Summary
Persistent drug-seeking behavior is hypothesized to co-opt the brain's natural reward-motivational system. Although ventral tegmental area (VTA) dopamine (DA) neurons represent a crucial component of this system, the synaptic adaptations underlying natural rewards and drug-related motivation have not been fully elucidated. Here we show that self-administration of cocaine, but not passive cocaine infusions, produced a persistent potentiation of VTA excitatory synapses, which was still present after 3 months abstinence. Further, enhanced synaptic function in VTA was evident even after 3 weeks of extinction training. Food or sucrose self-administration induced only a transient potentiation of VTA glutamatergic signaling. Our data show that synaptic function in VTA DA neurons is readily but reversibly enhanced by natural reward-seeking behavior, while voluntary cocaine self-administration induced a persistent synaptic enhancement that is resistant to behavioral extinction. Such persistent synaptic potentiation in VTA DA neurons may represent a fundamental cellular phenomenon driving pathological drug-seeking behavior.
Introduction
Dopamine (DA) neurons within the ventral tegmental area (VTA) are an integral part of the brain's natural reward circuit (Jones and Bonci, 2005; Hyman et al., 2006), and VTA neuron activity has been shown to be important in mediating a number of motivated behaviors (Fields et al., 2007). In particular, the involvement of a common pathway for both natural rewards and drugs of abuse have implicated VTA DA neurons in the development of drug addiction (Ikemoto and Wise, 2004; Kauer, 2004; Self, 2004). In addition, cues that predict natural or drug-related rewards can induce phasic DA neuron activation (Schultz 1998; Fields et al., 2007). Importantly, the transition to different firing modalities in the VTA, such as a shift from single spike firing mode to burst firing, can be modulated by glutamatergic afferents (White 1996; Mathon et al., 2003). A variety of brain regions, including the prefrontal cortex, lateral hypothalamus, bed nucleus of the stria terminalis, pedunculopontine nuclei and superior colliculus all send excitatory projections to the VTA (Fields et al., 2007; Geisler et al., 2007).
VTA DA neurons express different forms of synaptic glutamate plasticity, including long-term potentiation (LTP) and long-term depression (LTD; Bonci and Malenka, 1999; Jones et al., 2000; Thomas et al., 2000). In the hippocampus, these forms of long-term plasticity can contribute to the normal learning and memory processes associated with reward-related behaviors (Pastalkova et al., 2006; Whitlock et al., 2006). Importantly, in the VTA, transient increases in synaptic strength onto DA neurons can be produced by cocaine and other drugs of abuse (Ungless et al., 2001; Saal et al., 2003; Wolf et al., 2004, Hyman et al., 2006; Kauer and Malenka, 2007). However, in these previous studies, the synaptic plasticity was measured following experimenter-administered drugs rather than active voluntary self-administration. Because contingent, voluntary drug intake (e.g., through a self-administration paradigm) and non-contingent drug exposure (e.g., experimenter-administered or through a yoked design) can induce differential changes within the mesolimbic system (Hemby et al., 1997; Lu et al., 2003; McFarland et al., 2003; Tang et al., 2004; Hemby et al., 2005), changes that develop as a result of passive drug exposure may offer a narrow, incomplete picture about the development of addiction in humans, which likely involves voluntary intake and the learned association between drug and context (Leshner, 1997). Thus, previous studies revealed only the pharmacological effect of cocaine and not the consequences of the learned propensity to continually consume drugs.
Changes in synaptic plasticity in the VTA may serve to modulate learning and memory mechanisms necessary for natural reward-related behaviors, and long-term neuroadaptation of synapses may also facilitate responding for drugs as addiction develops. A growing interest has recently developed around the hypothesis that drug addiction results from maladaptive co-option of reward-related learning mechanisms that are normally involved in the pursuit of natural rewards (Hyman et al., 2006). However, while usurpation of learning and memory processes may support persistent seeking of abused drugs, common synaptic mechanisms of natural and drug reinforcement have not been demonstrated.
Results
Excitatory strength is increased in VTA DA neurons one day after self-administration of cocaine, food, or sucrose
The effects of voluntary self-administration on glutamatergic plasticity in VTA DA neurons were first examined in rats that self-administered cocaine, food, or sucrose. Rats were trained to self-administer cocaine (“Cocaine”, 0.25mg/kg/infusion), food (“Food”, 45 mg pellet), or sucrose (“Sucrose”, 45 mg pellet) under a fixed-ratio-1 (FR1) schedule of reinforcement for at least 14 days or until maintenance criteria were met (see Experimental Procedures and Supplemental Data). Behavior responses and earned reinforcers on the last day of responding in Cocaine, Food, and Sucrose groups are illustrated in Figure 1A. In vitro whole-cell recordings from VTA DA neurons in brain slices from each of the three groups were performed one day after the last self-administration session.
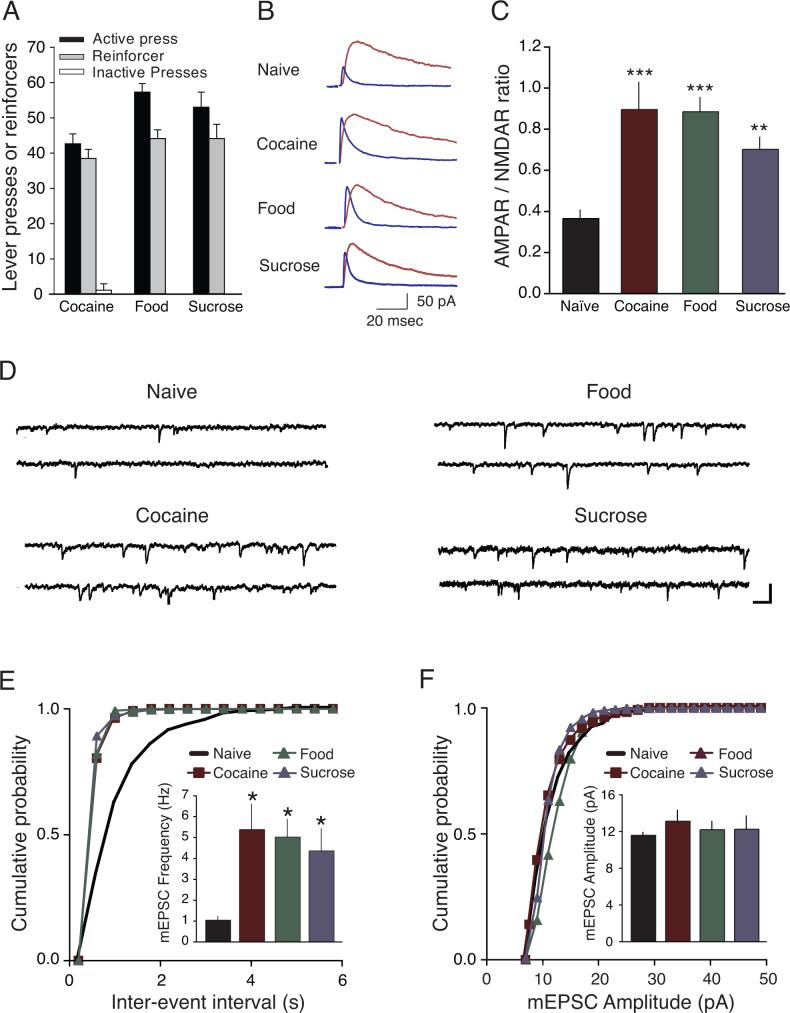
Figure 1.
