Abstract
Oligodendrocyte (OLG) damage leads to demyelination, which is frequently observed in ischemic cerebrovascular diseases. In this study, we investigated the effect of bone marrow stromal cells (BMSCs) on OLGs subjected to oxygen-glucose deprivation (OGD). N20.1 cells (mouse OLG cell line) were transferred into an anaerobic chamber for 3 hr in glucose-free and serum-free medium. After OGD incubation, OLG cultures were divided into the following groups: 1) OGD alone, 2) OLG cocultured with BMSCs, 3) treatment with the phosphoinostide 3-kinase (PI3k) inhibitor LY294002, 4) LY294002-treated OLGs with BMSC cocultured, and 5) anti-p75 antibody-treated OLGs. After an additional 3 hr of reoxygenation incubation, OLG viability and apoptosis were measured. The mRNA expression in the BMSCs and OLGs was analyzed using quantitative real-time PCR (RT-PCR). Serine/threonine-specific protein kinase (Akt), phosphorylated Akt (p-Akt), p75, and caspase 3 protein expressions in OLGs were measured by Western blot. Our results suggest that BMSCs produce growth factors, activate the Akt pathway, and increase the survival of OLGs. BMSCs also reduce p75 and caspase 3 expressions in the OGD-OLGs, which leads to decreased OLG apoptosis. BMSCs participate in OLG protection that may occur with promoting growth factors/PI3K/Akt and inhibiting the p75/caspase pathways. Our study provides insight into white matter damage and the therapeutic benefits of BMSC-based remyelinating therapy after stroke and demyelinating diseases.
Keywords: bone marrow stromal cells, oligodendrocytes, oxygen-glucose deprivation
Oligodendrocytes (OLGs) generate the myelin sheaths that enwrap axons and thus play a pivotal role in the proper execution of neural function (Shibata et al., 2000). OLGs are very vulnerable to hypoxic and ischemic insults (Lyons and Kettenmann, 1998; Pantoni et al., 1996); damage to them leads to demyelination, which is frequently observed in ischemic cerebrovascular diseases and contributes to neurological functional impairment (Shibata et al., 2000; Li et al., 2005, 2006; Gresle et al., 2006). The precise mechanisms underlying the ischemia-induced death of OLGs are currently unknown (Shibata et al., 2000). Many factors determine the vulnerability of OLGs after ischemia (Dirnagl et al., 1999), such as caspases (Shibata et al., 2000), excitotoxicity (Matute et al., 2007), and oxidative stress (Imai et al., 2001). Therapeutic interventions are needed to protect OLGs as well as neurons and thereby to reduce neurological deficits after stroke. Our previous studies found that bone marrow stromal cells (BMSCs) improved neurological functional recovery after stroke in rodents (Chopp and Li, 2002; Li et al., 2002; Zhang et al., 2004) and protected neural cells (i.e., neurons, astrocytes) from ischemic injury (Li et al., 2000, 2002, 2005, 2006; Chopp and Li, 2002; Gao et al., 2005). However, the effect of BMSCs on ischemic OLGs has not been investigated.
Growth factors bind to the tyrosine kinase receptors of neural cells, induce the production of phosphatidylinositol 3,4,5 [PtdIns(3,4,5)P3] by phosphoinostide 3-kinase (PI3K; Vlahos et al., 1994), activate the Akt cascade, and thereby play an important neurotrophic role in the central nervous system (CNS; Cantley, 2002). The pan-neurotrophin receptor p75 is a deathinducing receptor that belongs to the tumor necrosis factor receptor superfamily. p75 is a marker of cell damage (Coulson et al., 2000; Casha et al., 2001; Wang et al., 2001; Beattie et al., 2002). Caspases are evolutionarily conserved executioners of programmed cell death in normal development and are also implicated in a variety of pathological conditions, including cerebral ischemia (Nicholson and Thornberry, 1997). p75 induces apoptosis through its death domain. Caspases (e.g., caspase 3) are activated by p75 expression, indicating the activation of the common effector pathway of apoptosis (Casha et al., 2001; Wang et al., 2001). Caspases are involved in hypoxia- and ischemia-induced OLG death (Shibata et al., 2000). In this study, we investigated the effect of BMSCs on OLGs in an oxygen-glucose deprivation (OGD) condition and the molecular mechanisms underlying their protective effects.
MATERIALS AND METHODS
OLG and BMSC Cultures
An immortalized mouse OLG cell line (N20.1, generously provided by Dr. Anthony Campagnoni, University of California at Los Angeles) was employed in this study. N20.1 cells were obtained from mouse primary cultures of OLGs conditionally immortalized by transformation with a temperature-sensitive large T-antigen (Verity et al., 1993). N20.1 cells grow constantly in Dulbecco’s modified Eagle’s medium (DMEM)/F12 with 10% fetal bovine serum (FBS) and G418 (100 μg/ml) at 34°C (permissive temperature), and they differentiate into mature OLGs in DMEM/F12/1% FBS and G418 at 39°C (nonpermissive temperature; Paez et al., 2004). Therefore, in the present experiments, the N20.1 cell lines were placed in DMEM/F12 high glucose (Invitrogen, Carlsbad, CA), with 3.6 g/liter dextrose anhydrous, 3.38 g/liter HEPES, 2.16 g/liter sodium bicarbonate, 90 mg/liter gentamicin, 1% FBS, and 100 μg/ml G418 at 39°C (nonpermissive temperature) for 7 days.
