Abstract
Objective
To evaluate the methodology for exploring the specific aspects of functional impairment in multiple sclerosis (MS) through the pattern of forces exerted in various manipulation tasks.
Methods
Twelve mildly involved MS patients (EDSS 2.5–5.5) and 12 healthy controls performed various static and dynamic manipulation tasks with an instrumented device that recorded the grip (G; normal to the digit device contact area) and load force (L; tangential force that causes lifting).
Results
MS patients consistently displayed lower indices of task performance (as assessed by the ability to produce the required L profiles) and force coordination (as assessed by G/L ratio, coupling of G and L, and G modulation) than the healthy controls across all tested tasks.
Conclusions
The applied methodology could be sensitive enough to detect the hand dysfunction in mildly involved individuals with MS. Particularly recommended for future evaluations of the impairment of hand function could be a simple lifting task and the static task of tracing a gradually changing L, as well as the variables depicting both the task performance and G/L ratio.
Significance
The applied methodology could be developed into a standard clinical test for the assessment of hand function in MS and, possibly, in other neurological diseases.
Keywords: Grip, Load, Coordination, Performance, Impairment
Introduction
Independent living heavily depends on the ability to manipulate various objects in everyday life. Hand dysfunction regarding a limited manipulation ability is commonly seen in centrally or peripherally damaged neurological patients, such as in stroke (Nowak and Hermsdorfer, 2003a), Huntington’s (Serrien et al., 2002), Parkinson’s (Ingvarsson et al., 1997), motor neuron disease (Nowak and Hermsdorfer, 2002), or in peripheral neuropathy (Thonnard et al., 1997). In the clinical practice, hand dysfunction has been assessed either by simple quantitative tests, such as maximum grip strength, range of motion of the fingers or by timed simple actions, or by subjective qualitative assessments (for details see further text).
The research done over the last few decades suggests that the hand function can be assessed through the kinetic analysis of various functional tasks. Specifically, manipulation of fixed and free moving objects appears to be associated with high coordination of two particular force components. In a simple mechanical representation of lifting (e.g., lifting a glass of water), the load force (L) acts in parallel to the digits-object contact area and performs the lifting or holding of an object, but at the same time tends to cause slippage. Grip force (G) acts perpendicularly to the contact area. It helps in controlling the manipulated object hold within the grasp and also prevents the slippage. A consistent finding over a body of literature is that the changes in G are highly coordinated with the changes in L without any time lags between them and therefore, the coordination appears to be based on anticipatory neural control mechanisms ((Flanagan and Wing, 1995; Johansson and Westling, 1984). The final outcome of this coordination is a stable G/L ratio that is highly adjusted to the friction coefficient to provide G that is slightly above the minimal level that prevents the slippage (Johansson, 1998). In addition to G/L ratio, G and L coordination has been often assessed through a force coupling (as assessed by both high correlation coefficient and virtually no time lag between G and L (Flanagan and Tresilian, 1994; Zatsiorsky et al., 2005)) and a high G modulation with respect to changes in L (Flanagan et al., 1993). Taking into account the essential role of G and L in manipulation activities, it should not be surprising that neurological patients and other populations known for impaired hand function consistently show deteriorated force coordination when performing various manipulation tasks. For example, along with an elevated G (Nowak and Hermsdorfer, 2002, , 2003b; Rost et al., 2005; Serrien and Wiesendanger, 1999), uncoordinated changes in G and L have been consistently observed across neurological diseases (Fellows et al., 1998; Gordon et al., 2006; Hermsdorfer et al., 2003; Nowak et al., 2002; Nowak et al., 2003).
Interestingly, apart from G and L coordination, the coordination of L per se has been mainly neglected in the kinetic analysis of hand function in both healthy and various clinical populations. Namely, an accurate temporal pattern of L is required for precise manipulation, such as repositioning of an object, using a tool, or providing a postural support from an externally fixed object. Our recent findings suggest that, in addition to the above discussed G and L coordination, the accuracy of the exerted L pattern could reveal both the effect of hand dominance and the differences in manipulation performance between healthy participants and neurological patients (de Freitas et al., 2007; Ferrand and Jaric, 2006; Krishnan et al., 2008; Marwaha et al., 2006).
One of the most common neurological diseases of the central nervous system is multiple sclerosis (MS). It is a demyelinating, autoimmune disorder associated with sensory-motor disintegration, motor impairment, postural imbalance, intention tremor, ataxia and impaired motor coordination (Matthews, 1991). The socio economic impact of this disease is exceptionally high because of its high prevalence and incidence among young adults, long duration, physical disability and the need for assistance. A widely prevalent clinical symptom in MS is impairment of hand function that has been directly or indirectly assessed by various clinical tests. The most often applied clinical test for a general assessment of MS patients has been the Expanded Disability Status Scale (EDSS; (Kurtzke, 1983)) although its validity (Rossier and Wade, 2002) and reliability (Noseworthy et al., 1990; Whitaker et al., 1995) has been often questioned. A more specific Multiple Sclerosis Functional Composite Measure (MSFC) has been also developed (Cutter et al., 1999). An integral part of MSFC is the assessment of the upper extremity function by the 9-hole peg test (Grice et al., 2003) which is a timed test of a simple motor activity. Similarly, the Jebsen-Taylor test is based on a number of timed tests that mimic daily motor activities and it has been applied on a variety of neurological patients (Jebsen et al., 1969). Therefore, one could conclude that most of the contemporary clinical tests of hand function appear to be based either on the qualitative subjective assessment or on the timed simple motor actions. As a result, the above discussed elaborate force coordination that characterizes manipulation activities in healthy individual could provide an opportunity not only to assess specific aspects of impairment in various neurological diseases such as MS, but also to construct quantitative clinical tests of hand function.
We recently developed a methodology for the assessment of force coordination in uni- and bimanual static manipulation tasks (Jaric et al., 2006; Jaric et al., 2005; Krishnan et al., 2008; Marwaha et al., 2006). Both the indices of G and L coordination and the indices of task performance based on exertion of the prescribed L profile proved to be sensitive enough to detect various effects, such as of the rate of change of L and the type of the task performed (Jaric et al., 2006; Jaric et al., 2005), or of the hand dominance (de Freitas et al., 2007; Ferrand and Jaric, 2006). Of particular importance for the present study could be our recent findings obtained from mildly involved MS patients. Although most of them claimed that they had no problems in daily manipulation activities, their ability to accurately exert the instructed L profile proved to be impaired when compared to healthy controls, while their ability to coordinate G and L (excluding somewhat elevated G/L ratio) seemed to be mainly unaffected (Krishnan et al., 2008; Marwaha et al., 2006). Therefore, we concluded that in mildly involved MS patients "the deterioration in the ability for precise control of external forces and over-gripping could precede the decoupling of G and L and decreased G modulation in early phases of the disease" (Krishnan et al., 2008). Nevertheless, the results of both studies suggested that the methodological approach based on the force coordination in static manipulation tasks could be sensitive enough to be applied in the assessment of hand function in MS and probably, other neurological diseases.