Glutamatergic strength onto VTA DA neurons was enhanced one day after completion of cocaine, food, or sucrose self-administration. (A) Behavioral responses on the last day of training session in Cocaine, Food, and Sucrose groups. Active lever presses were 43 ± 3 presses for cocaine (n = 8 rats) 57 ± 2 presses for Food (n=11), and 53 ± 4 presses for Sucrose (n = 8). Cocaine rats earned 38 ± 3 infusions of cocaine (0.25 mg/kg/infusion), Food rats earned 44 ± 2 pellets of food, and Sucrose rats earned 44 ± 4 pellets of sucrose. Inactive lever responses averaged less than 3 presses for all groups. (B) Sample traces showing AMPAR- and NMDAR-mediated currents from Naïve rats and each of the self-administering groups. (C) Averaged AMPAR/NMDAR ratio from Naïve, Cocaine, Food, or Sucrose self-administrating rats. (D) Example traces of mEPSCs from Naïve, Cocaine, Food, and Sucrose groups. Scale bars, 20pA, 100 msec. Cumulative probability of frequency (E) and amplitude (F) of example cells from each of the four groups. Inset: averaged mEPSC frequencies were significantly increased in Cocaine, Food, and Sucrose rats compared with Naive rats, mEPSC amplitudes were not significantly different between the groups. *p < 0.05, **p < 0.01, ***p < 0.001 versus Naive.
We initially examined the ratio of AMPA receptor- to NMDA receptor-(AMPAR/NMDAR) mediated currents (Figure 1B), since changes in the ratio of AMPA to NMDA receptor-dependent currents can be a sensitive measure of synaptic plasticity at excitatory synapses (Malenka and Nicoll, 1999) and has been shown to be transiently enhanced after passive exposure to cocaine as well as other drugs of abuse (Ungless et al., 2001; Saal et al., 2003; Borgland et al., 2004).
In age-matched naïve rats (p80−150, “Naïve”), the AMPAR/NMDAR ratio was 0.36 ± 0.04 (n = 8, Figure 1C), similar to values previously observed in the VTA of young rats (Borgland et al., 2004). This suggests that, in the absence of behavioral or pharmacological manipulations, the baseline AMPAR/NMDAR ratio is similar in pre-adolescent (Borgland et al., 2004) and p80-p150 adult animals (present study), suggesting that AMPAR/NMDAR ratio remained consistent throughout a rat's lifetime. In contrast, self-administration of cocaine elicited a significant increase in AMPAR/NMDAR ratios compared to Naïve rats (0.89 ± 0.13, n = 8, p < 0.001, Figures 1B and 1C).
Similar to Cocaine rats, one day after the last training session, Food and Sucrose rats also showed significant increases in AMPAR/NMDAR ratio relative to Naïve rats, (Food: 0.88 ± 0.07, n = 10; Sucrose: 0.70 ± 0.10, n = 8; p < 0.001 and p < 0.01 for Naïve versus Food or Sucrose, respectively; Figures 1B and 1C). Together, these data showed that the AMPAR/NMDAR ratio was equally potentiated following cocaine, food, or sucrose self-administration.
Numerous brain regions send glutamatergic projections onto VTA DA neurons (Fields et al., 2007; Geisler et al., 2007), and changes in released glutamate can alter DA neuron activity. To further examine whether glutamatergic activity onto VTA DA neurons was affected by these behavioral training procedures, miniature excitatory post-synaptic currents (mEPSCs) were examined. Interestingly, increases in mEPSC frequency, suggesting an enhancement in glutamate release (Malenka and Nicoll, 1999), followed the same pattern as the AMPAR/NMDAR ratio data; rats that self-administered cocaine, food, or sucrose exhibited increased mEPSC frequency compared to Naïve rats (Naïve: 1.05 ± 0.17 Hz, n = 6; Cocaine: 5.39 ± 1.20 Hz, n = 8; Food: 5.02 ± 0.84 Hz, n = 6; Sucrose: 4.37 ± 1.05 Hz, n = 8; p < 0.05 for Cocaine, Food or Sucrose versus Naïve; Figures 1D and 1E). No differences were observed in mEPSC amplitudes between the self-administering groups (p > 0.05, Figures 1D and 1F). Taken together, the increases in AMPAR/NMDAR ratio and mEPSC frequencies suggest that self- administration of cocaine or natural rewards such as food or sucrose can transiently enhance glutamate transmission onto VTA DA neurons.
Non-contingent cocaine i.v. infusions do not potentiate excitatory transmission in VTA DA neurons
Previous studies have demonstrated that contingent, voluntary drug intake (e.g., through a self-administration paradigm) and non-contingent drug exposure (e.g., experimenter-administered intraperitoneal (i.p.) injection) can induce differential changes within the mesolimbic system (Hemby et al., 1997; Lu et al., 2003; McFarland et al., 2003; Tang et al., 2004; Hemby et al., 2005; You et al., 2007). Thus, we examined whether VTA glutamate function was affected by non-contingent drug delivery with electrophysiological experiments performed in rats receiving cocaine through a yoked design. In the first group, yoked rats received cocaine non-contingently for 14 days in a pattern and dosage (0.25mg/kg/infusion) similar to the Cocaine group. Cocaine infusions were not paired with cues. In vitro electrophysiological experiments were performed one day after the final cocaine exposure. The AMPAR/NMDAR ratio from yoked rats was not significantly different from Naïve rats, and despite having received similar cocaine doses as Cocaine rats, was significantly lower than Cocaine rats, (0.45 ± 0.07, n = 6, Figure 2A). Surprisingly, doubling the cocaine dosage (0.5mg/kg/infusion) did not enhance the AMPAR/NMDAR ratio (0.47 ± 0.04, n = 9, Figure 2A). The lack of glutamatergic potentiation following 14 days of cocaine infusion was unexpected because previous experiments have shown an enhanced AMPAR/NMDAR ratio following 1 or 7 days of daily i.p. cocaine injections (Ungless et al., 2001; Borgland et al., 2004). We also observed this effect in rats receiving multiple i.p. injections (once daily for 14 days, doses escalated from 3 mg/kg to 8 mg/kg to mirror the cocaine attained by self-administering rats and in yoked, 0.25mg/kg/infusion, rats). The AMPAR/NMDAR ratio measured one day after the last injection was 0.71 ± 0.05, significantly greater than Naïve rats and those from the yoked groups (n = 10, p < 0.01 versus Naïve, p < 0.05 versus yoked groups, Figure 2A).
