Abstract
Nod2 is an intracellular pattern recognition receptor that detects a conserved moiety of bacterial peptidoglycan and subsequently activates proinflammatory signaling pathways. Mutations in Nod2 have been implicated to be linked to inflammatory granulomatous disorders, such as Crohn's disease and Blau syndrome. Many phytochemicals possess anti-inflammatory properties. However, it is not known whether any of these phytochemicals might modulate Nod2-mediated immune responses and thus might be of therapeutic value for the intervention of these inflammatory diseases. In this report, we demonstrate that curcumin, a polyphenol found in the plant Curcuma longa, and parthenolide, a sesquiterpene lactone, suppress both ligand-induced and lauric acid-induced Nod2 signaling, leading to the suppression of nuclear factor-κB activation and target gene interleukin-8 expression. We provide molecular and biochemical evidence that the suppression is mediated through the inhibition of Nod2 oligomerization and subsequent inhibition of downstream signaling. These results demonstrate for the first time that curcumin and parthenolide can directly inhibit Nod2-mediated signaling pathways at the receptor level and suggest that Nod2-mediated inflammatory responses can be modulated by these phytochemicals. It remains to be determined whether these phytochemicals possess protective or therapeutic efficacy against Nod2-mediated inflammatory disorders.
Innate immunity provides the first line of defense against invading pathogens and is initiated by receptors that recognize pathogen-associated molecular patterns. Toll-like receptors (TLRs) are membrane-anchored pattern-recognition receptors (PRRs) that detect a variety of extracellular pathogen-associated molecular patterns and initiate innate immunity for host defense against pathogens (Takeda et al., 2003; Akira and Takeda, 2004). Another family of PRRs called nucleotide-binding oligomerization domain (NOD)-containing proteins recognize intracellular bacterial products and activates innate immune responses in the cytosol (Fritz et al., 2006; Meylan et al., 2006). Mammals have two closely related NOD family members, Nod1 and Nod2 (Bertin et al., 1999; Inohara et al., 1999; Ogura et al., 2001b). These NOD proteins consist of three distinct domains: a C-terminal LRR for autorepression and ligand sensing; a centrally located NACHT domain for self-oligomerization and the activation of the receptors, and an N-terminal effector domain for protein-protein interaction to initiate downstream signaling. Activation of Nod1 and Nod2 initiates proinflammatory signaling via NF-κB activation, necessary for clearance of infecting pathogens from the host (Carneiro et al., 2004; Inohara et al., 2005). These intracellular surveillance proteins recognize distinct motifs of bacterial peptidoglycan. Nod1 recognizes the peptide γ-d-glutamyl-meso-diaminopimelic acid (Chamaillard et al., 2003; Girardin et al., 2003a) and mainly acts as a sensor for Gram-negative bacteria. In contrast, Nod2 recognizes muramyl dipeptide MurNAc-l-Ala-d-isoGln (MDP) (Girardin et al., 2003b; Inohara et al., 2003; Tanabe et al., 2004), which is the minimal motif in all peptidoglycans, and therefore acts as a general sensor for both Gram-positive and Gram-negative bacteria.
The significance of Nod1 and Nod2 in immune responses is evident from the linkage of their mutations with inflammatory diseases in humans and the increased susceptibility of Nod1−/− and Nod2−/− mice to gastrointestinal bacterial infections. Mutations in Nod2 have been linked to susceptibility to inflammatory granulomatous disorders, such as Crohn's disease (Hugot et al., 2001; Ogura et al., 2001a) and Blau syndrome (Miceli-Richard et al., 2001). Nod2 mutations associated with Crohn's disease lead to defective NF-κB activation after stimulation with bacterial ligands (Girardin et al., 2003b; Inohara et al., 2003). Loss of Nod2 functions in Nod2 knockout mice abolishes the protective immunity mediated by Nod2 recognition of MDP (Kobayashi et al., 2005). In addition, Nod2 plays an important role in the activation of the adaptive immune system by acting as an adjuvant receptor for antibody production (Kobayashi et al., 2005). Nod2 mutations have also been associated with early-onset sarcoidosis, a rare but sporadic disease (Kanazawa et al., 2004, 2005). Therefore, Nod2 plays a critical role in protecting the host from bacterial infection, and loss of Nod2 functions leads to misfunctioning of the immune system and subsequently diseases.
It is now well recognized that chronic inflammation is one of the key etiological conditions for the development and progression of many chronic diseases, including cancer, atherosclerosis, and insulin resistance. Many phytochemicals, including polyphenols and sesquiterpene lactones, are known to possess anti-inflammatory properties. Curcumin, a yellow pigment present in the rhizome of turmeric (Curcuma longa L.) and related species, is one of the most extensively investigated phytochemicals, with regard to chemopreventive potential. Numerous studies have shown that curcumin is a potent anti-inflammatory agent (Aggarwal et al., 2007). Curcumin inhibits NF-κB activation induced by lipopolysaccharide, phorbol 12-myristate 13-acetate, TNF-α, and hydrogen peroxide through the inhibition of IKKβ that phosphorylates IκBα, leading to ubiquitinylation and degradation (Singh and Aggarwal, 1995; Pan et al., 2000). Curcumin inhibits TNF-α-induced cyclooxygenase-2 expression and NF-κB activation in human colonic epithelial cells (Plummer et al., 1999). Curcumin also suppresses TNF-α-induced nuclear translocation and DNA binding of NF-κB in a human myeloid leukemia cell line by blocking phosphorylation and subsequent degradation of IκB (Aggarwal et al., 2006). More recently, curcumin has been shown to inhibit TLR4 signaling by inhibiting TLR4 homodimerization (Youn et al., 2006).
