Abstract
Treatment of rodents after stroke with bone marrow stromal cells (BMSCs) improves functional outcome. However, the mechanisms underlying this benefit have not been ascertained. This study focused on the contribution of neurotrophic and growth factors produced by BMSCs to therapeutic benefit. Rats were subjected to middle cerebral artery occlusion and the ischemic brain extract supernatant was collected to prepare the conditioned medium. The counterpart normal brain extract from non-ischemic rats was employed as the experimental control. Using microarray assay, we measured the changes of the neurotrophin associated gene expression profile in BMSCs cultured in different media. Furthermore, real-time RT-PCR and fluorescent immunocytochemistry were utilized to validate the gene changes. The morphology of BMSCs, cultured in the ischemic brain-conditioned medium for 12 h, was dramatically altered from a polygonal and flat appearance to a fibroblast-like long and thin cell appearance, compared to those in the normal brain-conditioned medium and the serum replacement medium. Forty-four neurotrophin-associated genes in BMSCs were identified by microarray assay under all three culture media. Twelve out of the 44 genes (7 neurotrophic and growth factor genes, 5 receptor genes) increased in BMSCs cultured in the ischemic brain-conditioned medium compared to the normal brain-conditioned medium. Real time RT-PCR and immunocytochemistry validated that the ischemic brain-conditioned medium significantly increased 6/7 neurotrophic and growth factor genes, compared with the normal brain-conditioned medium. These six genes consisted of fibroblast growth factor 2, insulin-like growth factor 1, vascular endothelial growth factor A, nerve growth factor beta, brain-derived neurotrophic factor and epidermal growth factor. Our results indicate that transplanted BMSCs may work as ‘small molecular factories’ by secreting neurotrophins, growth factors and other supportive substances after stroke, which may produce therapeutic benefits in the ischemic brain.
Keywords: bone marrow stromal cell, brain ischemia, cell transplantation, growth factors, neurotrophins
Introduction
By stimulating neuroprotection and neurorestoration after central nervous system (CNS) disorders, cell transplantation may become a therapeutic option for patients with CNS diseases such as cerebral ischemia.1 Among several kinds of cell-based therapies, bone marrow stromal cell (BMSC) is a strong therapeutic candidate. BMSCs enriched in vitro by self-renewal after isolation from adult bone marrow, virtually eliminate the ethical, immunological and logistical problems associated with embryonic or adult neural stem cell therapies. Furthermore, there is increasing evidence from our laboratory and others showing that BMSCs survive, selectively migrate to injured areas and provide therapeutic benefits in a variety of CNS diseases, such as cerebral ischemia,2–4 traumatic brain injury,5 spinal cord injury6,7 and demyelinating disorders.8,9 These studies suggest the possibility of transplantation therapy using BMSCs for patients with various CNS disorders. However, the mechanisms by which BMSCs provide therapeutic benefits remain unclear. BMSCs, including stem and progenitor cells, are multipotent and capable of differentiation into mesodermal derivatives such as bone, cartilage, fatty tissue and even neural cells such as neurons.10,11 Although the transdifferentiation theory is attractive, it is inconsistent with in vivo data.12 Rodents after middle cerebral artery occlusion (MCAo) obtain therapeutic benefit within days, and very few BMSCs express neural markers.13 Clearly, weeks or months are needed for BMSCs to transdifferentiate into the lost neural cells and appropriately integrate into complex neural connections.14,15 Alternatively, orthotopic BMSCs naturally secrete a variety of cytokines and growth factors, which mainly support hematopoietic stem cells to differentiate into mature blood cells.16 Interestingly, the pattern and quantity of such functional secretion of BMSCs could be changed in response to their existing microenvironment.17 BMSCs in ischemic conditions increase the synthesis of some cytokines and growth factors.18–20 To expand the spectrum of neurotrophic genes secreted by BMSCs after transplantation into ischemic brain, microarray assay was employed in this study to characterize the change of the neurotrophic and growth factor gene expression profile in BMSCs cultured in ischemic brain-conditioned medium, normal brain-conditioned medium and serum replacement medium as a basic control. Real time RT-PCR and immunocytochemistry assay were employed to validate the expression of neurotrophic and growth factor genes.
Methods
All experimental procedures were approved by Institutional Animal Care and Use Committee of Henry Ford Hospital.
The transient MCAo model was induced by intraluminal vascular occlusion, as described previously.21 Briefly, male Wistar rats weighing 270–300 g (n = 12) were anesthetized with halothane in 70% N2O and 30% O2. The origin of the right middle cerebral artery was blocked by advancing a 4–0 monofilament nylon suture from the external carotid artery into the lumen of the internal carotid artery. Reperfusion was performed by withdrawal of the suture 2 h after operation.