The mouse BMSCs were isolated from C57/Bl6 mice and cultured as previously described (Li et al., 2006). Briefly, the marrow was removed from hind legs. The cells were plated at a density of 9 × 105/cm2 with alpha modified Eagle’s medium supplemented with 10% FBS and penicillin/streptomycin. After 2 days, the medium containing nonadherent cells was aspirated. Approximately 14 days later, the adherent stromal cells become confluent and were designated passage 0 (P0). The BMSCs were expanded and passaged to P3, at which point they were harvested and employed in the present study.
OGD Injury and Reoxygenation Experiments
OLG cultures were transferred into an anaerobic chamber (model 1025; Forma Scientific) for 3 hr in glucose-free and serum-free medium (OGD). After OGD incubation, OLG cultures were divided into: 1) the OGD-alone group (OGD-OLGs); 2) the BMSC coculture group (OLGs: BMSCs = 2:1); an insert (0.4 μm; BD Biosciences, San Jose, CA) was used to contain BMSCs. OLGs were plated on the base of the culture wells, and the upper transwell compartments were seeded with BMSCs (BMSC-OLGs); 3) the LY294002 [2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride, an inhibitor of PI3k; Calbiochem, La Jolla, CA; No. 440202; 10 μm]-treated group (LY-OLGs); 4) the LY294002-treated coculture of BMSC-OLG group (LY-BMSC-OLGs); and 5) the anti-p75 antibody (Chemicon, Temecula, CA; AB1554; 1:1,000)-treated group (anti-p75-OLGs; n = 4 in each group). To mimic the in vivo conditions, where the blood flow and oxygen supply increase gradually after stroke, OGD-OLGs with or without treatment were incubated in the reoxygenation incubator in medium with reduced glucose (1.69 g/liter) for an additional 3 hr.
Cell Viability Assay
After 3 hr post-OGD incubation, OLG viability was measured with a LIVE/DEAD Kit (Invitrogen, Molecular Probes). The OLG cultures were incubated with viability assay solution containing 2 μm calcein-AM and 4 μm ethidium homodimer-1 at room temperature for 30 min. After incubation, the cells were examined under a fluorescent microscope (Nikon, eclipse, TE2000-U). The extent of cell survival was calculated by counting in 10 random fields in each well with three wells per group. The results are presented as a percentage (survival cell divided by total cells).
Cell Apoptosis Assay
After 3 hr post-OGD incubation, cell apoptosis assays were performed with Hoechst 33342/propidium iodide (PI) double staining (Chemicon; Gao et al., 2005) and TUNEL method (Chemicon; ApopTag Fluorescein In Situ Apoptosis Detection Kit), respectively, according to the manufacturer’s instructions. The OLG cultures were incubated with Hoechst 33342 (1 μg/ml) and PI (1.25 μg/ml) for 5 min. In contrast to normal cells, the nuclei of apoptotic cells have highly condensed chromatin that is uniformly stained by Hoechst 33342. This can take the form of crescents around the periphery of the nucleus, or the entire nucleus can appear to be one or a group of featureless, bright, spherical beads. These morphological changes in the nuclei of apoptotic cells are visualized by fluorescence microscopy. Costaining of the cells with PI allows the discrimination of necrotic cells from apoptotic cells (Dive et al., 1992). The apoptotic cells were calculated from Hoechst 33342-stained cells with shrunken and highly condensed nuclei in 10 random fields in each well with three wells per group.
For the TUNEL method, briefly, cells were fixed in 1% paraformaldehyde and postfixed in ethanol:acetic acid (2:1) for 5 min at -20°C. After application of equilibration buffer, cells were incubated with TdT enzyme at 37°C for 1 hr. Incubated antidigoxigenin was conjugated with the cells for 30 min at room temperature. After washing with PBS, DAPI was applied to stain nuclei. Cell were rinsed with PBS and observed under a fluorescent microscope (Nikon eclipse, TE2000-U). The apoptotic cells were calculated by counting in 10 random fields in each well with three wells per group. The results are presented as a percentage (apoptotic cells divided by total cells).
Quantitative Real-Time PCR Analysis
After BMSCs were cocultured with OGD-OLGs for 3 hr, mRNA expression in BMSCs and OLGs was measured by using the SYBR Green RT-PCR method (Wang et al., 2005; Zhang et al., 2005). Total RNA was isolated from BMSCs and OLGs using the RNeasy Micro Kit (Qiagen, Valencia, CA). One microgram of RNA from each sample was used to produce cDNA, following the standard protocol supplied with the SuperScript III RTase (Invitrogen). qRT-PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA). Specificity of the produced amplification product was confirmed by examination of dissociation reaction plots. A distinct single peak indicated that a single DNA sequence will be amplified during PCR. PCR products were run on 2% agarose gels to confirm that correct molecular sizes. Each sample was tested in triplicate using qRT-PCR. Data analysis used the 2-ΔΔCT method (Livak and Schmittgen, 2001). The following primers for RT-PCR were designed as follows: glyceraldehyde-3-phosphate dehyrogenase (GAPDH; FWD AGAACATCATCCCTGCATCC, REV CACATT GGGGGTAGGAACAC), nerve growth factor (NGF; FWD CAAGGACGCAGCTTTCTATACTG, REV CTTCAGG GACAGAGTCTCCTTCT); glial cell-derived neurotrophic factor (GDNF; FWD GATATTGCAGCGGTTCCTGT, REV AACATGCCTGGCCTACTTTG), brain-derived neurotrophic factor (BDNF; FWD TACTTCGGTTGCATGAA GGCG, REV GTCAGACCTCTCGAACCTGCC), insulinlike growth factor (IGF-1; FWD GGCATTGTGGATGA GTGTTG, REV GTCTTGGGCATGTCAGTGTG), Akt (FWD GACCATGAACGAGTTTGAGTACCT, REV CTT GAGGATCTTCATGGCATAGTA), caspase 3 (FWD ATG GGAGCAAGTCAGTGGAC, REV CGTACCAGAGCGAG ATGACA), p75 (FWD CATCTCTGTGGACAGCCAGA, REV CAGCTTCTCGACCTCCTCAC), and tyrosine kinase receptor A (TrkA; FWD CGTCATGGCTGCTTTTATGG, REV ACTGGCGAGAAGG AGACAG).