Within the present study, we extended our previous research on individuals with MS by introducing several changes. First, we decided to evaluate simpler tasks regarding both the instructed force profiles and task conditions. Second, instead of a somewhat complex, expensive and bulky device used in previous studies, we designed a simplified and a relatively small and light device containing only two miniature single-axis force transducers. Finally, we intended to relate our findings with the assessments based on standard clinical tests. The motivation for both of these changes came from the above discussed need for a development of a clinical tool for quantitative testing of hand function. Various manipulation tasks were tested and standard dependent variables depicting both the task performance and the force coordination were obtained. In particular, the present study was designed in line with three main aims. The first one was to evaluate the impairment of the hand function of MS patients as assessed through the dependent variables depicting both the task performance and force coordination. Specifically, we hypothesized that the patients would demonstrate impaired force coordination across most of the dependent variables, but not only in the task performance and G/L ratio as the mildly involved patients had demonstrated in our previous studies. The second aim was to reveal both the manipulation tasks and the particular dependent variables that demonstrate the most prominent differences between the tested patients and healthy controls. The third aim was to evaluate the concurrent validity of the dependent variables with respect to frequently applied clinical tests of hand function. Therefore, the findings of the present study were expected to reveal specific aspects of the hand function impairment in MS and to evaluate the validity of the applied methodological approach. Note also that MS is the most diverse among neurological disorders both regarding the localization of damage of the neural tissue and regarding the associated clinical and functional symptoms. Therefore, the expected findings could be partly extended to other neurological diseases, providing a basis for developing a standard testing tool for assessment of hand function.
Materials and Methods
Participants
Considering the higher prevalence of MS in females (60–75%) (Whitacre 2001), a larger proportion of females when compared to males were selected for the experiment. Specifically, 9 female and 3 male MS patients (age range 39 to 65 years, mean ± SD 52.6 ± 7.1 ) and an equal number of age and gender matched healthy individuals (age range 33 to 67 years, 50.0 ± 8.4) participated in the study. MS patients were recruited from the MS Clinic at the Physical Therapy Department of the University of Delaware, while the healthy controls were recruited by public advertisement. The experimental procedure was approved by the Human Subjects Review Board of the University of Delaware and the participants provided their informed consent in accordance to the Declaration of Helsinki. All MS patients and healthy controls were right hand dominant, except for a single healthy control, as assessed by the Edinburgh Inventory (Oldfield, 1971).
An experienced neurologist screened the MS patients and evaluated them on the EDSS. In order to control for the heterogeneity of the expression of the disease, the following inclusion criteria were adopted: the patients were capable of independent living, they also had a normal or corrected to normal vision, the 9-hole peg test time was less than a minute and the Jebsen Taylor test time did not exceed 5 minutes. Patients were excluded if they had a history of psychiatric or other medical illnesses, drug or alcohol abuse, or if they were unable to perform the experimental tasks.
Experimental Device
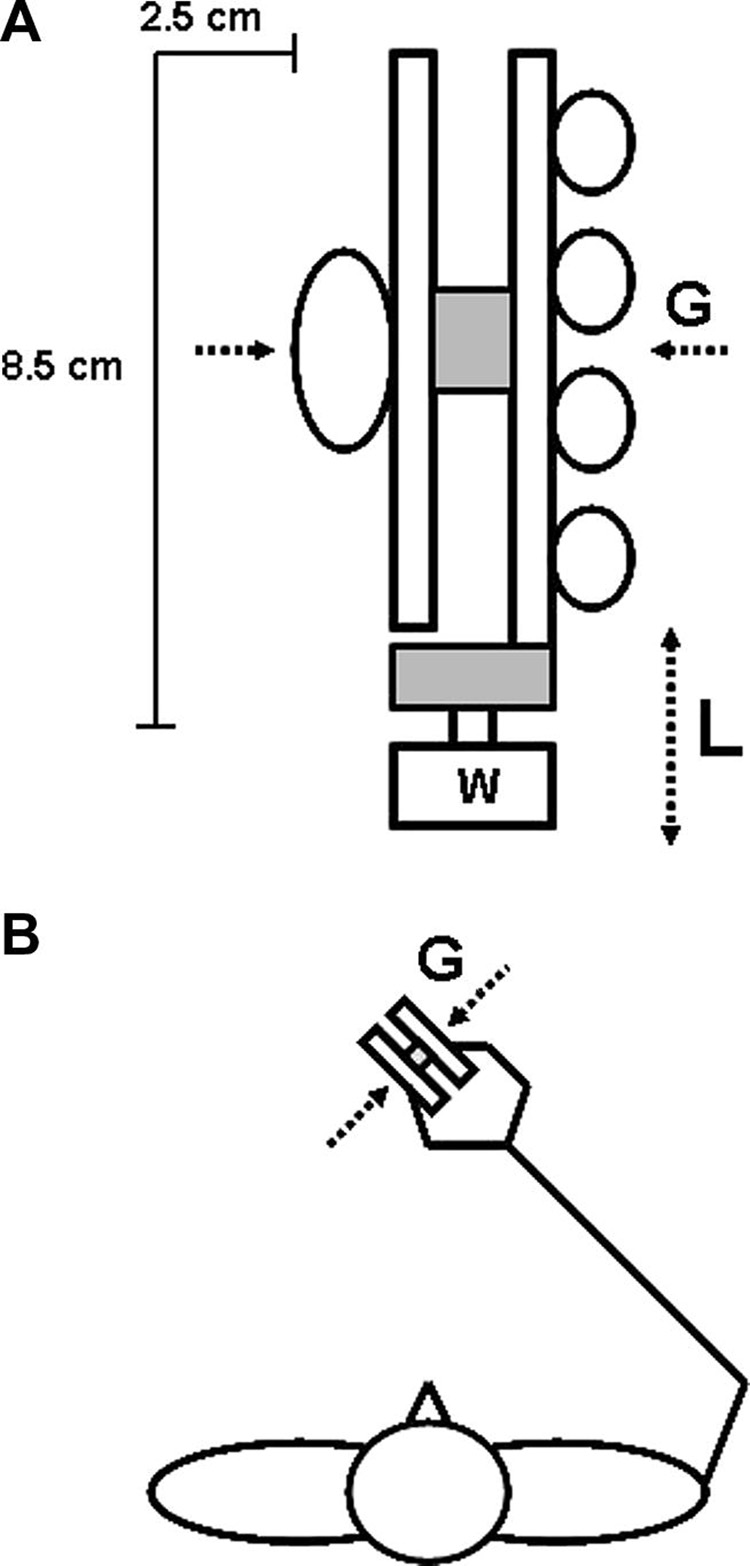
The experimental device used in the study consisted of a single handle (total length 8.5 cm and the grasping aperture 2.5 cm) that could be either externally fixed or free to move (see Fig. 1.A). The grasping surfaces were covered with rubber and the total weight was 1.5 N. A force transducer (Model 484B06, Piezotronics, Inc) located between the grasping surfaces recorded the grip force (G) of the fingers and the thumb applied perpendicularly against the opposing grasping surfaces. Another transducer was located below and recorded the load force (L) exerted tangentially to the grasping surfaces. Additional weights in steps of 100 g of mass served to adjust the total weight of the device to the prescribed Lmax (see further text for details). Note that due to moderately deficient hand control in some MS patients the 'slip point' (i.e., the minimum G/L ratio that prevents slippage; (Johansson and Westling, 1984)) was not measured. However, our pilot experiment performed on 4 healthy individuals revealed the slip point of about 0.40 which corresponds to the friction coefficient of 1.25.