Ischemic brain tissue extracts were obtained 2–3 days after MCAo.19,22 A standard block, centered at the territory of the MCAo (bregma −1 ∼ +1 mm23) was obtained by dissection on ice from the ipsilateral hemisphere and the counterpart normal brain tissue were collected from non-ischemia rats. Subsequently, the tissue pieces from each animal were homogenized by adding Dulbecco's modified Eagle medium (DMEM) (150 mg/mL). After centrifugation for 10 min at 10 000 × g at 4°C, the supernatant from brain tissue homogenate was collected and stored at −80°C for future treatment of mouse BMSCs.
The primary cultured mouse bone marrow stromal cells were provided by Cognate Inc. (Baltimore, MD, US). 1 × 106 mouse BMSCs were seeded in 35 mm dishes (three dishes per group) and precultured at 37°C in 5% CO2 for 24 h, and then treated with serum replacement DMEM (Knockout DMEM with 20% Knockout Serum Replacement, Gibco, New York, NY; Invitrogen, Carlsbad, CA, US) as the basic control group, or in addition to 20% of the supernatant collected from the normal brain extract or the ischemic brain extract.
After culture for the desired time, BMSCs were gently rinsed with phosphate buffered saline (PBS). Cells were subsequently observed under a phase-contrast microscope (Nikon, Eclipse, TE2000-U) with 20× magnification.
After 48 h culture, total RNA of mouse BMSCs in each group was isolated with the ArrayGrade Total RNA Isolated Kit (SuperArray Bioscience, Frederick, MD, US). RNA concentration and purity were measured by UV Spectrophotometry (UV160U, Shimadzu Corp, Kyoto, Japan). Using the Truelabeling-AMP Kit (SuperArray Bioscience), antisense RNAs were synthesized and labeled with biotinylated-UTP. After purification, the biotin-labeled cRNA target was hybridized with customized mouse Oligo GEArray microarray membranes with 113 oligonucleotide probes representing neurotrophin associated genes (SuperArray Bioscience). Afterward, the microarray membranes were bound with alkaline phosphatase-conjugated streptavidin. The chemiluminescent signal was recorded using X-ray film (BioMax MR Film, Kodak) and a flatbed desktop scanner (Power-look 1120, UMax). The web-based GEArray Expression Analysis Suite software (SuperArray Bioscience) was utilized to perform the image analysis and data acquisition. To gather reliable data, careful quality control of experiments, data extraction and standardization approaches were maintained throughout the experimental process.
For real time RT-PCR, total RNA was extracted from BMSCs using the RNeasy Mini Kit according to the manufacturer's manual (Qiagen, Germantown, MD, US). cDNA was obtained from total RNA using oligo(dT), dNTP mix, First-Strand Buffer, DTT, RnaseOUT, and Superscipt III (Invitrogen). Quantitative RT-PCR was performed using SYBR Green RT-PCR system on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA, US) with a three stage amplification program provided by the manufacturer as follows: 2 min at 5°C, 10 min at 95°C and then 40 cycles of amplification reaction, 15 s at 95°C and 1 min at 60°C. The specificity of the amplicons was confirmed by the examination of the dissociation plot, a single peak indicated that a single DNA sequence was amplified during PCR. Each sample was tested in triplicate, and the samples obtained from four independent experiments were employed for the analysis of relative transcription data using the 2−ΔΔCT method.
Immunocytochemistry was performed after 72 h culture in all experimental groups, as previously described.24 The cultured cells were fixed with 4% paraformaldehyde for 20 min, and incubated in the blocking buffer containing 1% bovine serum albumin, 0.1% Triton X-100 at 37°C for 1 h. Antibodies against fibroblast growth factor 2 (FGF2) (1:200, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), transforming growth factor beta 1 (TGFβ1) (1:200, R & D Systems, Minneapolis, MN, US), insulin-like growth factor 1 (IGF1) (1:200, Santa Cruz Biotechnology, Inc.), vascular endothelial growth factor A (VEGFa) (1:500, Santa Cruz Biotechnology, Inc.), nerve growth factor, beta (NGFB) (1:1000, Santa Cruz Biotechnology, Inc.), Brain derived neurotrophic factor (BDNF) (1:500, Santa Cruz Biotechnology, Inc.) and epidermal growth factor (EGF) (1:500, Santa Cruz Biotechnology, Inc.) were used for immunofluorescent staining of the BMSCs in different culture media. BMSCs were then washed in PBS for 3 times and treated with Cy3 conjugated secondary antibody. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, US). Cells were examined under a fluorescent microscope (Nikon, eclipse, TE2000-U). Micrography was performed using a 20× objective and images were processed with imaging software (MetaMorph Imaging System, Universal Imaging Corp, Downingtown, PA, US).
