Abstract
Infrasound (i.e., < 20 Hz for humans; < 100 Hz for chinchillas) is not audible, but exposure to high levels of infrasound will produce large movements of cochlear fluids. We speculated that high-level infrasound might bias the basilar membrane and perhaps be able to minimize noise-induced hearing loss. Chinchillas were simultaneously exposed to a 30 Hz tone at 100 dB SPL and a 4-kHz OBN at either 108 dB SPL for 1.75 h or 86 dB SPL for 24 h. For each animal, the tympanic membrane (TM) in one ear was perforated (~1 mm2) prior to exposure to attenuate infrasound transmission to that cochlea by about 50 dB SPL. Controls included animals that were exposed to the infrasound only or the 4-kHz OBN only. ABR threshold shifts (TSs) and DPOAE level shifts (LSs) were determined pre- and post-TM-perforation and immediately post-exposure, just before cochlear fixation. The cochleae were dehydrated, embedded in plastic, and dissected into flat preparations of the organ of Corti (OC). Each dissected segment was evaluated for losses of inner hair cells (IHCs) and outer hair cells (OHCs). For each chinchilla, the magnitude and pattern of functional and hair cell losses were compared between their right and left cochleae. The TM perforation produced no ABR TS across frequency but did produce a 10–21 dB DPOAE LS from 0.6–2 kHz. The infrasound exposure alone resulted in a 10–20 dB ABR TS at and below 2 kHz, no DPOAE LS and no IHC or OHC losses. Exposure to the 4-kHz OBN alone at 108 dB produced a 10–50 dB ABR TS for 0.5–12 kHz, a 10–60 dB DPOAE LS for 0.6–16 kHz and severe OHC loss in the middle of the first turn. When infrasound was present during exposure to the 4-kHz OBN at 108 dB, the functional losses and OHC losses extended much further toward the apical and basal tips of the OC than in cochleae exposed to the 4-kHz OBN alone. Exposure to only the 4-kHz OBN at 86 dB produces a 10–40 dB ABR TS for 3–12 kHz and 10–30 dB DPOAE LS for 3–8 kHz but little or no OHC loss in the middle of the first turn. No differences were found in the functional and hair-cell losses from exposure to the 4-kHz OBN at 86 dB in the presence or absence of infrasound. We hypothesize that exposure to infrasound and an intense 4-kHz OBN increases cochlear damage because the large fluid movements from infrasound cause more intermixing of cochlear fluids through the damaged reticular lamina. Simultaneous infrasound and a moderate 4-kHz OBN did not increase cochlear damage because the reticular lamina rarely breaks down during this moderate level exposure.
Keywords: Infrasound, noise, histopathology, ABR, DPOAE, chinchilla
1. Introduction
Many people ‘at risk’ for noise-induced hearing loss (NIHL) cannot or do not wear hearing protection (i.e., ear plugs, ear muffs) when exposed to noise (e.g., active military; workers who need to hear warning signals). Also, commonly available hearing protectors do not provide complete protection from developing NIHL for exposed individuals (e.g., Humes et al., 2006; Kopke, 2005). To improve hearing protection, alternative protection strategies must be developed.
Infrasound (i.e., < 20 Hz for humans) is not audible, but exposure to high levels of infrasound will produce large movements of cochlear fluids (Salt and DeMott, 1999). Although infrasound is inaudible, movements of cochlear fluids induce basilar membrane displacements, the magnitude of which is inversely proportional to infrasound frequency. We speculated that these displacements might shift the operating point of the basilar membrane and perhaps minimize NIHL from exposures to noise in the audible frequency range. Our hypothesis was that exposure to a non-damaging level of infrasound (e.g., 30-Hz tone for the chinchilla) at the same time as a damaging level of noise would protect the cochlea from hair-cell degeneration and hearing loss.
2. Methods
2.1 Animals
Eight 1–3-year-old chinchillas (Ryerson Chinchilla Ranch, Plymouth, OH) were used in this study. Four chinchillas were exposed to a potentially damaging level of noise [i.e., 4-kHz octave band of noise (OBN) at 86 or 108 dB sound pressure level (SPL)] plus infrasound [i.e., 30-Hz tone, 100 dB SPL]. In each of these chinchillas, one cochlea was exposed to the 4-kHz OBN and infrasound while the opposite cochlea was subjected to the 4-kHz OBN and highly attenuated infrasound as a result of perforating the tympanic membrane (TM) in that ear. This within-animal paradigm was designed to circumvent interanimal variability in response to noise. It is known that the magnitudes and locations of hair-cell losses in the two cochleae of a bilaterally noise-exposed chinchilla are highly correlated (Bohne et al., 1986). Thus, the within-animal paradigm allowed us to use a smaller sample size than would be required with a cross-animal design.
Because this study was focused on the primary effects of infrasound and noise exposure (i.e., the functional and hair-cell losses produced during the exposure), the animals were terminated within 3 h post-exposure. With longer recovery times, secondary losses of hair cells occur (e.g., Bohne and Harding, 2000) following exposure to the 4-kHz OBN at 108 dB SPL (e.g., Harding and Bohne, 2004a). With a recovery time of several weeks, it would not be possible to determine whether any change in the magnitude of hair-cell loss was the result of the presence of infrasound during the exposure or post-exposure degeneration.
The chinchillas were anesthetized with a mixture of ketamine, acepromazine and atropine (40 mg/kg, 1 mg/kg, and 0.04 mg/kg, respectively) for hearing testing, TM perforation, and in-vivo fixation of their cochleae. The protocol for animal use was approved by Washington University School of Medicine’s Animal Studies Committee (B.A. Bohne, PI).
2.2 TM perforation
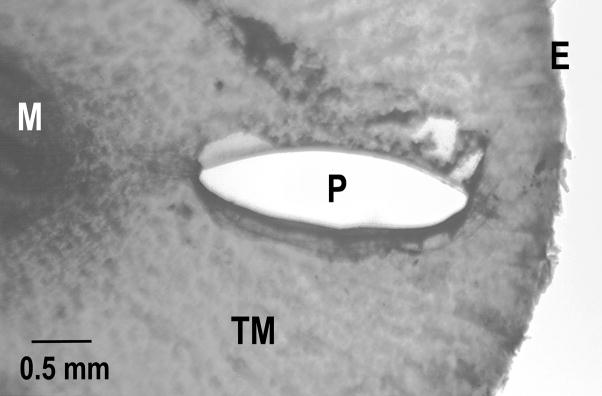
A pediatric ear speculum was used to visualize the inferior quadrant of the TM in the anesthetized chinchilla. A 1.0 mm2 perforation (Fig. 1) was made using a small, right-angle hook. The TM perforation attenuated the transmission of the 30 Hz signal by about 50 dB while audible signals around 4 kHz were unaffected (Voss et al., 2001).
Figure 1.
Dissection microscope view of a 1.00-mm2 perforation after fixation and removal of the TM. M - Tip of manubrium; P - Perforation; E - Edge of TM.
2.3 Exposure to infrasound and/or the OBN
All animals were exposed to the 4-kHz OBN, infrasound or the combination of the OBN and infrasound (Table 1) while awake. For their exposure, the animals were housed in a wire-mesh cage that was suspended in the middle of a reverberant sound-proof booth. To prevent the animals from lying on their side and occluding one external auditory canal, the animals were tethered to the top of the cage using a 20-cm long spring and an aluminum collar. Two chinchillas were exposed to the 30 Hz tone only for 1.75 and 24 h, respectively. Two chinchillas were exposed for 1.75 h to the 4-kHz OBN at 108 dB SPL. Two chinchillas were exposed for 1.75 h to the 30 Hz tone plus the 4-kHz OBN at 108 dB SPL. Two chinchillas were exposed for 24 h to the 30 Hz tone plus the 4-kHz OBN at 86 dB SPL.
Table 1.