We recently demonstrated that Nod2 signaling is differentially modulated by the types of fatty acids (Zhao et al., 2007). In this report, we investigated whether anti-inflammatory phytochemicals might inhibit Nod2 signaling and downstream proinflammatory gene expression. We found that curcumin and parthenolide both inhibited Nod2 signaling induced by its bacterial ligand MDP or saturated fatty acid lauric acid. The inhibition was mediated through the inhibition of Nod2 oligomerization, leading to suppression of NF-κB activation and proinflammatory gene expression. These results demonstrated that Nod signaling pathways are amenable to be modulated by dietary phytochemicals.
Materials and Methods
Reagents
Curcumin, parthenolide, helenalin, and epigallocatechin gallate (EGCG) were purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). Resveratrol and monoclonal antibodies against Flag, HA and β-actin were purchased from Sigma-Aldrich (St. Louis, MO). MDP was from Bachem Bioscience (King of Prussia, PA). Anti-HA affinity matrix was from Roche Applied Science (Indianapolis, IN). Polyclonal IκBα antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyclonal antibodies against NF-κB p65 and phospho-NF-κB p65 and monoclonal antibody against phospho-IκBα were purchased from Cell Signaling Technology Inc. (Danvers, MA).
Cell Culture
Human colonic epithelial cell line HCT116, purchased from American Type Culture Collection (Manassas, VA), was cultured in McCoy's 5A medium containing 10% (v/v) heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). HEK293T (a human embryonic kidney epithelial cell line provided by Sam Lee, Beth Israel Hospital, Boston, MA) was cultured in Dulbecco's modified Eagle's medium containing 10% (v/v) heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Plasmids
pcDNA3-HA-Nod2, pcDNA3-Flag-Nod2, and pcDNA3-Myc-RICK were described previously (Inohara et al., 2000, 2001; Ogura et al., 2001b). 2×NF-κB-luciferase reporter construct was provided by Frank Mercurio (Signal Pharmaceuticals, San Diego, CA). Human interleukin (IL)-8(−173bp)-Luc was from Marie Annick Buendia (Institut Pasteur, Paris, France). pRSV-β-galatosidase plasmid was from Jongdae Lee (University of California, San Diego, CA). Plasmid DNA from these expression vectors was prepared in large scale for transfection using the EndoFree Plasmid Maxi kit (QIAGEN, Valencia, CA).
Transfections and Luciferase Assays
Transient transfections were carried out using SuperFect transfection reagent (QIAGEN) according to the manufacturer's recommendations. HCT116 cells were seeded 5 to 10 × 104 cells/well in 24-well plates and cotransfected the following day with 0.2 μg of 2×NF-κB-Luc or 0.04 μg of IL-8(−173bp)-Luc and 0.1 μg of pRSV-β-galactosidase plasmid. For Nod2 overexpression experiments, 5 to 10 × 104 HEK293T cells were seeded. Four nanograms of pcDNA3-Nod2-HA was cotransfected with 40 ng of 2×NF-κB-Luc or 8 ng of IL-8(−173bp)-Luc and 20 ng of β-galactosidase control plasmid. For RICK overexpression experiments, 5 to 10 × 104 HEK293T cells were cotransfected with 20 ng of pcDNA3-Myc-RICK, 40 ng of 2×NF-κB-Luc or 8 ng of IL-8(−173bp)-Luc, and 20 ng of β-galactosidase control plasmid. The total amount of plasmids was equalized by supplementation with the corresponding empty vector to minimize differences in transfection efficiency among samples. The cells were pretreated with phytochemicals for 1 h and then coincubated with MDP or lauric acid for 6 h before lysis. Luciferase and β-galactosidase enzyme activities were determined using a luciferase assay system and β-galactosidase enzyme assay system (Promega, Madison, WI) according to the manufacturer's instructions. Luciferase activity was normalized by β-galactosidase activity to correct differences in transfection efficiency among samples. Each of the experiments was done at least three times.
Immunoblotting and Coimmunoprecipitation
These experiments were performed essentially as described previously (Zhao et al., 2007). We seeded 5 × 106 HCT116 cells per 100-mm dish. The following day, the cells were pretreated with phytochemicals for 1 h and then coincubated with MDP for the times indicated in the figure legends. The cells were rinsed with ice-cold phosphate-buffered saline twice and then lysed by sonication in cell lysis buffer (Cell Signaling Technology Inc.) containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin. For the NF-κB p65 translocation experiments, the nuclear and cytosolic fractions of proteins were prepared using a nuclear extract kit (Active Motif Inc., Carlsbad, CA) according to the supplier's protocol. The lysis supernatants were collected by centrifugation and subjected to 7.5 or 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). The membrane was blocked in 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 0.05% (v/v) Tween 20 containing 5% nonfat milk. The membrane was probed with primary antibody for 1 h at room temperature or at 4°C overnight followed by incubation with horseradish peroxidase-conjugated secondary antibody (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) for 1 h at room temperature. The proteins were detected by the enhanced chemiluminescence Western blot detection reagents (GE Healthcare) followed by exposure to an X-ray film (Carestream Health, Rochester, NY). For Nod2 oligomerization experiments, HEK293T cells were seeded at 5 × 106/100-mm dish. Twenty-four hours later, the cells were transfected with 3 μg of pcDNA3-HA-Nod2 and 3 μg of pcDNA3-Flag-Nod2. Twenty-four hours after transfection, the cells were rinsed twice with ice-cold phosphate-buffered saline and lysed in buffer containing 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, and a Halt protease inhibitor cocktail (Pierce Chemical, Rockford, IL) by passing the cells through a 18G1 needle 20 times. The lysate supernatants were collected by centrifugation. HA-Nod2 and Flag-Nod2 proteins in the supernatants were coimmunoprecipitated with anti-HA affinity matrix and detected by Western blotting with anti-Flag and anti-HA antibodies using the enhanced chemiluminescence Western Detection System as described above.