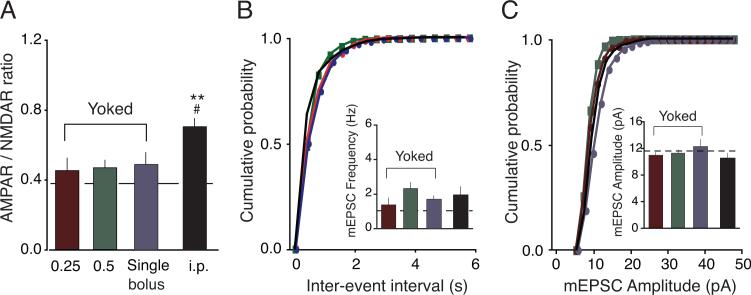
Figure 2.
Non-contingent cocaine delivery induced differential changes in glutamate function relative to voluntary cocaine self-administration. (A) Averaged AMPAR/NMDAR ratios from rats that received yoked cocaine (0.25 or 0.5 mg/kg/infusion, or a single i.v. bolus) were not significantly increased compared to Naïve rats. However, rats that received cocaine i.p. injections exhibited increased AMPAR/NMDAR ratios (B) Cumulative probability of frequency and amplitude (C) of example cells from each of the four groups. (Inset) Averaged mEPSC frequencies (B) and amplitudes (C) were not significantly different in rats that received cocaine noncontingently. Dotted lines represent averaged value from Naïve rats. **p < 0.01 versus Naïve, #p < 0.05 versus yoked groups.
The different effect of yoked and i.p. cocaine administration could be due to the rate of cocaine delivery. In the yoked experiments, cocaine was delivered in small doses over a 2-hr period, while for i.p. injections, a single, concentrated bolus of cocaine was delivered. To replicate the rate of i.p. injections but with intravenous (i.v.) delivery, another group of rats received a single i.v. bolus of cocaine each day for 14 days. The dose of cocaine used across days escalated from 3 mg/kg to 8 mg/kg, identical to the low-dose yoked group and the i.p. injection group. One day after the last i.v. bolus cocaine infusion, the AMPAR/NMDAR ratio was 0.49 ± 0.06 (n = 8, Figure 2A), similar to the ratios from the Naïve and the other yoked groups, but significantly less than the Cocaine group (p > 0.05 versus Naïve and other yoked groups, p < 0.01 versus Cocaine).
mEPSC analysis was also performed from all groups that received non-contingent cocaine. The mEPSC frequency was not significantly different from that observed in Naïve rats (yoked 0.25mg/kg/infusion: 1.38 ± 0.38 Hz, n = 6; yoked 0.5mg/kg/infusion: 2.32 ± 0.34, n = 12, i.v. bolus: 1.71 ± 0.19 Hz, n = 8, i.p. injection: 1.96 ± 0.46 Hz, n = 8, p > 0.05; Figures 2B and 2C). Surprisingly, the mEPSC frequency from i.p.-injected rats did not show an increase, even though the AMPAR/NMDAR ratio was potentiated in this group. As before, no differences were observed in mEPSC amplitudes across all groups (Figures 2C).
In summary, the results from these yoked groups show that the pharmacological effect of cocaine, via one of several rates and routes of administration, cannot alone account for the potentiation of glutamatergic synapses onto VTA DA neurons. These data suggest that a “strong” association between the drug and the self-administration environment must be present to potentiate VTA glutamatergic function, where a rat learns through response-reinforcer associating that its actions result in cocaine delivery. This interpretation is consistent with a previous microdialysis study showing that VTA glutamate release is a conditioned response dependent on an associative, reward-dependent process and is not a simple consequence of previous cocaine exposure (You et al., 2007).
Cocaine- but not Food or Sucrose self-administration induced long-lasting synaptic potentiation even after prolonged abstinence
Relapse to cocaine use is believed to result from enduring neuroadaptations within the brain's reward circuit and in particular, animal studies have suggested a critical role for the VTA (McFarland and Kalivas, 2001; Vorel et al., 2001) in relapse. Although experimenter-administered cocaine enhances synaptic strength in the VTA (Ungless et al., 2001; Borgland et al., 2004), this short-lived potentiation, lasting 5 but not 10 days after the last injection, cannot account for the persistence of drug-seeking behavior. However, it is not known whether voluntary cocaine self-administration could induce longer-lasting synaptic modifications that may be critical for relapse during abstinence. In addition, because cocaine, food, and sucrose self-administration induced similar potentiation of glutamatergic transmission onto VTA DA neurons one day after the completion of the training sessions, we also examined the duration of enhanced glutamate function in VTA DA neurons after food or sucrose self-administration.
To study the longevity of synaptic potentiation in the VTA, we examined whether glutamatergic function was enhanced after 7, 21 and 90 days of abstinence following 14 days of cocaine, food, or sucrose self-administration training. At 7 days of abstinence, AMPAR/NMDAR ratios in all three groups of rats were potentiated compared to Naïve rats (Cocaine: 0.66 ± .07, n = 9; Food: 0.74 ± 0.08, n = 9; Sucrose: 0.72 ± 0.10, n = 8; p < 0.05 for Cocaine or Sucrose versus Naïve, p < 0.01 for Food versus Naïve; Figure 3A). However, at 21 days of abstinence, only the Cocaine rats showed an enhanced AMPAR/NMDAR ratio whereas AMPAR/NMDAR ratio from Food and Sucrose rats had returned to Naïve levels (Cocaine: 0.68 ± 0.08, n = 6; Food: 0.42 ± 0.08, n =7; Sucrose: 0.44 ± 0.04, n = 10; p < 0.05 for Cocaine vs Naïve, p > 0.05 for Food or Sucrose vs Naïve; Figure 3A). Importantly, synaptic potentiation in Cocaine rats remained significantly elevated even after 90 days of abstinence from cocaine (0.64 ± 0.07, n = 7; p < 0.05; Figure 3A). Unlike rats that received non-contingent cocaine (Borgland et al., 2004), the voluntary choice to consume cocaine induced a significantly longer-lasting potentiation of AMPAR/NMDAR ratio.