Fig. 1.
(A) Schematic illustration of the device (not drawn to scale). The circles illustrate the position of the tips of the fingers and the thumb applying a precision grip against the handle. W indicates external load that could be attached to vary the total weight or, alternatively, fixation of the device to the table. The upper and lower shaded rectangles depict the force sensor that record grip force (G; perpendicular to device) and load force (L), respectively, applied against the device. (B) The stick diagram illustrates the horizontal projection of the subjects' body position while performing the tasks.
Experimental Procedure
The experimental procedure was conducted within a single session. Following the neurological examination and EDSS test (conducted only on MS patients), all participants were tested on the 9-hole peg and Jebsen-Taylor tests. Finally, they were tested on three manipulation tasks performed with the experimental device.
The participants were comfortably seated in a chair in front of the device. They were asked to keep their upper arm in a vertical position, elbow at approximately 90° of flexion, and forearms in mid-prone position. The device was vertically rotated 45° (see Fig 1B) to allow for a comfortable wrist position. This enabled the participants to grasp the device in an ergonomically natural way, as well as to preserve the prescribed position for the entire testing session without much deviation. The device was grasped by the tips of the digits applying, therefore, a precision grip.
The procedure started with cleaning the tips of their fingers and the thumb using an alcohol swab. Thereafter, the maximum voluntary G for each hand was recorded under the instruction to squeeze the handle of the device as hard as possible by applying the precision grip. According to our previous studies (Jaric et al., 2005), prolonged tasks requiring L below 15% of the maximum G were not expected to cause fatigue. In this study 10% of the maximum G of the weaker hand was prescribed as the maximum L (Lmax) exerted in each of the experimental tasks. As a result, Lmax was participant specific and ranged from 3 N to 10 N. Finally, the participants were tested on one 'dynamic' (i.e., lifting task) and two 'static' tasks (ramp-and-hold and oscillation task) performed uni-manually by each hand. The experimental tasks were explained and demonstrated and, thereafter, each task was practiced by the participants over three trials performed by each hand.
The lifting task was performed against the load consisting of the device and the attached weight that together closely corresponded to the prescribed Lmax. In particular, the participants were asked to prepare their hand for grasping the device by opening their fingers near the grasping area without touching them. Upon hearing the first computer generated beep, they grasped the device and lifted it approximately 3 cm above the table. They were instructed to hold it steady until the second beep (3 s later) prompted them to place the device back on the table and release it.
Two static tasks were expected to correspond either to a gradual increase and, thereafter, a steady L exertion (ramp-and-hold task), or to rapidly changing L (oscillatory task) against an externally fixed device. In the ramp-and-hold task, participants were asked to match a prescribed Lmax profile by pulling up the externally fixed device. A computer monitor placed in front of the seated participant displayed the prescribed Lmax, as well as the current value of L. The profile had the following three phases: zero L (duration 1 s), gradually increasing L (3 s), and constant L (3 s; see Fig. 2 for illustration). Four computer-generated auditory beeps marked the initiation of each phase and the termination of the last one. In the oscillatory task, the participants were instructed to exert a sinusoidal L by pulling the device vertically in a way that L minima and maxima corresponded to 0 N and the individually prescribed Lmax. The computer monitor displayed those horizontal lines depicting the prescribed minima and the maxima, as well as the current value of L. The task was paced by a metronome set at 1.33 Hz, while the duration of the trials was 8 s.
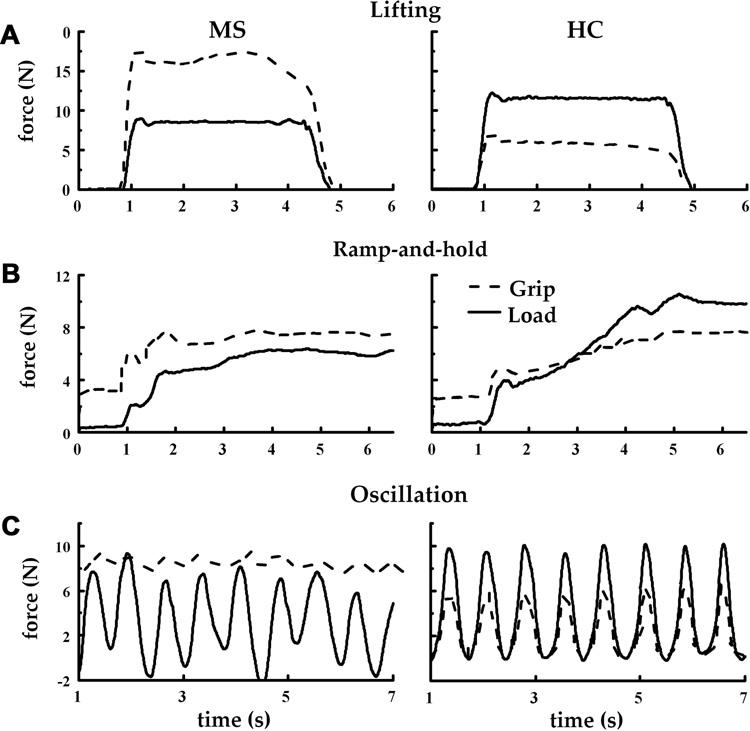
Fig. 2.
Grip and load force exerted against the device in the lifting task (A), ramp-and-hold task (B), and oscillation task (C) by a representative MS patient (left hand panels) and a healthy control (right hand panels).
A total of 4 trials of each task were performed with each hand separately. The last 3 trials were taken for further analysis. The sequence of tasks, as well as the sequence of hands within each task was randomized. Note that the participants were only focused on the movement task based on L exertion since G was never mentioned throughout the entire experimental session.
Data Processing
Both G and L signals were A/D converted and recorded at the sampling rate of 200 Hz. Custom made LabView (National Instruments, Austin, TX, USA) routines were used for the data acquisition and analysis. The signals were low-pass filtered at 10 Hz with a fourth order Butterworth filter. In the lifting task, the initiation of lifting – the lift phase (the time interval starting when L reaches 8% of the maximum and finishing when the maximum L is reached) and the hold phase (the 2 s interval following the instant 0.25 s after reaching the maximum L) were separately analyzed. In the ramp-and-hold task, the ramp phase (3 s) and the hold phase (the following 3 s) were also separately analyzed. Finally, to exclude the initial and final adjustments, only the middle 5 s of the oscillation task was analyzed (Freitas et al., 2007).
Two groups of dependent variables were selected. The task performance variables were expected to reveal the ability of the participants to exert the instructed pattern of L, while the force coordination variables assessed the relationship between the temporal profiles of G and L. However, note that both different tasks and the different phases of the same tasks provided different task performance variables. In particular, the coefficient of variation of L (CV) and the root mean square error of L (RMSE) were selected as presumably valid indices of the task performance while performing the steady holding phase of the lifting and of the ramp-and-hold task, respectively. Conversely, the constant (CE) and variable errors (VE) were expected to reveal the ability of the subject to reach the prescribed L peaks in the oscillation task.