Measurement of IL-8 Protein
The supernatants of HCT116 cells that were transiently transfected for reporter gene assays were collected, and the levels of IL-8 protein were determined using enzyme-linked immunosorbent assay (ELISA) kits (OptEIA ELISA kits; BD Biosciences Pharmingen, San Diego, CA) according to the supplier's instructions.
Data Analysis
Data from the reporter and ELISA assays were analyzed by a 2-tailed Student's t test.
Results
Curcumin Inhibited Nod2 Ligand-Induced NF-κB Activation and Target Gene IL-8 Expression
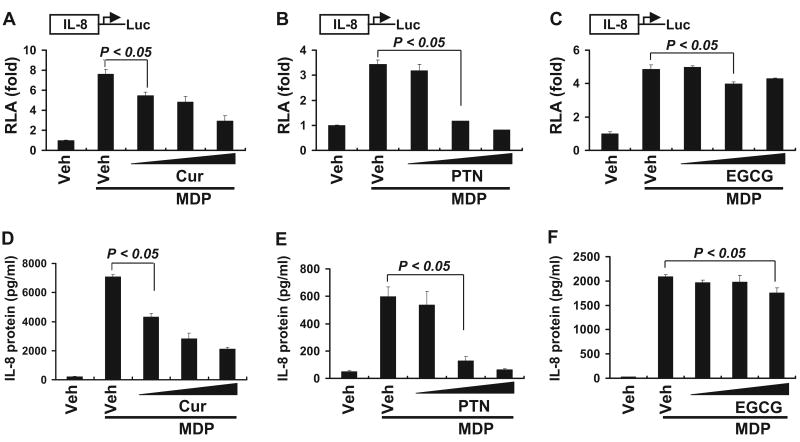
Nod2 signaling leads to the activation of the transcription factor NF-κB, which in turn induces the expression of proinflammatory genes (Strober et al., 2006). Many phytochemicals possess anti-inflammatory properties. We were interested in knowing whether any of these phytochemicals might inhibit Nod2 signaling, thereby suppressing the inflammatory responses. We chose a panel of phytochemicals consisting of curcumin, parthenolide, helenalin, EGCG, and resveratrol. Curcumin has been well documented to exhibit potent anti-inflammatory, antiviral, and antibacterial activities (Aggarwal et al., 2007). To test whether curcumin down-regulated Nod2 signaling, we looked at the effects of curcumin on Nod2 ligand-induced NF-κB activation and target gene IL-8 expression using NF-κB and IL-8 luciferase reporter genes as readouts. As shown in Fig. 1, curcumin inhibited MDP-induced NF-κB activation in a dose-dependent manner. Similar inhibitory effects on MDP-induced NF-κB activation were also observed for two other phytochemicals, parthenolide and helenalin. In contrast, EGCG (a polyphenol rich in green tea) and resveratrol (a polyphenol rich in grape) exhibited no inhibitory effects on MDP-induced NF-κB activation. In fact, resveratrol enhanced MDP-induced NF-κB activation. Consistent with NF-κB reporter assays, curcumin and parthenolide inhibited the transactivation of IL-8 expression. This inhibition was detected by both the reporter assays for transcription activation and ELISA assays for the IL-8 protein secreted from the cells (Fig. 2, A, B, D, and E). In contrast, EGCG showed little inhibition on IL-8 reporter transactivation and IL-8 protein expression (Fig. 2, C and F). These results demonstrate that curcumin and parthenolide negatively modulate MDP-induced NOD2 signaling, leading to the down-regulation of NF-κB activation and the suppression of proinflammatory gene expression.
Fig. 1.
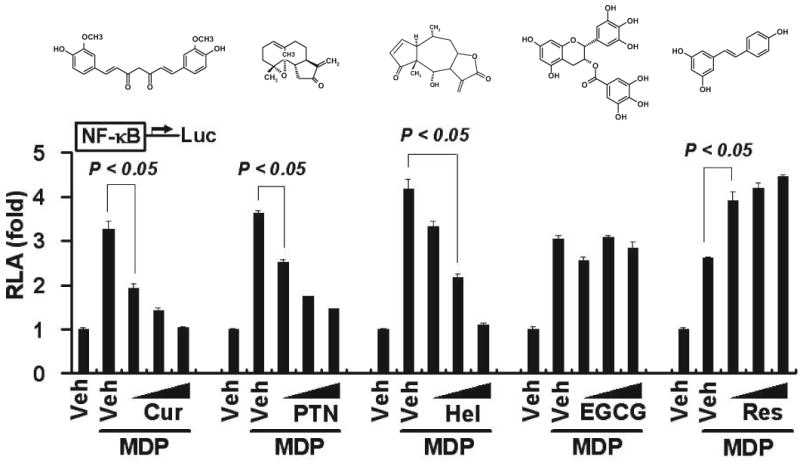
Curcumin inhibits MDP-induced NF-κB activation. HCT116 cells were transfected with NF-κB-luciferase and β-galactosidase reporters. After 24 h, the cells were pretreated with curcumin (10, 20, and 30 μM), parthenolide (5, 10, and 15 μM), helenalin (1, 3, and 5 μM), EGCG (10, 30, and 50 μM), or resveratrol (10, 30, and 50 μM) for 1 h and then coincubated with 50 μM MDP for additional 6 h. Cell lysates were prepared and luciferase and β-galactosidase enzyme activities measured as described under Materials and Methods. Relative luciferase activity (RLA) was normalized with β-galactosidase activity. Values are mean ± S.E.M (n = 3). Statistical significant difference (p < 0.05) is indicated between cells treated with MDP alone and cells treated with MDP plus the indicated phytochemicals. Cur, curcumin; PTN, parthenolide; Hel, helenalin; and Res, resveratrol. The molecular structure is shown above the reporter assay data for each of the phytochemicals.