Hair-cell losses in cochleae from present study
| Apical half of OC
|
Basal half of OC
|
|||||
|---|---|---|---|---|---|---|
| Ear # | Exposure | TM-perfa | % mIHC | % mOHC | % mIHC | %mOHC |
| 969L | Infrasoundb, 1.75 h | No | 0.6 | 2.9 | 0.5 | 0.8 |
| 969R | Infrasound, 1.75 h | Yes | 0.7 | 3.2 | 1.4 | 0.9 |
| 976L | Infrasound, 24 h | No | 0.5 | 3.1 | 0.1 | 1.1 |
| 976R | Infrasound, 24 h | Yes | 0.5 | 2.7 | 0.2 | 1.3 |
| 971R | 108 dB SPL, 1.75 h | No | 0.6 | 12.4 | 1.6c | 97.7 |
| 971L | 108 dB SPL, 1.75 h | Yes | 0.2 | 2.2 | 7.6c | 22.6 |
| 973L | 108 dB SPL, 1.75 h | No | 1.3 | 2.3 | 4.6c | 18.6c |
| 973R | 108 dB SPL, 1.75 h | Yes | 1.1 | 2.6 | 4.4 | 32.5 |
| 970R | 108 dB SPL, 1.75 h plus infrasound | No | 0.2 | 5.9 | 1.7 | 68.2 |
| 970L | 108 dB SPL, 1.75 h plus infrasound | Yes | 1.5 | 2.6 | 1.2 | 36.9 |
| 972R | 108 dB SPL, 1.75 h plus infrasound | No | 1.2 | 21.7 | 3.8 | 97.3 |
| 972L | 108 dB SPL, 1.75 h plus infrasound | Yes | 0.3 | 10.3 | 4.0 | 54.5 |
| 974L | 86 dB SPL, 24 h plus infrasound | No | 0.4 | 1.0 | 4.2 | 8.7 |
| 974R | 86 dB SPL, 24 h plus infrasound | Yes | 0.4 | 1.4 | 0.1 | 12.3 |
| 975R | 86 dB SPL, 24 h plus infrasound | No | 2.1 | 1.0 | 0.3 | 1.3 |
| 975L | 86 dB SPL, 24 h plus infrasound | Yes | 2.5 | 1.6 | 0.6 | 0.8 |
1.0 mm2 perforation of the tympanic membrane.
30 Hz pure tone, 100 dB SPL.
Includes pre-existing loss.
2.4 Functional testing
Hearing was tested three times at frequencies from 0.5–20 kHz with ABR thresholds and 2f1– f2 DPOAE magnitudes. These data were collected: pre-exposure to determine baseline auditory function; post-TM perforation to determine the amount of signal attenuation caused by the perforation; and immediately post-noise-exposure, just prior to cochlear fixation. Stimulus levels for DPOAEs were L1=75 dB SPL and L2=65 dB SPL with the f2/f1 ratio equal to 1.23 (Harding et al., 2002). ABR threshold shifts (TSs) and DPOAE level shifts (LSs) induced by TM perforation were calculated by subtracting post-perforation magnitudes from pre-perforation magnitudes at each ABR frequency and DPOAE frequency-pair tested. ABR TSs and DPOAE LSs induced by noise exposure were calculated by subtracting post-exposure magnitudes from post-perforation magnitudes in the TM-perforated ears and from pre-exposure magnitudes in the non-perforated ears. The differential noise floor for DPOAE measurement was determined by subtracting average pre- and post-treatment noise floor magnitudes from pre-treatment DPOAE magnitudes.
2.5 Tissue processing
Under deep anesthesia, the chinchillas had their cochleae surgically exposed and fixed in-vivo by perfusing a buffered solution of 1% osmium tetroxide through the scala tympani. After fixing both cochleae, the animals were decapitated, their temporal bones removed and immersed in cold fixative for two h. The following day, the cochleae were dehydrated in a graded series of ethanol followed by propylene oxide and then infiltrated with plastic (i.e., epoxy resin). The cochleae were embedded in a mold of plastic that was polymerized at 60° C for 48 h. After polymerization, the cochlear bone was removed with a sharpened pick and the cochlear ducts were dissected into short segments with small pieces of razor blades. In each segment, the plastic filling scala tympani was trimmed close and parallel to the basilar membrane. The trimmed segments were re-embedded in thin layers of plastic (Bohne, 1972; Bohne and Harding, 1993) which were examined as flat preparations by phase-contrast microscopy.
The TM on the perforated side was dissected from the tympanic ring, immersed in a cold, buffered solution of 1% osmium tetroxide for 10 minutes, rinsed in buffer and stored in 70% ethanol.
2.6 Data collection and processing
A dissection microscope (Nikon, SMZ-U) and a digital camera (Micropublisher, 5.0 RTV) were used to image all dissected segments of the OC from each cochlea. The camera was interfaced to a personal computer. The image of each dissected segment was captured at a magnification of 80X. OC length was measured to the nearest 0.01 mm along the junction of the heads of the inner and outer pillars using NIH ImageJ software.
Each segment of the cochlea from apex to base was evaluated by phase-contrast microscopy (Wild, M-20). Counts were made of the number of hair cells that were missing and had been replaced by mature phalangeal scars or immature phalangeal scars (Bohne, 1976a) and the number of hair cells that were degenerating (Bohne et al., 2007). The presence of mature scars indicates that the hair-cell loss occurred at least several days prior to cochlear fixation. The presence of degenerating cells indicates that the damage occurred within hours prior to fixation. The hair-cell counts included identification of focal hair-cell lesions [i.e., regions at least 0.03-mm in length in which 50% or more of the OHCs and/or IHCs were missing (Bohne et al., 1990)].
In three chinchillas, the area of the TM perforation as well as the area of the entire TM was measured with the digital imaging system described above.
For each cochlea, the functional data were overlaid according to the chinchilla frequency-place map (Eldredge et al., 1981) on a combined structural-functional cytocochleogram. These cytocochleograms show the percentage of missing and degenerating hair cells and degenerated peripheral processes of the spiral ganglion cells in individual cochleae as a function of percentage distance from cochlear apex and frequency-position. Plots of ABR TSs and DPOAE LSs are superimposed on the cytocochleograms as a function of frequency place. DPOAE level-shift data are plotted at f1 because earlier we showed that this produces the best alignment with detailed histopathology (Harding et al., 2002; Harding and Bohne, 2004b). For each chinchilla, the magnitude and pattern of hair-cell and functional losses were compared between their right and left cochleae as well as to data from control chinchillas and chinchillas exposed to the 4-kHz OBN alone (see below).
Data on hair-cell losses in non-noise-exposed controls (N=30) and in chinchillas exposed binaurally to the 4-kHz OBN at 108 dB SPL for 1.75 h (N=6) and at 86 dB SPL for 24 h (N=11) were available from our permanent collection of plastic-embedded cochleae from 1–3-year-old chinchillas. All noise-exposed cochleae had been fixed for microscopy by 3 h post-exposure.
3. Results
3.1. TM perforation
Figure 2 shows the typical ABR TSs and DPOAE LSs induced by a small TM perforation about 2% of the total TM area. There were essentially no ABR TSs; 5 dB is within the test-retest variability. However, the DPOAE LSs ranged from 10–21 dB for 0.6–2 kHz. The presence of DPOAE LSs below 0.6 kHz could not be determined due to the high noise floor.
Figure 2.