Regarding the variables depicting the force coordination, G/L ratio evaluated the magnitude of G with respect to the magnitude of L. It was calculated from the steady holding phases of the lifting and ramp-and-hold task, as well as from the averaged G and L of the oscillation task. The coupling of G and L was assessed by cross-correlation of the derivatives of G and L in the lifting task (Flanagan and Wing, 1997), and from the G and L of the remaining two tasks (Jaric et al., 2005). However, the time lags proved to be exceptionally small and inconsistent across all three tasks. Therefore, the Pearson's correlation coefficients were calculated instead (see (Jaric et al., 2006; Krishnan et al., 2008) for similar approach). Finally, G modulation with respect to changing L was assessed from G–L diagrams of the ramp phase of the ramp-and-hold task and from the oscillation task (de Freitas et al., 2007; Flanagan and Wing, 1993; Zatsiorsky et al., 2005). The regression lines provided the slope and intercept that were interpreted as gain and offset of G, respectively. In line with a number of previous studies, a high force coordination was expected to be revealed by a low G/L ratio, high force coupling (high correlation and low time lag between G and L), and high G modulation (high gain and low offset of G) (Blakemore et al., 1998; Blank et al., 2001; Flanagan and Tresilian, 1994; Jaric et al., 2005; Zatsiorsky et al., 2005). See Table 2 for a complete list of all task performance and force coordination variables obtained from particular tasks.
Table 2.
Correlations between the clinical tests and dependent variables obtained from individual tasks
| Tasks | Clinical Tests | Task Performance | Force Coordination | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CV | RMSE | CE | VE | G/L ratio | r | time lags | gain | offset | ||
| Lifting Task | 9-hole Peg | 0.26 | - | - | - | 0.66 | −0.66 | −0.53 | - | - |
| EDSS | 0.46 | - | - | - | 0.90* | −0.56 | −0.49 | - | - | |
| Jebsen-Taylor | −0.07 | - | - | - | 0.78* | −0.26 | −0.07 | - | - | |
| Ramp-and-hold task | 9-hole Peg | - | −0.29 | - | - | 0.53 | −0.14 | .35 | 0.49 | 0.20 |
| EDSS | - | 0.33 | - | - | 0.67 | −0.49 | 0.40 | 0.06 | 0.65* | |
| Jebsen-Taylor | - | 0.09 | - | - | 0.39 | −0.21 | 0.60 | 0.51 | 0.21 | |
| Oscillation task | 9-hole Peg | - | - | −0.03 | 0.02 | 0.37 | 0.27 | 0.07 | 0.39 | −0.31 |
| EDSS | - | - | 0.15 | 0.33 | 0.54 | 0.02 | 0.24 | 0.05 | 0.04 | |
| Jebsen-Taylor | - | - | 0.16 | −0.16 | 0.20 | 0.22 | 0.16 | 0.11 | −0.26 | |
CV - coefficients of variation of L; RMSE - root mean square error; CE - constant error; VE - variable error; r - Z-transformed correlation coefficients
p<0.017
The last 3 out of 4 experimental trials of each task performed with each hand were separately analyzed. The values of each dependent variable were averaged across three trials of each hand prior to the statistical analysis. Coefficients of correlation were Z-transformed prior to the averaging. However, a preliminary analysis did not reveal differences between the dominant and non-dominant hand regarding any of the dependent variables. Therefore, the data for two hands were averaged prior to the statistical analysis.
Statistical Analysis
The Kolmogorov-Smirnov test was applied to identify non-normally distributed variables. As a result, RMSE and offset needed to be log-transformed to fulfill the condition of normality. The same procedure could not be applied on time lags due to a number of negative values. Nevertheless, since the Wilcoxon rank-sum test provided the same finding regarding the effect of group, time lags were (together with other force coordination variables; see further text) analyzed by parametric techniques.
Since each task provided different indices of performance, the difference between two groups in individual task performance variables were assessed by independent t-test. Regarding the force coordination, G/L ratio, coupling of G and L and their respective time lags were calculated from all three tasks, while the gain and offset were calculated from the ramp-and-hold and oscillation tasks alone. Therefore, a mixed two-way MANOVA was used to assess the main effects of group (MS patients vs. healthy controls), and task (lifting vs. ramp-and-hold vs. oscillation) on G/L ratio,Z-transformed correlation coefficient of G and L and the corresponding time lags. Similar MANOVA was used to assess the main effect of group and task (ramp-and-hold and oscillation tasks) on gain and offset. Significant MANOVA were followed up by univariate ANOVAs. In line with the main aims of the study, we were particularly interested in the main effect of the group, as well as in the group × task interaction. The former was expected to expose the variables showing significant differences between the MS patients and healthy controls. The group × task interactions were expected to reveal the tasks that provide the highest differences in particular force coordination variables between two groups. The p-value was set to 0.05 Finally, linear regressions were calculated to assess the concurrent validity of the individual task performance and the force coordination variables was with respect to the applied clinical tests. Bonferroni correction for multiple comparisons was applied. Statistical analysis was performed in SPSS 10 for Windows (SPSS Inc., Chicago, USA).
Results
Table 1 shows results from the clinical testing. EDSS score averaged across the MS patients was (mean ± SD) 3.9 ± 1.1 (range 2.5–5.5). Note that regarding both the 9-hole peg and the Jebsen-Taylor test, neither group of participants revealed significant differences between the scores of two hands. Therefore, the data were averaged across the hands prior to the further statistical processing. As a result, the time to complete 9-hole peg test was 25.3 ± 4.7 s in MS patients, while in the healthy controls it was 16.9 ± 1.6 s. The difference proved to be significant (t=5.83, p<0.01; independent t-test). Regarding the Jebsen-Taylor test, the time to complete it was 40.9 ± 7.9 s and 27.1 ± 2.4 s in the MS patients and healthy controls, respectively (t=5.79, p<0.01).
Table 1.
Patients' data
| Patient | Sex | Age | EDSS | 9-hole peg D (s) | 9-hole peg ND (s) | Jebsen-Taylor D (s) | Jebsen-Taylor ND (s) |
|---|---|---|---|---|---|---|---|
| P1 | F | 46 | 4.0 | 21 | 21 | 38 | 45 |
| P2 | M | 56 | 3.5 | 25 | 23 | 35 | 37 |
| P3 | F | 43 | 2.5 | 19 | 16 | 28 | 27 |
| P4 | M | 53 | 5.0 | 25 | 38 | 42 | 44 |
| P5 | F | 45 | 3.5 | 18 | 38 | 45 | 31 |
| P6 | M | 57 | 5.5 | 22 | 31 | 32 | 41 |
| P7 | F | 39 | 3.0 | 26 | 39 | 41 | 51 |
| P8 | F | 54 | 3.5 | 22 | 25 | 44 | 47 |
| P9 | F | 55 | 5.5 | 27 | 35 | 45 | 68 |
| P10 | F | 65 | 3.5 | 25 | 23 | 45 | 44 |
| P11 | F | 58 | 5.0 | 24 | 24 | 43 | 49 |
| P12 | F | 60 | 2.5 | 21 | 19 | 33 | 27 |
| Mean±SD | 52.6±7.1 | 3.9±1.1 | 22.9±2.8 | 27.7±8.1 | 39.3±5.9 | 42.6±11.4 | |
| Range | 39–65 | 2.5–5.5 | 18–27 | 16–39 | 28–45 | 27–68 | |
D - dominant hand; ND - non dominant hand; EDSS - Expanded Disability Status Scale.