Fig. 2.
Curcumin inhibits MDP-induced transactivation of IL-8. HCT116 cells were cotransfected with IL-8-luciferase and β-galactosidase reporter constructs. After 24 h, the cells were pretreated with curcumin (10, 20, and 30 μM) (A and D), parthenolide (5, 10, and 15 μM) (B and E), or EGCG (10, 30, and 50 μM) (C and F) for 1 h and then coincubated with 50 μM MDP for additional 6 h. Cell lysates (A–C) were prepared and luciferase and β-galactosidase enzyme activities were measured as described under Materials and Methods. Medium supernatants (D–F) were collected from IL-8-luciferase-transfected cells, and the levels of IL-8 protein were measured as described under Materials and Methods. RLA was normalized with β-galactosidase activity. Values are mean ± S.E.M (n = 3). Statistical significant difference (p < 0.05) is indicated between cells treated with MDP alone and cells treated with MDP plus the indicated phytochemicals. Abbreviations are the same as described in Fig. 1.
Curcumin Inhibited Lauric Acid (C12:0)-Induced Nod2 Activation
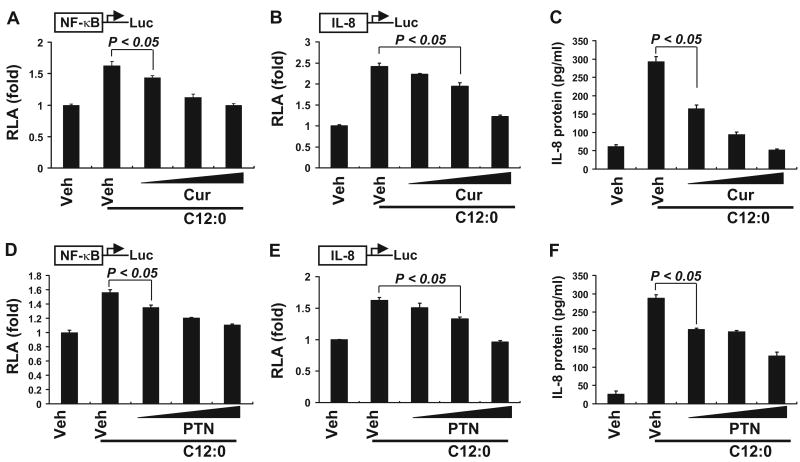
The innate immune systems were evolved to defend host against pathogenic infections. It has now been well documented that the activation of certain PRRs, including TLRs and NOD proteins (Nods), are also modulated by endogenous molecules, including fatty acids (Lee and Hwang, 2006; Zhao et al., 2007). For example, saturated fatty acids induce the activation of Nods signaling, whereas unsaturated fatty acids, particularly n-3 polyunsaturated fatty acids, inhibit the activation of Nods signaling induced by saturated fatty acids or Nod ligands (Zhao et al., 2007). To determine whether curcumin also inhibits the activation of Nod2 signaling induced by saturated fatty acids we coincubated HCT116 cells with saturated fatty acid lauric acid (C12:0) and curcumin. As shown in Fig. 3, curcumin dose-dependently inhibited C12:0-induced NF-κB activation (Fig. 3A). Curcumin also inhibited C12:0-induced transactivation of IL-8-Luc reporter and the expression of IL-8 protein (Fig. 3, B and C). Similar inhibition was observed for parthenolide on C12:0-induced NF-κB activation and IL-8 reporter transactivation and protein expression. Thus, curcumin and parthenolide inhibited not only the activation of Nod2 signaling induced by MDP, a Nod2 ligand of bacterial origin, but also the activation of Nod2 signaling induced by saturated fatty acid lauric acid (C12:0).
Fig. 3.
Curcumin inhibits C12:0-induced transactivation of NF-κB and IL-8 expression. HCT116 cells were cotransfected with NF-κB-luciferase or IL-8-luciferase and β-galactosidase reporter constructs. After 24 h, the cells were pretreated with curcumin (10, 20, and 30 μM) (A–C) or parthenolide (5, 10, and 15 μM) (D–F) for 1 h and then coincubated with C12:0 (100 μM) for additional 6 h. Cell lysates were prepared and luciferase and β-galactosidase enzyme activities were measured as described under Materials and Methods. Medium supernatants were collected from IL-8-luciferase-transfected cells, and the levels of IL-8 protein were measured as described under Materials and Methods. RLA was normalized with β-galactosidase activity. Values are mean ± S.E.M (n = 3). Statistical significant difference (p < 0.05) is indicated between cells treated with MDP alone and cells treated with MDP plus the indicated phytochemicals. Abbreviations are the same as described in Fig. 1.