Anova was used to evaluate differences between the experimental groups and the control in real time RT-PCR and immunocytochemistry assay. P < 0.05 was considered statistically significant.
Results
To mimic bone marrow stromal cell transplantation into the ischemic brain microenvironment, the BMSCs were treated with the brain supernatant conditioned medium. After 12 h culture in the serum replacement medium, the BMSCs showed a polygonal and flat appearance under the phase-contrast microscope (Fig. 1A). However, the morphology of most BMSCs in the ischemic brain-conditioned medium was altered to a fibroblast-like long and thin cell appearance with bipolar or multipolar processes (Fig. 1C). Only a small part of BMSCs in the normal brain-conditioned medium were protracted (Fig. 1B).
Fig. 1.
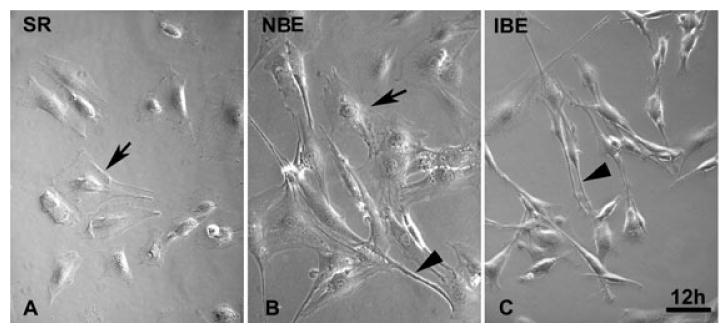
Microphotographs of the mouse bone marrow stromal cells (BMSCs) exposed to the serum replacement (SR) control medium (A), the normal brain extract (NBE) conditioned medium (B) and the ischemic brain extract (IBE) conditioned medium (C) for 12 h. Most BMSCs exhibited flat and polygonal appearances in SR control medium (A, arrow). The appearance of BMSCs changed to fibroblast-like cells with bipolar or multipolar ‘processes’ (C, arrowhead). A small part of BMSCs in NBE medium were protracted to from fibroblast-like cells (B, arrowhead). Scale bar = 100 μm.
Forty-four neurotrophic and growth factor genes were identified among 113 genes in BMSCs cultured in all three culture media (Fig. 2). Ratios of normalized signal intensities of the ischemic brain-conditioned medium group to both the normal brain-conditioned medium and the serum replacement control groups were evaluated for changes of gene expression in BMSCs. Using an arbitrary cut-off of 2.0-fold change, we found that, compared with the serum replacement medium, 14 genes (7 neurotrophic and growth factor genes and 7 receptor genes) and six genes (2 neurotrophic and growth factor genes and 4 receptor genes) increased in BMSCs in the ischemic brain-conditioned medium and the normal brain-conditioned medium, respectively (Table 1). Twelve genes (7 neurotrophic and growth factor genes and 5 receptor genes) increased in BMSCs subjected to the ischemic brain-conditioned medium, compared with the normal brain-conditioned medium (Table 1).
Fig. 2.
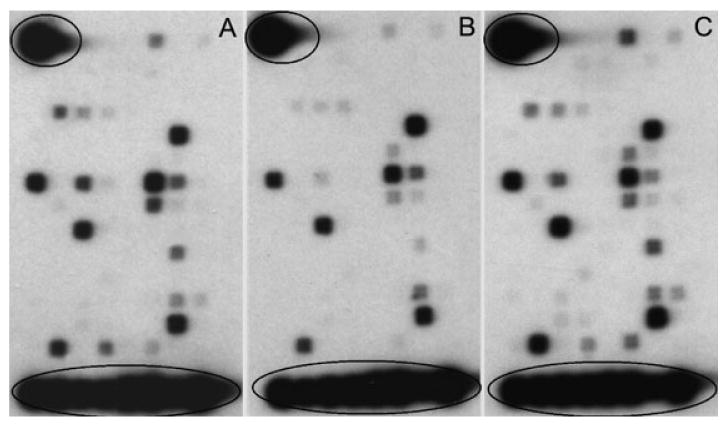
Image patterns of neurotrophic and growth factor gene changes in microarray membranes of bone marrow stromal cells (BMSCs) cultured in the serum replacement control medium (A) and the 20% supernatant of normal brain-conditioned medium (B) and the 20% supernatant of ischemic brain-conditioned medium (C) for 48 h. Both of the housekeeping genes and the biotinylated artificial sequence landmarkers are circled in the upper left corner and the lowest row. Other individual spots in the membranes represent specific single genes, with the density proportional to the gene expression level.
Table 1.
