Abstract
Overdoses of γ-hydroxybutyrate (GHB), a drug of abuse, result in coma, respiratory arrest, and death. The objective of this study was to evaluate a potential GHB detoxification strategy by inhibiting the monocarboxylate transporter (MCT)-mediated renal reabsorption of GHB in rats, using the MCT substrate L-Lactate. The use of the osmotic diuretic D-mannitol alone or combined with L-Lactate was also explored. GHB (208 mg/h/kg) was infused i.v. for 3 h in the absence or presence of L-Lactate (60.5, 121, and 302.5 mg h−1 kg−1), D-mannitol (0.5 g/kg), or L-Lactate (60.5 mg h−1 kg−1) combined with D-mannitol (0.5 g/kg). GHB in plasma and urine samples was determined along with blood pH, electrolytes, glucose, and L-Lactate. Administration of L-Lactate, or the combination of L-Lactate and D-mannitol, but not D-mannitol alone, significantly increased the renal and total clearances of GHB in rats. Blood pH and electrolyte concentrations exhibited small changes with GHB, GHB/lactate, and GHB/mannitol treatments, although most values remained within their normal range. The concomitant administration of lactated Ringer's solution (28 mM L-Lactate) at 300 µl/min with mannitol (0.5 g/kg) resulted in a significant increase in GHB clearance and a decrease in sleep time after an i.v. dose of 1 g/kg. Overall, our results indicated the following: 1) the use of the MCT inhibitor L-Lactate can increase the renal and total clearances of GHB, and 2) the combination of lactated Ringer's solution and D-mannitol significantly alters GHB toxicokinetics and toxicodynamics and represents a potential clinical detoxification strategy for the treatment of GHB overdoses.
The presence of γ-hydroxybutyrate (GHB) has been demonstrated in the mammalian brain, where it is primarily formed from GABA (Fishbein and Bessman, 1964). Endogenous GHB has also been detected in various tissues other than the brain, including heart, kidney, liver, lung, muscle, and gastrointestinal tract (Nelson et al., 1981; Tedeschi et al., 2003). GHB has been proposed to be a neurotransmitter or neuromodulator with its own specific receptor identified in the brain (Andriamampandry et al., 2003). Clinically, GHB has been used to treat alcohol and heroin dependence (Gallimberti et al., 2000) and sleep disorders in Europe (Mamelak et al., 1986), and it has been approved in the United States for the treatment of cataplexy attacks and to reduce excessive daytime sleepiness in patients with narcolepsy. However, GHB has been extensively used as a popular steroid alternative by body builders, as a recreational drug at night-clubs and rave parties, and as a means of drug-facilitated sexual assaults (Schwartz et al., 2000; Okun et al., 2001). Serious adverse effects have been associated with GHB overdoses including coma, seizure, and even death (Mason and Kerns, 2002). GHB or its prodrugs, γ-butyrolactone and 1,4-butanediol, are especially toxic when mixed with alcohol and/or other recreational drugs to increase its euphoric effects, and this has led to significant morbidity and mortality (Okun et al., 2001). There were over 7100 reports of GHB overdoses and 65 GHB related deaths in the United States from 1990 to 2000 (Shannon and Quang, 2000). The treatment of GHB overdoses consists mainly of supportive care, and no specific antidotes have been reported for clinical use (Mason and Kerns, 2002).
The physiological, pharmacological, and toxicological effects of GHB are believed to be mediated by its own receptor and the GABAB receptor (Maitre, 1997). Although contradictory reports exist in the literature, there is increasing evidence suggesting that the hypnotic effect of GHB is mediated by the GABAB receptor by two putative pathways: directly acting on the GABAB receptor as a partial agonist (Carai et al., 2001) and indirectly acting on GABAB receptor by interconverting GHB to GABA (Hechler et al., 1997). The affinity of GHB for the GABAB receptor is very low, and the IC50 value for GHB displacing GABA is above 100 µM (Bernasconi et al., 1992). It is believed that GABA converted from exogenous GHB is sufficient to induce the hypnotic effects associated with GHB (Hechler et al., 1997).
Nonlinear pharmacokinetics of GHB have been reported for rats (Lettieri and Fung, 1979) and humans (Ferrara et al., 1992), and the mechanisms underlying the nonlinear pharmacokinetics of GHB include capacity-limited metabolism (Lettieri and Fung, 1979; Ferrara et al., 1992), capacity-limited absorption (Arena and Fung, 1980), and capacity-limited reabsorption in the proximal tubules mediated by pH- and sodium-dependent monocarboxylate transporters (Morris et al.,2005; Wang et al., 2006). Because the metabolic clearance is saturated after high doses of GHB, the renal clearance represents a significant route of elimination at these doses, and the renal clearance of GHB can be increased by inhibiting the renal reabsorption of GHB (Morris et al., 2005). L-Lactate is a typical substrate of monocarboxylate transporter (MCT) 1 to 4 (Halestrap and Price, 1999) and can significantly inhibit the uptake of GHB in membrane vesicles isolated from rat proximal tubule cells (Wang et al., 2006). We have reported previously that a high dose of L-Lactate can reduce the renal reabsorption of GHB in rats in vivo (Morris et al., 2005). In that same study, we also reported that a high dose of the osmotic diuretic D-mannitol is effective in increasing GHB renal clearance (Morris et al., 2005). However, the effects of more clinically relevant lower doses of L-Lactate and mannitol and that of the combination of L-Lactate and mannitol on the toxicokinetics (TK) of GHB have not been examined. Also, studies have not previously examined the effects of these treatments on the toxicodynamics (TD) of GHB. Our hypothesis is that increasing the renal clearance of GHB after overdoses will increase its overall clearance and decrease its toxicity, representing a potential strategy to treat overdoses. The objectives of this study were to: 1) characterize the dose-dependent effects of L-lactate, D-mannitol, and the combined treatment of L-lactate and D-mannitol on the steady-state TK of GHB; and 2) determine the effects of lactated Ringer's solution and mannitol on the TK and TD (hypnotic effect) of GHB after a single i.v. bolus dose of GHB (1 g/kg). L-Lactate was administered as lactated Ringer's solution to evaluate the potential use of this clinically available and widely used form of L-lactate.
Materials and Methods
Chemicals and Reagents
GHB (as sodium salt), L-(+)-lactate (as sodium salt), formic acid, anthrone, inulin, and trichloroacetic acid were purchased from Sigma-Aldrich (St.Louis, MO). The internal standard sodium GHB-D6 (1 mg/ml) was purchased from Cerilliant Corporation (Round Rock, TX). Lactated Ringer's solution was purchased from Henry Schein (Melville, NY). Methanol and high-performance liquid chromatography-grade water were purchased from Thermo Fisher Scientific (Waltham, MA). Concentrated H2SO4 (93%) was purchased from Mallinckrodt Baker, Inc. (Phillipsburg, NJ).
Animals and Surgery
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) were used as our animal model. The body weights of the rats were 240 to 280 and ∼200 g for the infusion study with L-lactate and the infusion study using lactated Ringer's solution, respectively. The rats were randomly assigned to study groups and kept in individual cages after surgery. The animal housing room had controlled environmental conditions, with temperature and relative humidity of approximately 20 ± 2°C and 40 to 70%, respectively, and artificial lighting, alternating on a 12-h light/dark cycle. All care and experiments were approved by the Institutional Animal Care and Use Committee at the University at Buffalo. The rats had cannulas implanted as previously described (Morris et al., 2005). Briefly, rat right jugular veins, left femoral veins and bladders were cannulated under anesthesia after an i.m. injectionof 90 mg/kg ketamine and 9 mg/kg xylazine (Henry Schein). The animals were allowed 3 to 4 days for recovery from surgery before the experiment.