Western Blot Analysis
After 3 hr of post-OGD treatment, proteins from OLG cultures were extracted in 200 μl RIPA lysis buffer. Equal amounts of proteins, as determined using the bicinchoninic acid (BCA) protocol (Pierce, Rockford, IL), were loaded on 12% Bis-Tris gels (Invitrogen) after being denatured. The proteins were then transferred to Invitrogen PVDF membranes (Invitrogen), which were blocked for 1 hr with 5% bovine serum albumin (BSA) in TBS-T (10 mM Tris-HCl, pH 7.6, and 150 mM NaCl, 0.1% Tween-20). Afterward, the membranes were incubated with primary antibodies against Akt (Cell Signaling, Beverly, MA; 9272; 1:1,000), phosphorylated Akt (p-Akt; Cell Signaling; 9271; 1:1,000), p75 (Chemicon; AB1554; 1:1,000), and caspase 3 (Cell Signaling; 9661; 1:1,000) in 3% BSA at 4°C overnight. The membranes were washed with TBS-T and incubated for 1 hr at room temperature with horseradish peroxidase (HRP)-conjugated secondary antibodies (Bio-Rad Laboratories, Hercules, CA). After washing, the immunoblots were detected using a SuperSignal West Pico Chemiluminescent Substrate kit (Pierce). The experiment was repeated in triplicate. β-Actin was used as the internal control, and the densities of bands were analyzed in Scion Image (Scion Corp., Frederick, MD).
Statistical Analysis
The percentages of live cells and apoptotic cells were measured among four groups. Comparisons were made between treatment groups using analysis of variance (ANOVA). If the overall group difference was significant (P < 0.05), pairwise comparisons were made. Measures of NGF, GDNF, IGF-1, and BDNF mRNA expression were compared between normal BMSCs and BMSCs treated with OGD using two-sample t-tests. ANOVA was used to compare mRNA and protein expression among normal OLGs and OLGs treated with OGD and BMSCs. Pairwise comparisons were made if the overall treatment group difference was significant (P < 0.05). Data are presented as mean ± SD.
RESULTS
BMSCs Improved OLG Viability After OGD Injury
By using the LIVE/DEAD Kit to measure OLG viability, we found that approximately 20.8% of OLGs were dead in the OGD group after 3 hr of OGD injury and an additional 3 hr of post-OGD incubation (Fig. 1a). Cell survival was significantly increased in the BMSC-OLG group compared with the OGD-OLG group (P < 0.01; Fig. 1b), indicating the benefit of BMSCs on the survival of OLGs after OGD injury. The PI3K/Akt pathway is pivotal for cell survival (Cantley, 2002; Shah et al., 2005). In the present study, we employed LY294002, which is a potent and selective PI3K inhibitor (Vlahos et al., 1994). After LY294002 treatment, there was no significant decrease cell survival in LY-OLGs compared with the OGD-OLGs (Fig. 1c). Treatment with LY294002, blocked the BMSC survival benefits on OLGs after OGD injury (Fig. 1d,e), the cell survival of LY-BMSC-OLGs was significantly decreased compared with BMSC-OLGs, suggesting that the PI3K/Akt pathway plays an important role in the BMSC protective benefits on OLGs after OGD injury.
Fig. 1.
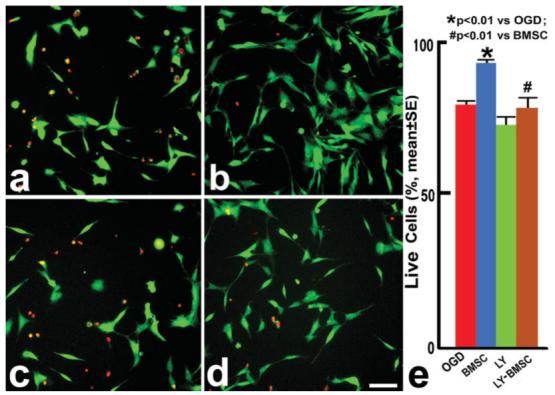
Cell viability assay. OLGs were transferred into an anaerobic chamber for 3 hr in glucose-free and serum-free DMEM conditions. After OGD incubation, OLG cultures were divided into an OGD control group (OGD-OLGs; a); a BMSC coculture group (BMSC-OLGs; b); LY294002-treated OLGs (LY-OLGs; c); and LY294002-treated BMSC cocultured OLGs (LY-BMSC-OLGs; d). The OLGs in the four groups were incubated in reoxygenation incubator in medium with reduced glucose. After 3 hr of post-OGD incubation, the OLG viability was measured with a LIVE/DEAD Kit. The red fluorescein labels dead cells, the green labels live cells. Quantitative data (e) show that 20.8% of OLGs were dead after OGD injury, the survival was increased after coculture with BMSCs (P < 0.01); however, additional LY294002 can block the BMSC beneficial effects on OLGs. Scale bar = 50 μm.