Curcumin Inhibited MDP-Induced Phosphorylation of IκBα and NF-κB p65
The activation of Nod2 signaling leading to the expression of proinflammatory genes is mediated by the phosphorylation of a cascade of effector proteins, including IκBα and NF-κB p65 (Strober et al., 2006). Phosphorylation of these effector proteins leads to the release of the active form of NF-κB, which in turn translocates to the nucleus and activates the expression of its target proinflammatory genes. MDP induced IκBα phosphorylation with a peak at 2 h after MDP treatment. Treatment of the cells with curcumin dramatically inhibited MDP-induced phosphorylation of this effector protein (Fig. 4A). During the 2-h incubation, curcumin dose-dependently inhibited MDP-induced IκBα phosphorylation (Fig. 4B). In contrast, EGCG showed no significant effect on MDP-induced IκBα phosphorylation (Fig. 4B). The inhibitory effect by curcumin on phosphorylation of IκBα was confirmed by its inhibition on IκBα degradation (data not shown). Curcumin treatment also significantly inhibited MDP-induced phosphorylation of the effector protein NF-κB p65 in a similar dose-dependent manner, whereas EGCG had no inhibitory effect (Fig. 4, C and D). Likewise, treatment of the cells with parthenolide also inhibited MDP-induced phosphorylation of IκBα and NF-κB p65 in a dose-dependent manner (Fig. 4, E and F). Consistent with the inhibition of NF-κB p65 phosphorylation, curcumin dose-dependently decreased the levels of NF-κB p65 translocated to the nucleus (Fig. 4G). Similar inhibitory effects on p65 translocation were observed for parthenolide (data not shown).
Fig. 4.
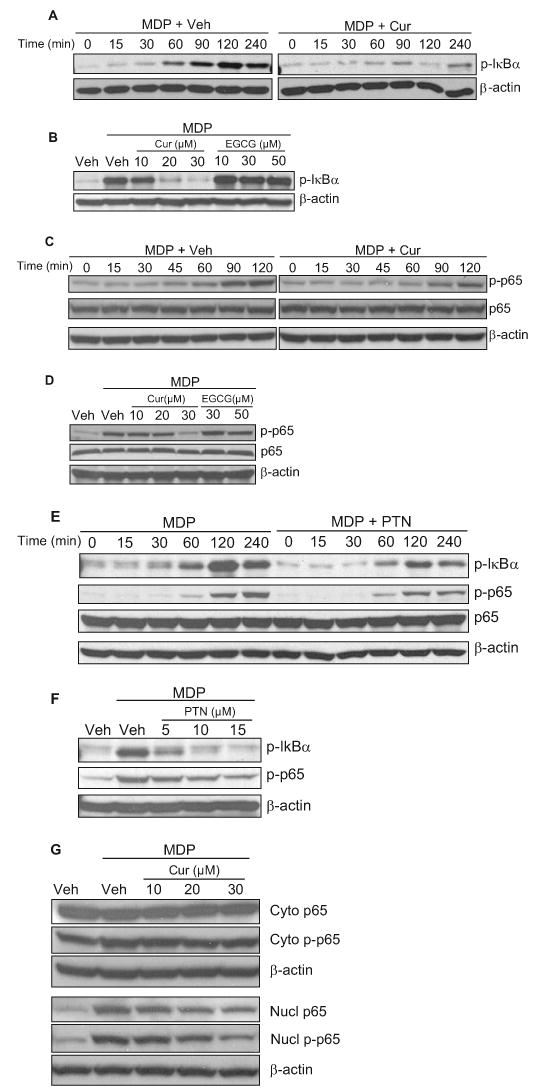
Curcumin inhibits MDP-induced phosphorylation of IκBα and NF-κB p65. A to F, HCT cells were pretreated with 30 μM curcumin (Cur) (A and C) or 15 μM parthenolide (PTN) (E) for 1 h and then coincubated with 50 μM MDP for 0 to 240 min or pretreated with curcumin (10, 20, 30 and μM) (B and D), EGCG (10, 30, and 50 μM) (B and D), or parthenolide (5, 10, and 15 μM) (F) for 1 h and then coincubated with 50 μM MDP for 2 h. The protein lysates were prepared and analyzed by Western blotting using anti-phospho-IκBα (p-IκBα), anti-phospho–NF-κB p65 (p-p65), and anti-NF-κB p65 (p65) antibodies as described under Materials and Methods. G, HCT cells were pretreated with curcumin (10, 20, and 30 μM) for 1 h and then coincubated with 50 μM MDP for 2 h. Nuclear (nucl) and cytosolic (cyto) fractions of protein lysates were prepared and analyzed by Western blotting using anti-phospho-NF-κB p65 (p-p65) and anti-NF-κB p65 (p65) antibodies as described under Materials and Methods. β-Actin was used as loading controls.