Infusion Studies and Sample Collection
The rats were placed in metabolism cages for collection of urine during the study period. GHB, inulin, L-lactate, and/or D-mannitol dissolved in sterile water were administered via an i.v. bolus injection followed by an i.v. infusion through the femoral veins to rats (n ≥ 3 each group). Before the infusion, the solutions were sterilized by passing them through 0.2-µm filters. All the infusion solutions were prepared to maintain a total osmolarity of 320 mOsm and pH of 7.4. The components of the infusion solution for GHB only (control) were sodium GHB (159 mM) and inulin (1.9 mM). The components of the infusion solutions for L-lactate treatments were sodium GHB (121, 97, and 62 mM for L-lactate doses of 60.5, 121, and 302.5 mg h−1 kg−1, respectively), sodium L-lactate (40, 64, and 102 mM for L-lactate doses of 60.5, 121, and 302.5 mg h−1 kg−1, respectively), and inulin (1.5, 1.2, and 0.8 mM for L-lactate dose of 60.5, 121, and 302.5 mg h−1 kg−1, respectively). The infusion rate was different for different doses of L-lactate to use iso-osmotic infusion solutions. Blood samples for the measurement of GHB (100 µl) were withdrawn from the jugular vein at different time points and placed in heparinized 0.6-ml microcentrifuge tubes. For the measurement of blood pH and electrolytes, the blood samples (300 µl each) were withdrawn into 1-ml heparinized syringes and stored on ice (<30 min) before analysis (ABL 605; Radiometer, Copenhagen, Denmark). The plasma was separated from whole blood by centrifugation at 2000g for 5 min at 4°C. For urine collection at 1 or 3 h, the bladder was rinsed with warm normal saline (2 ml) at the end of each collection. Urine pH and volume were measured right after the collection. For the brain samples, the animals were sacrificed at the end of the 3-h infusion period, and whole brains were harvested for drug concentration analysis. All plasma, urine, and brain samples were stored at −80°C until analysis by liquid chromatography (LC)-tandem mass spectrometry (MS/MS) (Fung et al., 2004) to determine GHB concentrations.
Experimental Design
Effects of L-lactate and/or mannitol on the steady-state TK of GHB
Based on our previous study (Morris et al., 2005), a single dose of GHB, 400 mg/kg i.v. bolus followed by 208 mg h−1 kg−1, was selected to represent a high dose of GHB. Three different doses of L-lactate were used in this study: 130 mg/kg i.v. bolus followed by 60.5 mg h−1 kg−1, 330 mg/kg i.v. bolus followed by 121 mg h−1 kg−1, and 330 mg/kg i.v. bolus followed by 302.5 mg h−1 kg−1 to achieve three steady-state L-lactate blood concentration elevation of ∼0.1, ∼0.2, and ∼0.4 mM, respectively. An i.v. dose of 0.5 g/kg D-mannitol was used to study the effect of osmotic diuresis. A combined dosing regimen of 0.5 g/kg D-mannitol by i.v. bolus and L-lactate 60.5 mg h−1 kg−1 by i.v. infusion was used to determine the effect of combining the two treatments. The glomerular filtration rate (GFR) was determined from the clearance of inulin, as previously described (Morris et al., 2005). The 60 mg/kg i.v. bolus dose of inulin, followed by a 100 mg h−1 kg−1 i.v. infusion, was used in these studies (Darling and Morris, 1991). Blood samples were collected at 0 (blank), 10, 20, 30, 60, 90, 120, and 180 min and urine samples at 0 (blank) and between 1 and 3 h.
Effect of lactated Ringer's solution and/or mannitol on GHB TK and TD
For lactated Ringer's solution infusion studies, a single dose of GHB (1000 mg/kg i.v. bolus) was given to all rats (control, D-mannitol alone, lactated Ringer's solution alone, and D-mannitol/lactated Ringer's solution combination group). In the control group, saline was infused at the rate of 300 µl/min, starting immediately after the i.v. bolus of GHB. In the D-mannitol treatment group, an i.v. bolus dose of 0.5 g/kg D-mannitol followed by an i.v. infusion of saline, administered immediately after the i.v. bolus of GHB, was used to study the effect of osmotic diuresis on GHB disposition. In the lactated Ringer's solution treatment group, lactated Ringer's solution was infused at the rate of 300 µl/min immediately after the i.v. bolus of GHB and a loading dose of sodium L-lactate (0.4 g/kg). In the combined dosing regimen group, a dose of 0.5 g/kg D-mannitol by i.v. bolus and lactated Ringer's solution by i.v. infusion was administered to rats immediately after the i.v. bolus of GHB. Blood samples were collected at 0 (blank), 5, 15, 30, 60, 120, 180, and 240 min, and urine samples were collected at 0 (blank) and between 2 and 4 h. The hypnotic effect of GHB after various treatments was determined by the sleep time, which was measured as the difference between the time of loss of the righting reflex (LRR) and regaining of the righting reflex (RRR). LRR and RRR are the indicators for the onset and offset of sleep induced by GHB, respectively.
Determination of TK Parameters
The total clearance of GHB (CL) was determined from its plasma concentration at steady state (Css) and infusion rate (k0), using the equation k0/Css. In the GHB i.v. bolus study, CL was determined from dose/AUC, where AUC is the area under the plasma concentration-time curve. Renal clearance (CLR) was determined by dividing the urinary excretion rate (dAe/dt) by the mean plasma concentration of GHB (Css) or Ae,∞/AUC, where Aeis the amount of GHB excreted in the urine, and Ae,∞is the amount of GHB excreted in the urine from time 0 to ∞. The fraction of the dose eliminated by renal excretion (fe) was determined by CLR/CL or Ae,∞/dose. GFR was determined from the inulin clearance (k0,IN/Css,IN), where k0,IN and Css,IN represent the infusion rate and plasma concentration at steady state of inulin. The renal filtration rate of GHB was determined as the product of GFR and the unbound (free) plasma concentration (Cu), where Css is approximately equal to Cubecause GHB is negligibly protein bound in rat plasma (Morris et al., 2005). The renal tubular reabsorption rate of GHB was calculated by the difference between renal filtration rate and urinary excretion rate, assuming there is negligible renal secretion of GHB. A lack of renal secretion has been reported for the ketone bodies of β-hydroxybutyrate (the congener of GHB with the hydroxyl group at C-3) and acetoacetate, even at high plasma concentrations of 15 mM (Ferrier et al., 1992). Because both GHB and inulin reached steady state by 1 h, the average steady-state concentrations for 1 to 3 h were used for Cssand Cmid in the calculations of GFR, CLR, and CL. The metabolic clearance (CLm) could be calculated indirectly from our data using the equation CLm = CL − CLR, assuming that total plasma clearance of GHB is equal to renal clearance plus metabolic clearance.
LC/MS/MS Assay
GHB in rat plasma, urine, and brain samples was measured by a validated LC/MS/MS assay as previously described (Fung et al., 2004), with some modifications. Briefly, the LC/MS/MS system consisted of a PE Sciex API 3000 triple-quadruple tandem mass spectrometer (Applied Biosystems, Foster City, CA) equipped with a turbo ion spray (PerkinElmer-Sciex Instruments, Boston, MA), a Series 200 PE micropump (PerkinElmer Life and Analytical Sciences, Waltham, MA), and a Series 200 PE autosampler (PerkinElmer Life and Analytical Sciences). The separation by reversed-phase high-performance liquid chromatography was performed using an Aqua C-18 5-µm, 125-Å column (150- × 4.6-mm i.d.; Phenomenex, Torrance, CA) protected by a C-18 5-µm guard column cartridge system (Phenomenex). Compounds were eluted isocratically with a mobile phase consisting of 5 mM formic acid/methanol (33:67, v/v). The flow rate was 0.75 ml/min, and the injection volume was 10 µl (the flow rate was achieved by a splitter, so the actual flow-to-mass spectrum was 0.25 ml/min, and the injection volume was 3.3 µl). The mass spectrometer was operated in a positive ionization mode with an optimized ion yield setting for GHB. The interface temperature was set at 400°C. The declustering potential and collision energy for fragmentation were set at 30 and 13 eV, respectively. Multiple reaction monitoring was used for specific detection of GHB and GHB-D6 by MS/MS measuring the ion pair transitions of m/z 105 (parent ion) to 87 (product ion) and of m/z 111 (parent ion) to 93 (product ion), respectively. The retention time of GHB and GHB-D6 was 2.6 min, and there were no interfering peaks in plasma, brain, and urine samples. The Analyst software version 1.4.1 (PerkinElmerSciex Instruments) was used for instrument control and data analysis.