BMSCs Reduced OLG Apoptosis After OGD Injury
To test the BMSCs effect on the apoptosis of OLGs, we employed Hoechst 33342/PI double staining and TUNEL staining 3 hr post-OGD, respectively. With Hoechst 33342/PI double staining, approximately 51.9% of OLGs underwent apoptosis in the OGD group with shrunken or condensed bright blue nuclei, in the form of crescents around the periphery of the nucleus or the entire nucleus, which appear to be featureless, bright, spherical beads (Fig. 2a). After LY294002 treatment, there was no significant increase in cell apoptosis compared with the OGD group (Fig. 2b). Apoptotic OLGs were significantly decreased after BMSC coculture (Fig. 2c) and after p75 blocking antibody treatment (Fig. 2d) compared with the OGD group (P < 0.01; Fig. 2e). With the TUNEL method, similar results were obtained (Fig. 3a-e). The protection of OLGs from apoptosis by BMSCs was more effective than that of single p75 antibody treatment (P < 0.05), suggesting that the benefits of BMSCs on apoptotic OLGs may be partially mediated by p75.
Fig. 2.

Hoechst 33342/PI staining after 3 hr of post-OGD incubation. The blue fluorescein labels OLG nuclei, the red fluorescein necrotic OLG nuclei. Arrows indicate apoptotic OLGs in the OGD-OLG group (a), LY-OLG group (b), BMSC-OLG group (c), and anti-p75-OLG group (d). Quantification of OLG apoptosis (e): 51.9% OLGs underwent apoptosis after OGD injury treatment. The apoptotic OLGs were significantly decreased after coculture with BMSCs or anti-p75 blocking antibody treatment compared with the OGD group (P < 0.01). Scale bar = 50 μm.
Fig. 3.
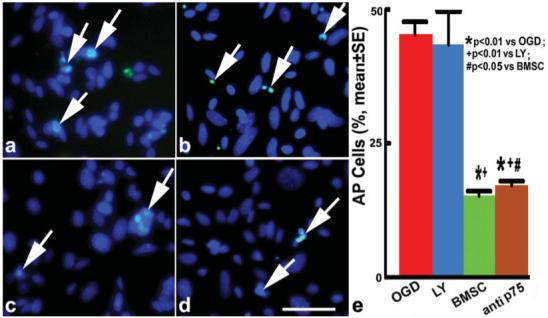
TUNEL staining after 3 hr of post-OGD incubation. The green fluorescein labels apoptotic OLG nuclei, the blue fluorescein OLG nuclei. Arrows indicate apoptotic OLGs in the OGD-OLG group (a), LY-OLG group (b), BMSC-OLG group (c), and anti-p75-OLG group (d). Quantification of OLG apoptosis (e): the apoptotic OLGs were significantly decreased after coculture with BMSCs or anti-p75 blocking antibody treatment compared with the OGD group (P < 0.01). Scale bar = 50 μm.
BMSCs Expressed Growth Factor mRNA and Increased TrkA and Akt mRNA Expression and Decreased p75 and Caspase 3 Expression in OLGs After OGD Injury
The PI3K/Akt pathway is activated by growth factors that promote cell survival. Therefore, in the present study, we measured the growth factor expression in BMSCs and investigated the mechanisms underlying the BMSC benefits on OGD-OLGs. qRT-PCR data revealed that normal BMSCs expressed mRNA of NGF, GDNF, IGF-1, and BDNF. After coculture with the OGD-OLGs in the reduced glucose and serum medium, NGF mRNA level was significantly increased, GDNF mRNA level did not change, and the expression of IGF-1 and BDNF mRNA was significantly decreased in BMSCs compared with the normal BMSCs (Fig. 4A). These data revealed that the BMSC growth factor gene expression is sensitive to the medium condition and is altered when cocultured with damaged OLGs. Thus, there may be feedback mechanisms of gene expression in BMSCs that foster protection of OLGs after OGD injury.
Fig. 4.
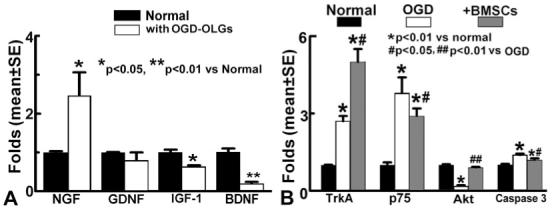
A: qRT-PCR analysis shows the mRNA expression of growth factors in BMSCs. mRNA expression of NGF, GDNF, IGF-1, and BDNF was present in the normal BMSCs. After coculture with the OGD-OLGs in the reduced-glucose and -serum medium, NGF mRNA level was significantly increased, GDNF mRNA level did not change, and the expression of IGF-1 and BDNF mRNA was significantly decreased in BMSCs compared with normal BMSCs. B: qRT-PCR analysis shows that TrkA, p75, and caspase 3 mRNA expressions were significantly increased, and Akt mRNA level was significantly decreased in the OGD-OLGs compared with normal OLGs (P < 0.01). After treatment with BMSCs, TrkA and Akt mRNA levels in the OLGs were significantly increased, and p75 and caspase 3 mRNA levels were decreased compared with OGD-OLGs.