Figure 2 shows the force profiles obtained from a representative MS patient and a healthy control in three tested tasks. Note that the patient demonstrates somewhat deteriorated task performance revealed through a less regular ramp profile, as well as through the highly variable maxima and minima of L exerted in the oscillation task. The patient also consistently applies a higher grip force (G) relative to the load force (L). Finally, the modulation of G is lower in the MS patient, which should lead to a relatively low G gain and high offset, as well as to a low correlation between G and L.
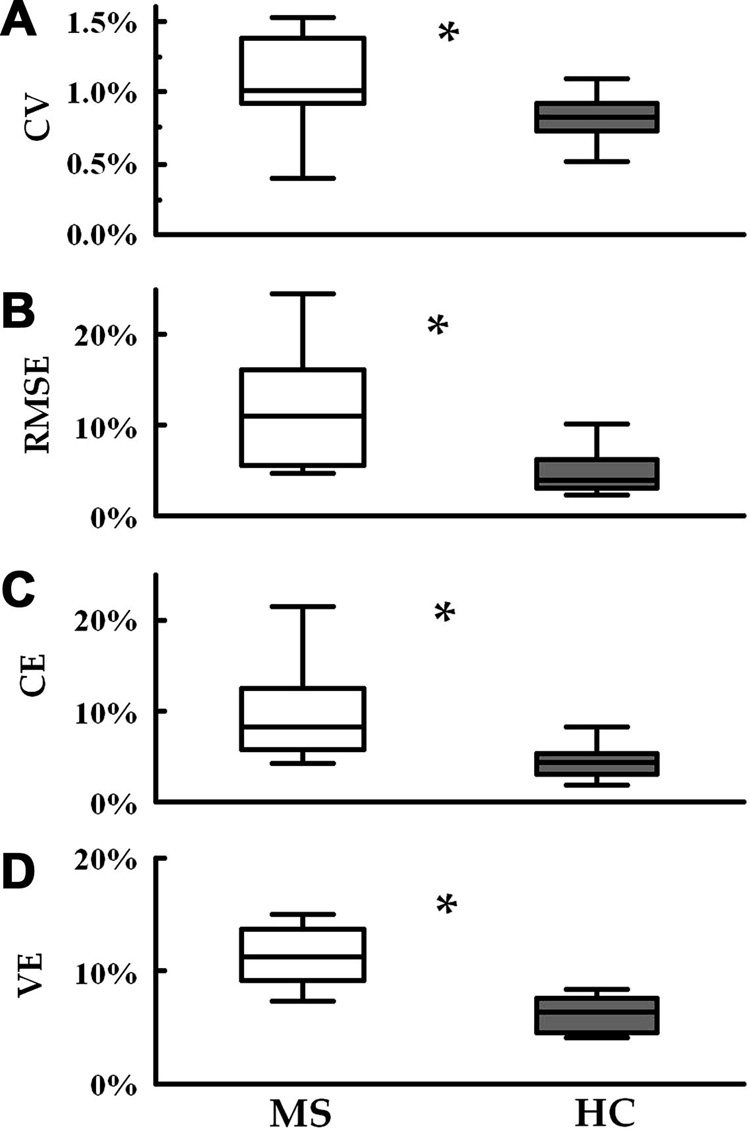
Figure 3 shows the task performance variables averaged across the participants. In particular, the upper panels illustrate smoothness of G and L in the tasks where the participants were instructed either to hold steadily the device (the hold phase of the lifting task) or to exert the instructed constant L against the externally fixed device (the hold phase of the ramp-and-hold task; Fig. 3A and 3B). Both the coefficient of variation (CV; t=2.7, p<0.05) and the root mean square error (RMSE; t=3.3, p<0.05) of L were higher in MS patients than in healthy controls. The same was true for the constant error (CE; t=3.4, p<0.05) and the variable error (VE; t=4.4, p<0.05) obtained from the maxima and minima of the oscillation task (Fig. 3C and 3D).
Fig. 3.
Task performance variables averaged across the participants. CV is the coefficients of variation of L (lifting task), RMSE is the root mean square error (ramp-and-hold task), while CE and VE are the constant and variable errors, respectively (oscillation task). The box plots represent the 25th to the 75th percentile of the distribution and the middle line represents the median.
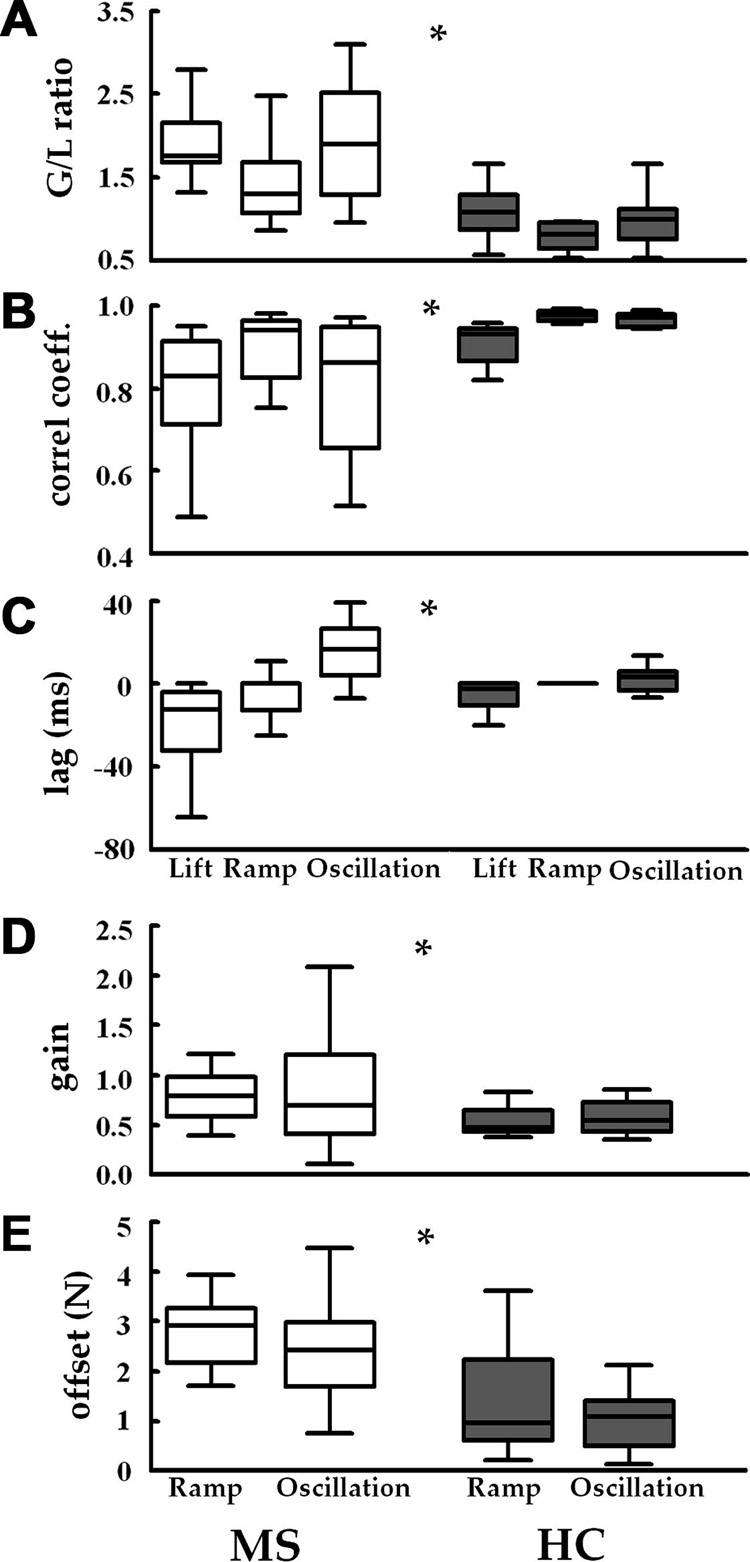
Figure 4 shows the averaged across the participants indices of G and L coordination obtained from MS patients and healthy controls. A mixed 2-way MANOVA (group × task) applied on G/L ratio, Z-transformed coefficients of correlation and their respective time lags revealed a significant main effect of group [Wilks’ Lambda = 0.383, F(3,20)=10.72, p<0.001, η2=0.62] and task [Wilks’ Lambda=0.172, F(6,17)=13.66, p<0.001, η2=0.83]. The univariate analysis of G/L ratio revealed both a main effect of group (higher G/L ratio in MS patients; F(1,22)=28.2, p<0.001) and a main effect of task (F(2,44)=10.31, p<0.001); (see Fig. 4A) with no interaction. The Z-transformed coefficients of correlation were lower in MS patients than in healthy controls (F(1,22)=25.6, p<0.001) with a main effect of task (F(2,44)=11.1, p<0.001) and no interaction (Fig. 4B). Regarding the G modulation, the MANOVA applied on gain and offset revealed a significant main effect of group [Wilks’ Lambda = 0.393, F(2,21)=16.22, p<0.001, η2=0.61] and no effect of task [Wilks’ Lambda = 0.956, F(2,21)=0.48, p>0.05, η2=0.04]. In the subsequent univariate analysis, both the gain (F(1,22)=5.4, p<0.05) and offset (F(1,22)=20.92, p<0.001) were higher in MS patients than in healthy controls (Figs. 4D & 4F). Neither the main effect of the task nor the group × task interactions were significant.
Fig. 4.
Force coordination variables averaged across the participants for lifting, ramp (ramp-and-hold) and oscillation tasks. Box plots represent the 25th to the 75th percentile of the distribution and the middle line represents the median.
Finally, we assessed the relationship between the individual dependent variables and each of the three clinical tests (i.e., EDSS, 9-hole peg, and Jebsen-Taylor test). Due to both a limited number of the tested patients and the Bonferroni correction for multiple comparisons applied, only two significant relationships were observed. In particular, the G/L ratio obtained from the lifting task was positively related with both the EDSS and Jebsen-Taylor test (p<0.05). However, note that several other values depicting force coordination of the lifting and ramp-and-hold task, albeit non-significant, suggested that approximately 35–45% of variance of the clinical tests could be explained by individual force coordination variables.
Discussion