Plasma, Brain, and Urine Sample Preparation and Assay
To each blank plasma, brain, or urine sample (50 µl; appropriately diluted with H2O), GHB-D6 stock solution (6 mM, 5 µl) and varying concentrations of GHB stock solution were added to prepare standards of GHB (60, 240, 600, 1200, 2400, 4800, and 7200 µM as final concentrations) for the calibration curve. The internal standard GHB-D6 was added in the same concentration and volume to each plasma, brain, or urine sample. The proteins present in plasma, brain, and urine were precipitated using 50% methanol (1 volume of 100% methanol to 1 volume sample). After centrifugation at 22,000g for 20 min, the supernatant was collected for the LC/MS/MS assay. Quality control samples at low (240 µM), medium (1200 µM), and high (4800 µM) concentrations of GHB were prepared by the same procedures. The linearity of the calibration curve was evaluated by regression analysis of peak height ratios (GHB/GHB-D6) to GHB concentrations in blank plasma and urine samples, respectively. The accuracy was determined by comparing the calculated concentration using calibration curves of known concentrations. The precision was determined by the coefficient of variation (CV %). Within-day variability was assessed through the analysis of quality controls in triplicate, and between-day variability was determined through the analysis of quality controls on 4 to 5 separate days.
Inulin Assay
The inulin concentrations in plasma and urine samples were determined by a colorimetric method based on the procedure of Davidson and Sackner (1963), as previously described (Morris et al., 2005). Briefly, inulin (10 µl) was added to blank plasma (10 µl) or urine sample (10 µl; diluted with H2O) to prepare a series of standards of inulin (5, 10, 20, 40, 60, 80, and 100 µg/ml as final concentrations) for the calibration curve. The protein content of each plasma (10 µl) or urine (10 µl; appropriately diluted with H2O) sample was precipitated by incubating with 40 µl of trichloroacetic acid (1.0 N) for 30 min. After centrifugation at 11,000g for 5 min, the supernatant (20 µl) was mixed with 120 µl of anthrone reagent (0.08%, w/v) that was prepared by dissolving anthrone in H2O/H2SO4 (10:53, v/v). The mixture was vortexed for 5 s, chilled on ice for 1 min, and incubated at 38°C in a water bath for 50 min. At the end of the incubation, the mixture was chilled on ice for 2 min and allowed to stand at room temperature for 20 min to equilibrate. The mixture (100 µl) was transferred to a 96-well microplate, and the absorbance was determined by microplate spectrometer Spectra Max 340PC (Molecular Devices, Sunnyvale, CA) at 623 nm at 25°C. The color was stable for as long as 3 h. The assay was validated for sensitivity, linearity, accuracy, and precision based on intraplate and interplate analysis.
Blood Ion and Metabolite Measurements
The measurements of ion and metabolite concentrations in rat venous blood were conducted using a radiometer (ABL 605), which was equipped with multiple electrodes for measurement of Na+, K+, Ca2+, Cl−, pH, pCO2, pO2, glucose, and L-lactate. Venous blood samples, withdrawn from the right jugular vein at 0 and 3 h, were analyzed using the ABL 605 radiometer. The blood samples were stored on ice right after sampling, and the samples were analyzed within 30 min.
Statistical Analysis
Statistical comparisons among more than two treatments employed one-way ANOVA (Prism 3.0 software; GraphPad Software Inc., San Diego, CA) followed by a Dunnett's post hoc test. A paired Student's t test was used for comparing two treatments. Differences were considered to be significant when p < 0.05.
Results
LC/MS/MS Assay for GHB in Plasma, Brain, and Urine
The lower limit of quantitation was determined to be 60 µM. The endogenous concentrations of GHB in blood, brain, and urine are in the range of 10 to 40 µM, as determined previously (Nelson et al., 1981; Fung et al., 2004), and the blood, urine, and brain concentrations of GHB after GHB administration were in the range of 1 to 20 mM. Therefore, the endogenous concentration of GHB was negligible in samples and omitted in the calculation of GHB concentration. The calibration curve for plasma was linear from 60 to 7200 µM based on the regression analysis (r2 > 0.99) of peak height ratios (GHB/GHB-D6) versus GHB concentrations. The accuracy and precision (CV %) for plasma samples was 96 to 112% and 2.4 to 12.7%, respectively. The calibration curve for urine was linear from 240 to 4800 µM based on the regression analysis (r2 > 0.992) of peak height ratios (GHB/GHB-D6) versus GHB concentrations. The accuracy and precision (CV %) for urine samples was 95 to 119% and 3.0 to 8.7%, respectively. The calibration curve for brain was linear from 60 to 1200 µM based on the regression analysis (r2 > 0.998) of peak height ratios (GHB/GHB-D6) versus GHB concentrations. The accuracy and precision (CV %) for brain samples was 93 to 111% and 3.4 to 7.6%, respectively.
Dose-Dependent Effects of L-Lactate on GHB TK
Our studies demonstrated that the plasma concentrations of GHB reach an apparent steady state after 1 h of administration. The steady-state plasma concentrations of L-lactate determined at the end of a 3 h-infusion were dose-dependent and increased with the L-lactate doses (Table 1). The plasma concentration of GHB was significantly decreased after the administration of L-lactate, and the lowest concentration achieved was 0.38 mg/ml at a dose of L-lactate of 302.5 mg/ml. The steady-state renal clearances of GHB were significantly increased after the administration of L-lactate at all three doses (Fig. 1), and the highest renal clearance of GHB occurred after the infusion of L-lactate 302.5 mg h−1 kg−1. The total clearance of GHB was also significantly increased after the administration of L-lactate, with the highest values observed after the administration of L-lactate 302.5 mg h−1 kg−1 (Fig. 1). However, the metabolic clearance values, which were calculated as total clearance minus renal clearance, were not significantly different from that of the control (Fig. 1). The GFR values were not significantly changed after L-lactate dosing (Table 1). The amount of GHB reabsorbed in the kidney was significantly decreased after L-lactate dosing in a dose-dependent manner (Table 1).
TABLE 1.
Effects of L-lactate, mannitol, and combined mannitol/L-lactate administrations on GHB toxicokinetics GHB (208 mg/h/kg) was administered by i.v. infusion to reach a steady-state concentration. L-Lactate (60.5, 121, and 302 mg/h/kg) was administered by i.v. infusion for 3 h. D-Mannitol (0.5 g/kg) was administered by i.v. bolus. For the combined treatment, L-lactate (60.5 mg/h/kg) was administered by i.v. infusion for 3 h, and D-mannitol (0.5 g/kg) was administered by i.v. bolus. The ratio of brain to plasma was calculated using the brain concentrations divided by the last plasma concentration of GHB at the end of the study. [L-Lactate] change represents the concentration difference between the beginning and the end of the treatments; the mean L-lactate concentrations are given in Table 2. One-way ANOVA followed by Dunnett's test, n = 3 to 8, mean ± S.D.
| Control | L-Lactate (60.5 mg/h/kg) | L-Lactate (121 mg/h/kg) | L-Lactate (302.5 mg/h/kg) | D-Mannitol (0.5 g/kg) | D-Mannitol and L-Lactate | |
|---|---|---|---|---|---|---|
| Plasma [GHB] (mg/ml) | 0.62 ± 0.11 | 0.48 ± 0.04** | 0.46 ± 0.01** | 0.38 ± 0.02** | 0.57 ± 0.08 | 0.45 ± 0.06** |
| GHB fe (%) | 32.5 ± 5.2 | 36.6 ± 9.5 | 48.7 ± 2.7 | 41.8 ± 4.4 | 31.9 ± 6.5 | 39.8 ± 14.5 |
| Plasma [inulin] (mg/ml) | 0.19 ± 0.02 | 0.23 ± 0.04 | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.02 | 0.19 ± 0.04 |
| GFR (ml/h/kg) | 584 ± 111 | 482 ± 54 | 603 ± 30 | 549 ± 16 | 623 ± 114 | 538 ± 87 |
| Percentage of GHB reabsorbed | 80.3 ± 2.6 | 66.3 ± 10.2** | 63.59 ± 5.1** | 61.1 ± 4.8** | 83.1 ± 6.5 | 65.1 ± 13.3* |
| Brain [GHB] (mg/g) | 0.08 ± 0.04 | 0.10 ± 0.02 | 0.09 ± 0.01 | 0.07 ± 0.02 | 0.10 ± 0.04 | 0.06 ± 0.02 |
| [L-Lactate] change (mM) | 0 ± 0.08 | 0.27 ± 0.15 | 0.43 ± 0.06** | 0.70 ± 0.08** | 0.00 ± 0.01 | 0.28 ± 0.23 |
p < 0.05.
p < 0.01.
