Abstract
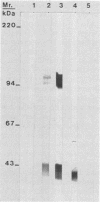
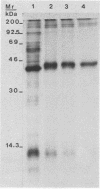
Purified elementary bodies (EBs) of Chlamydia trachomatis serovar L2 were analyzed by chemical cross-linking with disuccinimidyl selenodipropionate. The effect of the cross-linking was analyzed by immunoblotting sodium dodecyl sulfate-polyacrylamide gel electrophoresis-separated components which were reacted with monoclonal antibodies against major outer membrane protein (MOMP) and lipopolysaccharide (LPS). It was shown that in EBs, MOMP was cross-linked to the LPS component of the outer membrane. Migration analysis of the cross-linked components showed that with extensive cross-linking, most of the MOMP became cross-linked to LPS, changing the migration rate from 40 to 42.5 kilodaltons. A small fraction of MOMP associated with LPS was shown to be present in bands with migration rates of 100 and 110 kilodaltons. No association of MOMP or LPS to other proteins, or to dimer or multimer forms of MOMP without LPS, was observed. A totally different membrane structure must be present in reticulate bodies, since there, MOMP was so heavily cross-linked that it did not enter the polyacrylamide gel and thus became impossible to analyze. Furthermore, the monoclonal antibody, which reacted with LPS associated with MOMP in the cross-linked EBs, did not react with reticulate bodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batteiger B. E., Newhall W. J., 5th, Jones R. B. Differences in outer membrane proteins of the lymphogranuloma venereum and trachoma biovars of Chlamydia trachomatis. Infect Immun. 1985 Nov;50(2):488–494. doi: 10.1128/iai.50.2.488-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Ohlin A., Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984 May;44(2):479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade H., Brade L., Nano F. E. Chemical and serological investigations on the genus-specific lipopolysaccharide epitope of Chlamydia. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2508–2512. doi: 10.1073/pnas.84.8.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade L., Nurminen M., Mäkelä P. H., Brade H. Antigenic properties of Chlamydia trachomatis lipopolysaccharide. Infect Immun. 1985 May;48(2):569–572. doi: 10.1128/iai.48.2.569-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchardt O., Elsner H. I., Nielsen P. E., Petersen L. C., Suenson E. Protein crosslinking reagents containing a selenoethylene linker are cleaved by mild oxidation. Anal Biochem. 1986 Oct;158(1):87–92. doi: 10.1016/0003-2697(86)90593-2. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Hitchcock P. J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984 May;44(2):306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A. Medical chlamydiology--a position paper. Scand J Infect Dis Suppl. 1982;32:3–8. [PubMed] [Google Scholar]
- Nano F. E., Caldwell H. D. Expression of the chlamydial genus-specific lipopolysaccharide epitope in Escherichia coli. Science. 1985 May 10;228(4700):742–744. doi: 10.1126/science.2581315. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., Jones R. B. Disulfide-linked oligomers of the major outer membrane protein of chlamydiae. J Bacteriol. 1983 May;154(2):998–1001. doi: 10.1128/jb.154.2.998-1001.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen M., Leinonen M., Saikku P., Mäkelä P. H. The genus-specific antigen of Chlamydia: resemblance to the lipopolysaccharide of enteric bacteria. Science. 1983 Jun 17;220(4603):1279–1281. doi: 10.1126/science.6344216. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Wahlström E., Kleemola M., Leinonen M., Saikku P., Mäkelä P. H. Immunologically related ketodeoxyoctonate-containing structures in Chlamydia trachomatis, Re mutants of Salmonella species, and Acinetobacter calcoaceticus var. anitratus. Infect Immun. 1984 Jun;44(3):609–613. doi: 10.1128/iai.44.3.609-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Ripa K. T. Biological principles of the culture of Chlamydia trachomatis in cell monolayers. Scand J Infect Dis Suppl. 1982;32:25–29. [PubMed] [Google Scholar]
- Salari S. H., Ward M. E. Polypeptide composition of Chlamydia trachomatis. J Gen Microbiol. 1981 Apr;123(2):197–207. doi: 10.1099/00221287-123-2-197. [DOI] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Mullenbach G., Sanchez-Pescador R., Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986 Dec;168(3):1277–1282. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Stähli C., Staehelin T., Miggiano V. Spleen cell analysis and optimal immunization for high-frequency production of specific hybridomas. Methods Enzymol. 1983;92:26–36. doi: 10.1016/0076-6879(83)92006-2. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Zamze S. E., Byrne M. D., Lusher M., Evans R. T. Properties of monoclonal antibodies to the genus-specific antigen of Chlamydia and their use for antigen detection by reverse passive haemagglutination. J Gen Microbiol. 1985 Jan;131(1):7–15. doi: 10.1099/00221287-131-1-7. [DOI] [PubMed] [Google Scholar]
- Uchiumi T., Kikuchi M., Ogata K. Cross-linking study on protein neighborhoods at the subunit interface of rat liver ribosomes with 2-iminothiolane. J Biol Chem. 1986 Jul 25;261(21):9663–9667. [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Interaction between major outer membrane protein (O-8) and lipopolysaccharide in Escherichia coli K12. Eur J Biochem. 1980 Jan;103(1):209–218. doi: 10.1111/j.1432-1033.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- Yu F., Mizushima S. Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4. J Bacteriol. 1982 Aug;151(2):718–722. doi: 10.1128/jb.151.2.718-722.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]