The aims of our study were (1) to assess the specific aspects of impairment of hand function in MS patients through the standard dependent variables depicting task performance and force coordination, (2) to reveal both the manipulation tasks and particular dependent variables that demonstrate the most prominent differences between the tested patients and healthy controls, and (3) to evaluate the concurrent validity of the dependent variables with respect to the standard clinical tests of hand function. The most important finding of the study could be a consistently observed impairment of hand function in the tested MS patients observed across the tasks regarding all performance and most of the force coordination variables. However, the relationship between the same dependent variables and the standard clinical tests were relatively weak and inconsistent. The following paragraphs will be mainly focused on the comparison of these findings with the previous studies in understanding the impairment of hand function in MS, on the importance of these findings for future assessments of hand function in MS and, possibly, other neurological diseases.
Regarding our first aim, the present study consistently revealed differences between the MS patients and healthy controls across both the tasks and the dependent variables. Of particular importance could be that the tested patients were only mildly involved and fully able to live an independent and, in the most cases, a professionally active life. Nevertheless, they revealed a marked deterioration of their ability to exert an accurate pattern of L (as assessed by the task performance variables), as well as to coordinate G with the ongoing changes in L (as assessed by the force coordination variables). These finding are not only in line with the finding obtained from other neurological patients (see Introduction for details), but also indirectly justify the applied approach of the assessment of hand function through the force coordination.
The differences between the findings of the present and our previous studies based on similar methodology could deserve particular attention. Namely, our previous studies performed on mildly involved MS patients revealed the differences between the patients and healthy controls only regarding the task performance and G/L ratio (Krishnan et al., 2008; Marwaha et al., 2006). Therefore, we concluded that the decoupling of G and L recorded in other neurological diseases (Mai et al., 1988; Nowak et al., 2003) could be associated with the advanced phases of the disease. In the present study we intended to test more involved MS patients, although still able to perform simple tasks without major difficulties. However, following the preliminary neurological screening and rejection of several patients due to their inability to perform tasks properly (mainly the oscillation and, occasionally, the ramp-and-hold task) our patients on average appeared to be not much more involved than the patients tested in our previous studies. For example, the average involvement of our current patients as assessed by EDSS was 3.9 (range 2.5–5.5), while in the previous studies it was 3.0 (range 1.5–4) (Marwaha et al., 2006) and 3.2 (range 1–5) (Krishnan et al., 2008). Similarly, the score on the 9-hole peg test averaged across the hands in the present study was 25.3 s, while in the previous two studies the score was 23.2 s and 20.3 s, respectively. For a comparison, Fey and co-workers tested the hand function of the MS patients that scored as high as 8 (range 6–8) on EDSS (Feys et al., 2005), as well as 180 s (range 31–180) on 9-hole peg test (Feys et al., 2007; Feys et al., 2003). Therefore, one could ask why the present study provided such consistent and prominent differences in the variables depicting both the task performance and force coordination, as compared with our previous studies. One possible explanation could be that the somewhat higher level of involvement of the current group of MS patients corresponds to the stage of the disease where the ability both to exert the needed L profile and to coordinate G with L start rapidly to deteriorate. Alternatively, one could also speculate on the possible effects of the partly novel methodology applied in the present study. In particular, the device was smaller and simpler, the bi-directional oscillation task and the tasks performed without visual feedback were excluded, while a simple and 'ecological' lifting task was added. We do not have data that could support either of the proposed explanations. Nevertheless, the prominent differences between the tested groups observed despite a relatively low level of the patient involvement generally suggest that the methodology applied in the present study could be sensitive for the assessment of hand function in MS.