Fig. 1.
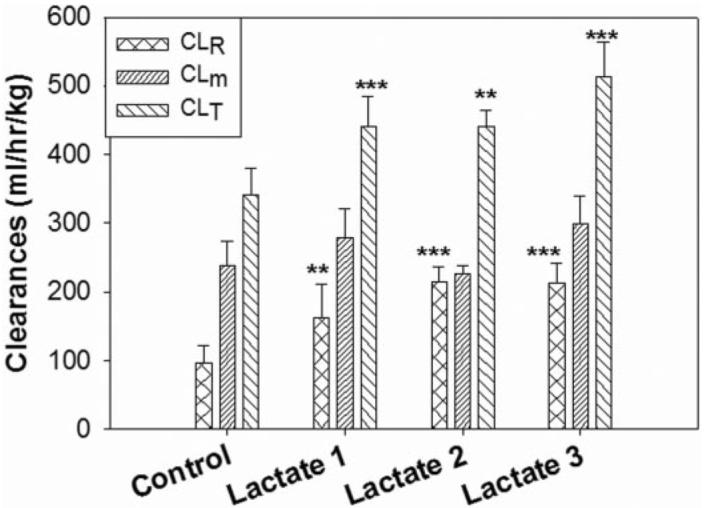
The renal clearance (CL R), metabolic clearance (CLm), and total clearance (CLT) of GHB determined after the infusion of different doses of L-lactate. The control was administered GHB (208 mg/kg/h) alone. L-Lactate-1, L-lactate-2, and L-lactate-3 represent the doses of 60.5, 121, and 302.5 mg h−1 kg−1, respectively. Results are plotted as mean ± S.D., n = 3 to 5. Statistical differences were compared with the control (**, p < 0.01; ***, p < 0.001), using a one-way ANOVA followed by a Dunnett's post hoc test.
The total brain concentrations of GHB after a 3-h infusion were determined and expressed as milligrams per gram of tissue (Table 1). Because the plasma concentration of GHB reached steady state by 1 h, it is likely that the 3-h brain concentration also represents a steady-state concentration. There were no significant differences among the brain concentrations of control and L-lactate-treated groups.
pH and Electrolyte Concentrations in Blood after GHB and L-Lactate Treatments
The blood pH of animals receiving GHB treatment was not significantly changed after a 3-h infusion; however, the blood pH was increased after a 3-h infusion of GHB with each of the three doses of L-lactate. The greatest pH change was observed with the highest L-lactate dose, with the mean pH values changing from 7.46 to 7.53 (Table 2). The concentrations of Na+, K+, and Ca2+ were not significantly changed after a 3-h infusion of GHB in both control and treatment groups (Table 2). The Cl− concentrations were slightly decreased after a 3-h GHB infusion in all groups, but the concentrations were still within the normal range (Table 2). Anion gaps were significantly increased with the infusion of GHB alone and with the infusion of GHB/L-lactate 302.5 mg h−1 kg−1 (Table 2). Anion gap refers to the difference between the concentration of cations other than Na+ and the concentration of anions other than Cl− and HCO3− in the plasma, and it usually increases with the accumulation of organic anions such as lactate in the blood. Glucose and lactate concentrations were also altered, with an apparent increase of both compounds in the GHB/L-lactate infusions of 121 and 302.5 mg h−1 kg−1; however, the concentrations were all within the normal ranges (Table 2).
TABLE 2.
Blood pH and electrolyte concentrations before and after GHB administration alone or with concomitant L-lactate and/or D-mannitol treatments GHB (208 mg/h/kg) was administered by i.v. infusion to reach steady-state concentration. L-Lactate (60.5, 121, and 302 mg/h/kg) was administered by i.v. infusion for 3 h. D-Mannitol (0.5g/kg) was administered by i.v. bolus. For the combined treatment, L-lactate (60.5 mg/h/kg) was administered by i.v. infusion for 3 h, and D-mannitol (0.5g/kg) was administered by i.v. bolus. Blood pH and electrolyte concentrations before and after the infusion were measured using an ABL 605 Radiometer. The results are expressed as mean ± S.D., n = 3 to 5. A paired Student's t test was used to detect statistical significance.
| GHB Only | L-Lactate (60.5 mg/h/kg) | L-Lactate (121 mg/h/kg) | L-Lactate (302.5 mg/h/kg) | D-Mannitol (0.5 g/kg) | D-Mannitol + L-Lactate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 min | 180 min | 0 min | 180 min | 0 min | 180 min | 0 min | 180 min | 0 min | 180 min | 0 min | 180 min | |
| pH | 7.44 ± 0.02 | 7.48 ± 0.02 | 7.43 ± 0.01 | 7.52 ± 0.01** | 7.46 ± 0.01 | 7.52 ± 0.02* | 7.46 ± 0.01 | 7.53 ± 0.01*** | 7.46 ± 0.01 | 7.48 ± 0.03* | 7.44 ± 0.01 | 7.49 ± 0.01*** |
| K+ (mM) | 4.1 ± 0.5 | 3.7 ± 0.4 | 4.2 ± 0.4 | 4.0 ± 0.4 | 4.5 ± 0.7 | 4.0 ± 0.7 | 3.8 ± 0.2 | 3.5 ± 0.2 | 4.0 ± 0.2 | 3.4 ± 0.2* | 4.2 ± 0.5 | 3.6 ± 0.3* |
| Na+ (mM) | 145 ± 2 | 147 ± 2 | 145 ± 2 | 145 ± 2 | 145 ± 2 | 145 ± 2 | 145 ± 2 | 146 ± 3 | 141 ± 1 | 143 ± 1 | 142 ± 1 | 143 ± 2 |
| Cl− (mM) | 108 ± 2 | 99 ± 2** | 107 ± 2 | 97 ± 2** | 107 ± 3 | 97 ± 2* | 108 ± 4 | 95 ± 1** | 104 ± 2 | 100 ± 1 | 106 ± 4 | 96 ± 3*** |
| Ca2+ (mM) | 0.98 ± 0.20 | 0.96 ± 0.12 | 0.90 ± 0.17 | 0.77 ± 0.20 | 0.79 ± 0.14 | 0.80 ± 0.04 | 0.82 ± 0.09 | 0.96 ± 0.31 | 1.22 ± 0.06 | 1.17 ± 0.04 | 1.25 ± 0.09 | 1.16 ± 0.17 |
| Anion gap (mM) | 14.9 ± 2.4 | 19.8 ± 3.0* | 17.5 ± 2.2 | 19.5 ± 2.0 | 16.7 ± 2.1 | 18.3 ± 1.8 | 14.2 ± 2.7 | 17.1 ± 3.7* | 13.3 ± 0.8 | 16.7 ± 0.3 | 12.7 ± 0.9 | 16.2 ± 2.4* |
| Glucose (mM) | 7.5 ± 0.6 | 9.5 ± 1.6* | 8.1 ± 0.3 | 7.3 ± 1.2 | 7.0 ± 0.2 | 9.3 ± 0.8* | 6.5 ± 0.7 | 8.9 ± 0.7** | 8.4 ± 1.8 | 10.0 ± 1.2 | 6.7 ± 0.8 | 9.5 ± 2.0* |
| L-Lactate (mM) | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.4 ± 0.5 | 1.0 ± 0.1 | 1.4 ± 0.1** | 0.8 ± 0.1 | 1.4 ± 0.2** | 1.2 ± 0.4 | 0.9 ± 0.1 | 1.0 ± 0.3 | 1.3 ± 0.4 |
p < 0.05.
p < 0.01.
p < 0.001.
