Abstract
SUMO modification of nuclear receptors, including the constitutively active receptor steroidogenic factor 1 (SF-1; NR5A1), is proposed to repress their transcriptional activity. We examined the functional and structural consequences of SF-1 sumoylation at two conserved lysines (Lys119 and Lys194) that reside adjacent to the DNA-binding domain (DBD) and ligand-binding domain (LBD), respectively. Surprisingly, while previous loss-of-function studies predicted that sumoylation at Lys194 would greatly impact SF-1 function, the conformation and coregulator recruitment of fully sumoylated SF-1 LBD protein was either unchanged or modestly impaired. Sumoylation at Lys194 also modestly reduced Ser203 phosphorylation. In contrast to these findings, sumoylation of the DBD at Lys119 resulted in a marked and selective loss of DNA binding to noncanonical SF-1 targets, such as inhibinα; this binding deficit was extended to all sites when the sumoylated human mutant (R92Q) protein, which exhibits lower activity, was used. Consistent with this result, the K119R mutant, compared to wild-type SF-1, was selectively recruited to a “SUMO-sensitive” site in the endogenous inhibinα promoter, leading to increased transcription. DNA binding and sumoylation of Lys119 appeared to be mutually exclusive, suggesting that once SF-1 is bound to DNA, sumoylation may be less important in regulating SF-1 activity. We propose that sumoylation of nuclear receptors imposes an active posttranslational mark that dampens recognition of SUMO-sensitive target genes to restrain their expression.
Posttranslational modification with ubiquitin-like proteins has emerged as an important regulatory mechanism in many aspects of cellular function (29). Indeed, the small ubiquitin-like modifier (SUMO) conjugate modifies many transcription factors and results in marked transcriptional repression (27). Sumoylation occurs on lysines within consensus ϕKxE sites through an enzymatic mechanism analogous to ubiquitination (6). While the requirement for an obligate SUMO E3 ligase is still debated, several proteins exhibit SUMO E3 ligase activity in cells, with the largest group belonging to the protein inhibitors of activated STATs (PIAS) family (47). Sumoylation is easily reversible by the action of SUMO isopeptidases (SUSPs or SENPs), with seven members identified in humans thus far (40).
While recent structural studies have helped to elucidate the enzymatic details of SUMO conjugation and substrate recognition (44, 45), the mechanisms underlying transcriptional repression by sumoylation are less clear. Identification of a SUMO-interacting motif found in the PML protein and transcriptional repressors such as Daxx has helped to establish how the SUMO conjugate might function as a passive molecular mark to attenuate gene expression (28, 39). However, it is also plausible that the SUMO conjugate functions as an active mark to modify either protein-protein or protein-DNA interactions. Evidence for this latter hypothesis remains controversial. For instance, sumoylation of thymine-DNA glycosylase (TGD) was shown to alter its conformation and promote dissociation from DNA (3, 49), and several other studies suggest that transcription factor DNA binding is completely abrogated after sumoylation (2, 10, 52). On the other hand, structural analysis of sumoylated Ets-1 revealed a “beads-on-a-string” conformation, where SUMO1 and Ets-1 behaved as two independent domains (38). More recently, it has been suggested that SUMO-dependent repression of the glucocorticoid receptor actually requires DNA binding to multiple GREs (25). Thus, a comprehensive analysis that compares the functions of fully sumoylated and nonsumoylated protein variants is needed to further define how this posttranslational event represses gene expression.
Many nuclear hormone receptors are sumoylated in vitro and possess multiple sumoylation sites within or near their DNA-binding domain (DBD) or ligand-binding domain (LBD). As with other transcription factors, mutating sumoylation sites in nuclear hormone receptors increases their activity and can also relieve transrepression (16, 19, 33, 43). Regulation via sumoylation is particularly important for the constitutively active subset of nuclear receptors whose activity is not modulated by a switchlike ligand. Such is the case for members of the NR5A subfamily that includes steroidogenic factor 1 (SF-1; NR5A) and liver receptor homolog 1 (LRH-1; NR5A2) (32, 33).