Curcumin Inhibited Nod2 Oligomerization
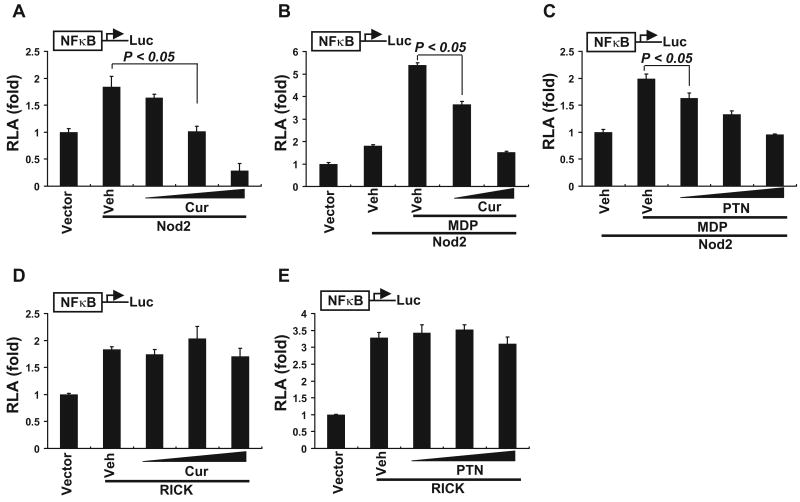
The inhibition of MDP-induced phosphorylation of IκBα and NF-κB p65 by curcumin and parthenolide suggested that these phytochemicals might directly inhibit the phosphorylation of these effector proteins. Alternatively, they might inhibit a target protein upstream of these effector proteins, the suppression of which in turn led to the inhibitory effects on the phosphorylation of these effector proteins. To identify the target for the inhibition of Nod2 signaling by curcumin and parthenolide, we used a reconstituted system in which 293T cells had been transiently transfected with Nod2. 293T cells express only a trace amount of Nod2 (Zhao et al., 2007). As shown in Fig. 5, overexpression of Nod2 in 293T cells stimulated Nod2 signaling, leading to increased NF-κB activation. Treatment of the cells with curcumin inhibited Nod2 overexpression-induced NF-κB activation in a dose-dependent manner (Fig. 5A). MDP treatment of 293T cells overexpressing Nod2 further enhanced NF-κB activation, and curcumin dose-dependently inhibited this MDP-induced NF-κB activation (Fig. 5B). Similar to curcumin, parthenolide also inhibited MDP-induced NF-κB reporter expression in a dose-dependent manner in Nod2-overexpressing 293T cells (Fig. 5C). These data indicated that curcumin and parthenolide also inhibited NF-κB activation induced by Nod2 overexpression or MDP stimulation in 293T cells overexpressing Nod2.
Fig. 5.
Curcumin does not inhibit RICK-induced NF-κB activation. A to C, HEK293T cells were cotransfected with NF-κB-luciferase and β-galactosidase reporter constructs and Nod2 expression vector. Twenty-four hours after transfection, the cells were treated with curcumin (10, 20, and 30 μM) for 6 h (A) or pretreated with curcumin (10 and 20 μM) for 1 h and then coincubated with 200 ng/ml MDP for an additional 6 h (B) or pretreated with parthenolide (5, 10, and 15 μM) for 1 h and then coincubated with 200 ng/ml MDP for additional 6 h (C). D and E, HEK293T cells were cotransfected with RICK expression vector and NF-κB-luciferase and β-galactosidase reporters. Twenty-four hours after transfection, the cells were treated with curcumin (10, 20, and 30 μM) (D) or parthenolide (5, 10, and 15 μM) (E) for 6 h. Cell lysates were prepared and luciferase and β-galactosidase enzyme activities were measured as described under Materials and Methods. RLA was normalized with β-galactosidase activity. Values are mean ± S.E.M (n = 3). Statistical significant difference (p < 0.05) is indicated between cells treated with vehicle and cells treated with curcumin (A) or between cells treated with MDP alone and cells treated with MDP plus curcumin (B) or parthenolide (C). Abbreviations are the same as described in Fig. 1.
Next, we examined whether curcumin and parthenolide inhibited RICK-induced Nod2 signaling in 293T cells that overexpress RICK. As shown in Fig. 5, D and E, overexpression of RICK in 293T cells stimulated Nod2 signaling, resulting in enhanced NF-κB activation. However, treatment of the cells with curcumin or parthenolide had no significant effects on the induction of NF-κB reporter expression by RICK overexpression. These data suggest that the target for the inhibition of Nod2 signaling by curcumin and parthenolide might be located upstream of Nod2-RICK interaction instead of downstream of RICK.
To test whether curcumin inhibited Nod2 oligomerization, we cotransfected 293T cells with HA-tagged Nod2 and Flag-tagged Nod2 cDNA expression vectors. The resulting Nod2 proteins were coimmunoprecipitated with anti-HA antibody matrix and detected by immunoblotting with anti-Flag and anti-HA antibodies (Fig. 6). MDP induced Nod2 oligomerization, with a peak induction at 100 ng/ml MDP and 15 min after MDP treatment (Zhao et al., 2007). These conditions were used for the coimmunoprecipitation experiments. As shown in Fig. 6A, curcumin inhibited MDP-induced Nod2 oligomerization in a dose-dependent manner. Likewise, curcumin also inhibited Nod2 oligomerization induced by lauric acid in a dose-dependent manner (Fig. 6B). Similar inhibition of Nod2 oligomerization was observed for parthenolide (data not shown). The fact that curcumin inhibited Nod2 oligomerization, the first step of Nod2 signaling, but not RICK overexpression-induced NF-κB activation, the downstream event of Nod2 signaling, strongly suggests that the target for the inhibition of Nod2 signaling by curcumin and parthenolide is Nod2 oligomerization.
Fig. 6.