Urine pH Changes after GHB and L-Lactate Treatments
The blank urine pH values averaged 6.6 in the absence of treatment but increased after GHB administration (control), from 6.88 ± 0.26 at 1 h to 7.53 ± 0.36 at 3 h. The urine pH after administering GHB plus L-lactate at the three different doses was also increased significantly from that after GHB treatment alone; the changes in urine pH after L-lactate treatment were dose-dependent. The urine pH values measured after administration of the three lactate doses (lowest to highest) were 7.65 ± 0.25, 8.08 ± 0.18, and 7.73 ± 0.19 at 1 h and 8.19 ± 0.13, 7.81 ± 0.63, and 8.56 ± 0.18 at 3 h.
Effects of Combined D-Mannitol/L-Lactate Treatment on GHB Toxicokinetics
The steady-state plasma concentrations of GHB were significantly decreased after the administration of L-lactate 60.5 mg h−1 kg−1 and the coadministration of D-mannitol and L-lactate (60.5 mg h−1 kg−1); however, the steady-state plasma concentration of GHB after the administration of D-mannitol was not different from that of the control (Table 1). The total and renal clearances of GHB in the combined D-mannitol and L-lactate treatment group were similar to those of the L-lactate group but were significantly higher than those of the control group (Fig. 2). The metabolic clearances and GFR values were not significantly different in all groups (Fig. 2). Consistent with the changes in renal clearance, the amount of GHB reabsorbed was significantly decreased after L-lactate treatment and combined D-mannitol and L-lactate treatment but not after D-mannitol treatment (Table 1). The total brain concentrations of GHB after the 3-h infusion were determined (Table 1). There were no significant differences among the brain concentrations observed in the control and treatment groups.
Fig. 2.
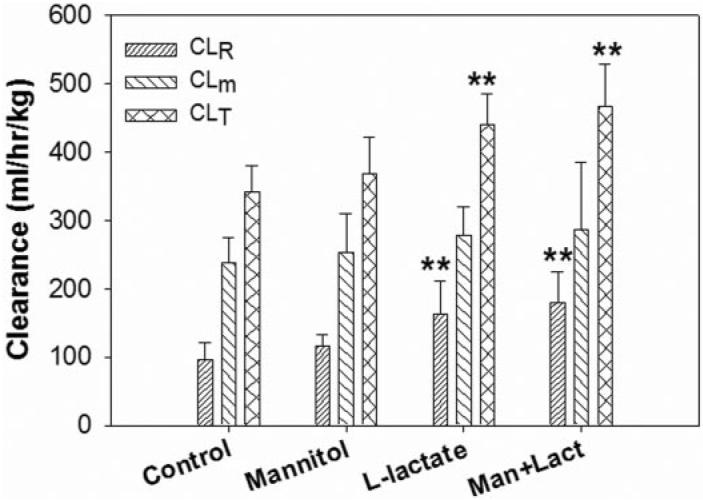
The renal clearance (CLR), metabolic clearance (CLm), and total clearance (CLT) of GHB determined after the concomitant treatment with D-mannitol, L-lactate, or D-mannitol/L-lactate. The control was administered with GHB (208 mg h−1 kg−1) only. L-Lactate was administered at a dose of 60.5 mg h−1 kg−1 by i.v. infusion, D-mannitol was administered as an i.v. bolus dose of 0.5 g/kg, and Man + Lact represents the combined treatment with D-mannitol and L-lactate. One-way ANOVA followed by Dunnett's test, n = 3-8, mean ± S.D. **, p < 0.01.
pH and Electrolyte Concentrations in Blood after Combined L-Lactate/D-Mannitol Treatment
The blood pH for the GHB treatment group was not changed significantly; however, the blood pH of the combined L-lactate/D-mannitol group was increased significantly, from pH 7.44 to 7.49 (Table 2). The concentrations of Na+, K+, and Ca2+ were not significantly changed with a 3-h infusion of GHB alone (control group). The Cl− concentration was significantly decreased after a 3-h GHB infusion, but the concentration was still within the normal range. D-mannitol, L-lactate, and combined L-lactate/D-mannitol treatments had similar effects on the concentrations of Na+, Cl−, K+, and Ca2+ as observed in the control group (Table 2). Anion gaps and glucose concentrations were slightly increased with the infusion of GHB alone and the combination groups, but the concentrations were still within the normal range (Table 2).
Urine pH and Volumes Change after Combined L-Lactate/D-Mannitol Treatment
The urine pH values in the D-mannitol treatment group at 1 and 3 h were 6.92 ± 0.41 and 7.48 ± 0.32, respectively (Fig. 3). The urine pH values at 1 and 3 h after the treatment with L-lactate/D-mannitol at 1 and 3 h were 7.34 ± 0.53 and 8.02 ± 0.24, respectively, which were higher than that of the control (Fig. 3A). The urine volumes obtained in the L-lactate alone or D-mannitol-lactate combined groups were significantly higher than that of the control group (Fig. 3B), likely because of the large volumes of fluid administrated during the experiments (the total volumes of infusion solution were 9.6, 9.6, 12.6, and 12.6 ml for control, D-mannitol only, L-lactate only, and D-mannitol-L-lactate groups, respectively). There was no significant change in urine volume after D-mannitol administration compared with that in the control group (Fig. 3B).
Fig. 3.
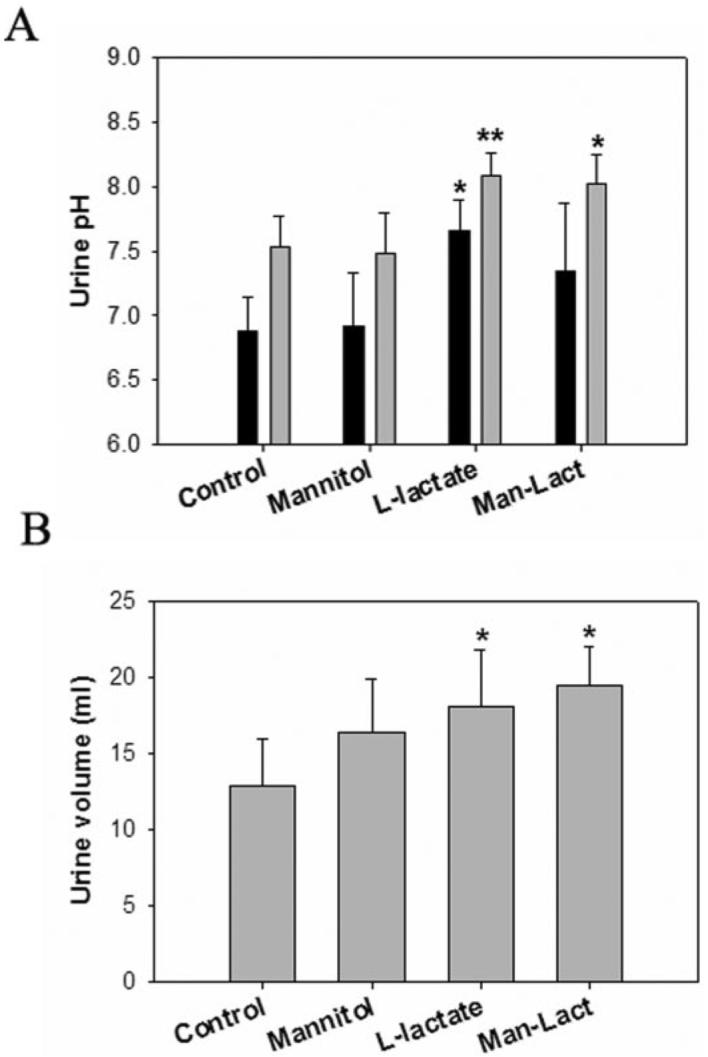
Effect of treatment with GHB alone or GHB with L-lactate, GHB with D-mannitol, or GHB with the combination of D-mannitol/L-lactate on urine pH and volume. Urine pH changes (A) and urine volume changes (B) after administration of 60.5 mg h−1 kg−1 L-lactate, 0.5 g/kg D-mannitol, and the combination of D-mannitol and L-lactate. One-way ANOVA followed by Dunnett's test, n = 3 to 5, mean ± S.D. *, p < 0.05; **, p < 0.01.