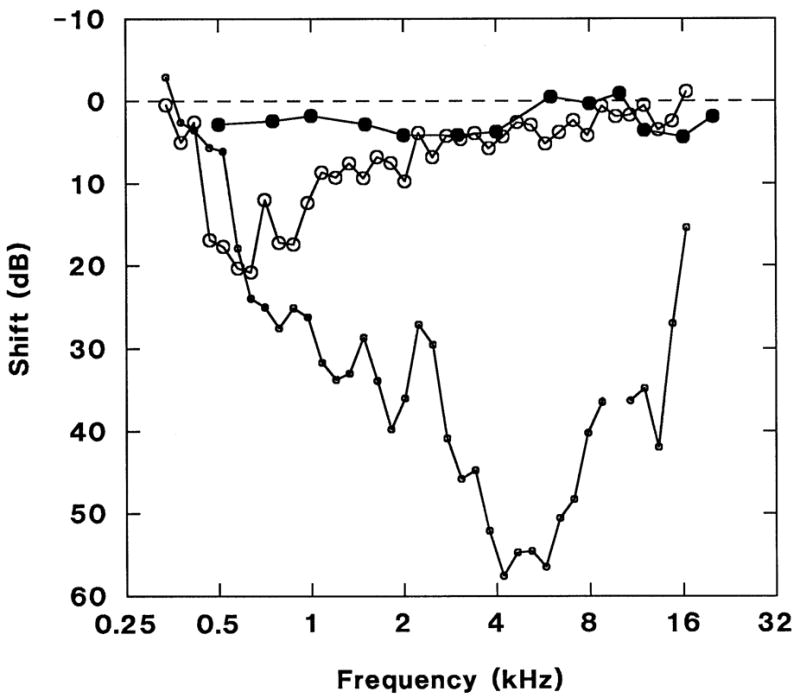
ABR threshold shifts (solid circles; n=6, no post-perforation ABR was obtained in 1 animal) and DPOAE level shifts (large open circles; n=5, no pre-perforation DPOAE was obtained in 2 animals) following TM perforation. There are essentially no ABR threshold shifts (<5 dB, within test-retest variability) but 10–21 dB DPOAE level shifts from 0.6 to 2 kHz. Differential DPOAE noise floor (small open circles). Error bars omitted for clarity; standard deviations ranged from 1–7 dB. In this and subsequent graphs, DPOAE data are plotted at f1 and when present, an artifact in the differential noise floor at 10 kHz has been deleted.
3.2. Damage from exposure to infrasound only
Figure 3 shows combined cytocochleograms with functional results overlaid according to the chinchilla frequency place map (Eldredge et al., 1981) on the histopathological findings from an animal exposed to infrasound only for 24 h. These cochleae had minimal hair-cell losses (Table 1) and no nerve-fiber losses. All missing hair cells were replaced by mature phalangeal scars so it is very unlikely that any of the hair-cell losses were due to the infrasound. Perforation of the TM resulted in a 10-dB ABR TS at 0.8 kHz only and 10-dB DPOAE LSs from 0.4–0.9 kHz. Thus, exposure to infrasound had little effect on cochlear function. On the TM-intact side, the exposure resulted in 10–20 dB ABR TSs for frequencies at and below 2 kHz, but no shifts for higher frequencies and there were no DPOAE LSs in this cochlea. Structural and functional data from the animal exposed to infrasound for 1.75 h were very similar to the data shown in Figure 3.
Figure 3.
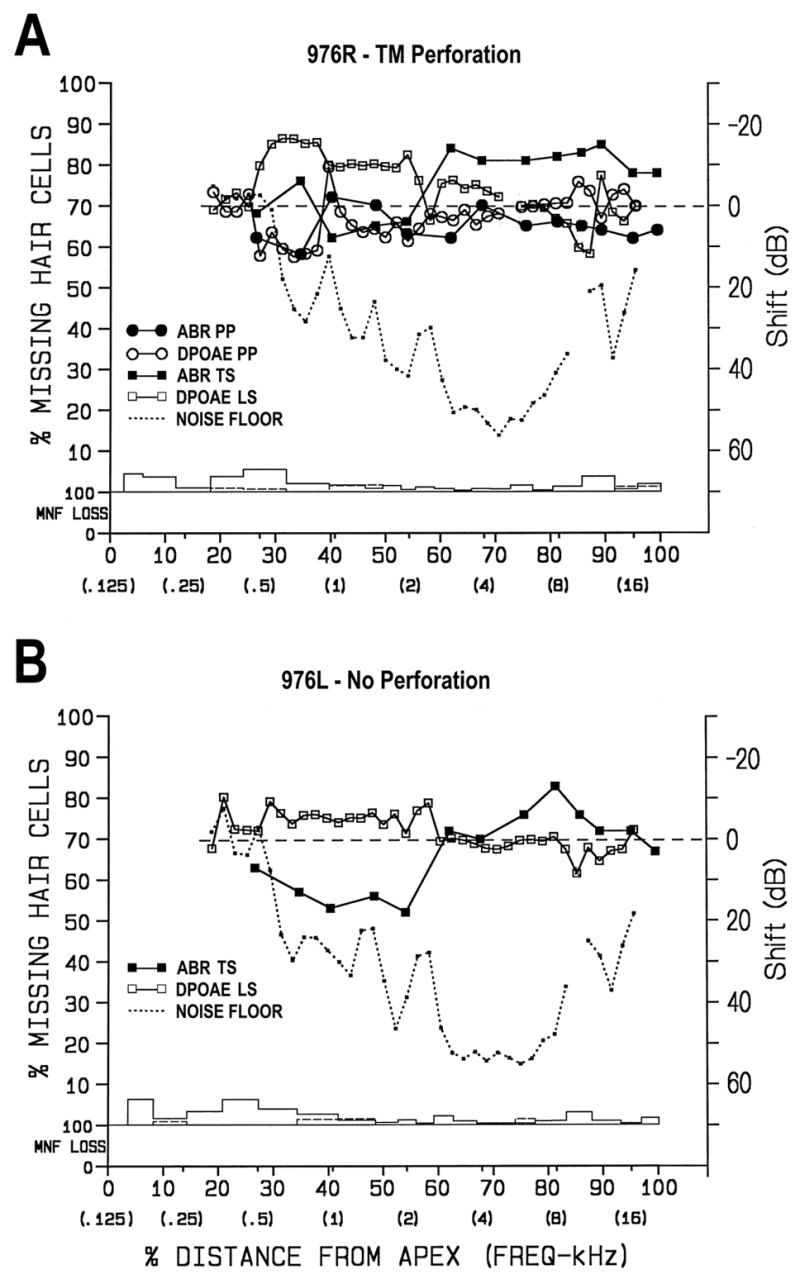
Combined structural-functional cytocochleograms for chinchilla exposed to infrasound alone (i.e., 30-Hz tone at 100 dB SPL). The cytocochleograms show the percentage of missing and degenerating hair cells (IHC – dashed line; OHC – solid line) and nerve fibers (MNF LOSS) in individual cochleae (on the left y-axis) as a function of percentage distance from cochlear apex and frequency-position (on the x-axis). MNF loss means degeneration of the myelinated peripheral processes of spiral ganglion cells. Plots of ABR threshold shifts post-perforation (PP) and post-exposure (TS) and DPOAE level shifts (PP, LS; right y-axis; see key for symbols) are superimposed on the cytocochleograms as a function of frequency place. DPOAE level-shift data are plotted at f1 because this produces the best alignment with detailed histopathology (Harding et al., 2002; Harding and Bohne, 2004). There were minimal losses of IHCs and OHCs throughout both the perforated (A) and non-perforated (B) ears. All missing hair cells had been replaced by mature phalangeal scars so none of this loss could be attributed to the infrasound exposure. A) Perforation of the TM caused minimal functional loss (i.e., 10 dB at 0.5–0.8 kHz). The infrasound had no effect on ABR TSs or DPOAE LSs. An artifact in the DPOAE results at 5 kHz has been removed from graph; B) On the non-perforated side, the exposure resulted in a 10–20 dB ABR TS for frequencies at and below 2 kHz, but no shifts for higher frequencies. There was no DPOAE LS. In A and B, an artifact in the noise floor at 10 kHz was removed.
3.3. Damage from 4-kHz OBN at 108 dB SPL for 1.75 h
Chinchillas exposed to the 4-kHz OBN at 108 dB SPL for 1.75 h (# 971 and 973) sustained moderate to large losses of OHCs in the basal half of the OC around the 4-kHz region (Table 1). All cochleae had at least one sizeable hair-cell lesion in the basal half of the OC. For example, Figure 4 shows combined cytocochleograms from chinchilla 973. Both the left and right cochleae had focal lesions. The cochlea on the perforated side (Fig. 4A) had three focal lesions around 68% distance from the apex (i.e., 4 kHz): 0.25-mm OHC lesion; 2.5-mm combined (i.e., OHC and IHC) lesion; and 0.13-mm OHC lesion. The cochlea on the non-perforated side (Fig. 4B) had five focal lesions: 0.11-mm and 0.77-mm OHC lesions around 72 % distance; 0.22-mm and 0.31-mm combined lesions; and 0.13-mm OHC lesion around 88% distance (8–12 kHz). The 0.31-mm lesion included a region of complete loss of the OC, which had been replaced on the basilar membrane with an epithelial scar, and degeneration of corresponding myelinated nerve fibers in the osseous spiral lamina (i.e., black bars in MNF LOSS section). The three lesions around 88% distance were most likely pre-existing because both the epithelial scar on the basilar membrane and the advanced degeneration of myelinated nerve fibers require about 14–21 days to develop. Also, there was a lack of a notch in the ABR TS (e.g., Nordmann et al., 2000) at this location. Thus, the functional data also indicate that the cochlear damage around 10 kHz was pre-existing.