For SF-1, which coordinates endocrine organ development and steroidogenesis (36, 42), transcriptional activity in cells appears to be potently repressed by sumoylation (8, 31, 33), possibly through the recruitment of corepressors (33). The close proximity of the two SF-1 sumoylation sites, at Lys119 and Lys194, to either the DBD or LBD, respectively (Fig. 1a) predicts that sumoylation might also directly influence the functional properties of these two domains to either diminish DNA binding, decrease coactivator recruitment, or hamper the binding of a phospholipid ligand. In addition, the presence of a phospho-sumoyl switch site (ϕKXEXXSP) (22, 33, 58) that includes phosphoserine Ser203 near the LBD potentially sets up a regulatory relationship between SF-1 sumoylation and phosphorylation (54, 57, 58).
FIG. 1.
In vitro sumoylation of recombinant SF-1 protein. (a) A schematic of mouse SF-1 protein constructs is shown with SUMO sites at Lys119 (K119, S) and Lys194 (K194, S), the A-box loop region, and corresponding amino acids indicated. HLBD, Hinge and ligand-binding domain. (b) Coomassie blue-stained gels of unmodified SF-1 (−E1), sumoylated SF-1 treated with Ulp1 enzyme (+E1, +Ulp1), or sumoylated SF-1 (+E1) proteins (10 μl in vitro reaction) are shown for the SF-1 constructs depicted in panel a. The asterisk denotes wild-type SF-1 degradation product. SF-1, unmodified SF-1; Su-SF-1, sumoylated SF-1; 2XSu-SF-1, SF-1 with two SUMO1 conjugated; Su-Ubc9, sumoylated Ubc9.
Despite convincing evidence that sumoylation is an important posttranslational modification in transcriptional regulation, understanding the mechanism of repression by sumoylation has largely been limited to loss-of-function or overexpression approaches in cellular studies (33). Here, we used a combination of biochemical and structural methods to examine the molecular effects of sumoylation on SF-1 LBD structure and function, as well as DNA-binding activity. We exploited the fact that both the LBD and DBD DNA structures have been determined for SF-1 and LRH-1 to ask how sumoylation might affect each of these functional domains (32, 34, 35, 48, 55). Our results provide evidence that sumoylation serves as an active mark that modulates the ability of SF-1 to regulate SUMO-sensitive target genes.
MATERIALS AND METHODS
Plasmids.
Mouse SF-1 fragment containing SF-1 LBD (amino acids [aa] 178 to 462, cysteine mutant, described previously [32]) was cloned using BamHI-XhoI sites into the bacterial expression vector pBH4 (modified pET-based vector, described previously [13]) and the glutathione S-transferase (GST) fusion vector pGEX6P2 (Clontech). For maltose-binding protein (MBP) fusion proteins, mouse SF-1 full length (aa 1 to 462), Hinge-LBD (aa 106 to 462), and DBD (aa 1 to 122) forms were cloned into the pMALp2X vector (New England Biolabs) modified to contain N-terminal tobacco etch virus protease cleavage site using EcoRI/XbaI (full-length SF-1), EcoRI-HindIII (Hinge-LBD), and EcoRI (DBD) sites. Human SUMO1 (aa 1 to 97) and mouse Ubc9 (aa 1 to 158) were cloned into the bacterial expression vector pBH4 using BamHI-XhoI sites. S203D and S203E SF-1 phosphomimics were created using pBH4-SF-1 LBD as a template, and K100D/R103D and R92Q SF-1 DBD point mutants were created using pMAL-SF-1 DBD as a template by PCR mutagenesis (QuikChange site-directed mutagenesis kit; Stratagene). All DNA concentrations were measured by using a spectrophotometer (NanoDrop Technologies), and the validity of the constructs was verified by DNA sequencing.
Recombinant protein expression.