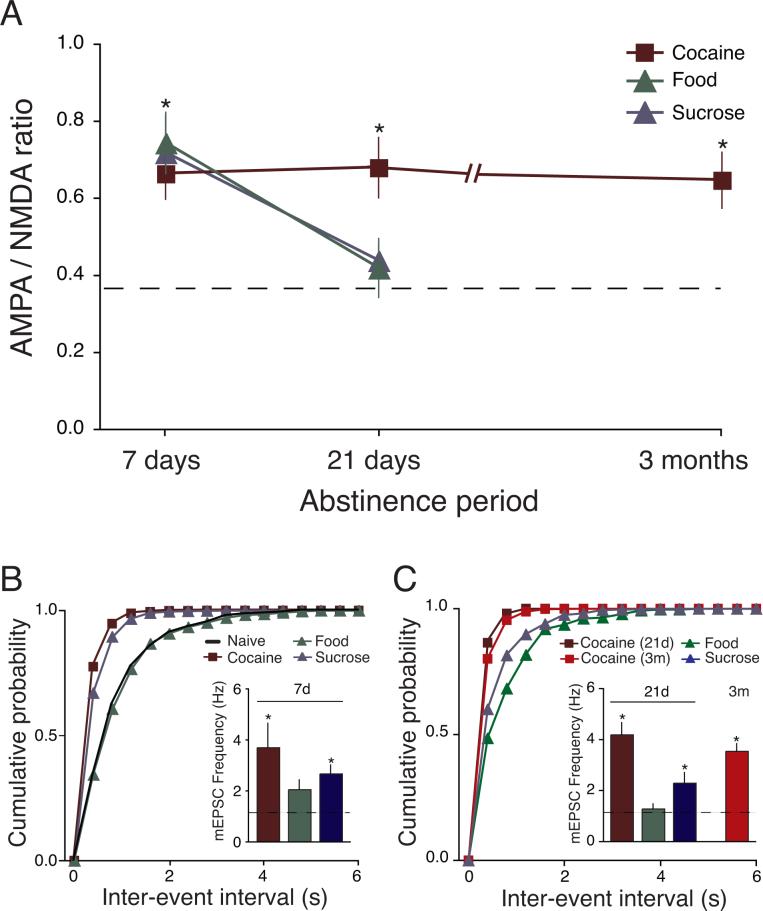
Figure 3.
Cocaine self-administration produced a significantly longer potentiation of glutamatergic transmission than food or sucrose self-administration. (A) AMPAR/NMDAR ratio in cocaine self-administering rats remained enhanced after 90 days of abstinence. However, in food and sucrose self-administering rats, increased AMPAR/NMDAR ratio is observed following 7d of abstinence but returned to Naïve levels by 21 days of abstinence. (B,C) Following 7d and 21d abstinence, mEPSC frequencies from Cocaine and Sucrose groups remained significantly enhanced relative to Naïve. In Food rats, (B), the mEPSC frequency was not significantly different than Naïve rats by 7 days of abstinence and remained unchanged at 21 days of abstinence (C). mEPSC frequencies from Cocaine continues to be significantly enhanced relative to Naïve at 3m abstinence. Dotted line represents averages from Naïve rats. * p < 0.05 versus Naive.
The continual increases in AMPAR/NMDAR ratios in Cocaine rats were matched by an identical pattern of enhancement in the mEPSC frequencies. mEPSC frequencies from Cocaine rats were significantly elevated at 7, 21 and 90 days of abstinence (7d: 3.69 ± 1.02 Hz, n = 8; 21d: 4.19 ± 0.50 Hz, n = 8; 90 days: 3.54 ± 0.28 Hz, n = 8; p < 0.05 versus Naïve; Figures 3B and 3C). In contrast, even though Food rats also exhibited an increase in AMPAR/NMDAR ratio at 7 days of abstinence, no significant increases in mEPSC frequencies were observed at the 7d or the 21d abstinence time point. (2.05 ± 0.37 Hz, n = 10; 21d: 1.28 ± 0.19 Hz, n = 7, p > 0.05; Figures 3B and 3C). However, mEPSC frequencies from rats that had self-administered the more palatable sucrose were significantly increased at both the 7d and 21d abstinence time points (7d: 2.66 ± 0.34 Hz, n = 11; 21d: 2.29 ± 0.40, n = 7, p < 0.05 versus Naïve; Figures 3B and 3C). mEPSC amplitudes were not significantly different between the three groups at all time points (data not shown). Thus, self-administration of food or sucrose showed transient increases in AMPAR/NMDAR ratio, lasting only 7 days, compared to 90 days in Cocaine rats. In addition, mEPSC frequency from Food rats was no longer elevated after 7 days of abstinence. In contrast, mEPSC frequency from Sucrose rats continues to be elevated even at 21 days of abstinence, at which point the AMPAR/NMDAR ratio had returned to Naïve levels. Taken together with results from cocaine self-administering rats, glutamatergic function in VTA DA neurons showed a very similar, transient pattern of enhancement (increased AMPAR/NMDAR ratio and increased mEPSC frequency) after self-administration of either a drug or natural reward, suggesting a similar cellular substrate of drug- and natural-reward-related learning. However, only self-administration of the cocaine drug reward was able to induce a long-lasting enhancement of glutamatergic function onto VTA DA neurons.
In order to further examined how the duration of training could affect glutamate synaptic plasticity in VTA DA neurons, rats self-administered cocaine for 7 days and glutamate function was examined after 21 days of abstinence. The AMPAR/NMDAR ratio was potentiated compared to Naïve rats (0.61 ± 0.06, n = 5, p < 0.05 versus Naïve rats; data not shown). However, mEPSC frequency and amplitude were similar to values from Naïve rats (Frequency: 1.73 ± 0.5 Hz, n = 6; Amplitude: 10.53 ± 0.5, n = 6 p > 0.05 versus Naïve; data not shown). Thus, the AMPAR/NMDAR ratio was potentiated 21d after 7d or 14d of cocaine self-administration, but the mEPSC frequency was only potentiated from rats that had 14 days of cocaine self-administration. This suggests that the duration of enhanced glutamate function may be dependent in part on the number of training days.
LTP is occluded in Cocaine rats after 21 days of abstinence
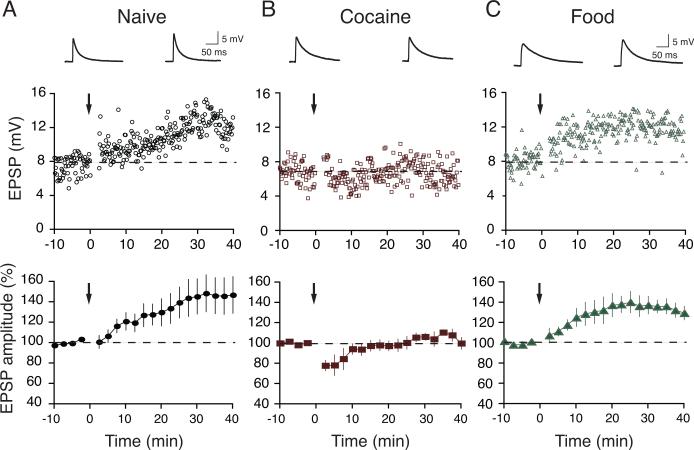
An enhanced AMPAR/NMDAR ratio in VTA DA neurons has been associated with occlusion of LTP induction, suggesting that excitatory synapses in VTA DA neurons have become saturated and thus that no further plasticity could be induced (Ungless et al., 2001, but see Liu et al., 2005). Therefore, we hypothesized that if the history of cocaine self-administration induced a prolonged in vivo potentiation of synaptic strength, as suggested by a persistently enhanced AMPAR/NMDAR ratio, then in vitro induction of LTP should also be occluded exclusively in Cocaine rats after 21 days of abstinence. LTP was induced using a spike-timing protocol consisting of 4 bouts of EPSP-spike pairs delivered 5 sec apart. Each bout consisted of a burst of 5 paired stimuli delivered at 100-msec intervals with the onset of EPSPs preceding the peak of the postsynaptic spike by 3 msec (Liu et al., 2005). This protocol induced a robust LTP in Naïve rats (147 ± 18% of baseline, n = 6, p < 0.01, Figure 4A). In contrast, LTP could not be generated in Cocaine rats after 21d of abstinence (100 ± 6% of baseline, n = 5, p > 0.05, Figure 4B). Interestingly, LTP was successfully induced in the Food-abstinent rats (130 ± 7% of baseline, n = 6, p < 0.01, Figure 4C), where the AMPAR/NMDAR ratios were low (Figure 3A). Taken together, our data strongly support the hypothesis that voluntary self-administration of natural rewards does not persistently alter VTA glutamatergic plasticity, and that only rats that had undergone learning in relation to voluntary drug self-administration showed persistent enhancements in glutamatergic signaling in VTA DA neurons.