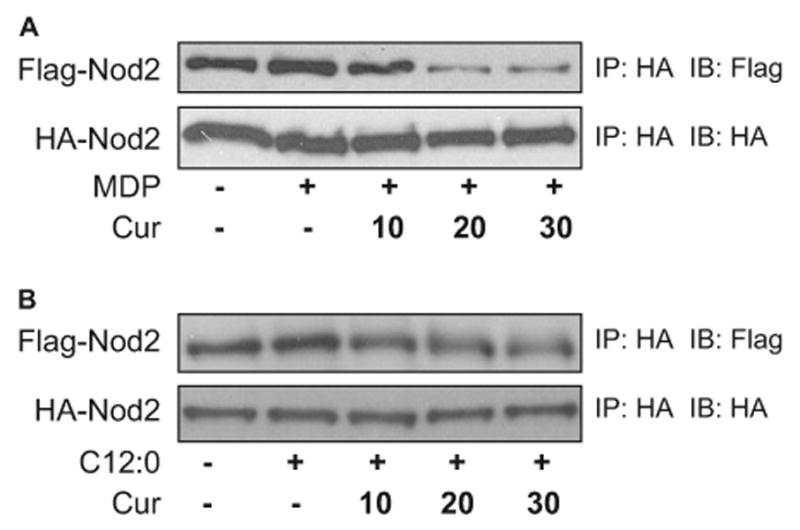
Curcumin inhibits Nod2 oligomerization induced by MDP or C12:0. HEK293T cells were cotransfected with HA-Nod2 and Flag-Nod2 cDNA expression vectors. After 24 h, cells were pretreated with curcumin (10, 20, and 30 μM) for 1 h and then coincubated with 100 ng/ml MDP (A) or 100 μM C12:0 (B) for 15 min. Cell lysates were prepared, Nod2 proteins were immunoprecipitated with anti-HA affinity matrix, and HA-Nod2 and Flag-Nod2 proteins were detected by Western blotting using anti-HA and anti-Flag antibodies as described under Materials and Methods. IP, immunoprecipitation; and IB, immunoblotting.
Discussion
We show here that dietary phytochemical compounds curcumin (a polyphenol) and parthenolide (a sesquiterpene lactone) inhibited NF-κB activation and target gene IL-8 expression. The inhibitory effects were mediated through the inhibition of Nod2 oligomerization and subsequent inhibition of downstream signaling.
Numerous studies have demonstrated the inhibitory effects of curcumin on NF-κB pathway as one of the key mechanisms underlying its anti-inflammatory and anticancer properties (Singh and Aggarwal, 1995; Jobin et al., 1999; Plummer et al., 1999; Pan et al., 2000; Shishodia et al., 2003, 2005; Kang et al., 2004). For example, it was reported that curcumin inhibited cytokine IL-1β-induced NF-κB activation and proinflammatory gene expression by inhibiting IKK activity (Jobin et al., 1999). However, the inhibition of IKK activity by curcumin was not mediated by direct interference with IKK activity, implying that the inhibition occurs rather at the upstream signal event(s), leading to reduced IKK activity. Moreover, curcumin inhibited both ligand-induced and ligand-independent dimerization of TLR4, a prerequisite step in TLR4 activation, leading to inhibition of lipopolysaccharide (TLR4 agonist)-induced NF-κB activation (Youn et al., 2006). Here, we show that curcumin also inhibits ligand-dependent Nod2 oligomerization, leading to reduced downstream NF-κB activation and target gene expression.
As a member of NOD-LRR family, Nod2 has recently been implicated to be involved in cytosolic innate recognition of bacteria and host defense (Inohara et al., 2005). Moreover, mutations of the Nod2 gene have been associated with susceptibility to inflammatory diseases. Because of the deficiency in sensing bacterial peptidoglycan or synthetic MDP, the three major Nod2 mutations, R702W, G908R, and L1007fsinsC, that have been associated with Crown's disease, a chronic relapsing inflammatory disease of the bowel, represent loss-of-function mutations (Hugot et al., 2001; Ogura et al., 2001a). In contrast, the Nod2 mutations R334Q, R334W, and L469F that have been associated with the development of Blau syndrome, a dominantly inherited disease characterized by early-onset granulomatous arthritis, uveitis, and skin rashes, exhibit constitutive NF-κB activity compared with the wild-type Nod2. Thus, these mutations represent gain-of-function mutations (Miceli-Richard et al., 2001). Furthermore, Nod2 has been shown to mediate “sterile” inflammation induced by nonmicrobial molecules (Zhao et al., 2007). Therefore, it is of worth to determine whether curcumin and parthenolide are of therapeutic value in treating these Nod2-mediated inflammatory conditions, particularly Blau syndrome, which seems to be induced by constitutive activation of Nod2 in the absence of pathogenic infection.
The mechanisms by which curcumin and parthenolide inhibit Nod2 receptor oligomerization remain to be determined. Many polyphenol compounds including curcumin containing α,β-unsaturated carbonyl group can react with biological nucleophiles such as sulfhydryl group by Michael addition (Rüngeler et al., 1999; Dinkova-Kostova et al., 2001; Siedle et al., 2004; Fang et al., 2005). For example, curcumin has been shown to bind to catalytically active cysteine residue of thioredoxin reductase by Michael addition to inhibit the enzyme activity (Fang et al., 2005). Nod2 contains a centrally located NOD domain that mediates self-oligomerization, in addition to a C-terminal LRR domain for ligand recognition and an N-terminal caspase recruitment domain for effector binding. Sequence analysis reveals several cysteine residues (Cys333, Cys353, 354, and Cys395) in the NOD domain, which might mediate Nod2 oligomerization by forming disulfide bonds. Whether curcumin inhibits Nod2 oligomerization by disrupting the cysteine-mediated disulfide bonds between the NOD domains remains to be determined. The results that curcumin inhibits both TLR4- and Nod2-mediated signaling pathways by inhibiting their oligomerization provide new insight into the mechanisms that underlie the anti-inflammatory effects of curcumin.