List of 44 neurotrophic and growth factor genes of mouse bone marrow stromal cells detected by microarray analysis and alteration of their expression after the normal brain extract (NBE) and ischemic brain extract (IBE) conditioned medium treatment compared with serum replacement control medium (SR)
| Genebank | Symbol | Gene title | SR | NBE | IBE |
|---|---|---|---|---|---|
| NM_198962 | Hcrtr2ठ| Hypocretin (orexin) receptor 2 | 0.17 | 0.26 | 0.77 |
| NM_008006 | Fgf2ठ| Fibroblast growth factor 2 | 0.10 | 0.09 | 0.34 |
| NM_010113 | Egfठ| epidermal growth factor | 0.44 | 0.25 | 1.46 |
| NM_007762 | Crhr1ठ| Corticotropin releasing hormone receptor 1 | 0.11 | 0.12 | 0.32 |
| NM_020014 | Gfra4†‡ | Glial cell line derived neurotrophic factor family receptor alpha 4 | 0.18 | 0.51 | 0.50 |
| NM_013609 | Ngfbठ| Nerve growth factor, beta | 0.49 | 0.23 | 1.34 |
| NM_009953 | Crhr2ठ| Corticotropin releasing hormone receptor 2 | 0.10 | 0.09 | 0.26 |
| NM_010280 | Gfra3†‡ | Glial cell line derived neurotrophic factor family receptor alpha 3 | 0.08 | 0.26 | 0.21 |
| NM_184052 | Igf1ठ| insulin-like growth factor 1 | 0.25 | 0.29 | 0.57 |
| NM_009505 | Vegfaठ| vascular endothelial growth factor A | 0.42 | 0.33 | 0.97 |
| NM_011577 | Tgfb1ठ| Transforming growth factor, beta 1 | 1.21 | 1.07 | 2.70 |
| NM_007540 | Bdnfठ| Brain derived neurotrophic factor | 1.00 | 1.03 | 2.18 |
| NM_009911 | Cxcr4ठ| Chemokine (C-X-C motif) receptor 4 | 0.21 | 0.18 | 0.46 |
| NM_016673 | Cntfrठ| Ciliary neurotrophic factor receptor | 0.65 | 0.63 | 1.32 |
| NM_010168 | F2† | Coagulation factor II | 0.26 | 0.52 | 0.46 |
| NM_008115 | Gfra2 | Glial cell line derived neurotrophic factor family receptor alpha 2 | 0.15 | 0.13 | 0.25 |
| NM_198959 | Hcrtr1† | Hypocretin (orexin) receptor 1 | 0.13 | 0.34 | 0.20 |
| NM_022024 | Gmfg† | Glia maturation factor, gamma | 0.46 | 1.32 | 0.70 |
| NM_007407 | Adcyap1r1 | Adenylate cyclase activating polypeptide 1 receptor 1 | 9.05 | 10.33 | 13.42 |
| NM_010279 | Gfra1 | Glial cell line derived neurotrophic factor family receptor alpha 1 | 0.18 | 0.30 | 0.25 |
| NM_205769 | Crh | Corticotropin releasing hormone | 0.52 | 0.49 | 0.66 |
| NM_008742 | Ntf3 | Neurotrophin 3 | 8.55 | 9.28 | 10.10 |
| NM_144939 | Frs3† | Fibroblast growth factor receptor substrate 3 | 1.36 | 2.94 | 1.54 |
| NM_010275 | Gdnf | Glial cell line derived neurotrophic factor | 0.40 | 0.43 | 0.44 |
| NM_009750 | Ngfrap1 | Nerve growth factor receptor associated protein 1 | 27.13 | 38.06 | 29.43 |
| NM_177798 | Frs2 | Fibroblast growth factor receptor substrate 2 | 3.57 | 2.38 | 3.56 |
| NM_019678 | Tfg | Trk-fused gene | 31.08 | 43.71 | 30.08 |
| NM_011611 | Tnfrsf5 | Tumor necrosis factor receptor superfamily, member 5 | 1.53 | 1.26 | 1.41 |
| NM_022023 | Gmfb | Glia maturation factor, beta | 29.27 | 57.01 | 26.67 |
| NM_033217 | Ngfr | Nerve growth factor receptor | 0.75 | 0.32 | 0.58 |
| NM_178591 | Nrg1 | Neuregulin 1 | 0.54 | 0.39 | 0.40 |
| NM_008954 | Pspn | Persephin | 1.07 | 0.46 | 0.73 |
| XM_283871 | Ntrk1 | Neurotrophic tyrosine kinase, receptor, type 1 | 0.62 | 0.34 | 0.42 |
| NM_032002 | Nrg4 | Neuregulin 4 | 0.50 | 0.20 | 0.32 |
| NM_010936 | Nr1i2 | Nuclear receptor subfamily 1, group I, member 2 | 0.58 | 0.35 | 0.37 |
| NM_008745 | Ntrk2 | Neurotrophic tyrosine kinase, receptor, type 2 | 0.64 | 0.36 | 0.39 |
| BF471031 | Nrg2 | Strain CD−1 neuregulin 2-beta | 0.70 | 0.48 | 0.43 |
| NM_013603 | Mt3 | Metallothionein 3 | 0.52 | 0.37 | 0.31 |
| NM_001002927 | Penk1 | Preproenkephalin 1 | 0.49 | 0.55 | 0.30 |
| NM_198190 | Ntf5 | Neurotrophin 5 | 0.68 | 0.66 | 0.40 |
| NM_009312 | Tac2 | Tachykinin 2 | 1.04 | 0.71 | 0.58 |
| NM_009311 | Tac1 | Tachykinin 1 | 0.62 | 0.55 | 0.35 |
| NM_008964 | Ptger2 | Prostaglandin E receptor 2 (subtype EP2) | 0.83 | 0.52 | 0.44 |
NBE versus SR;
IBE versus SR;
IBE versus NBE (an arbitrary cut-off >2.0-fold change).