Figure 4.
LTP was occluded exclusively in Cocaine rats after 21 days of abstinence. Example (top) and summary (bottom) of normalized EPSP amplitude before and after paired stimulation. (A) Naïve rats exhibited robust LTP while (B) LTP was not observed in Cocaine rats after 21 days of abstinence. (C) LTP was induced in Food rats after 21 days of abstinence. Arrows indicate time of LTP induction protocol.
Pre-clinical data suggest that enhanced motivation to seek drugs during abstinence can facilitate craving and relapse (Grimm et al., 2001; Kalivas and McFarland, 2003; Lu et al., 2004). Thus, we examined responding under a progressive-ratio schedule to determine the motivational state in Cocaine, Food, and Sucrose rats after 21 days of abstinence. Progressive ratio responding in Cocaine, Food, and Sucrose rats showed no significant difference in active lever responding, reinforcers obtained or breakpoints (see Supplemental Figure 1), suggesting that at 21 days of abstinence, rats in each group exhibited similar motivation to obtain their respective reinforcer. Our results are consistent with previous reports showing that motivation for rewarding substances is observed for natural and drug rewards (Grimm et al., 2002; Lu et al., 2004). Thus, increased glutamate function in the VTA may be critical exclusively in cocaine-seeking behaviors.
Excitatory transmission is potentiated even when drug-seeking behavior is extinguished
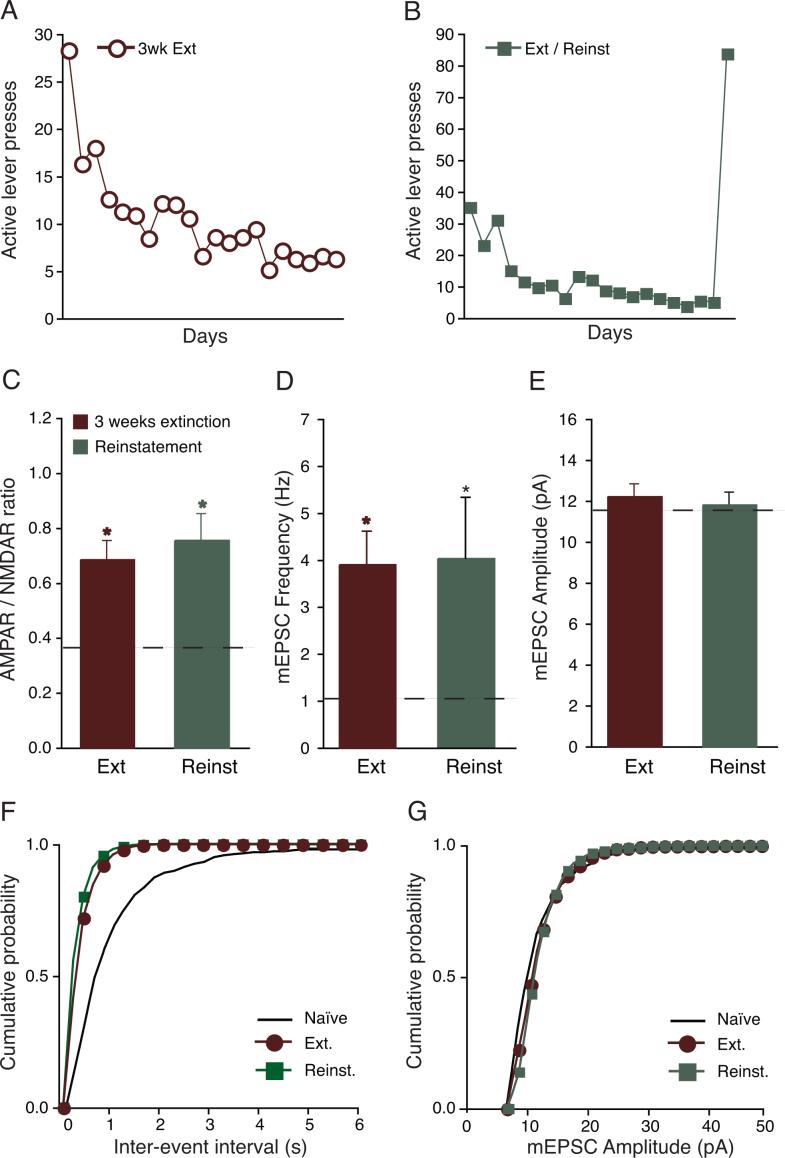
The animal reinstatement model (de Wit and Stewart, 1981) has been used to investigate mechanisms underlying relapse to drugs because it replicates many of the features associated with relapse in humans (Shaham et al., 2003). However, it is unknown whether the persistent potentiation of glutamate function in VTA DA neurons could contribute to resumption of drug-seeking behavior. To this end, two cocaine groups were trained using the reinstatement model. Both groups were trained to self-administer cocaine for 14 days, followed by 3 weeks of daily 2-hr extinction training. During extinction, depression of the active lever, which was previously paired with cocaine delivery, led instead to saline infusions, and also was not paired with compound tone-light cues. One day after the last extinction session, one group of rats (“Ext”) was sacrificed and electrophysiological experiments were performed. The second group underwent a single, 2-hr cue-induced reinstatement session (“Reinst”) one day after the last extinction session. Active lever presses during the reinstatement session led to saline infusions and activation of the compound tone-light cues previously paired with cocaine delivery. Reinst rats were sacrificed immediately after the reinstatement session for electrophysiological experiments. Figures 5A and 5B show active lever presses in the Ext and Reinst groups, respectively.
Figure 5.