Figure 4.
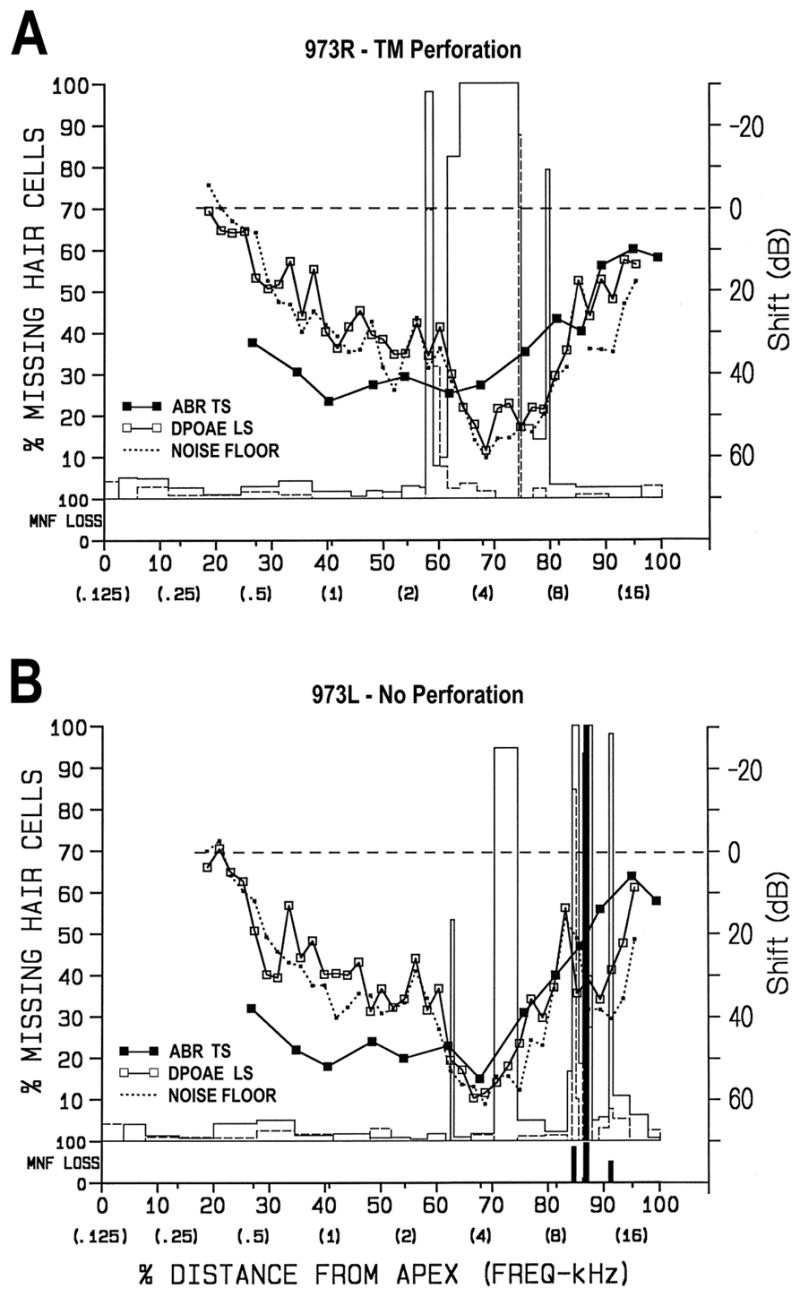
Combined structural-functional cytocochleograms for ears exposed for 1.75 h to 4-kHz OBN at 108 dB SPL. Both cochleas had focal lesions. A) The cochlea on the perforated side had three focal lesions: 0.25-mm and 2.5-mm combined lesions and 0.13-mm OHC lesion around 4 kHz; B) The cochlea on the non-perforated side had five focal lesions: 0.11-mm and 0.77-mm OHC lesions around 4 kHz, and 0.22-mm and 0.31-mm combined lesions and 0.13-mm OHC lesion at 10–12 kHz. The latter three lesions were pre-existing, as evidenced by the loss of myelinated nerve fibers in the osseous spiral lamina (i.e., vertical black bars in MNF LOSS section). In both ears, there was a 10–50 dB ABR TS from 0.5–12 kHz. The DPOAE LS was at or near the noise floor from 0.4–8 kHz in the perforated ear (A) and 0.4–12 kHz in the non-perforated ear. No functional data were obtained post-perforation in this animal. In A, an artifact in the noise floor at 10 kHz was removed. See legend for Figure 3 for description of axes.
In both ears, there were 10–55 dB ABR TSs from 0.5–12 kHz and 10–60 dB DPOAE LSs from 0.6–16 kHz. At and below 8 kHz, the DPOAE LS was in or near the noise floor. No functional data were obtained post-perforation in chinchilla 973. As a consequence, functional shifts were referenced to pre-exposure levels in this animal only. Further examples of acute functional changes from this exposure can be found in Harding et al. (2002).
The functional changes in chinchilla 971 were similar, with the exception that DPOAE LSs in the TM-perforated cochlea had completely recovered for frequencies from 0.4–1.2 kHz post-noise-exposure. In the TM-perforated ear had a pre-existing 0.98-mm combined focal lesion with associated loss of myelinated nerve fibers centered around 6 kHz. The non-perforated ear had an unusually large loss of OHCs in the basal half of the cochlea (Table 1).
3.4. Damage from 4-kHz OBN at 108 dB SPL for 1.75 h plus infrasound
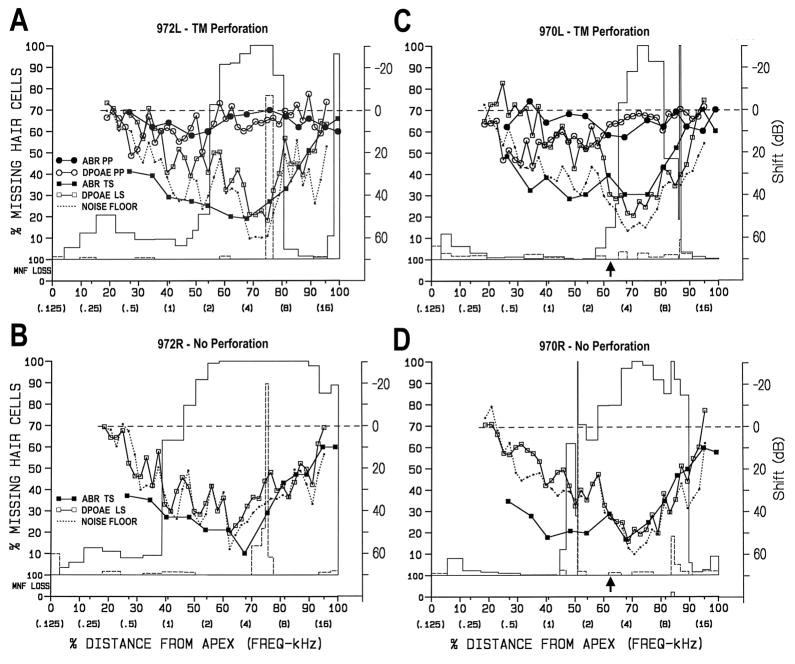
Cochleae exposed for 1.75 h to the 4-kHz OBN at 108 dB SPL plus infrasound sustained moderate to severe losses of OHCs in the basal half of the cochlea. In animals # 970 and 972, the cochlea on the non-perforated side had substantially larger basal OHC losses (i.e., 31.3% and 42.8%, respectively) than on the perforated side (Table 1).