Regarding our second aim, consistent differences between the patients and healthy controls were observed across both the tested tasks and the dependent variables. In addition, the group × task interactions failed to reveal the tasks that provide particularly prominent differences between the tested MS patients and healthy controls regarding individual force coordination variables. Therefore, one could conclude that none of the tested tasks and the evaluated variables can be particularly recommended for future assessment of hand function in MS. Nevertheless, our direct experience with the tested patients suggests that the lifting task could be recommended over the two static tasks, as well as that, between two static tasks, the ramp-and-hold could be recommended over the oscillation task. The first recommendation is based on the task simplicity and the potential 'ecological validity'. For the tested patients this task was the easiest both to understand and to perform, where a part of the 'easiness' could have come from its close resemblance to the daily lifting tasks. In addition, only the variables obtained from this task revealed significant relationship with the standard clinical assessments. Regarding the second recommendation, when compared with the oscillation task that was heavily based on the predictive feed-forward control mechanisms (Jaric et al., 2006; Marwaha et al., 2006), the feedback based ramp-and-hold task also proved to be easier both to understand and to perform. In general, the advantage of the tasks that prove to be easier both to understand and perform could be even more important if more involved patients were tested. Finally, note that among the force coordination variables, the G/L ratio, gain and offset are not mutually independent variables. Specifically, a high G/L ratio could be a consequence either of a high gain, or of a high offset, or both (Krishnan et al., 2008; Zatsiorsky et al., 2005). Taking into account the simplicity of the assessment of G/L ratio, a consistently elevated G/L ratio across the neurological patients (Fellows et al., 1998; Nowak and Hermsdorfer, 2005), as well as its concurrent validity (see next paragraph), we could recommend G/L ratio as a particularly valid and sensitive force coordination variable for testing hand function.
With respect to our third aim, the main finding is that the relationships between the dependent variables and the three clinical tests proved to be inconsistent and mainly non-significant. The only exception was a positive relationship between G/L ratio and all three clinical tests. Having in mind both a relatively low validity of some of the applied clinical tests (see Introduction) and the obtained weak relationships among them, the first finding could not be considered as surprising. The second finding, however, could suggest that the G/L ratio obtained from the 'most natural' manipulation task tested could also be the most valid force coordination variable for the assessment of hand impairment in MS. However, we believe that the relatively weak relationships of the evaluated kinetic variables with the standard clinical tests could be of limited importance. Namely, having in mind the justification of the functional importance of G and L in daily manipulation tasks (see Introduction), it could be hard to argue that the timed actions evaluated in the standard clinical tests (such as in the 9-hole peg and Jebsen-Taylor tests) could be more valid for assessing hand function than the kinetic variables evaluated in the present study.
In conclusion, the present study revealed that a brief experimental protocol based on simple manipulation tasks and a relatively simple and inexpensive device recording of only single components of G and L can detect the differences between mildly involved individuals with MS and healthy controls. The differences were consistently observed not only across the variables depicting a deteriorated G and L coordination frequently studied in other neurological patients over the last two decades, but also across the variables depicting the MS patients' deteriorated ability to accurately exert prescribed patterns of L. When compared with our previous study, the prominent differences observed between the tested groups could be explained by a somewhat higher level of patient involvement and/or a simpler device and less demanding tasks. Despite on average a relatively weak relationship with the clinical tests, the presumed face validity and apparent sensitivity to detect mild level of impairment of hand function advocate the applied methodology for future use. The ramp-and-hold task and, in particular, simple lifting and holding could be particularly recommended tasks, as well as the indices of task performance variables and G/L ratio as a variable depicting force coordination. Due to impaired bimanual function caused by demyelinization frequently recorded within the corpus callosum of MS patients (Mendez, 1995; Tsolaki et al., 1994), development of bimanual tests could also be of importance. Of particular importance could be the development of the applied methods into a standard clinical test of impairment of hand function.
ACKNOWLEDGMENT
We thank Dr. S. Radosavljevic Jaric for clinical evaluation of MS patients. The study was supported in part by a grant from the National Multiple Sclerosis Society to S. Jaric.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: the role of sensorimotor context estimation. J Neurosci. 1998;18:7511–7518. doi: 10.1523/JNEUROSCI.18-18-07511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank R, Breitenbach A, Nitschke M, Heizer W, Letzgus S, Hermsdorfer J. Human development of grip force modulation relating to cyclic movement-induced inertial loads. Exp Brain Res. 2001;138:193–199. doi: 10.1007/s002210000622. [DOI] [PubMed] [Google Scholar]
- Cutter GR, Baier ML, Rudick RA, Cookfair DL, Fischer JS, Petkau J, Syndulko K, Weinshenker BG, Antel JP, Confavreux C, Ellison GW, Lublin F, Miller AE, Rao SM, Reingold S, Thompson A, Willoughby E. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(Pt 5):871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- de Freitas PB, Krishnan V, Jaric S. Force coordination in static manipulation tasks: effects of the change in direction and handedness. Exp Brain Res. 2007;183:487–497. doi: 10.1007/s00221-007-1064-3. [DOI] [PubMed] [Google Scholar]
- Fellows SJ, Noth J, Schwarz M. Precision grip and Parkinson's disease. Brain. 1998;121(Pt 9):1771–1784. doi: 10.1093/brain/121.9.1771. [DOI] [PubMed] [Google Scholar]
- Ferrand L, Jaric S. Force coordination in static bimanual manipulation: Effect of handedness. Motor Control. 2006;10:359–370. doi: 10.1123/mcj.10.4.359. [DOI] [PubMed] [Google Scholar]
- Feys P, Helsen W, Prinsmel A, Ilsbroukx S, Wang S, Liu X. Digitised spirography as an evaluation tool for intention tremor in multiple sclerosis. J Neurosci Methods. 2007;160:309–316. doi: 10.1016/j.jneumeth.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Feys P, Helsen WF, Lavrysen A, Nuttin B, Ketelaer P. Intention tremor during manual aiming: a study of eye and hand movements. Mult Scler. 2003;9:44–54. doi: 10.1191/1352458503ms863oa. [DOI] [PubMed] [Google Scholar]