Extinction training and reinstatement did not reduce synaptic potentiation in VTA DA synapses after cocaine self-administration. Behavioral responses in rats that underwent (A) 3 weeks of extinction training or (B) 3 wks extinction followed by 2 hr of cue-induced reinstatement. Error bars were removed for clarity. AMPAR/NMDAR ratios (C) and mEPSC frequency (D) remained elevated after 3 weeks of extinction and extinction/reinstatement. (E) mEPSC amplitude was not altered after extinction and extinction/reinstatement. Cumulative probability of frequency (F) and amplitude (G) of example cells from Ext and Reinst groups in comparison with Naive rats. Dotted line represents averages from Naive rats. *p < 0.05 versus Naïve rats.
After 3 weeks of daily extinction training, at the end of which rats exhibited significantly fewer active lever presses, the AMPAR/NMDAR ratio was still elevated relative to Naïve and was not significantly different from rats after 21 days of abstinence from cocaine (0.69 ± 0.07, n = 6, p < 0.05 versus Naïve, p > 0.05 versus Cocaine and 3 weeks abstinence, Figure 5C). Additionally, mEPSC frequency but not amplitude was also elevated after extinction training (Freq: 3.91 ± 0.7 Hz, n = 6, p > 0.05 versus Cocaine and 3 wks abstinence, p < 0.05 versus Naïve; Amplitude: 12.23 ± 0.6 pA, n = 6, p > 0.05; Figures 5D, 5E, 5F and 5G).
Similar to Ext rats, cue-induced reinstatement did not alter excitatory synapses onto VTA DA neurons, which remained at an elevated level in Reinst rats. The AMPAR/NMDAR in Reinst rats was 0.76 ± 0.1 (n = 7, p > 0.05 versus Ext or Cocaine and 3 wks abstinence, p < 0.05 versus Naïve; Figure 5C) and the mEPSC frequency was 4.04 ± 1.30 Hz (n=5, p > 0.05 versus Ext or Cocaine and 3 wks abstinence, p < 0.05 versus Naïve; Figures 5D, 5E, 5F and 5G). No significant changes were observed in the mEPSC amplitude in the Reinst group. Our data suggest that even when drug-seeking behaviors are extinguished, the enhancement in glutamate function induced by voluntary cocaine self-administration remains potentiated, and thus may be important in the resumption of the previously extinguished behavior.
Discussion
The present study showed that rats that learned to self-administer cocaine, food, or sucrose exhibited an enhancement of glutamatergic function in VTA DA neurons. Increased glutamatergic strength was evident as an increased AMPAR/NMDAR ratio and an increase in mEPSC frequency that was independent of mEPSC amplitude.
Self-administration of cocaine or natural rewards (food or sucrose) was equally potent in transiently enhancing synaptic potentiation in the VTA. However, the increase in glutamatergic strength induced by cocaine self-administration was significantly longer than that induced after food or sucrose self-administration, since only Cocaine rats showed enhanced AMPAR/NMDAR ratio after 21 days and 3 months of abstinence. Finally, VTA synaptic function remained persistently elevated even after extinction of lever-pressing behavior, and after cue-induced reinstatement. Taken, together, our data showed that excitatory synaptic strength onto VTA DA neurons was similarly but transiently potentiated by the performance of a learned appetitive behavior to obtain cocaine, food, or sucrose. However, in rats that were trained to self-administer cocaine, the potentiation of VTA glutamatergic function was significantly prolonged, lasting for up to 3 months and importantly, was not affected even when drug-seeking behavior was extinguished. An interesting finding from this study was that rats receiving repeated i.v. cocaine under one of several yoked designs did not exhibit potentiation of VTA glutamatergic function. These results suggest that cocaine-related changes were not simply due to pharmacological effects of cocaine, but instead could be due to an associative process (Everitt and Robbins, 2005; Hyman et al., 2006) acquired during self-administration and experimenter-administered sessions. In this regard, our data are in agreement with a previous study showing that VTA glutamate release was associated with cocaine self-administration but not with rats receiving yoked cocaine (You et al., 2007). In fact, the absence of potentiation in the Yoked group cannot be attributed to the cocaine dose injected because cocaine infusions at levels twice that seen in self-administering animals did not affect synaptic potentiation. Thus, our data show that the pharmacological effect of cocaine may not by itself be sufficient to potentiate glutamate transmission in the VTA, and that a strong pairing between the drug and a cue is necessary to induce this form of synaptic plasticity. The differential changes induced by passive drug administration versus voluntary drug consumption shown here and in previous studies (Hemby et al., 1997; Lu et al., 2003; McFarland et al., 2003; Tang et al., 2004; Hemby et al., 2005) highlight the need for careful interpretation of data obtained using these different experimental procedures. Importantly, we also observed, as has been reported previously, that i.p. injections of cocaine enhanced AMPAR/NMDAR ratio in the VTA (Ungless et al., 2001; Borgland et al., 2004; Bellone and Lücher, 2006). It is plausible to hypothesize that, during i.p. injections, the experimenter provides a strong, predictive cue for the cocaine injections, whereas in yoked experiments, cocaine administrations were not paired with any explicit cues, and thus cocaine was delivered at unpredictable times throughout the 2-hr session. While the operant chambers during yoked cocaine delivery could act as a cue to the rats, because passive cocaine delivery occurs across a dispersed time and comprised a small portion of the total time in the chamber, the salience of the behavioral apparatus is very likely diminished. In contrast, when an animal receives an i.p. injection, a strong pairing of the event occurs between the operator and the ‘high’ that the rats feel following the cocaine injection. This distinct pairing may be necessary to potentiate glutamatergic synapses in the VTA. In support of this conclusion, preliminary data shows that when Yoked cocaine delivery is paired with a compound tone-light cue, an increased AMPAR/NMDAR ratio is observed (see Supplemental Figure 2).
The potentiation of glutamatergic transmission onto VTA DA neurons seen in Cocaine, Food, or Sucrose rats one day after the completion of operant training may reflect an initial, common synaptic response to reward-related learning. Consistent with the present findings, blockade of VTA ionotropic glutamate receptors attenuates lever pressing for cocaine and sucrose (Sun et al., 2005 but see You et al., 2007). Since the VTA can be critical in initiating reward-seeking behavior (Yun et al., 2004), enhanced synaptic efficacy could increase VTA DA neuron firing to signal motivationally-salient stimuli. However, it is advantageous for this synaptic response to remain labile and not permanently saturated, so that an animal can form new, adaptive behaviors. For example, when synapses in the hippocampus are fully saturated by tetanic stimulation, spatial and associative learning is greatly impaired (Moser et al., 1998; Gruart et al., 2006). Thus, the transient potentiation of excitatory transmission in Food and Sucrose rats may reflect the normal resetting of excitatory synapses in VTA to allow processing of future stimuli.