Figure 5 shows the combined cytocochleograms for both animals in this group. In the perforated ear of animal 972 (Fig. 5A), there was a 5.09-mm combined lesion from 55–81% distance from the apex and a 0.39-mm OHC lesion at the basal tip (97–100%). At the primary lesion site (i.e., 76% distance) all OHCs and some IHCs were already missing. Surrounding this area, many OHCs were in the process of degenerating. Post TM-perforation, there were slight (10 dB) ABR TSs from 1.5–2 kHz and larger (15 dB) DPOAE LSs from 0.5–2 kHz. Post noise-exposure, ABR TSs ranged from 10–50 dB for 0.5–12 kHz and DPOAE LSs ranged from 10–50 dB for 1–12 kHz, much of the latter being at the noise floor.
Figure 5.
Combined structural-functional cytocochleograms for ears exposed for 1.75 h to 4-kHz OBN at 108 dB SPL plus infrasound. A and B) Data from perforated and non-perforated ear of chinchilla # 972. A) There was a 5.09-mm combined lesion from 55–81% distance from the apex and a 0.39-mm OHC lesion at the basal tip. At the primary lesion site (i.e., around 76%) all OHCs and some IHCs were already missing. Surrounding this area, many OHCs were in the process of degenerating. Post TM-perforation, there were a slight (10 dB) ABR TS from 0.8–2 kHz and larger (15 dB) DPOAE LS from 0.5–2 kHz. Post noise-exposure, the ABR TS ranged from 10–50 dB for 0.5–12 kHz and DPOAE LS ranged from 10–50 dB for 1–12 kHz; B) There was a 12.34-mm combined lesion from 39–100% distance from the apex. Degenerating OHCs were found much further apically and basally compared to the perforated ear (A). The ABR TS ranged from 10–60 dB and DPOAE LS was at or near the noise floor across all frequencies below 14 kHz. C and D) Data from perforated and non-perforated ear of chinchilla # 970. C) In the perforated ear, there was a 3.08-mm OHC lesion from 65–81 % distance and a 0.11-mm OHC lesion at 86%; D) In the non-perforated ear, there was a 0.63-mm OHC lesion from 47 to 50 % distance and a 7.55-mm combined lesion from 51–90% distance, considerably larger than in the TM perforated ear. ABR TSs and DPOAE LSs were quite similar to those in A and B. Arrows in C and D indicate locations where photomicrographs in Figure 6 were taken. See legend for Figure 3 for description of axes.
On the non-perforated side of 972 (Fig 5B), there was a 12.34-mm combined lesion from 39–100% distance from the apex; 2.3 times larger than the lesion in the TM-perforated ear. The vast majority of this lesion involved loss of OHCs. IHC loss was localized at 75% distance (6 kHz). Degenerating OHCs were found much further apically and basally compared to that in the perforated ear. Post-exposure, ABR TSs ranged from 10–60 dB over all frequencies tested and DPOAE LSs were at or near the noise floor across all frequencies below 14 kHz.
Figures 5C and 5D show the combined cytocochleograms from animal 970. ABR TSs and DPOAE LSs (Figs. 5C and 5D) were quite similar to those in Figures 5A and 5B, respectively. In the perforated ear (Fig. 5C), there was a 3.08-mm OHC lesion from 65 to 81 % distance and another 0.11-mm OHC lesion at 86%. In the non-perforated ear (Fig. 5D), there was a 0.63-mm OHC focal lesion from 47 to 50 % distance and a 7.55-mm OHC focal lesion from 51 to 90% distance; 2.4 times larger than the lesion in the perforated ear. There was no loss of myelinated nerve fibers in the cochlea from the perforated ear, but there was beginning nerve fiber degeneration associated with the IHC loss at 83% distance in the non-perforated ear. In the regions of substantial OHC loss, OHC-sized openings in the reticular lamina could be seen by phase contrast microscopic examination of the flat preparations (Bohne, 1976b). Note that conclusive demonstration of open holes in the reticular lamina would have required the in vivo introduction of a tracer into scala media and TEM examination (Ahmad et al., 2002).
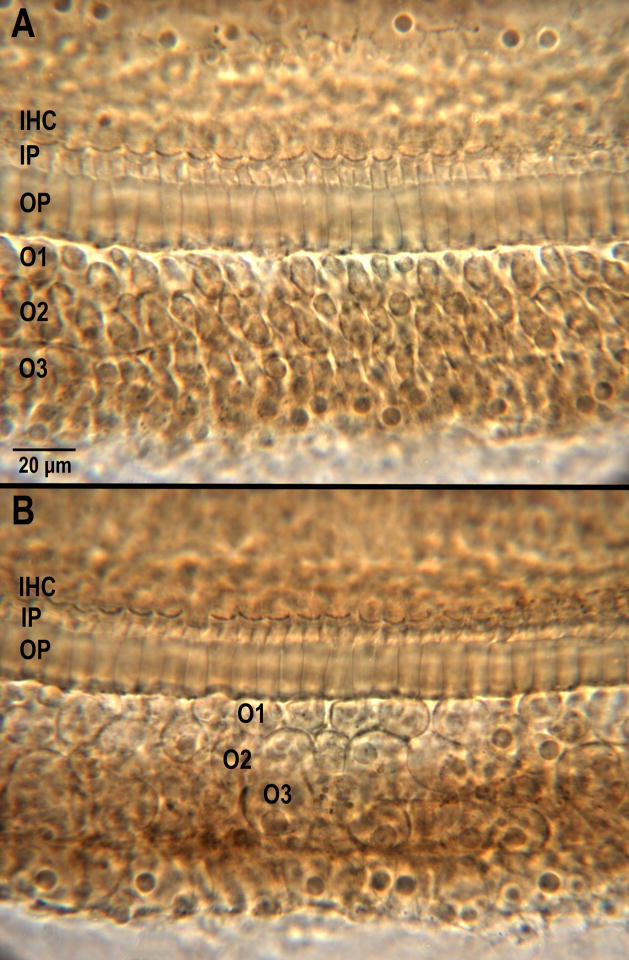
Figure 6 shows photomicrographs of the damage to the OC from Figures 5C and 5D (at arrows). Figure 6A shows the damage in the perforated ear and Figure 6B shows the damage at the same percentage distance from the apex in the non-perforated ear. At 62% distance from the apex, OHCs are out-of-position but near normal in shape in A and grossly swollen (i.e., oncotic) in B. This finding indicates that secondary damage spread from the initial injury site much further apically and basally in ears exposed to the 4-kHz OBN plus infrasound than ears exposed to the 4-kHz OBN only (Fig. 5A and C).
Figure 6.
Phase-contrast photomicrographs of OC at 62% distance from the apex in animal # 970. A) Left (perforated) ear. Inner hair cells (IHC), inner pillars (IP) and outer pillars (OP) are intact; outer hair cells in all three rows (O1, O2, O3) are out-of-position but near normal in shape; B) Right (non-perforated) ear. IHC, IP and OP are present. Outer hair cells in all three rows are grossly swollen.