Proteins were expressed by using Escherichia coli BL21 (λDE3) cells grown in LB medium and lysed by using a Microfluidizer (Microfluidics, Inc). His6-15N-SUMO1 was expressed in M9 medium containing 1 g of 15NH4Cl (Cambridge Isotope Labs)/liter. His6-13C ILV methyl-SF-1 LBD was expressed in M9 medium containing 1 g of unlabeled NH4Cl/liter with 100 mg of γ-13C-α-ketoisovalerate (Cambridge Isotope Labs)/liter and 50 mg of γ-13C-α-ketobuterate (Cambridge Isotope Labs)/liter added to the culture 30 min prior to induction with IPTG (isopropyl-β-d-thiogalactopyranoside). His6-hSUMO1 and wild-type and phosphomimic His6-SF-1 LBD proteins were purified by Talon chromatography (Clontech), followed by anion-exchange chromatography using a HiTrapQ column (GE Healthcare) in a buffer containing 20 mM HEPES (pH 7.5), 1 mM EDTA, and 2 mM CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}. The proteins were then eluted with an ammonium acetate gradient and concentrated by ultrafiltration. All MBP fusion proteins were purified by using amylose resin (New England Biolabs) according to the manufacturer's protocol, except the MBP buffer contained 400 mM NaCl and 50 μM ZnCl2 for SF-1 DBD-containing constructs. Full-length MBP-SF-1 and MBP-Hinge-LBD SF-1 were subsequently purified by size-exclusion chromatography in the MBP buffer using Superdex 200 column (GE Healthcare) and concentrated by ultrafiltration. Wild-type and mutant SF-1 DBD proteins were further purified by anion-exchange chromatography as described above. Recombinant His6-hE1 (SAE1/SAE2) and recombinant His6-mUbc9 were expressed and purified as described previously except that Talon chromatography (Clontech) was used, and His6 tags were not removed (59). GST fusion proteins were expressed and purified as described previously (13). Sumoylated His6-SF-1 LBD protein was purified by anion-exchange chromatography as described above for His6-SF-1 LBD, followed by size exclusion chromatography in a buffer containing 50 mM HEPES (pH 7.0), 150 mM NaCl, 50 mM arginine, 50 mM glutamine, 2% glycerol (vol/vol), 2 mM CHAPS, and 2 mM dithiothreitol (DTT) using a Superdex 200 column (GE Healthcare).
In vitro sumoylation assays.
SF-1 sumoylation assays were carried out in 30 μl (small scale) or 10 ml (large scale) with 0.1 μM E1, 10 μM Ubc9, and 30 μM SUMO1 in a sumoylation buffer containing 50 mM Tris-HCl (pH 8.0), 100 mM NaCl, 10 mM MgCl2, 10 mM ATP, and 2 mM DTT at 4°C overnight. For DNA inhibition assays, DNA at various concentrations was incubated with SF-1 DBD protein in the sumoylation buffer at room temperature for 30 min, followed by the addition of E1, Ubc9, and SUMO1 at the concentrations given above and incubation overnight at 4°C. Reactions were resolved on 4 to 12% NuPAGE Bis-Tris gel (Invitrogen) in NuPAGE morpholinepropanesulfonic acid (MOPS) buffer (Invitrogen), stained either with Coomassie blue or with Sypro Red dye (Molecular Probes) according to the manufacturer's protocol, and visualized by using a Typhoon laser scanner (Molecular Dynamics). The signal was quantified by using NIH ImageJ and plotted by using GraphPad Prism software. GST-SF-1 protein bound to glutathione-agarose 4B beads (Pharmacia) was sumoylated overnight at 4°C in the sumoylation buffer containing 2.5 μM E1, 8 μM Ubc9, and 40 μM SUMO1, and the extent of sumoylation was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining. Sumoylated GST-SF-1 was extensively washed with a buffer containing 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, 10% glycerol (vol/vol), 0.01% NP-40 (vol/vol), 1 mM EDTA, and 1 mM DTT to remove sumoylation enzymes and free SUMO1.