Next, we measured the expression of TrkA (the high-affinity NGF receptor) and other important genes involved in the PI3K/Akt and p75/caspase pathways in normal OLGs, OGD-OLGs, and BMSC-OLGs. TrkA, p75, and caspase 3 mRNA expressions were significantly increased and Akt mRNA level was significantly decreased in the OGD-OLGs group compared with normal OLGs (P < 0.01; Fig. 4B). After treatment with BMSCs, TrkA and Akt mRNA levels in the OLGs were significantly increased, and p75 and caspase 3 mRNA levels were siginificantly decreased compared with OGD-OLGs.
BMSCs Increased p-Akt Expression and Decreased p75 and Caspase 3 Expression in OLGs After OGD Injury
To confirm our hypothesis that the protective effect of BMSCs on OGD-OLGs is mediated via PI3K/Akt and p75/caspase 3 pathways, we employed Western blots to measure p-Akt, p75, and caspase 3 expression. Western blots showed that p-Akt levels were significantly decreased and protein levels of p75 and caspase 3 were significantly increased in OLGs after 3 hr of OGD injury and an additional 3 hr post-OGD incubation. These data suggest that the ability of the OLG survival decreased, and apoptosis pathways are activated in OLGs after OGD. However, BMSC treatment increased p-Akt protein expression and inhibited increased p75 and caspase 3 expression levels in the OGD-OLGs (Fig. 5). These data are consistent with the cell viability and apoptosis data.
Fig. 5.
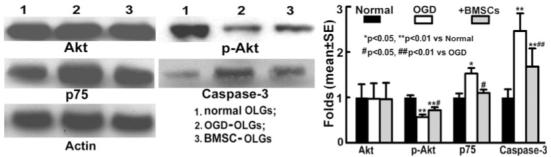
Western blot analysis shows Akt, p-Akt, and p75 protein expression in OLGs. p-Akt level was significantly decreased and p75 and caspase 3 protein levels were significantly increased in OGD-OLGs compared with normal OLGs; however, BMSC treatment increased p-Akt protein expression and inhibited increased p75 and caspase 3 expression levels in OLGs subjected to OGD.
DISCUSSION
OLGs are important components in the CNS and are the only myelin-producing cells that allow rapid electrical conduction of impulses (Wilkins et al., 2003). The lack of oxygen and glucose and free radicals contribute to OLG damage after stroke (Beckman and Koppenol, 1996; Shibata et al., 2000). BMSC treatment of stroke in rodents improves neurological recovery and enhances reactive OLG- and astrocyte-related axonal remodeling (Li et al., 2006). The present study revealed that BMSC treatment increased the viability and reduced apoptosis of OLGs after 3 hr of OGD injury and an additional 3 hr post-OGD incubation. These benefits were associated with growth factors expressed by BMSCs and increased p-Akt and decreased p75 and caspase 3 in OLGs cocultured with BMSCs.
The OGD model provides a standard and widely accepted, simplified in vitro model for extracting mechanistic information on focal stroke (Mielke et al., 2006; Thompson et al., 2006; McCarran and Goldberg, 2007). After occlusion of the middle cerebral artery in the animal or stroke in the patient, cerebral blood flow and thereby glucose deprivation persist for hours. Hypoperfusion of the brain after ischemic injury is somewhat simulated in vitro by the delay in reinstituting glucose. Therefore, we used reduced-glucose (1.69 g/liter) medium post-OGD. Glucose added to the culture medium (in concentrations up to 25 mM) prevented hypoxiainduced cell death in a concentration-dependent manner (Yoshioka et al., 2000). Moreover, when we double the glucose level in the medium after OGD, OLG damage was reduced after 3 hr post-OGD injury (data not shown). Our objective was to provide a proof of principle that BMSCs reduce OLG damage in a stressed, ischemic environment, e.g., OGD, by multiple mechanisms. Simulating the in vitro situation, the complexity of the in vivo conditions of ischemia, reperfusion, and glucose levels, is beyond the goals of the present study. However, we demonstrate for the first time the neuroprotective effects of BMSCs on OLGs.
BMSCs are a mixed cell population, including multipotential mesenchymal stem and precursor cells, and appear to have broad therapeutic applications to neurological disease. Functional recovery was evident after BMSC treatment in rodents with stroke (Chen et al., 2001; Li et al., 2002), experimental autoimmune encephalomyelitis (EAE; Zappia et al., 2005; Zhang et al., 2005), traumatic brain injury (Mahmood et al., 2001), and Parkinson’s disease (Li et al., 2001). We also found that the BMSC treatment of stroke in rodents reduced apoptosis in the penumbral zone of the lesion (Li et al., 2002; Chen et al., 2003) and protected against astrocyte apoptosis after OGD and reperfusion injury in vitro (Gao et al., 2005). Expression of neurotrophic and growth factors, i.e., NGF (Mahmood et al., 2004; Zhang et al., 2006), BDNF (Wang et al., 2004), IGF-1 (Zhang et al., 2004), VEGF (Chen et al., 2002b), and bFGF (Chen et al., 2002a), stimulated by BMSCs likely contribute to the beneficial effects of the treatment (Chopp and Li, 2002).