3.5. Damage from 4-kHz OBN at 86 dB SPL for 24 h plus infrasound
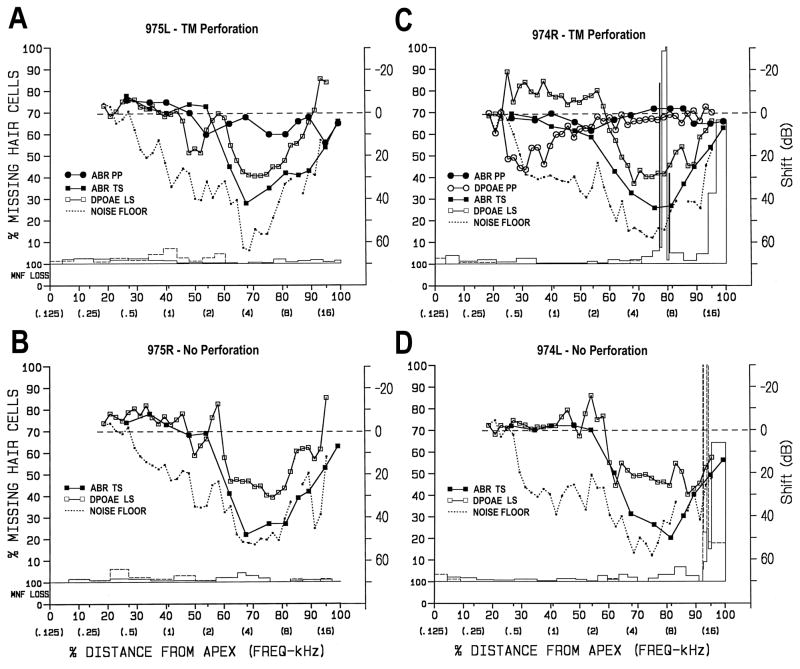
Cochleae exposed for 24 h to the 4-kHz OBN at 86 dB SPL plus infrasound sustained small losses of OHCs throughout the cochlea. The histopathological findings in the two animals in this group were somewhat different (i.e., # 974 and 975; Table 1). In chinchilla # 975, losses of IHCs and OHCs were minimal in both the perforated and non-perforated ears (Figs. 7A and 7B, respectively). All degenerated hair cells were replaced by mature phalangeal scars so the losses could not be attributed to the 4-kHz OBN exposure. Neither cochlea had a loss of myelinated nerve fibers in the osseous spiral lamina.
Figure 7.
Combined structural-functional cytocochleograms for ears exposed for 24 h to 4-kHz OBN at 86 dB SPL plus infrasound. A and B) Data from perforated and non-perforated ear of chinchilla # 975. There was minimal hair-cell loss in both cochleae. All degenerated hair cells had been replaced by mature phalangeal scars so the loss could not be attributed to the 4-kHz OBN exposure. A) There were minimal ABR TSs immediately post TM-perforation. Post-perforation DPOAEs could not be obtained. Post-exposure, this cochlea had a 10–43 dB ABR TS for 3–16 kHz and a 10–30 dB DPOAE LS from 3–10 kHz; B) The ABR TS and DPOAE LS were almost identical to those in the TM-perforated ear. C and D) Data from perforated and non-perforated ear of chinchilla # 974. C) In the TM-perforated ear, there was a 0.05-mm and 0.36-mm focal OHC lesion around 78 % distance as well as a 0.72-mm OHC lesion at the basal tip (i.e., 97 to 100%). D) In the non-perforated ear, there was a 0.05-mm and 0.10-mm IHC lesion around 93% distance and a 0.99-mm OHC lesion at the basal tip (i.e., 95 to 100%), much like that in the other ear. ABR TSs and DPOAE LSs were quite similar to those in A and B. An artifact in the noise floor at 10 kHz was removed. See legend for Figure 3 for description of axes.
In the perforated ear of chinchilla # 974, there were three focal OHC lesions: 0.05 mm at 77% distance; 0.36 mm at 78–80% distance; and 0.72 mm at 96–100% distance (Fig. 7C). The first two lesions were recent whereas the basal tip lesion was longstanding. In the non-perforated ear of chinchilla # 974, there were two focal IHC lesions: 0.05 mm lesion at 93% distance and 0.10 mm at 94% distance, both of which were recent; and a 0.99 mm focal OHC lesion at 95–100% distance that was longstanding (Fig. 7D). Neither cochlea had lost myelinated nerve fibers in the osseous spiral lamina.
There were minimal (10 dB or less) ABR TSs immediately post-TM-perforation. Pre-exposure DPOAEs could not be collected in chinchilla # 974 due to technical difficulties. Thus, the effect of TM perforation on DPOAE LSs could not be assessed. Post-exposure, the cochlea on the perforated side (Fig. 7A) had 10–43 dB ABR TSs from 3–16 kHz and 10–30 dB DPOAE LSs from 1.5–1.8 kHz and 3–10 kHz. On the non-perforated side (Fig. 7B), ABR TSs and DPOAE LSs were nearly identical to those on the perforated side. ABR TSs and DPOAE LSs (Figs. 7C and 7D) were quite similar to those in Figures 7A and 7B, respectively.
3.6 Comparison of hair-cell losses in present study with losses in cochleae from our permanent collection
In the present study, the sample size for perforated and non-perforated ears within each exposure group was too small to calculate probabilities for significant differences in hair-cell losses from the corresponding group in Table 2. Therefore, qualitative comparisons among groups are made below.
Table 2.
Hair-cell losses in cochleae from permanent collection
| Apical half of OC
|
Basal half of OC
|
||||
|---|---|---|---|---|---|
| Exposure | N | %mIHC | %mOHC | %mIHC | %mOHC |
| Non-noise-exposed; | |||||
| Mean | 30a | 0.6 | 1.6 | 0.6 | 1.0 |
| SD | 0.5 | 1.2 | 0.5 | 1.1 | |
| Range | 0.1–2.3 | 0.4–4.7 | 0.0–2.1 | 0.2–6.0 | |
| 4-kHz OBN, 108 dB SPL, 1.75 h; | |||||
| Mean | 6b | 0.5 | 4.3 | 2.7 | 35.5 |
| SD | 0.3 | 3.6 | 2.7 | 30.0 | |
| Range | 0.2–1.1 | 1.5–10.9 | 0.6–6.4 | 3.7–85.7 | |
| 4-kHz OBN, 86 dB SPL, 24 h; | |||||
| Mean | 11b | 0.8 | 1.3 | 0.9 | 1.8 |
| SD | 0.8 | 0.6 | 0.6 | 1.5 | |
| Range | 0.1–2.7 | 0.7–2.2 | 0.0–2.0 | 0.3–4.2 | |
1–3-year-old chinchillas.
1–3-year-old chinchillas fixed by 3 h post-exposure.
Mean (and SD) hair-cell losses in the apical and basal halves of the OC are shown in Table 2 for control and noise-exposed chinchillas from our permanent collection. Hair-cell losses in the perforated (# 969R, # 976R) and non-perforated (# 969L, # 976L) cochleae of chinchillas exposed to infrasound only (Table 1) were very similar to those in non-noise-exposed controls (Table 2).
In the present study, three of four cochleae (i.e., # 971L, # 973L, # 973R; Table 1) exposed for 1.75 h to the 4-kHz OBN at 108 dB SPL only, hair-cell losses in the apical and basal halves of the OC were comparable to those shown in Table 2 for the same exposure. However, in cochlea # 971R, OHC losses were somewhat greater in the apical half and considerably greater in the basal half than is typical with this exposure. We have no explanation for this apparent increased susceptibility to noise in this cochlea.
Hair-cell losses in the perforated ears of animals exposed for 1.75 h to the 4-kHz OBN at 108 dB SPL plus infrasound (i.e., # 970L, # 972L; Table 1) were fairly similar to those in cochleae exposed to the same OBN alone (Table 2). However, OHC losses in the apical and basal halves of # 972L were somewhat greater than average for this exposure.
Hair-cell losses in the perforated ears of animals exposed for 24 h to the 4-kHz OBN at 86 dB SPL plus infrasound (i.e., # 974R, # 975L; Table 1) were fairly similar to those in cochleae exposed for 24 h to the same OBN alone (Table 2). However, OHC loss in the basal half of # 974R was somewhat greater than average because of the development of a focal lesion (Fig. 7C).
4. Discussion
4.1 Pre-existing hair-cell losses in controls from our permanent collection
Our permanent collection of dissected cochleae includes sixty-two cochleae from 1–3 y-old chinchillas not exposed to noise or any other ototraumatic agent. Fourteen of these cochleae had small, pre-existing focal lesions (i.e., 0.03–0.12 mm in length involving 3–12 IHCs). One animal had 1.0 mm, pre-existing lesions in both cochleae. Some of these pre-existing lesions may have been congenital. However, others could have been the result of exposure to noise at the commercial chinchilla facility or during transport to our animal quarters. Pre-existing focal lesions were found in three cochleae in the present study (i.e., 971L, 971R and 973L, Table 1). The pre-existing focal lesions found in these cochleae were comparable to those seen in control cochleae from our permanent collection.