In order to validate the microarray results, real time RT-PCR with specific primers (Table 2) was used to evaluate the expression profile of the seven neurotrophic and growth factors. The results showed that compared with the serum replacement medium control, the mRNA levels of seven genes from BMSCs were significantly up-regulated after 48 h of exposure to the ischemic brain-conditioned medium and four genes were significantly increased in BMSCs cultured in the normal brain-conditioned medium (n = 4, P < 0.05, Fig. 3). Six out of the seven genes, except tgfb1, increased in the BMSCs subjected to the ischemic brain-conditioned medium, compared with the normal brain-conditioned medium. The gene expression of tgfb1 in BMSCs significantly increased in both the ischemic and normal brain-conditioned media compared with the serum replacement medium.
Table 2.
Sequences of primers for RT-PCR
| Gene title | Symbol | Genebank | Size | Primer sequence (from 5′- to 3′) | |
|---|---|---|---|---|---|
| Fibroblast growth factor 2 | Fgf2 | NM_008006 | 71 | Forward | CCCACCAGGCCACTTCAA |
| Reverse | GATGGATGCGCAGGAAGAA | ||||
| Epidermal growth factor | Egf | NM_010113 | 130 | Forward | ACGGTTTGCCTCTTTTCCTT |
| Reverse | GTTCCAAGCGTTCCTGAGAG | ||||
| Transforming growth factor, beta 1 | Tgfb1 | NM_011577 | 100 | Forward | GGACTCTCCACCTGCAAGAC |
| Reverse | GACTGGCGAGCCTTAGTTTG | ||||
| Insulin-like growth factor 1 | Igf1 | NM_184052 | 130 | Forward | CCTCTGGGATACGGCACTTA |
| Reverse | GAAGGTCTTGGTGGCATGTT | ||||
| Vascular endothelial growth factor A | Vegfa | NM_009505 | 136 | Forward | GAAAATCACTGTGAGCCTTGTTC |
| Reverse | TGCAAGTACGTTCGTTTAACTCA | ||||
| Nerve growth factor, beta | Ngfb | NM_013609 | 149 | Forward | CAAGGACGCAGCTTTCTATACTG |
| Reverse | CTTCAGGGACAGAGTCTCCTTCT | ||||
| Brain derived neurotrophic factor | Bdnf | NM_007540 | 137 | Forward | TACTTCGGTTGCATGAAGGCG |
| Reverse | GTCAGACCTCTCGAACCTGCC | ||||
Fig. 3.
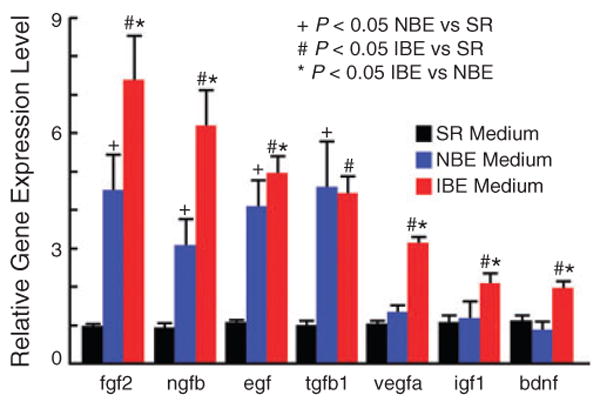
Changes of mRNA levels for seven neurotrophic and growth factors in mouse bone marrow stromal cells (BMSCs) after 48 h culture by real-time RT-PCR. The mRNA levels of seven and four genes from BMSCs were significantly up-regulated after 48 h of exposure to the ischemic brain-conditioned medium (IBE) and the normal brain-conditioned medium (NBE), respectively, compared with the serum replacement (SR) control medium (n = 4, P < 0.05). Compared with the NBE medium group, 6/7 genes, except tgfβ1, increased in the BMSCs subjected to the ischemic brain-conditioned medium.