In conclusion, we show that curcumin and parthenolide inhibit Nod2-mediated NF-κB activation and target gene expression by inhibiting Nod2 oligomerization. Our results suggest a possibility that curcumin and parthenolide might be beneficial in treating Nod2-mediated inflammatory conditions, including Blau syndrome.
Acknowledgments
This work was supported by National Institutes of Health grants DK064007, DK41868, and CA75613; United States Department of Agriculture (USDA) grant 2001-35200-10721; American Institutes for Cancer Research grant 01A095Rev; and program funds from the Western Human Nutrition Research Center, USDA Agricultural Research Service.
Abbreviations
- TLR
Toll-like receptor
- PRR
pattern recognition receptor
- NOD
the nucleotide-binding oligomerization domain family
- LRR
leucine-rich repeat domain
- NACHT
domain present in NAIP (neuronal apoptosis inhibitory protein)
- CIITA
MHC class II transcription activator
- HET-E
incompatibility locus protein from Podospora anserine
- TP1
telomerase-associated protein
- NF-κB
nuclear factor-κB
- MDP
muramyl dipeptide MurNAc-l-Ala-d-isoGln
- IKK
IκB kinase complex
- IκB
inhibitor of NF-κB
- EGCG
epigallocatechin gallate
- HA
hemagglutinin
- HEK
human embryonic kidney
- IL
interleukin
- Luc
luciferase
- RICK
Rip-like interacting CLARP kinase
- ELISA
enzyme-linked immunosorbent assay
- RLA
relative luciferase activity
References
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IκBα kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Bertin J, Nir WJ, Fischer CM, Tayber OV, Errada PR, Grant JR, Keilty JJ, Gosselin ML, Robison KE, Wong GH, et al. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-κB. J Biol Chem. 1999;274:12955–12958. doi: 10.1074/jbc.274.19.12955. [DOI] [PubMed] [Google Scholar]
- Carneiro LA, Travassos LH, Philpott DJ. Innate immune recognition of microbes through Nod1 and Nod2: implications for disease. Microbes Infect. 2004;6:609–616. doi: 10.1016/j.micinf.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci U S A. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem. 2005;280:25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003a;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003b;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Inohara N, Chamaillard M, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J Biol Chem. 1999;274:14560–14567. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- Inohara N, Koseki T, Lin J, del Peso L, Lucas PC, Chen FF, Ogura Y, Nunez G. An induced proximity model for NF-κB activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem. 2000;275:27823–27831. doi: 10.1074/jbc.M003415200. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Chen FF, Muto A, Nunez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem. 2001;276:2551–2554. doi: 10.1074/jbc.M009728200. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- Kanazawa N, Matsushima S, Kambe N, Tachibana T, Nagai S, Miyachi Y. Presence of a sporadic case of systemic granulomatosis syndrome with a CARD15 mutation. J Invest Dermatol. 2004;122:851–852. doi: 10.1111/j.0022-202X.2004.22341.x. [DOI] [PubMed] [Google Scholar]
- Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S, Fuji A, Yuasa T, Manki A, Sakurai Y, et al. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood. 2005;105:1195–1197. doi: 10.1182/blood-2004-07-2972. [DOI] [PubMed] [Google Scholar]
- Kang G, Kong PJ, Yuh YJ, Lim SY, Yim SV, Chun W, Kim SS. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappaB bindings in BV2 microglial cells. J Pharmacol Sci. 2004;94:325–328. doi: 10.1254/jphs.94.325. [DOI] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- Lee JY, Hwang DH. The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Mol Cells. 2006;21:174–185. [PubMed] [Google Scholar]
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, Chamaillard M, Zouali H, Thomas G, Hugot JP. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001a;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J Biol Chem. 2001b;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol. 2000;60:1665–1676. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- Rüngeler P, Castro V, Mora G, Goren N, Vichnewski W, Pahl HL, Merfort I, Schmidt TJ. Inhibition of transcription factor NF-kappaB by sesquiterpene lactones: a proposed molecular mechanism of action. Bioorg Med Chem. 1999;7:2343–2352. doi: 10.1016/s0968-0896(99)00195-9. [DOI] [PubMed] [Google Scholar]
- Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003;24:1269–1279. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]
- Siedle B, Garcia-Pineres AJ, Murillo R, Schulte-Monting J, Castro V, Rüngeler P, Klaas CA, Da Costa FB, Kisiel W, Merfort I. Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-kappaB. J Med Chem. 2004;47:6042–6054. doi: 10.1021/jm049937r. [DOI] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Chamaillard M, Ogura Y, Zhu L, Qiu S, Masumoto J, Ghosh P, Moran A, Predergast MM, Tromp G, et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 2004;23:1587–1597. doi: 10.1038/sj.emboj.7600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn HS, Saitoh SI, Miyake K, Hwang DH. Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem Pharmacol. 2006;72:62–69. doi: 10.1016/j.bcp.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Zhao L, Kwon MJ, Huang S, Lee JY, Fukase K, Inohara N, Hwang DH. Differential modulation of Nods signaling pathways by fatty acids in human colonic epithelial HCT116 cells. J Biol Chem. 2007;282:11618–11628. doi: 10.1074/jbc.M608644200. [DOI] [PubMed] [Google Scholar]