4.2 Effect of TM perforation on cochlear function
LeBourgeois et al. (2000) reported that perforations up to 25% of the area of the TM allowed DPOAEs to be detected over a 2–6 kHz in guinea pigs. Hatzopoulos et al. (2002) found that DPOAEs were little affected over 5–10 kHz in rats with TM perforations of unspecified size. However, testing over 0.5–10 kHz in guinea pigs, Ueda et al. (1998) found that TM perforations of 1–5% of total TM area reduced DPOAE levels as much as 22 dB at and below 1 kHz. Large perforations (i.e., 30% of TM area) significantly reduced DPOAE levels at all frequencies. Our results from small TM perforations were very similar to those found by Ueda et al. (1998). The TM perforations in the present study were about 2% of the total TM area. ABR TSs were little changed (<5 dB) across the tested frequencies (0.5–20 kHz). Post-perforation DPOAE LSs were essentially the same (1–6 dB) in the 2–20 kHz range as those from the non-perforated ears. However, below 2 kHz, DPOAE LSs were as much as 21 dB.
4.3 Effect of infrasound on cochlear pathology and hearing levels
Although von Gierke and Parker (1976) determined the gross effects of infrasound on the TM and middle ear, Lim et al. (1982) were first to look at detailed histopathology in the middle and inner ears following exposure to very intense infrasound (i.e., 150–170 dB SPL). Lim et al. studied the effects of short-duration exposure (i.e., 7.5–10 min) to continuous and intermittent infrasound (i.e., at 1, 10 or 20 Hz) in chinchillas. They found a number of pathological changes including: rupture of the TM; middle-ear bleeding; rupture of Reissner’s membrane; endolymphatic hydrops; hair-cell losses; and stria-vascularis damage. In the present study, exposure to infrasound alone at 100 dB SPL did not produce middle-ear or inner-ear damage. A 24-hour exposure to infrasound alone had a minor effect (i.e., 10–20 dB) on ABR TSs at and below 2 kHz and no effect on DPOAE LSs.
4.4 Effect of exposure to 4-kHz OBN at 108 dB SPL for 1.75 h on cochlear pathology and hearing levels
The six cochleae in our permanent collection (i.e., from six chinchillas) that had the same exposure and recovery time had a variable amount of OHC loss. The cochlea with the least damage was missing 3.7% OHCs while the cochlea with the most damage was missing 85.7% OHCs in the basal half of the OC. Despite this large variability, all cochleae sustained at least one lesion having concentrated loss of OHCs. A similar range in OHC losses was found in the present study. This large variability in hair-cell losses following the same noise exposure suggests that there were considerable differences in noise susceptibility across animals.
Usually, noise-induced hair cell losses in the left and right cochleae of binaurally exposed chinchillas are highly correlated (i.e., 0.93 for IHC loss and 0.97 for OHC loss; Bohne et al., 1986). Infrequently, asymmetrical hair-cell losses like that seen in animal # 971 have been found (e.g., see Fig. 2; Harding et al., 2002). We have no explanation for this asymmetry, particularly in view of the tethering system that was used during the exposure. In the present study, the ABR TSs and DPOAE LSs were typical for what was previously reported for this exposure (Harding et al., 2002).
It is possible that perforation of the TM still allows sound to be transmitted to the cochlea via bone conduction. Clark and Bohne (1987) showed that in the chinchilla, sound transmitted by bone conduction averages 65 dB less over a frequency range of 125 Hz to 16 kHz than that from air conduction via the tympanic membrane and ossicular chain. At 125 Hz, the attenuation was 42 dB. A study by Mills (1973) indicated that no detrimental effect on chinchilla hearing would be expected from a 4-kHz OBN at exposure levels below 48 dB SPL. Carder and Miller (1972) concluded that no detrimental effect on chinchilla hearing would be expected from a 0.5-kHz OBN at exposure levels below 65 dB SPL. Via bone conduction, transmission of a 30 Hz tone at 100 dB SPL would stimulate the cochlea at about 58 dB and transmission of a 4-kHz OBN at 108 dB SPL would stimulate the cochlea at about 43 dB. It is very unlikely that these levels of sound transmitted via bone conduction would have caused hearing loss and hair-cell loss.
Exposure to the 4-kHz OBN at 108 dB SPL would likely have produced a permanent threshold shift (PTS) after several weeks of recovery. Examples of the typical temporary threshold shift (TTS) at 0 d of recovery and PTS after 28 d of recovery caused by exposure to the 4-kHz OBN at 108 dB SPL can be found in Harding et al. (2002). In Harding et al.’s study, the amount of recovery from TTS depended upon how much hair-cell loss had occurred and where this loss was located. In general, there was complete recovery at and below 1 kHz. Above 1 kHz, there was 20–30 dB of recovery. However, all ears were left with 20–50 dB PTS for 2–8 kHz.
4.5 Effect of exposure to 4-kHz OBN at 86 dB SPL for 24 h on cochlear pathology and hearing levels
The 11 cochleae from our permanent collection that had been exposed to this noise had minimal hair-cell loss. In most cases, OHC losses were scattered in the basal half of the cochlea. Two of 11 cochleae had a small focal OHC lesion by 3 h post-exposure. Because this exposure is borderline for producing a permanent threshold shift, focal lesions may occur in one cochlea of a particular animal but not the other (e.g., Nordmann et al., 2000). In the present study, the ABR TSs and DPOAE LSs were typical for what was previously reported for this exposure (Harding and Bohne, 2004b).
Examples of the typical TTS from exposure to the 4-kHz OBN at 86 dB SPL can be found in Nordmann et al. (2000) and Harding and Bohne (2004b) and the PTSs after 27 days of recovery in Nordmann et al. (2000). In general, there was complete recovery at all frequencies with the exception of a 10–30 dB notch located at focal lesions that included substantial IHC and nerve fiber losses.
4.6 Interaction of infrasound with the 4-kHz OBN
It was surprising to find that the combination of infrasound and a high-level OBN produced 2.4 times more OHC loss than the 4-kHz OBN alone. Ahmad et al. (2002) showed that a 1.75-h exposure to a 4-kHz OBN at 108 dB SPL produced holes in the reticular lamina which allows endolymph to enter the fluid spaces of the OC. The presence of endolymph in the OC produced secondary damage which spread apically and basally from the initial sites of hair-cell loss. We speculate that the presence of high-level infrasound increased the intermixing of endolymph and perilymph across the damaged reticular lamina.
The sample size in the present study was small. However, because the clear results from the two chinchillas exposed at 108 dB SPL disproved our hypothesis, there was no need to continue with this exposure. A larger sample will be needed to confirm the finding that the 86 dB SPL exposure showed essentially no difference in hair-cell losses between the perforated and non-perforated ears. On the other hand, damage to the reticular lamina has rarely been observed in our binaural chinchillas exposed to the 4-kHz OBN at 86 dB SPL. Thus, secondary hair-cell loss from intermixing of cochlear fluids is not likely with this exposure.
The combination of intense infrasound and intense noise can be found in a number of workplaces such as large ships, submarines, offshore oil and gas platforms and around large combustion sources (e.g., Leventhall, 2006; Alves-Pereira and Castelo Branco, 2006). This combination may also occur in non-workplace settings such as automobiles, airplanes and concerts. The present findings suggest that exposure to intense noise plus infrasound produces more damage to the OC than an intense noise alone.
5. Conclusions
Small TM perforations had no effect on ABR TSs and a modest (10–20 dB) effect upon DPOAE LSs in the 0.6–2 kHz range and with no effect above 2 kHz.
Exposure to infrasound (30 Hz, 100 dB SPL) resulted in 10–20 dB ABR TSs for frequencies </= 2 kHz. No histopathological damage was found immediately post-exposure. Thus, infrasound alone did not have a permanent deleterious effect on the cochlea.
Exposure to a 4-kHz OBN at 108 dB SPL for 1.75 h resulted in 10–60 dB ABR TSs for 1–16 kHz; DPOAE LSs generally fell to the noise floor at and below 8 kHz. Substantial OHC loss was found in the middle of the first turn immediately post-exposure. Focal losses of IHCs were sometimes present.