Effects of Lactated Ringer's Solution and D-Mannitol Administration on GHB TK and TD
To further develop a clinically relevant detoxification treatment, lactated Ringer's solution, which consists of 28 mM lactate, was given to rats by i.v. infusion (300 µl/min), either alone or together with D-mannitol (0.5 g/kg). GHB (1000 mg/kg) was given as an i.v. bolus to all groups to study the effect of interventions on the toxicodynamics (hypotic effect) of GHB. As shown in Fig. 4, compared with the control group (GHB infused with saline), administration of lactated Ringer's solution, D-mannitol, or lactated Ringer's solution/D-mannitol all resulted in a decrease in GHB plasma concentrations. However, a significant decrease in the GHB AUC was only observed in the combined treatment group (109 ± 24.1 in control versus 69.6 ± 1.00 mg ml−1 min, p < 0.05). The total and renal clearances of GHB in the D-mannitol and combined treatment groups, but not the lactated Ringer's solution group, were significantly higher than those of the control group (Table 3). Compared with the control group, the metabolic clearance value in the combined treatment group was significantly increased also (355 ± 56.6 in control versus 484 ± 19.6 mg h−1 kg−1, p < 0.05) (Table 3).
Fig. 4.
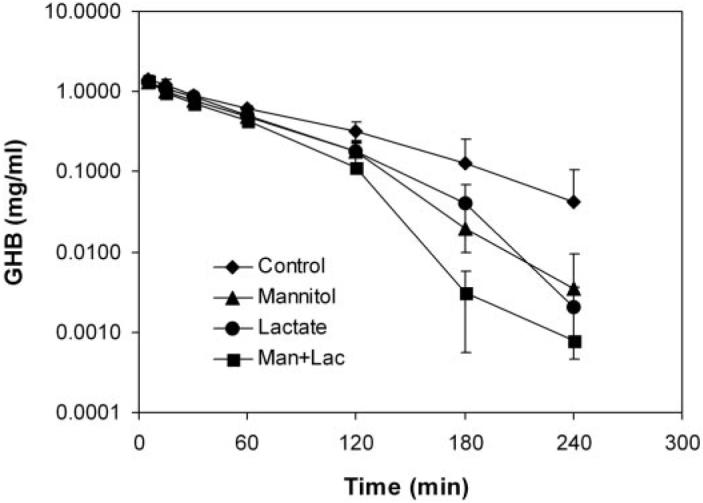
Plasma concentrations of GHB after treatment with GHB alone or with concomitant lactated Ringer's solution, D-mannitol, or lactated Ringer's solution/D-mannitol administration. The control was administered GHB (1000 mg/kg, i.v. bolus) only, followed by i.v. infusion of saline at the rate of 300 µl/min; L-lactate represents the i.v. infusion of lactated Ringer's solution at the rate of 300 µl/min; D-mannitol represents the 0.5g/kg bolus dose of D-mannitol; and Man + Lact represents the combined treatment with D-mannitol 0.5g/kg and i.v. infusion of lactated Ringer's solution at the rate of 300 µl/min, n = 3 to 4 per group.
TABLE 3.
Effects of combined D-mannitol (0.5 g/kg i.v. bolus) and lactated Ringer's solution (300 µl/min i.v. infusion) on GHB toxicokinetics and toxicodynamics GHB (1000 mg/kg) was administered by i.v. bolus. Saline or lactated Ringer's solution was administered by i.v. infusion at the rate of 300 µl/min for 4 h. D-Mannitol (0.5 g/kg) was administered by i.v. bolus. For the combined treatment group, lactated Ringer's solution was administered by i.v. infusion (300 µl/min) for 4 h, and D-mannitol (0.5 g/kg) was administered by i.v. bolus. One-way ANOVA followed by Dunnett's test was used to detect statistical significance, n = 3 to 4, mean ± S.D.
| Control | D-Mannitol (0.5 g/kg) | Lactated Ringer's Solution (300 µl/min) | D-Mannitol and Ringer's Solution | |
|---|---|---|---|---|
| CRRR, plasma concentration of GHB at the time point of RRR. | ||||
| AUC [GHB] (mg ml-1 min) | 109 ± 24.1 | 77.5 ± 9.24 | 83.5 ± 13.5 | 69.6 ± 1.00* |
| Total clearance (ml/h/kg) | 569 ± 113 | 781 ± 87.3* | 733 ± 120 | 862 ± 12.3** |
| Renal clearance (ml/h/kg) | 215 ± 64.9 | 356 ± 25.6* | 329 ± 85.0 | 378 ± 8.61* |
| Metabolic clearance (ml/h/kg) | 355 ± 56.6 | 425 ± 64.8 | 404 ± 41.0 | 484 ± 19.6* |
| GHB Fe(%) | 37.2 ± 5.17 | 45.8 ± 2.62 | 44.4 ± 4.83 | 43.8 ± 1.53 |
| LRR (min) | 3 ± 1 | 4 ± 1 | 7 ± 3 | 13 ± 2** |
| RRR (min) | 131 ± 40 | 93 ± 3 | 93 ± 17 | 75 ± 3* |
| Sleep time (min) | 128 ± 41 | 89 ± 4 | 86 ±20 | 62 ± 2* |
| CRRR (mg/ml) | 0.322 ± 0.052 | 0.315 ± 0.040 | 0.315 ± 0.014 | 0.352 ± 0.005 |
p < 0.05.
p < 0.01.
In the control group, the time of LRR (an indicator of the onset of sleep), the time of RRR (an indicator of the offset of sleep), and total sleep time of rats were 3 ± 1, 131 ± 40, and 128 ± 41 min, respectively. Compared with the control group, administration of lactated Ringer's solution or D-mannitol or lactated Ringer's solution/D-mannitol delayed the onset of GHB-induced sleep and decreased total sleep time. However, statistically significant changes were detected only for the combined treatment group (Table 3). At the return of righting reflex time, there was no significant difference in GHB plasma concentrations among all control and treatment groups (∼0.32 mg/ml) (Table 3), indicating this concentration might represent the wake-up threshold concentration in rats. This further suggested that the hypnotic effect of GHB was closely related to its plasma concentration.
Discussion
GHB is an endogenous fatty acid, which is fully ionized at physiological pH. The transport of GHB across various biological barriers and cellular membranes, such as the blood-brain barrier, brain cells, and proximal tubule cells, requires specific transporters (Benavides et al., 1982; Bhattacharya and Boje, 2004; Wang et al., 2006). The renal transport of GHB has been studied previously, and MCT1 represents an important transporter for GHB transport across proximal tubule cells (Wang et al., 2006). Most of the GHB filtered into kidney tubules is reabsorbed back into blood circulation in a capacity-limited manner (Morris et al., 2005); hence, the renal clearance of GHB may be increased by inhibiting its reabsorption using specific transporter inhibitors such as L-lactate and pyruvate (Morris et al., 2005). Overdoses of GHB result in coma, seizure, and death; there are no specific antidotes for the treatment of GHB overdoses. Our approach is to devise a mechanistic strategy for the treatment of GHB overdoses using specific inhibitors for MCTs, membrane transporters that are involved in the renal reabsorption of GHB. Therefore, in this study, we evaluated the dose-dependent effects of L-lactate or lactated Ringer's solution, specific inhibitors of MCT-mediated transport, on the TK and TD of GHB. The effects of administering the osmotic diuretic D-mannitol concomitantly with a low dose of L-lactate were also evaluated.
