer of Ad4BP/SF-1 gene. Mol. Endocrinol. 192812-2823. [DOI] [PubMed] [Google Scholar]
- 30.Shima, Y., M. Zubair, T. Komatsu, S. Oka, C. Yokoyama, T. Tachibana, T. A. Hjalt, J. Drouin, and K. I. Morohashi. 2008. Pituitary homeobox 2 regulates adrenal4 binding protein/steroidogenic factor-1 gene transcription in the pituitary gonadotrope through interaction with the intronic enhancer. Mol. Endocrinol. 221633-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 2170-71. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki, T., M. Kasahara, H. Yoshioka, K. Morohashi, and K. Umesono. 2003. LXXLL-related motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Mol. Cell. Biol. 23238-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uotila, U. 1940. The early embryological development of the fetal and permanent adrenal cortex in man. Anat. Res. 76183-203. [Google Scholar]
- 34.Val, P., K. Jeays-Ward, and A. Swain. 2006. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev. Biol. 299250-256. [DOI] [PubMed] [Google Scholar]
- 35.Val, P., J. P. Martinez-Barbera, and A. Swain. 2007. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development 1342349-2358. [DOI] [PubMed] [Google Scholar]
- 36.Wilhelm, D., and C. Englert. 2002. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 161839-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson, D. G., and M. A. Nieto. 1993. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 225361-373. [DOI] [PubMed] [Google Scholar]
- 38.Yu, R. N., M. Ito, T. L. Saunders, S. A. Camper, and J. L. Jameson. 1998. Role of Ahch in gonadal development and gametogenesis. Nat. Genet. 20353-357. [DOI] [PubMed] [Google Scholar]
- 39.Zajicek, G., I. Ariel, and N. Arber. 1986. The streaming adrenal cortex: direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J. Endocrinol. 111477-482. [DOI] [PubMed] [Google Scholar]
- 40.Zubair, M., S. Ishihara, S. Oka, K. Okumura, and K. Morohashi. 2006. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol. Cell. Biol. 264111-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.