On the other hand, in rats that self-administered cocaine, the AMPAR/NMDAR ratios remained significantly enhanced and LTP induction was occluded following 21 days of abstinence, suggesting that a history of voluntary cocaine self-administration may have produced a persisting LTP-like increase in AMPAR function or surface expression (Lisman and Raghavachari, 2006). Enhanced glutamatergic activity in the VTA can lead to an increase in spike-firing frequency during pacemaker-like activity as well as within episodes of burst firing (Canavier and Landry, 2006). Pacemaker activity has been hypothesized to underlie tonic DA release, while bursts of activity may be more closely related to phasic DA release (Gonon, 1988). Thus, it is tempting to speculate that enhancement of both firing modalities would promote greater DA release in VTA target regions, such as the prefrontal cortex (Carr and Sesack, 2000), which could in turn excite the glutamate projection to the nucleus accumbens and promote pathological drug-seeking behavior (Kalivas and McFarland, 2003; McFarland et al., 2003). In addition, long-lasting neuroadaptations within the VTA induced by voluntary cocaine self- administration may be especially harmful because saturation of excitatory synaptic responses in the VTA could preclude future responding for and learning in relation to other salient stimuli, and could explain the reduced responsiveness to non-drug rewards seen in rats and human addicts (Kalivas et al., 2005).
Of particular interest is the role of VTA glutamate signaling in the animal reinstatement model, which is used to model human relapse conditions because it recapitulates many hallmarks of human drug-seeking behaviors (Shaham et al., 2003). Glutamate plays an important role in reinstatement of cocaine-seeking behavior because inhibition of ionotropic and metabotropic glutamate receptors in the VTA attenuates both cocaine- and cue-induced reinstatement (Bäckström and Hyytiä, 2006; Sun et al., 2005; You et al., 2007). However, how glutamate synaptic plasticity is affected by extinction and reinstatement training is unknown. In the present study, we showed that excitatory transmission in VTA DA neurons remains heightened even when lever responses for cocaine were extinguished, since measures of synaptic strength (AMPAR/NMDAR ratio and mEPSC frequencies) after extinction were similar to rats that had undergone abstinence but not extinction. Additionally, this heightened synaptic enhancement was not further increased following the restoration of the lever response during a cue-induced reinstatement session. This persistent strengthening of the glutamate transmission supports the hypothesis that extinction training is not “unlearning” of old behavior but rather, is a new form of learning that leaves the original memories intact (Bouton 2004; Myers and Davis, 2007). Thus, neuroadaptations induced by drug self-administration form a powerful ‘memory’ that can be activated by drug-associated cues.
In conclusion, this study provides evidence for common, short-lasting neuroadaptations in VTA DA neurons following extended operant responding for cocaine or natural rewards. Importantly, synaptic strengthening in the VTA following natural reward consumption returned to Naïve levels following 21 days of abstinence, while cocaine self-administration resulted in persistent synaptic enhancement of glutamatergic input onto VTA DA neurons, despite protracted abstinence from the drug or extinction of drug-seeking behaviors. Equally important is that the synaptic enhancements in Cocaine rats were not due to the pharmacological effects of cocaine since passive exposure to cocaine did not potentiate these same synapses. How a drug but not natural reward could induce enduring changes is not currently known. Although studies have revealed neurochemical changes in the VTA following cocaine self-administration (Grimm et al., 2003; Lu et al., 2004), future in vivo and in vitro studies will be required to identify the exact mechanisms through which drugs of abuse alter neural circuitry that is normally accessed by naturally-reinforcing events, but is usurped by cocaine to persistently cement these synaptic adaptations, perhaps ultimately leading to pathological drug-seeking behavior.
Experimental procedures
Rats and housing
Male, Sprague Dawley rats (Charles River; P80-P140) were used for all experiments. All rats were singly housed in a climate-controlled facility on a 12-hr light/dark cycle. Experiments followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Rats, and were performed during the light cycle between 10:00 and 17:00 hr.
General Behavioral Methods
Behavioral experiments were performed in standard operant chambers (Coulbourn Inst., Allentown, PA) with two retractable levers set 5 cm above the floor. A cue light was positioned 6 cm above each lever. The two levers flanked a food hopper. One lever was paired with reinforcer delivery (the active lever) while the other lever had no programmed consequence (the inactive lever). The right and left orientation of active and inactive levers were randomly assigned to individual rats, but fixed for each rat throughout experimentation. Each chamber was also equipped with a house light and tone-generator. The house light was illuminated throughout each training session. All sessions continued for 2 hrs or until 50 reinforcers were earned, and were performed 7 days a week for 14−19 days. Details of cocaine, food, and sucrose self-administration and yoked procedures are provided in Supplemental Data.
Catheter surgery
Rats receiving intravenous cocaine were implanted with a chronically-indwelling intravenous catheter, as previously described (Carelli et al., 2000; Martin et al., 2006). Briefly, rats were anesthetized with pentobarbital (60 mg/kg, i.p, Sigma, St. Louis, MO) and unilateral, intrajugular catheters inserted 2.7−3.0 cm. The catheter was flushed daily with sterile saline and heparin (100 IU/0.2 ml) to help maintain catheter patency. Following surgery, rats were allowed to recover for one week and were then randomly assigned to the different groups used in this study. During the recovery period, rats were housed in home cages and had ad libitum access to food and water.
Intraperitoneal cocaine injection
Rats in this group received sham surgeries and followed the same food schedule (see Supplemental Data). After recovery from surgery, i.p. injections were given once daily for 14 days in their home cage. The cocaine dose used escalated from 3 mg/kg to 8 mg/kg to match the 0.25mg/kg/infusion yoked group. One day after the last i.p. injection, rats were sacrificed for electrophysiological experiments.
Intravenous bolus cocaine administration
After recovery from catheter surgery, rats in this group experienced a 15-hr, overnight session in the operant chamber. During the overnight session, both levers were extended but were inactive, and presses on the levers had no programmed consequences. The next day, I.V. bolus rats were placed in the operant chamber for 2 hrs, where they received a single, non-contingent cocaine infusion. At the start of the session, cocaine infusion was delivered after a random delay selected by the computer. Cocaine was delivered in a single 50 μl volume, the cocaine dose used escalated from 3 mg/kg to 8 mg/kg to match the 0.25mg/kg/infusion yoked group. Cocaine infusions were not paired with light or tone. Thus, Yoked rats never associated discrete stimuli or operant responding with cocaine delivery.
Progressive ratio
Cocaine, food, and sucrose self-administering rats were tested for progressive-ratio responding after 21 days of abstinence. Responding on the previously active lever was reinforced under a progressive-ratio schedule. The progressive ratio schedule used is described by the exponential function: 5e(infusion number × 0.1)-5 (Richardson and Roberts 1996). With this procedure, the response requirement to obtain a cocaine infusion (0.25 mg/kg/infusion) increased progressively throughout the session in the following steps: 1, 1, 2, 2, 3, 4, 5, 6, 7, 9, 10, 12, 13, 15, 17, 20, 22, 25, 28, 32, 36, 40, 45, 50, 56, 62, etc. The session lasted 2 hrs. The breaking point was defined by last completed ratio achieved.