In the present investigation, we determined the concentration-dependent effects of L-lactate to evaluate the in vivo relationship between plasma lactate concentration and MCT inhibition. A 10-fold lower dose of L-lactate than used previously (Morris et al., 2005) was found to be effective in increasing the renal (70% increase; 163 ± 48 versus 96 ± 26 ml h−1 kg−1 in control group, p < 0.01) and total (30% increase; 441 ± 44 versus 340 ± 39 ml h−1 kg−1 in control group, p < 0.01) clearances of GHB in this study. When GHB was given by i.v. infusion, the increase of GHB total clearance after the administration of L-lactate was mainly because of an increase in the renal clearance of GHB, and there was no change in the metabolic clearance of GHB. We had reported previously an increase in the metabolic clearance of GHB after L-lactate treatment (Morris et al., 2005), but this was after the administration of a lower dose of GHB by i.v. infusion than used in the present study. With this lower dose of GHB, the capacity-limited metabolic clearance was changed from ∼250 to ∼500 ml h−1 kg−1 as the plasma concentration of GHB decreased from 0.40 to 0.25 mg/ml. In contrast, in the present study, the plasma concentration of GHB decreased from 0.60 to 0.40 mg/ml, and the metabolic clearance changed from ∼240 to ∼290 mg h−1 kg−1, indicating that metabolism remained saturated over this concentration range. However, when GHB was given as an i.v. bolus in the current study, a small but significant increase was observed in the metabolic clearance in the L-lactate/D-mannitol treatment group (355 ± 56.6 in control versus 484 ± 19.6 ml h−1 kg−1, p < 0.05). This is likely because of the greater decrease in the plasma concentration of GHB that occurred with this combined treatment.
The administration of L-lactate or combined L-lactate/D-mannitol produced increases in both blood and urine pH. There were small changes in blood electrolytes, glucose, and lactate concentrations, but most values remained within the normal range of values. Miller et al. (2002) reported that the exogenous administration of L-lactate does not alter the endogenous production and metabolic clearance of L-lactate. The clearance of L-lactate in humans at rest has been reported to be 22.7 to 27 ml min−1 kg−1 (Mazzeo et al., 1986; Stanley et al., 1988), which is much lower than that in the rat at rest (180.6 ml min−1 kg−1) (Donovan and Brooks, 1983). A blood L-lactate concentration of 4 mM has been reported after the exogenous administration of 2.76 to 2.98 mg min−1 kg−1 in humans (Miller et al., 2002), and this dose is close to the intermediate L-lactate dose used in this study.
Although a significant increase of urine pH after L-lactate treatment was observed, it is unlikely that this change contributed significantly to the inhibition of GHB reabsorption because the urine pH after GHB in the presence or absence of L-lactate was greater than or equal to 7.2; at this pH value, GHB is fully ionized (>99%), so very limited passive reabsorption would be expected. The urine volumes of the L-lactate treatment groups were also significantly higher than that of the controls; however, there were no differences in urine volume among the three L-lactate dose groups, although there were concentration-dependent decreases in GHB renal clearance. Overall, the results suggest that the increase in GHB clearance by L-lactate was because of, at least in part, the inhibition of renal transport by MCTs.
In this investigation, the infusion of three doses of L-lactate resulted in changes in the plasma concentrations but no change in brain concentrations of GHB. These findings suggest that total brain GHB concentrations are not correlated with GHB concentrations at the site of action. Brain and CSF concentrations have not been extensively examined, but Snead et al. (1980) reported a good association between plasma or CSF concentrations and electroencephalographic and behavioral effects after GHB administration in cats. In previous studies, we have reported that plasma concentrations correlate with the RRR after GHB administration in rats (Wang et al., 2008). Additionally, Kapadia et al. (2007) reported that GHB brain extracellular fluid concentrations may correlate with GHB hypnotic effects. Also, it is possible that a different neurotransmitter, such as GABA, which is formed from GHB in the brain (Hechler et al., 1997), may better correlate with RRR after GHB administration and therefore should be monitored when investigating concentration-effect relationships.
D-mannitol is an osmotic diuretic agent and the dose of D-mannitpol (0.5 g/kg) used in this study should provide plasma concentrations of D-mannitol necessary for its diuretic effect. Clinically, D-mannitol is administered via i.v. bolus or infusion, with doses ranging from 0.25 to 1.0 g/kg body weight, administered as 15 or 20% solutions (Knapp, 2005). In this investigation, the urine volumes of the D-mannitol treatment groups were not significantly different from those of the control groups. This finding may be a result of our study protocol, where a relatively large volume of infusion solution was administered, resulting in diuresis. The renal and total clearances of GHB were not significantly increased by D-mannitol. We have reported previously that higher doses of D-mannitol can result in increases in the renal and total clearances of GHB (Morris et al., 2005); therefore, osmotic diuresis may be effective in increasing the renal clearance of GHB at higher doses of D-mannitol. Interestingly, when GHB was given as an i.v. bolus, a small but significant increase in the renal and total clearances of GHB was observed in the D-mannitol treatment group but not the lactated Ringer's solution treatment group (Table 3). This is probably because of the different dosing regimen of GHB in these studies (i.v. infusion to steady state versus i.v. bolus injection).
An interesting phenomenon was observed when we combined D-mannitol (0.5 g/kg) with lactated Ringer's solution: the AUC of GHB and sleep time were significantly decreased when compared with the control group. However, these effects were not statistically significant when only lactated Ringer's Solution or D-mannitol was administered. One possible explanation is that L-lactate and D-mannitol acted additively or synergistically to increase GHB renal clearance. As a result, GHB plasma concentrations are significantly decreased, which may reflect changes in GHB at the site of action in the brain. Additionally, other mechanisms may also contribute to the additive/synergistic effects of mannitol and L-lactate on GHB TD, including changes in GHB uptake into the brain and changes in L-lactate disposition. The mechanism underlying the enhanced effects on the TK and TD of GHB is unknown.
In summary, we have characterized the effects of administering an MCT inhibitor, L-lactate (or lactated Ringer's solution), on GHB toxicokinetics and toxicodynamics. The administration of L-lactate (or lactated Ringer's solution), alone and with concomitant D-mannitol, resulted in a significant effect on GHB toxicokinetics and toxicodynamics. The strategies used in this study may represent potential clinical approaches for the treatment of GHB overdoses and a general strategy to treat overdoses of other small molecular weight organic anions.
Acknowledgments
We thank David Soda and Sunmi Fung for technical support.
This study was supported in part by the National Institutes of Health (Grant DA023223).