The gene expression changes at the protein level of the seven genes in BMSCs were further evaluated by fluorescent immunocytochemistry. Compared with the serum replacement medium, seven and one genes increased in BMSCs cultured in the ischemic brain-conditioned medium and the normal brain-conditioned medium, respectively. Except TGFB1, 6/7 neurotrophic factors showed significantly enhanced immunostaining in BMSCs in the ischemic brain-conditioned medium compared with the normal brain-conditioned medium (P < 0.05, Fig. 4).
Fig. 4.
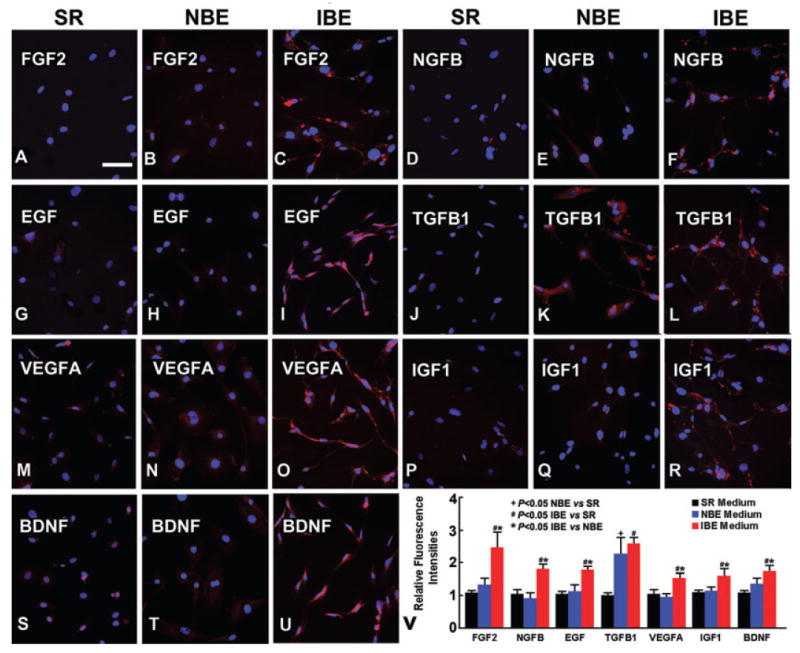
Fluorescent immunostaining showed that at the protein level in bone marrow stromal cells (BMSCs), the seven neurotrophic and growth factors significantly increased when cultured with the ischemic brain extract (IBE) conditioned medium for 72 h (C, F, I, L, O, R, U) compared with the serum replacement (SR) control medium (A, D, G, J, M, P, S). And 6/7 proteins in the BMSCs significantly increased when cultured with the IBE medium, compared with the normal brain extract (NBE) conditioned medium (B, E, H, K, N, Q, T). A, B, C for FGF2; D, E, F for NGFB; G, H, I for EGF; J, K, L for TGFB1; M, N, O for VEGFA; P, Q, R for IGF1 and S, T, U for BDNF. Scale bar = 100 μm (from A to U).
Discussion
We are the first to use the mouse oligo-microarray assay to investigate the effect of ischemic brain tissue extract on cultured mouse BMSCs. The present study demonstrates that 44 neurotrophin-associated genes (Table 2) among 113 genes in the microarray membrane are identified by microarray assay from BMSCs cultured in ischemic brain-conditioned medium, normal brain-conditioned medium, and serum replacement medium. Using an arbitrary cut-off 2.0-fold change, several genes, consisting of neurotrophic and growth factor genes and receptor genes are up-regulated when BMSCs are cultured in the ischemic brain-conditioned medium compared with the normal brain-conditioned medium and the serum replacement medium. Real-time RT-PCR and immunocytochemistry confirmed that the ischemic brain-conditioned medium significantly increased gene production of seven neurotrophic and growth factors compared with the serum replacement medium. These seven genes consist of fibroblast growth factor 2 (fgf2), transforming growth factor beta 1 (tgfβ1), insulin-like growth factor 1 (igf1), vascular endothelial growth factor A (vegfa), nerve growth factor beta (ngfβ), brain derived neurotrophic factor (bdnf), and epidermal growth factor (egf). Six out of the seven genes are significantly increased in the BMSCs cultured with the ischemic brain-conditioned medium compared with the normal brain-conditioned medium, except tgfβ1, which increases in BMSCs cultured in both the ischemic and normal brain-conditioned media, compared with the serum replacement medium. These findings suggest that the gene expression pattern of neurotrophic and growth factors in BMSCs is altered in response to the surrounding brain ischemic microenvironment, and this may contribute to the therapeutic benefit of MSC transplantation after stroke.