NMR spectroscopy.
15N-HSQC spectra for His6-SUMO1 (aa 1 to 97) and sumoylated His6-13C ILV methyl-SF-1 LBD were recorded on a Bruker 800 MHz (1H frequency) spectrometer equipped with a triple-resonance cryogenic probe. The samples were in a buffer containing 50 mM HEPES (pH 7.0), 150 mM NaCl, 50 mM arginine, 50 mM glutamine, 2% glycerol (vol/vol), 2 mM CHAPS, 2 mM TCEP, and 10% (vol/vol) D2O. The final protein concentration was estimated to be 200 μM. Nuclear magnetic resonance (NMR) data were processed with NMRPipe, and spectral analysis was carried out by using Sparky (20). Assignments for residues in SUMO1 were previously reported (4, 11, 37).
AlphaScreen assay.
Unmodified or sumoylated His6-SF-1 LBD at 30 nM and 30 nM N-term biotinylated mouse DAX1 peptide N-QGSILYSLLTSAQQ-OH (BioSynthesis) were incubated in a buffer containing 50 mM MOPS (pH 7.5), 50 mM NaCl, 5 mM DTT, and 1 mg of bovine serum albumin/ml at 4°C overnight. Unlabeled competitor mouse DAX1 peptide was added at various concentrations. An AlphaScreen assay was performed in triplicate in a final volume of 25 μl using 384-well ProxiPlate Plus plates (Perkin-Elmer) using a histidine detection kit (Perkin-Elmer) according to the manufacturer's protocol. The plates were read on an AlphaQuest HTS microplate analyzer, and the data were processed by using GraphPad Prism software (San Diego, CA). Peptide purity was > 95% by high-pressure liquid chromatography, and the quality was checked by mass spectrometry.
In vitro kinase assay.
Purified recombinant in vitro sumoylated or unmodified His6-SF-1 LBD protein was incubated with 20 U of Erk2 kinase (New England Biolabs) in buffer containing 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 2 mM DTT, 0.01% Brij 35, 200 μM [γ-32P]ATP, and excess cold ATP to a final specific activity of 500 μCi/μmol. Reactions were performed at 30°C for 0, 10, 20, 40, 80, and 160 min; stopped by the addition of SDS loading buffer; and resolved by SDS-PAGE. The gel was dried, exposed to film (Pierce) or phosphorimager, and quantified by using a Storm 860 (Molecular Dynamics). The data were plotted and curve fitted using GraphPad Prism software.
EMSAs.
Electrophoretic mobility shift assay (EMSAs) were performed to measure the DNA-binding ability of sumoylated or unmodified SF-1 using in vitro sumoylation reactions containing purified recombinant SF-1 DBD and double-stranded oligonucleotides taken from the promoters of SF-1 target genes (see Table S1 in the supplemental material for sequence information). In short, 2 to 5 μl of the in vitro sumoylation reaction (300 to 700 nM unmodified SF-1 DBD) was incubated in a total volume of 20 μl at room temperature for 30 min in a buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 10 mM MgCl2, 10 mM DTT, 10 mM ATP, and a 1 μM concentration of double-stranded oligonucleotide. Ulp1 samples were subsequently treated with 0.5 μl of recombinant Ulp1 (LifeSensors) at room temperature for 20 min. Then, 10 μl of the EMSA reaction was loaded onto a native 6% polyacrylamide gel, and electrophoresis was carried out in 1× TBE buffer at room temperature. The gel was analyzed by using a fluorescence-based EMSA kit (Molecular Probes). To detect total protein, the remaining 10 μl of the EMSA reaction was added to SDS-PAGE loading dye, boiled, resolved on a 4 to 12% NuPAGE Bis-Tris gel (Invitrogen) in NuPAGE MOPS buffer (Invitrogen), and stained with Sypro Red dye (Molecular Probes) according to the manufacturer's protocol. Gels stained with fluorescent dyes were visualized by using a Typhoon laser scanner.