Akt is a serine/threonine kinase and plays a critical regulatory role in diverse cellular processes (Brazil et al., 2002; Shiojima and Walsh, 2002). Akt is a major regulator of insulin signaling and glucose metabolism (Hajduch et al., 2001) and regulates cell growth and proliferation. Akt also mediates growth factor-associated cell survival (Shah et al., 2005) by inhibiting apoptosis through its ability to phosphorylate and inactivate several targets, including Bad and Forkhead transcription factors (Coffer et al., 1998; Brunet et al., 1999). Akt is activated downstream from PI3K (Cantley, 2002; Vivanco and Sawyers, 2002), which appears to be centrally involved with growth factor signal transduction (Vlahos et al., 1994; Ulrich et al., 1998; Vaillant et al., 1999), including NGF (Xie et al., 2000; Itakura et al., 2005; Rahbek et al., 2005; Lin et al., 2006), BDNF (Bhave et al., 1999; Dolcet et al., 1999; Foulstone et al., 1999; Rajagopal et al., 2004), IGF-1 (Kulik et al., 1997; Fujio et al., 2000; Mockridge et al., 2000; Politi et al., 2001; Yamaguchi et al., 2001; Itakura et al., 2005), and GDNF (Mograbi et al., 2001; Focke et al., 2003; Veit et al., 2004; Anitha et al., 2006; Hauck et al., 2006; Tsui et al., 2006; Villegas et al., 2006; Lee et al., 2007; Braydich-Stolle et al., 2007; Wang et al., 2007). p-Akt is activatedAkt (Shah etal., 2005). Akt is therefore an important therapeutic target for the treatment of stroke and neurodegenerative diseases. Our data show that BMSCs expressed growth factors, i.e., NGF, BDNF, IGF-1, and GDNF, although the expression of some growth factors in the medium with reduced glucose and serum was lower than that of normal medium. The mRNA level of TrkA, the high-affinity receptor of NGF, was significantly increased in BMSC-OLGs compared with OGD-OLGs. These growth factors bind to the tyrosine kinase receptors of the OLGs subjected to OGD and, thereby, activate the Akt cascade via PI3K, which induced p-Akt, leading to improved OLG survival and reduced the apoptosis of OGD-OLGs. The PI3K inhibitor LY 294002 blocked this survival benefit of BMSCs on OGD-OLGs, implying that BMSCs improved OLG survival via the PI3K/Akt cascade.
The pan-neurotrophin receptor p75 has been implicated in damage-induced cell death (Casaccia-Bonnefil et al., 1996; Yoon et al., 1998; Coulson et al., 2000; Syroid et al., 2000; Casha et al., 2001; Wang et al., 2001; Beattie et al., 2002). p75 is required for the death of neural cells after CNS injury (Lee et al., 2001; Beattie et al., 2002). OLGs undergoing apoptosis expressed p75, and the absence of p75 resulted in a decrease in the number of apoptotic OLGs and increased survival of OLGs (Beattie et al., 2002). Our results also show that a p75 blocking antibody decreased apoptotic OLGs. Caspase 3 is activated by p75 expression and induces the common apoptotic pathway (Wang et al., 2001). The present study revealed that, although OGD injury induced p75 and caspase 3, treatment with BMSCs significantly inhibited transcriptional activity of p75, caspase-3-like activation, and OLG death.
The present data in concert with our previous studies indicate that the BMSC capacity to increase expression of growth and trophic factors may be key to the neuroprotective and neurorestorative benefits found in neural cells after ischemic injury (Chen et al., 2002b; Chopp and Li, 2002; Zhang et al., 2004; Gao et al., 2005). Growth and trophic factors bind to the tyrosine kinase receptors of neural cells, activate the PI3K/Akt cascade (Vlahos et al., 1994), and thereby play an important neurorestorative role in the CNS (Cantley, 2002). Moreover, the present study also found that BMSC can inhibit p75/caspase pathways and reduce apoptosis of OLGs after OGD and reperfusion injury. These data provide insight into white matter damage and the therapeutic benefits of BMSC-based remyelinating therapy after stroke (Chopp and Li, 2002; Li et al., 2005, 2006) and demyelinating diseases (Zhang et al., 2005).
ACKNOWLEDGMENTS
The authors thank Dr. Mark Katakowski and Qinge Lu for their technical assistance and Deborah Jewell for secretarial support.
Contract grant sponsor: Benson Ford Foundation; Contract grant sponsor: NIH; Contract grant number: PO1 NS42345; Contract grant number: RO1 NS45041.