To test the morphological changes of BMSCs, the ischemic brain-conditioned medium is used to mimic the complicated ischemic brain microenvironment, as previously reported.19,22 After 12 h culture in the ischemic brain-conditioned medium, the morphology of most BMSCs is dramatically altered from a polygonal and flat appearance to a fibroblast-like long and thin cell appearance with bipolar or multipolar ‘processes’ (Fig. 1). The similar morphological change is only found in a small part of BMSCs in the normal brain-conditioned medium. These morphological changes of BMSCs indicate that BMSCs respond to the ambient environment, reprogram the cellular organization and modulate gene and protein expression. More work is required to clarify the mechanisms underlying the morphological changes, which may be related to a restructuring of the cytoskeleton of BMSCs cultured in the ischemic brain-conditioned medium.
Neurotrophic and growth factors have been shown to support the survival and differentiation of many neural populations of the CNS during fetal development25 or repair after cerebral injury in the adult.8 FGF2 has potent survival-promoting and protective effects against neural loss,26 and FGF2 also amplifies neurogenesis in the adult brain after injury.27 Cerebral ischemia induced in FGF2−/− mice results in enlarged infarct volume and loss of BDNF mRNA induction compared to wild type.28 NGF promotes the survival and differentiation of sensory and sympathetic neurons both in the development and the repair period after stroke.25 NGF/Trk A is neuroprotective following various forms of brain injury.8,29 EGF is a polypeptide involved in directed cell migration30 and connexin 43 gap junction communications.31 Following cerebral ischemia, TGFβ1 is strongly up-regulated in the CNS.32 TGFβ1 shows neuroprotective activity against ischemia-induced neuronal death through suppressing the expression and phosphorylation of proapoptosis factors such as Bad, and activating the MAPK/Erk signal pathway.32 TGFβ1 also regulates the fate of multipotential neural or mesenchymal stem cells by modulating the expression or function of tissue-specific transcription factors, as well as selectively regulating the expression of required growth factors and their receptors.33 Interestingly, we found that the gene tgfβ1 significantly increased in BMSCs in both the ischemic and normal brain-conditioned media, compared with the serum replacement medium. The increased expression of tgfβ1 in BMSCs may be caused by the changed culture environment, independent of ischemia. VEGF is an angiogenesis protein with therapeutic potential in stroke.34 Endogenous VEGF increases in the postischemic brain and represents a natural neuroprotective mechanism improving survival after stroke.35 Exogenous VEGF, administered pharmacologically or genetically, reduces infarct size and apoptosis, improves neurological performance and enhances the survival of newborn neurons35 and after stroke in the adult.34 IGF1 is a neurotrophic polypeptide that improves somatosensory function by reducing the size of cortical infarction and protecting neurons from ongoing progressive death after ischemia,36 and increases myelination and glial numbers in white matter after ischemia in near-term fetal sheep by suppressing apoptosis and boosting proliferation of the oligodendrocytes.37 BDNF, an abundantly expressed neurotrophin in the mature CNS, is involved in long-term survival of newborn neurons,38 biochemical differentiation and modulation of synaptic plasticity.39
In this study, we found that compared with the normal brain-conditioned medium, 6/7 neurotrophic factors were significantly up-regulated in BMSCs placed in the ischemic brain-conditioned medium. We have previously reported that BMSCs increased the production of BDNF, NGF, VEGF and HGF at the protein level when cultured in the supernatant from ischemic or traumatic brain tissue extract.19,22 The increased level of neurotrophic and growth factors directly from transplanted BMSCs could enhance the repair of injured cells around the ischemic brain tissue by increasing their viability and proliferation, or by decreasing apoptosis of the injured neural cells. BMSC production of neurotrophic and growth factors may be a major mechanisms underlying the BMSC-based therapeutic benefits.
Co-culture of BMSCs with ischemic astrocytes significantly enhances the gene expression of bdnf, vegfa and fgf2 from the injured astrocytes and dramatically protects these injured astrocytes from apoptotic cell death and increases cell survival and proliferation.24 Co-culture of BMSCs with astrocytes pretreated in oxygen and glucose-deprived medium significantly increased bone morphogenetic protein (BMP) 2/4 expression of ischemic astrocytes, which subsequently promoted subventricular zone neural stem/progenitor cells to differentiate into a glial fibrillary acidic protein (GFAP) positive linage.40 The above studies also showed that BMSCs could indirectly enhance the injured astrocytes to increase the secretion of bioactive factors, neurotrophic and growth factors, which may also contribute to BMSC-based therapeutic benefits.
All these findings suggest that BMSCs may work as ‘small molecular factories’ by secreting cytokines, neurotrophins, growth factors, and other supportive substances at least acutely after stroke which activates the restorative properties on endogenous brain parenchymal cells. Altered neurotrophic and growth factor gene expression may be the initial spark for therapeutic progress, which may produce therapeutic benefits in the ischemic brain.