- Feys P, Helsen WF, Liu X, Nuttin B, Lavrysen A, Swinnen SP, Ketelaer P. Interaction between eye and hand movements in multiple sclerosis patients with intention tremor. Mov Disord. 2005;20:705–713. doi: 10.1002/mds.20382. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Tresilian J, Wing AM. Coupling of grip force and load force during arm movements with grasped objects. Neurosci Lett. 1993;152:53–56. doi: 10.1016/0304-3940(93)90481-y. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Tresilian JR. Grip-load force coupling: a general control strategy for transporting objects. J Exp Psychol Hum Percept Perform. 1994;20:944–957. doi: 10.1037//0096-1523.20.5.944. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. Modulation of grip force with load force during point-to-point arm movements. Exp Brain Res. 1993;95:131–143. doi: 10.1007/BF00229662. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. The Stability of Precision Grip Forces during Cyclic Arm Movements with a Hand-Held Load. Exp Brain Res. 1995;105:455–464. doi: 10.1007/BF00233045. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci. 1997;17:1519–1528. doi: 10.1523/JNEUROSCI.17-04-01519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas PB, Jr, Krishnan V, Jaric S. Elaborate force coordination of precision grip could be generalized to bimanual grasping techniques. Neurosci Lett. 2007;412:179–184. doi: 10.1016/j.neulet.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Charles J, Steenbergen B. Fingertip force planning during grasp is disrupted by impaired sensorimotor integration in children with hemiplegic cerebral palsy. Pediatric Research. 2006;60:587–591. doi: 10.1203/01.pdr.0000242370.41469.74. [DOI] [PubMed] [Google Scholar]
- Grice KO, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available nine hole peg test for finger dexterity. American Journal of Occupational Therapy. 2003;57:570–573. doi: 10.5014/ajot.57.5.570. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Hagl E, Nowak DA, Marquardt C. Grip force control during object manipulation in cerebral stroke. Clinical Neurophysiology. 2003;114:915–929. doi: 10.1016/s1388-2457(03)00042-7. [DOI] [PubMed] [Google Scholar]
- Ingvarsson PE, Gordon AM, Forssberg H. Coordination of manipulative forces in Parkinson's disease. Exp Neurol. 1997;145:489–501. doi: 10.1006/exnr.1997.6480. [DOI] [PubMed] [Google Scholar]
- Jaric S, Collins JJ, Marwaha R, Russell E. Interlimb and within limb force coordination in static bimanual manipulation task. Exp Brain Res. 2006;168:88–97. doi: 10.1007/s00221-005-0070-6. [DOI] [PubMed] [Google Scholar]
- Jaric S, Knight CA, Collins JJ, Marwaha R. Evaluation of a method for bimanual testing coordination of hand grip and load forces under isometric conditions. J Electromyogr Kinesiol. 2005;15:556–563. doi: 10.1016/j.jelekin.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- Johansson RS. Sensory input and control of grip. Novartis Found Symp. 1998;218:45–59. doi: 10.1002/9780470515563.ch4. discussion 59–63. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56:550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Krishnan V, de Freitas PB, Jaric S. Impaired object manipulation in mildly involved individuals with multiple sclerosis. Motor Control. 2008;12:3–20. doi: 10.1123/mcj.12.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke JF. Rating Neurologic Impairment in Multiple-Sclerosis - an Expanded Disability Status Scale (Edss) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Mai N, Bolsinger P, Avarello M, Diener HC, Dichgans J. Control of isometric finger force in patients with cerebellar disease. Brain. 1988;111(Pt 5):973–998. doi: 10.1093/brain/111.5.973. [DOI] [PubMed] [Google Scholar]
- Marwaha R, Hall SJ, Knight CA, Jaric S. Load and grip force coordination in static bimanual manipulation tasks in multiple sclerosis. Motor Control. 2006;10:160–177. doi: 10.1123/mcj.10.2.160. [DOI] [PubMed] [Google Scholar]
- Matthews W. Multiple sclerosis and other demyelinating diseases: clinical features. In: M S, J O, editors. Clinical Neurology. New York: Churchill Livingstone; 1991. pp. 1106–1116. [Google Scholar]
- Mendez MF. The Neuropsychiatry of Multiple-Sclerosis. Int J Psychiatry Med. 1995;25:123–135. doi: 10.2190/NK8F-MTUW-QHH1-0531. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Vandervoort MK, Wong CJ, Ebers GC. Interrater variability with the Expanded Disability Status Scale (EDSS) and Functional Systems (FS) in a multiple sclerosis clinical trial. The Canadian Cooperation MS Study Group. Neurology. 1990;40:971–975. doi: 10.1212/wnl.40.6.971. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J. Impaired coordination between grip force and load force in amyotrophic lateral sclerosis: a case-control study. Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3:199–207. doi: 10.1080/146608202760839005. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J. Deficits of predictive grip force control during object manipulation in acute stroke. Journal of Neurology. 2003a;250:850–860. doi: 10.1007/s00415-003-1095-z. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J. Selective deficits of grip force control during object manipulation in patients with reduced sensibility of the grasping digits. Neurosci Res. 2003b;47:65–72. doi: 10.1016/s0168-0102(03)00182-2. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J. Grip force behavior during object manipulation in neurological disorders: Toward an objective evaluation of manual performance deficits. Movement Disorders. 2005;20:11–25. doi: 10.1002/mds.20299. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J, Marquardt C, Fuchs HH. Grip and load force coupling during discrete vertical arm movements with a grasped object in cerebellar atrophy. Exp Brain Res. 2002;145:28–39. doi: 10.1007/s00221-002-1079-8. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J, Topka H. When motor execution is selectively impaired: Control of manipulative finger forces in amyotrophic lateral sclerosis. Motor Control. 2003;7:304–320. doi: 10.1123/mcj.7.3.304. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rossier P, Wade DT. The Guy's Neurological Disability Scale in patients with multiple sclerosis: a clinical evaluation of its reliability and validity. Clin Rehabil. 2002;16:75–95. doi: 10.1191/0269215502cr447oa. [DOI] [PubMed] [Google Scholar]
- Rost K, Nowak DA, Timmann D, Hermsdorfer J. Preserved and impaired aspects of predictive grip force control in cerebellar patients. Clin Neurophysiol. 2005;116:1405–1414. doi: 10.1016/j.clinph.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Burgunder JM, Wiesendanger M. Control of manipulative forces during unimanual and bimanual tasks in patients with Huntington's disease. Exp Brain Res. 2002;143:328–334. doi: 10.1007/s00221-001-0992-6. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Wiesendanger M. Grip-load force coordination in cerebellar patients. Exp Brain Res. 1999;128:76–80. doi: 10.1007/s002210050820. [DOI] [PubMed] [Google Scholar]
- Thonnard JL, Detrembleur C, VandenBergh PYK. Assessment of hand function in a patient with chronic sensory demyelinating neuropathy. Neurology. 1997;49:253–257. doi: 10.1212/wnl.49.1.253. [DOI] [PubMed] [Google Scholar]
- Tsolaki M, Drevelegas A, Karachristianou S, Kapinas K, Divanoglou D, Routsonis K. Correlation of dementia, neuropsychological and MRI findings in multiple sclerosis. Dementia. 1994;5:48–52. doi: 10.1159/000106694. [DOI] [PubMed] [Google Scholar]
- Whitaker JN, McFarland HF, Rudge P, Reingold SC. Outcomes assessment in multiple sclerosis clinical trials: a critical analysis. Mult Scler. 1995;1:37–47. doi: 10.1177/135245859500100107. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Gao F, Latash ML. Motor control goes beyond physics: differential effects of gravity and inertia on finger forces during manipulation of hand-held objects. Exp Brain Res. 2005;162:300–308. doi: 10.1007/s00221-004-2152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]