REFERENCES
- Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. Pro-NGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Bhave SV, Ghoda L, Hoffman PL. Brain-derived neurotrophic factor mediates the anti-apoptotic effect of NMDA in cerebellar granule neurons: signal transduction cascades and site of ethanol action. J Neurosci. 1999;19:3277–3286. doi: 10.1523/JNEUROSCI.19-09-03277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103:203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen X, Katakowski M, Li Y, Lu D, Wang L, Zhang L, Chen J, Xu Y, Gautam S, Mahmood A, Chopp M. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res. 2002a;69:687–691. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002b;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson EJ, Reid K, Murray SS, Cheema SS, Bartlett PF. Role of neurotrophin receptor p75NTR in mediating neuronal cell death following injury. Clin Exp Pharmacol Physiol. 2000;27:537–541. doi: 10.1046/j.1440-1681.2000.03295.x. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dive C, Gregory CD, Phipps DJ, Evans DL, Milner AE, Wyllie AH. Analysis and discrimination of necrosis and apoptosis (programmed cell death) by multiparameter flow cytometry. Biochim Biophys Acta. 1992;1133:275–285. doi: 10.1016/0167-4889(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Dolcet X, Egea J, Soler RM, Martin-Zanca D, Comella JX. Activation of phosphatidylinositol 3-kinase, but not extracellular-regulated kinases, is necessary to mediate brain-derived neurotrophic factor-induced motoneuron survival. J Neurochem. 1999;73:521–531. doi: 10.1046/j.1471-4159.1999.0730521.x. [DOI] [PubMed] [Google Scholar]
- Focke PJ, Swetlik AR, Schilz JL, Epstein ML. GDNF and insulin cooperate to enhance the proliferation and differentiation of enteric crest-derived cells. J Neurobiol. 2003;55:151–164. doi: 10.1002/neu.10204. [DOI] [PubMed] [Google Scholar]
- Foulstone EJ, Tavare JM, Gunn-Moore FJ. Sustained phosphorylation and activation of protein kinase B correlates with brain-derived neurotrophic factor and insulin stimulated survival of cerebellar granule cells. Neurosci Lett. 1999;264:125–128. doi: 10.1016/s0304-3940(99)00166-4. [DOI] [PubMed] [Google Scholar]
- Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Li Y, Chopp M. Bone marrow stromal cells increase astrocyte survival via up-regulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005;136:123–134. doi: 10.1016/j.neuroscience.2005.06.091. [DOI] [PubMed] [Google Scholar]
- Gresle MM, Jarrott B, Jones NM, Callaway JK. Injury to axons and oligodendrocytes following endothelin-1-induced middle cerebral artery occlusion in conscious rats. Brain Res. 2006;1110:13–22. doi: 10.1016/j.brainres.2006.06.111. [DOI] [PubMed] [Google Scholar]
- Hajduch E, Litherland GJ, Hundal HS. Protein kinase B (PKB/Akt)—a key regulator of glucose transport? FEBS Lett. 2001;492:199–203. doi: 10.1016/s0014-5793(01)02242-6. [DOI] [PubMed] [Google Scholar]
- Hauck SM, Kinkl N, Deeg CA, Swiatek de Lange M, Schoffmann S, Ueffing M. GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol Cell Biol. 2006;26:2746–2757. doi: 10.1128/MCB.26.7.2746-2757.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke. 2001;32:2149–2154. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- Itakura M, Yamamori S, Kuwahara R, Sekiguchi M, Takahashi M. Two distinct regulatory mechanisms of neurotransmitter release by phosphatidylinositol 3-kinase. J Neurochem. 2005;94:502–509. doi: 10.1111/j.1471-4159.2005.03242.x. [DOI] [PubMed] [Google Scholar]
- Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neurosci Lett. 2001;316:67–70. doi: 10.1016/s0304-3940(01)02384-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- Li Y, McIntosh K, Chen J, Zhang C, Gao Q, Borneman J, Raginski K, Mitchell J, Shen L, Zhang J, Lu D, Chopp M. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol. 2006;198:313–325. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Lin DC, Quevedo C, Brewer NE, Bell A, Testa JR, Grimes ML, Miller FD, Kaplan DR. APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol Cell Biol. 2006;26:8928–8941. doi: 10.1128/MCB.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 - Delta Delta C(T) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lyons SA, Kettenmann H. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J Cereb Blood Flow Metab. 1998;18:521–530. doi: 10.1097/00004647-199805000-00007. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1203. discussion1203-1194. [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21:33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, Sanchez Gomez MV, Perez-Samartin A, Rodriguez-Antiguedad A, Perez-Cerda F. Excitotoxic damage to white matter. J Anat. 2007;210:693–702. doi: 10.1111/j.1469-7580.2007.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarran WJ, Goldberg MP. White matter axon vulnerability to AMPA/kainate receptor-mediated ischemic injury is developmentally regulated. J Neurosci. 2007;27:4220–4229. doi: 10.1523/JNEUROSCI.5542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke JG, Taghibiglou C, Wang YT. Endogenous insulin signaling protects cultured neurons from oxygen-glucose deprivation-induced cell death. Neuroscience. 2006;143:165–173. doi: 10.1016/j.neuroscience.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Mockridge JW, Benton EC, Andreeva LV, Latchman DS, Marber MS, Heads RJ. IGF-1 regulates cardiac fibroblast apoptosis induced by osmotic stress. Biochem Biophys Res Commun. 2000;273:322–327. doi: 10.1006/bbrc.2000.2934. [DOI] [PubMed] [Google Scholar]