Acknowledgments
This work was supported by NINDS grants PO1 NS23393 and RO1 NS45041.
References
- 1.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 2.Zhao LR, Duan WM, Reyes M, et al. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- 3.Shen LH, Li Y, Chen J, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 5.Mahmood A, Lu D, Qu C, et al. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J Neurosurg. 2006;104:272–277. doi: 10.3171/jns.2006.104.2.272. [DOI] [PubMed] [Google Scholar]
- 6.Chopp M, Zhang XH, Li Y, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 7.Himes BT, Neuhuber B, Coleman C, et al. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20:278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Li Y, Chen J, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002;22:6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 11.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 12.Castro RF, Jackson KA, Goodell MA, et al. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Chen J, Chopp M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant. 2001;10:31–40. [PubMed] [Google Scholar]
- 14.Brazelton TR, Rossi FM, Keshet GI, et al. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 15.Mezey E, Chandross KJ, Harta G, et al. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 16.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Li Y, Wang L, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 20.Tokumine J, Kakinohana O, Cizkova D, et al. Changes in spinal GDNF, BDNF, and NT-3 expression after transient spinal cord ischemia in the rat. J Neurosci Res. 2003;74:552–561. doi: 10.1002/jnr.10760. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Katakowski M, Li Y, et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res. 2002;69:687–691. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd. Sydney and Orlando, FL: Academic Press; 1986. [Google Scholar]
- 24.Gao Q, Li Y, Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005;136:123–134. doi: 10.1016/j.neuroscience.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 25.Semkova I, Krieglstein J. Neuroprotection mediated via neurotrophic factors and induction of neurotrophic factors. Brain Res Brain Res Rev. 1999;30:176–188. doi: 10.1016/s0165-0173(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Sanchez MT, Novelli A. Basic fibroblast growth factor protects cerebellar neurons in primary culture from NMDA and non-NMDA receptor mediated neurotoxicity. FEBS Lett. 1993;335:124–131. doi: 10.1016/0014-5793(93)80453-2. [DOI] [PubMed] [Google Scholar]
- 27.Alzheimer C, Werner S. Fibroblast growth factors and neuroprotection. Adv Exp Med Biol. 2002;513:335–351. doi: 10.1007/978-1-4615-0123-7_12. [DOI] [PubMed] [Google Scholar]
- 28.Kiprianova I, Schindowski K, von Bohlen und Halbach O, et al. Enlarged infarct volume and loss of BDNF mRNA induction following brain ischemia in mice lacking FGF-2. Exp Neurol. 2004;189:252–260. doi: 10.1016/j.expneurol.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 30.Le Stunff H, Mikami A, Giussani P, et al. Role of sphingosine-1-phosphate phosphatase 1 in epidermal growth factor-induced chemotaxis. J Biol Chem. 2004;279:34290–34297. doi: 10.1074/jbc.M404907200. [DOI] [PubMed] [Google Scholar]
- 31.Cameron SJ, Malik S, Akaike M, et al. Regulation of epidermal growth factor-induced connexin 43 gap junction communication by big mitogen-activated protein kinase1/ERK5 but not ERK1/2 kinase activation. J Biol Chem. 2003;278:18682–18688. doi: 10.1074/jbc.M213283200. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Yang GY, Ahlemeyer B, et al. Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage. J Neurosci. 2002;22:3898–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moses HL, Serra R. Regulation of differentiation by TGF-beta. Curr Opin Genet Dev. 1996;6:581–586. doi: 10.1016/s0959-437x(96)80087-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan J, Miller OT, Waugh KM, et al. Insulin-like growth factor-1 improves somatosensory function and reduces the extent of cortical infarction and ongoing neuronal loss after hypoxia-ischemia in rats. Neuroscience. 2001;105:299–306. doi: 10.1016/s0306-4522(01)00145-2. [DOI] [PubMed] [Google Scholar]
- 37.Cao Y, Gunn AJ, Bennet L, et al. Insulin-like growth factor (IGF) -1 suppresses oligodendrocyte caspase-3 activation and increases glial proliferation after ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2003;23:739–747. doi: 10.1097/01.WCB.0000067720.12805.6F. [DOI] [PubMed] [Google Scholar]
- 38.Sairanen M, Lucas G, Ernfors P, et al. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tolwani RJ, Buckmaster PS, Varma S, et al. BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience. 2002;114:795–805. doi: 10.1016/s0306-4522(02)00301-9. [DOI] [PubMed] [Google Scholar]
- 40.Xin H, Li Y, Chen X, et al. Bone marrow stromal cells induce BMP2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res. 2006;83:1485–1493. doi: 10.1002/jnr.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]