In the animals exposed to the 4-kHz OBN at 108 dB SPL, there were larger functional losses and much greater hair-cell loss in the cochlea that was simultaneously exposed to infrasound. We hypothesize that the presence of infrasound during this intense 4-kHz OBN exposure increased cochlear damage because infrasound caused more intermixing of cochlear fluids through the damaged reticular lamina than that which occurred during the intense 4-kHz OBN exposure alone.
The presence of infrasound during a 4-kHz OBN exposure at 86 dB SPL did not increase hair-cell loss and functional losses. We hypothesize that the combined exposure did not increase structural damage or functional losses because the reticular lamina is rarely injured during moderate-level, 4-kHz OBN exposures.
Acknowledgments
This study was supported by NIOSH grant OH-03973, NIDCD grant DC-00071, and the Dept. of Otolaryngology, Washington University School of Medicine. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of CDC or NIOSH.
Abbreviations
- ABR
Auditory Brainstem Response
- DPOAE
Distortion Product Otoacoustic Emission
- IHC(s)
Inner Hair Cell(s)
- LS(s)
Level Shift(s)
- NIHL
Noise-Induced Hearing Loss
- OBN
Octave Band of Noise
- OC
Organ of Corti
- OHC(s)
Outer Hair Cell(s)
- PTS(s)
Permanent Threshold Shift(s)
- SPL
Sound Pressure Level
- TM
Tympanic Membrane
- TS(s)
Threshold Shift(s)
- TTS(s)
Temporary Threshold Shift(s)
References
- Ahmad M, Bohne BA, Harding GW. An in vivo tracer study of noise-induced damage to the reticular lamina. Hear Res. 2002;175:82–100. doi: 10.1016/s0378-5955(02)00713-x. [DOI] [PubMed] [Google Scholar]
- Alves-Pereira M, Castelo Branco NAA. Vibroacoustic disease: Biological effects of infrasound and low-frequency noise explained by mechanotransduction cellular signaling. Prog Biophys Mol Biol. 2006 doi: 10.1016/j.pbiomolbio.2006.07.011. In press. [DOI] [PubMed] [Google Scholar]
- Bohne BA. Location of small cochlear lesions by phase contrast microscopy prior to thin sectioning. Laryngoscope. 1972;82:1–16. doi: 10.1002/lary.5540820101. [DOI] [PubMed] [Google Scholar]
- Bohne BA. Healing of the noise-damaged inner ear. In: Hirsh SK, Eldredge DH, Hirsh IJ, Silverman SR, editors. Hearing and Davis: Essays Honoring Hallowell Davis. Washington University Press; St. Louis, MO: 1976a. pp. 85–96. [Google Scholar]
- Bohne BA. Mechanisms of noise damage in the inner ear. In: Henderson D, Hammernik RP, Dosanjh DS, Mills JH, editors. Effects of Noise on Hearing. Raven Press; New York: 1976b. p. 4168. [Google Scholar]
- Bohne BA, Harding GW. Combined organ of Corti/modiolus technique for preparing mammalian cochleas for quantitative microscopy. Hear Res. 1993;71:114–124. doi: 10.1016/0378-5955(93)90027-x. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: Primary versus secondary events. Am J Otol. 2000;21:505–509. [PubMed] [Google Scholar]
- Bohne BA, Bozzay DG, Harding GW. Interaural correlations in normal and traumatized cochleas: length and sensory cell loss. J Acoust Soc Am. 1986;80:1729–1736. doi: 10.1121/1.394285. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Gruner MM, Harding GW. Morphological correlates of aging in the chinchilla cochlea. Hear Res. 1990;148:79–92. doi: 10.1016/0378-5955(90)90200-9. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Harding GW, Lee SC. Death pathways in noise-damaged outer hair cells. Hear Res. 2007;223:61–70. doi: 10.1016/j.heares.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Carder HM, Miller JD. Temporary threshold shifts from prolonged exposure to noise. J Speech Hear Res. 1972;15:603–623. doi: 10.1044/jshr.1503.603. [DOI] [PubMed] [Google Scholar]
- Clark WW, Bohne BA. Attenuation and protection provided by ossicular removal. J Acoust Soc Am. 1987;81:1093–1099. doi: 10.1121/1.394629. [DOI] [PubMed] [Google Scholar]
- Eldredge DE, Miller JD, Bohne BA. A frequency-position map for the chinchilla cochlea. J Acoust Soc Am. 1981;69:1091–1095. doi: 10.1121/1.385688. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA. Noise-induced hair-cell loss and total energy: Analysis of a large data set. J Acoust Soc Am. 2004a;115:2207–2220. doi: 10.1121/1.1689961. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA. Temporary DPOAE level shifts, ABR threshold shifts and histopathological damage following below-critical-level noise exposures. Hear Res. 2004b;196:94–108. doi: 10.1016/j.heares.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, Ahmad M. DPOAE level shifts and ABR threshold shifts compared to detailed analysis of histopathological damage from noise. Hear Res. 2002;174:158–171. doi: 10.1016/s0378-5955(02)00653-6. [DOI] [PubMed] [Google Scholar]
- Hatzopoulos S, Petruccelli J, Laurell G, Finesso M, Martini A. Evaluation of anesthesia effects in a rat model using otoacoustic emission protocols. Hear Res. 2002;170:12–21. doi: 10.1016/s0378-5955(02)00448-3. [DOI] [PubMed] [Google Scholar]
- Humes LE, Joellenback LM, Durch JS. Implications for Hearing Loss and Tinnitus. The National Academies Press; 2006. Noise and Military Service; pp. 163–170. [Google Scholar]
- Kopke RD. Combating hearing loss in the military. Hearing Health. 2005 Fall;:26–30. [Google Scholar]
- LeBourgeois HW, 3rd, Anand VK, McAuley JR, Dickman JD, Malphurs O., Jr Effects of tympanic perforations on the detection of distortion-product otoacoustic emissions. Ear Nose Throat J. 2000;79:610–618. [PubMed] [Google Scholar]
- Leventhall G. What is infrasound? Prog Biophys Mol Biol. 2006 doi: 10.1016/j.pbiomolbio.2006.07.006. In press. [DOI] [PubMed] [Google Scholar]
- Lim DJ, Dunn DE, Johnson DL, Moore TJ. Trauma of the ear from infrasound. Acta Otolaryngol. 1982;94:213–231. doi: 10.3109/00016488209128907. [DOI] [PubMed] [Google Scholar]
- Mills JH. Temporary and permanent threshold shifts produced by nine-day exposures to noise. J Speech Hear Res. 1973;16:426–438. doi: 10.1044/jshr.1603.426. [DOI] [PubMed] [Google Scholar]
- Nordmann AS, Bohne BA, Harding GW. Histopathological differences between temporary and permanent threshold shift. Hear Res. 2000;139:13–30. doi: 10.1016/s0378-5955(99)00163-x. [DOI] [PubMed] [Google Scholar]
- Salt AN, DeMott JE. Longitudinal endolymph movements and endocochlear potential changes induced by stimulation at infrasonic frequencies. J Acoust Soc Am. 1999;106:847–856. doi: 10.1121/1.427101. [DOI] [PubMed] [Google Scholar]
- Ueda H, Nakata S, Hoshino M. Effects of effusion in the middle ear and perforation of the tympanic membrane on otoacoustic emissions in guinea pigs. Hear Res. 1998;122:41–46. doi: 10.1016/s0378-5955(98)00084-7. [DOI] [PubMed] [Google Scholar]
- von Gierke HE, Parker DE. Handbook of Sensory Physiology, Auditory System: Clinical and Special Topics. Springer-Verlag; Berlin: 1976. Infrasound; pp. 585–624. [Google Scholar]
- Voss SE, Rosowski JJ, Merchant SN, Peake WT. How do tympanic-membrane perforations affect human middle-ear sound transmission? Acta Otolaryngol. 2001;121:169–173. doi: 10.1080/000164801300043343. [DOI] [PubMed] [Google Scholar]