- Mograbi B, Bocciardi R, Bourget I, Busca R, Rochet N, Farahi-Far D, Juhel T, Rossi B. Glial cell line-derived neurotrophic factorstimulated phosphatidylinositol 3-kinase and Akt activities exert opposing effects on the ERK pathway: importance for the rescue of neuroectodermic cells. J Biol Chem. 2001;276:45307–45319. doi: 10.1074/jbc.M101220200. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Paez PM, Garcia CI, Davio C, Campagnoni AT, Soto EF, Pasquini JM. Apotransferrin promotes the differentiation of two oligodendroglial cell lines. Glia. 2004;46:207–217. doi: 10.1002/glia.20001. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. discussion 1647. [DOI] [PubMed] [Google Scholar]
- Politi LE, Rotstein NP, Salvador G, Giusto NM, Insua MF. Insulin-like growth factor-I is a potential trophic factor for amacrine cells. J Neurochem. 2001;76:1199–1211. doi: 10.1046/j.1471-4159.2001.00128.x. [DOI] [PubMed] [Google Scholar]
- Rahbek UL, Dissing S, Thomassen C, Hansen AJ, Tritsaris K. Nerve growth factor activates aorta endothelial cells causing PI3K/Akt- and ERK-dependent migration. Pflugers Arch. 2005;450:355–361. doi: 10.1007/s00424-005-1436-0. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Swain WA, Richardson D, Edwards J, Stewart DJ, Richardson CM, Swinson DE, Patel D, Jones JL, O’Byrne KJ. Phospho-akt expression is associated with a favorable outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11:2930–2936. doi: 10.1158/1078-0432.CCR-04-1385. [DOI] [PubMed] [Google Scholar]
- Shibata M, Hisahara S, Hara H, Yamawaki T, Fukuuchi Y, Yuan J, Okano H, Miura M. Caspases determine the vulnerability of oligodendrocytes in the ischemic brain. J Clin Invest. 2000;106:643–653. doi: 10.1172/JCI10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- Syroid DE, Maycox PJ, Soilu-Hanninen M, Petratos S, Bucci T, Burrola P, Murray S, Cheema S, Lee KF, Lemke G, Kilpatrick TJ. Induction of postnatal schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy. J Neurosci. 2000;20:5741–5747. doi: 10.1523/JNEUROSCI.20-15-05741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- Tsui CC, Shankland SJ, Pierchala BA. Glial cell line-derived neurotrophic factor and its receptor ret is a novel ligand-receptor complex critical for survival response during podocyte injury. J Am Soc Nephrol. 2006;17:1543–1552. doi: 10.1681/ASN.2005080835. [DOI] [PubMed] [Google Scholar]
- Ulrich E, Duwel A, Kauffmann-Zeh A, Gilbert C, Lyon D, Rudkin B, Evan G, Martin-Zanca D. Specific TrkA survival signals interfere with different apoptotic pathways. Oncogene. 1998;16:825–832. doi: 10.1038/sj.onc.1201842. [DOI] [PubMed] [Google Scholar]
- Vaillant AR, Mazzoni I, Tudan C, Boudreau M, Kaplan DR, Miller FD. Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival. J Cell Biol. 1999;146:955–966. doi: 10.1083/jcb.146.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit C, Genze F, Menke A, Hoeffert S, Gress TM, Gierschik P, Giehl K. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64:5291–5300. doi: 10.1158/0008-5472.CAN-04-1112. [DOI] [PubMed] [Google Scholar]
- Verity AN, Bredesen D, Vonderscher C, Handley VW, Campagnoni AT. Expression of myelin protein genes and other myelin components in an oligodendrocytic cell line conditionally immortalized with a temperature-sensitive retrovirus. J Neurochem. 1993;60:577–587. doi: 10.1111/j.1471-4159.1993.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Villegas SN, Njaine B, Linden R, Carri NG. Glial-derived neurotrophic factor (GDNF) prevents ethanol (EtOH) induced B92 glial cell death by both PI3K/AKT and MEK/ERK signaling pathways. Brain Res Bull. 2006;71:116–126. doi: 10.1016/j.brainresbull.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wang HJ, Cao JP, Yu JK, Gao DS. Role of PI3-K/Akt pathway and its effect on glial cell line-derived neurotrophic factor in midbrain dopamine cells. Acta Pharmacol Sin. 2007;28:166–172. doi: 10.1111/j.1745-7254.2007.00494.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang L, Gang Zhang Z, Lan Zhang R, Chopp M. Activation of the PI3-K/Akt pathway mediates cGMP enhanced-neurogenesis in the adult progenitor cells derived from the subventricular zone. J Cereb Blood Flow Metab. 2005;25:1150–1158. doi: 10.1038/sj.jcbfm.9600112. [DOI] [PubMed] [Google Scholar]
- Wang X, Bauer JH, Li Y, Shao Z, Zetoune FS, Cattaneo E, Vincenz C. Characterization of a p75NTR apoptotic signaling pathway using a novel cellular model. J Biol Chem. 2001;276:33812–33820. doi: 10.1074/jbc.M010548200. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Tisi MA, Yeo TT, Longo FM. Nerve growth factor (NGF) loop 4 dimeric mimetics activate ERK and AKT and promote NGF-like neurotrophic effects. J Biol Chem. 2000;275:29868–29874. doi: 10.1074/jbc.M005071200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Tamatani M, Matsuzaki H, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J Biol Chem. 2001;276:5256–5264. doi: 10.1074/jbc.M008552200. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka A, Yamaya Y, Saiki S, Kanemoto M, Hirose G, Beesley J, Pleasure D. Non-N-methyl-D-aspartate glutamate receptors mediate oxygen-glucose deprivation-induced oligodendroglial injury. Brain Res. 2000;854:207–215. doi: 10.1016/s0006-8993(99)02359-8. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M, Chopp M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004;1030:19–27. doi: 10.1016/j.brainres.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Lu M, Cui Y, Chen J, Noffsinger L, Elias SB, Chopp M. Bone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis mice. J Neurosci Res. 2006;84:587–595. doi: 10.1002/jnr.20962. [DOI] [PubMed] [Google Scholar]