ABBREVIATIONS
- GHB
γ-hydroxybutyrate
- MCT
monocarboxylate transporter
- TK
toxicokinetics
- TD
toxicodynamics
- LC
liquid chromatography
- MS/MS
tandem mass spectrometry
- GFR
glomerular filtration rate
- LRR
loss of the righting reflex
- RRR
regaining of the righting reflex
- AUC
area under the plasma concentration-time curve
- CV %
coefficient of variation
- ANOVA
analysis of variance
References
- Andriamampandry C, Taleb O, Viry S, Muller C, Humbert JP, Gobaille S, Aunis D, Maitre M. Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator gamma-hydroxybutyrate (GHB) FASEB J. 2003;17:1691–1693. doi: 10.1096/fj.02-0846fje. [DOI] [PubMed] [Google Scholar]
- Arena C, Fung HL. Absorption of sodium gamma-hydroxybutyrate and its prodrug gamma-butyrolactone: relationship between in vitro transport and in vivo absorption. J Pharm Sci. 1980;69:356–358. doi: 10.1002/jps.2600690331. [DOI] [PubMed] [Google Scholar]
- Benavides J, Rumigny JF, Bourguignon JJ, Wermuth CG, Mandel P, Maitre M. A high-affinity, Na+-dependent uptake system for gamma-hydroxybutyrate in membrane vesicles prepared from rat brain. J Neurochem. 1982;38:1570–1575. doi: 10.1111/j.1471-4159.1982.tb06634.x. [DOI] [PubMed] [Google Scholar]
- Bernasconi R, Lauber J, Marescaux C, Vergnes M, Martin P, Rubio V, Leonhardt T, Reymann N, Bittiger H. Experimental absence seizures: potential role of gamma-hydroxybutyric acid and GABAB receptors. J Neural Transm Suppl. 1992;35:155–177. doi: 10.1007/978-3-7091-9206-1_11. [DOI] [PubMed] [Google Scholar]
- Bhattacharya I, Boje KM. GHB (gamma-hydroxybutyrate) carrier-mediated transport across the blood-brain barrier. J Pharmacol Exp Ther. 2004;311:92–98. doi: 10.1124/jpet.104.069682. [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Brunetti G, Melis S, Serra S, Vacca G, Mastinu S, Pistuddi AM, Solinas C, Cignarella G, et al. Role of GABA(B) receptors in the sedative/hypnotic effect of gamma-hydroxybutyric acid. Eur J Pharmacol. 2001;428:315–321. doi: 10.1016/s0014-2999(01)01334-6. [DOI] [PubMed] [Google Scholar]
- Darling IM, Morris ME. Evaluation of “true” creatinine clearance in rats reveals extensive renal secretion. Pharm Res. 1991;8:1318–1322. doi: 10.1023/a:1015820316660. [DOI] [PubMed] [Google Scholar]
- Davidson WD, Sackner MA. Simplification of the anthrone method for the determination of inulin in clearance studies. J Lab Clin Med. 1963;62:351–356. [PubMed] [Google Scholar]
- Donovan CM, Brooks GA. Endurance training affects lactate clearance, not lactate production. Am J Physiol. 1983;244:E83–E92. doi: 10.1152/ajpendo.1983.244.1.E83. [DOI] [PubMed] [Google Scholar]
- Ferrara SD, Zotti S, Tedeschi L, Frison G, Castagna F, Gallimberti L, Gessa GL, Palatini P. Pharmacokinetics of gamma-hydroxybutyric acid in alcohol dependent patients after single and repeated oral doses. Br J Clin Pharmacol. 1992;34:231–235. doi: 10.1111/j.1365-2125.1992.tb04129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier B, Martin M, Janbon B, Baverel G. Transport of beta-hydroxybutyrate and acetoacetate along rat nephrons: a micropuncture study. Am J Physiol. 1992;262:F762–F769. doi: 10.1152/ajprenal.1992.262.5.F762. [DOI] [PubMed] [Google Scholar]
- Fishbein WN, Bessman SP. Gamma-hydroxybutyrate in mammalian brain: reversible oxidation by lactic dehydrogenase. J Biol Chem. 1964;239:357–361. [PubMed] [Google Scholar]
- Fung HL, Haas E, Raybon J, Xu J, Fung SM. Liquid chromatographic-mass spectrometric determination of endogenous gamma-hydroxybutyrate concentrations in rat brain regions and plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;807:287–291. doi: 10.1016/j.jchromb.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Gallimberti L, Spella MR, Soncini CA, Gessa GL. Gamma-hydroxybutyric acid in the treatment of alcohol and heroin dependence. Alcohol. 2000;20:257–262. doi: 10.1016/s0741-8329(99)00089-0. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- Hechler V, Ratomponirina C, Maitre M. Gamma-hydroxybutyrate conversion into GABA induces displacement of GABAB binding that is blocked by valproate and ethosuximide. J Pharmacol Exp Ther. 1997;281:753–760. [PubMed] [Google Scholar]
- Kapadia R, Böhlke M, Maher TJ. Detection of gamma-hydroxybutyrate in striatal microdialysates following peripheral 1,4-butanediol administration in rats. Life Sci. 2007;80:1046–1050. doi: 10.1016/j.lfs.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Knapp JM. Hyperosmolar therapy in the treatment of severe head injury in children: mannitol and hypertonic saline. AACN Clin Issues. 2005;16:199–211. doi: 10.1097/00044067-200504000-00011. [DOI] [PubMed] [Google Scholar]
- Lettieri JT, Fung HL. Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J Pharmacol Exp Ther. 1979;208:7–11. [PubMed] [Google Scholar]
- Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Mamelak M, Scharf MB, Woods M. Treatment of narcolepsy with gamma-hydroxybutyrate: a review of clinical and sleep laboratory findings. Sleep. 1986;9:285–289. doi: 10.1093/sleep/9.1.285. [DOI] [PubMed] [Google Scholar]
- Mason PE, Kerns WP., 2nd Gamma hydroxybutyric acid (GHB) intoxication. Acad Emerg Med. 2002;9:730–739. doi: 10.1111/j.1553-2712.2002.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Brooks GA, Schoeller DA, Budinger TF. Disposal of blood [1-13C]lactate in humans during rest and exercise. J Appl Physiol. 1986;60:232–241. doi: 10.1152/jappl.1986.60.1.232. [DOI] [PubMed] [Google Scholar]
- Miller BF, Fattor JA, Jacobs KA, Horning MA, Navazio F, Lindinger MI, Brooks GA. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol. 2002;544:963–975. doi: 10.1113/jphysiol.2002.027128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Hu K, Wang Q. Renal clearance of gamma-hydroxybutyric acid in rats: increasing renal elimination as a detoxification strategy. J Pharmacol Exp Ther. 2005;313:1194–1202. doi: 10.1124/jpet.105.083253. [DOI] [PubMed] [Google Scholar]
- Nelson T, Kaufman E, Kline J, Sokoloff L. The extraneural distribution of gamma-hydroxybutyrate. J Neurochem. 1981;37:1345–1348. doi: 10.1111/j.1471-4159.1981.tb04689.x. [DOI] [PubMed] [Google Scholar]
- Okun MS, Boothby LA, Bartfield RB, Doering PL. GHB: an important pharmacologic and clinical update. J Pharm Pharm Sci. 2001;4:167–175. [PubMed] [Google Scholar]
- Schwartz RH, Milteer R, LeBeau MA. Drug-facilitated sexual assault (“date rape”) South Med J. 2000;93:558–561. [PubMed] [Google Scholar]
- Shannon M, Quang LS. Gamma-hydroxybutyrate, gamma-butyrolactone, and 1,4-butanediol: a case report and review of the literature. Pediatr Emerg Care. 2000;16:435–440. doi: 10.1097/00006565-200012000-00017. [DOI] [PubMed] [Google Scholar]
- Snead OC, 3rd, Bearden LJ, Pegram V. Effect of acute and chronic anticonvulsant administration on endogenous gamma-hydroxybutyrate in rat brain. Neuropharmacology. 1980;19:47–52. doi: 10.1016/0028-3908(80)90165-3. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Wisneski JA, Gertz EW, Neese RA, Brooks GA. Glucose and lactate interrelations during moderate-intensity exercise in humans. Metabolism. 1988;37:850–858. doi: 10.1016/0026-0495(88)90119-9. [DOI] [PubMed] [Google Scholar]
- Tedeschi L, Carai MA, Frison G, Favretto D, Colombo G, Ferrara SD, Gessa GL. Endogenous gamma-hydroxybutyric acid is in the rat, mouse and human gastrointestinal tract. Life Sci. 2003;72:2481–2488. doi: 10.1016/s0024-3205(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Wang Q, Darling IM, Morris ME. Transport of gamma-hydroxybutyrate in rat kidney membrane vesicles: role of monocarboxylate transporters. J Pharmacol Exp Ther. 2006;318:751–761. doi: 10.1124/jpet.106.105965. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang Q, Morris ME. Pharmacokinetic interaction between the flavonoid luteolin and gamma-hydroxybutyrate in rats: potential involvement of monocarboxylate transporters. AAPS J. 2008;10:47–55. doi: 10.1208/s12248-007-9001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