Extinction/Reinstatement
Two groups of cocaine self-administering rats underwent either extinction or extinction followed by cue-induced reinstatement. In one group, rats self-administered cocaine (0.25mg/kg/infusion) for 14 days, followed by 21 days of daily, 2-hr extinction sessions. During the extinction sessions, active lever presses resulted in the delivery of saline instead of cocaine and tone + light cues previously paired with cocaine infusions were turned off. One day after the last extinction session, rats were sacrificed for electrophysiological experiments. The second group of rats underwent identical extinction training followed by a single, 2-hr cue-induced reinstatement session one day after the last extinction session. A single, non-contingent presentation of the cue (tone + light) previously paired with active lever presses was given at the start of the reinstatement session to reinitiated lever pressing. Rats that did not reinitiate lever presses after the cue presentation were not used in this study. Active lever presses (on an FR-1 schedule) resulted in the presentation of the tone-light cue previously paired to cocaine administration, however cocaine was not administered to these rats. Rats were sacrificed immediately after the cue-induced reinstatement session for electrophysiological experiments.
Slice preparation
Rats were sacrificed one day or 19 to 23 days after reaching acquisition criteria (Cocaine, Food) or after 14−18 days exposure to the operant chamber (Yoked). Rats were anesthetized with 40 mg/kg pentobarbital (i.p.) and transcardially perfused with ∼30 ml of nearly frozen (∼0°C) modified artificial cerebro spinal fluid (aCSF) at a rate of ∼20 ml/min. The modified aCSF for perfusion contained (in mM): 225 sucrose, 119 NaCl, 2.5 KCl, 1.0 NaH2PO4, 4.9 MgCl2, 0.1 CaCl2, 26.2 NaHCO3, 1.25 glucose; 3 kynurenic acid, 1 mM ascorbic acid. After perfusion, the brain was quickly removed and placed into ice-cold aCSF for 1−2 min. Horizontal sections containing the VTA (230 μm) were prepared with a vibratome (Leica, Nussloch, Germany). Slices were placed in a holding chamber (containing aCSF with 1 mM ascorbic acid added 15 min before brain dissection), and allowed to recover for at least 1 hr before being placed in the recording chamber and superfused with a bicarbonate-buffered solution saturated with 95% O2 and 5% CO2 and containing (in mM): 119 NaCl, 2.5 KCl, 1.0 NaH2PO4, 1.3 MgCl2, 2.4 CaCl2, 26.2 NaHCO3, and 11 glucose (at 32−34°C).
Electrophysiology
General voltage-clamp setup and procedures were the same as previously described (Ungless et al., 2001). VTA DA neurons were identified by the presence of a large Ih current (Lacey et al., 1990; Johnson and North 1992) and in some cases, tyrosine hydroxylase (TH) labeling. 60 Ih-positive neurons were filled with Lucifer Yellow during recording and stained for TH. Of the 60 cells, 57 cells were positive for TH. This high correlation (95%) between the presence of Ih and TH assured us that the majority of neurons recorded were dopaminergic.
EPSCs and EPSPs were evoked using a bi-polar stimulating electrodes positioned ∼100μm rostral to the recording neuron. AMPAR/NMDAR ratio was calculated by averaging 12 EPSCs at +40mV before and after application of the NMDAR blocker AP-5 (50 μM) for 5 min. NMDAR responses were calculated by subtracting the average response in the presence of AP-5 (AMPAR only) from that seen in its absence.
AMPAR mEPSCs were recorded in cells voltage-clamped at −70 mV and in the continual presence of lidocaine (500 μM), APV (50 μM), and sucrose (100mM; Borgland et al., 2006). Detection criteria were set at >7 pA. mEPSC traces were recorded while sampling every 10 μs; the images were filtered at 1 kHz.
LTP was induced using a spike-timing protocol (Liu et al., 2005). This protocol consisted of 4 bouts of EPSP-spike pairs delivered 5 sec apart. Each bout consisted of a burst of 20 paired stimuli delivered at 100-msec intervals and the onset of EPSPs preceding the peak of the postsynaptic spike by 3 msec.
Data analysis and statistics
All values are expressed as mean ± SEM. Statistical significance in AMPAR/NMDAR ratio and mEPSC amplitude and frequencies were assessed using one-way ANOVA for multiple group comparisons, with a Bonferroni post hoc test to test significant differences between groups.
For spike-timing-dependent LTP, peak EPSP amplitudes were calculated by taking the mean of a 1−2-ms window around the peak and comparing this with the mean of an 8-ms window immediately before the stimulation artifact. EPSP data were analyzed in 2.5-min bins. LTP was assessed 40 min after the last pairing stimulation, by comparing the averaged EPSPs from the last 2.5 min of baseline before LTP induction with the last 2.5 min of averaged EPSPs at the end of 40 min, and assessing for statistical significance using paired Student's t-test (Martin et al., 2006).
Supplementary Material
Acknowledgements
This research was supported by NIDA DA020236 to B.T.C. and DA15096-01 to A.B. The authors would like to thank L. Daitch for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bäckström P, Hyytiä P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharm. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat. Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J. Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J. Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Canavier CC, Landry RS. An increase in AMPA and a decrease in SK conductance increase burst firing by different mechanisms in a model of a dopamine neuron in vivo. J. Neurophysiol. 2006;96:2549–2563. doi: 10.1152/jn.00704.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J. Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J. Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharm. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579.. Review. Erratum in: Nat Neurosci. 2006 Jul;9(7):979
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu. Rev. Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J. Neurosci. 2007;27:5730–43. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19028. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav. Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruart A, Munoz MD, Delgado-Garcia JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J. Neurosci. 2006;26:1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentration in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Res. 2005;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47:190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plsticity and drug addiction. Curr. Opin. Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jones S, Kornblum JL, Kauer JA. Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J. Neurosci. 2000;20:5575–5580. doi: 10.1523/JNEUROSCI.20-15-05575.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. NOT. [DOI] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu. Rev. Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Actions of cocaine on rat dopaminergic neurones in vitro. Br. J. Pharmacology. 1990;99:731–735. doi: 10.1111/j.1476-5381.1990.tb12998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner IA. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci. STKE. 2006;356:1–15. doi: 10.1126/stke.3562006re11. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;13:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J. Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat. Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- Mathon DS, Kamal A, Smidt MP, Ramakers GMJ. Modulation of cellular activity and synaptic transmission in the ventral tegmental area. Eur. J. Pharmacol. 2003;480:97–115. doi: 10.1016/j.ejphar.2003.08.097. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;25:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;20:577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47:242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharm. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharm. 2005;30:2073–2081. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Tang W, Wesley M, Feeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaina self-administration and withdrawal in rats. J. Neurochem. 2004;89:1021–1033. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC, Bonci A. Modulation of long-term depression by dopamine in the mesolimbic system. J. Neurosci. 2000;20:5581–5586. doi: 10.1523/JNEUROSCI.20-15-05581.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;11:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- White FJ. Synaptic regulation of mesocorticolimbic dopamine neurons. Ann Rev Neurosci. 1996;19:405–436. doi: 10.1146/annurev.ne.19.030196.002201. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J. Neurosci. 2007;27:10546–10555. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J. Neurosci. 2004;24:2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.