Cell lines.
Stable tetracycline-inducible Flp-in T-Rex HEK293 cell lines (Invitrogen) expressing 3× Flag-tagged wild-type mSF-1 or K119R mutant were generated by transfection of pcDNA5/TOFRT-3× Flag mouse wild-type or K119R SF-1 by FuGene6 (Roche) and selection in 200 μg of hygromycin (Invitrogen)/ml. The cells were maintained in Dulbecco modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 50 μg of blasticidin (Invitrogen)/ml, and 200 μg of hygromycin/ml in a humidified incubator with 5% CO2.
Real-time qPCR and ChIP-qPCR.
HEK 293 Flp-In Trex cell lines were treated for 24 h with 0.1 nM tetracycline to induce wild-type or K119R mSF-1 expression. Total RNA was isolated the following day with TRIzol (Invitrogen). Real-time PCR was conducted with primers and methods described previously (50). For chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR), cells were cross-linked for 10 min at 37°C with 1% formaldehyde, washed, and collected in phosphate-buffered saline, followed by a 10-min incubation on ice with 200 μl of lysis buffer (1% SDS, 5 mM EDTA, and 50 mM Tris [pH 8.0] plus one protease inhibitor cocktail tablet and 20 mM NEM). Lysates were sonicated three times for 15 s each time, using an output control setting of 4 with a Branson Sonifier 250, and then centrifuged for 10 min at 13,000 rpm. Shearing was determined to be between 300- to 600-bp fragments. Supernatants were diluted 1:5 with dilution buffer (1% Triton X-100, 2 mM EDTA, 150 mM NaCl, and 20 mM Tris [pH 8.0] plus a cocktail tablet). Chromatin was measured by NanoDrop, and 0.7 μg was first precleared with mouse immunoglobulin G; 2 μg of salmon sperm was adsorbed to protein G-agarose and subsequently immunoprecipitated overnight with anti-Flag antibody (M2; Sigma) preconjugated to protein G-agarose. Chromatin immunoprecipitates were washed for 10 min each as described previously (21). To determine SF-1 protein immunoprecipitation, the immunoconjugates were boiled for 5 min in 2× DTT Laemmli sample buffer, separated by SDS-7.5% PAGE, and Western blotted with anti-SF-1 antibody (Upstate). For qPCR, samples were incubated for 3 h at 55°C in elution buffer (200 μg of proteinase K/ml, 10 mM Tris [pH 8.0], 1 mM EDTA, 0.5% SDS), and cross-links were reversed by incubation at 65°C overnight. Chromatin was recovered by phenol-chloroform extraction and resuspended in 100 μl of Tris-EDTA, and 4 μl was used in qPCRs. The sequences of each primer pair were as follows: inhibinα forward, 5′-GGTGTTGTATGTTTGCATGTGTGA-3′; inhibinα reverse, 5′-TCTTCTACCCTTCTCATCCAGTCTTC-3′; CYP11A1 forward, 5′-AGGAGCTGTCTGCGGGTTT-3′; and CYP11A1 reverse, 5′-CCACCAGGGCCAAGATTATAAC-3′.
RESULTS
Sumoylation does not alter the SF-1 LBD structure but impairs phosphorylation.
To understand how sumoylation affects SF-1, we in vitro sumoylated SF-1 variant proteins containing either one or both sumoylation sites at Lys119 near the DBD or Lys194 near the LBD (Fig. 1a) using a modified reaction that lacks a SUMO E3 ligase (59). As shown previously in cellular and in vitro assays, we found that SF-1 is efficiently sumoylated on two sites, Lys119 and Lys194 (Fig. 1b) (8, 31, 33). All proteins containing the SF-1 DBD were expressed as N-terminal MBP fusions to enhance protein solubility and stability (48). SF-1 sumoylation is easily reversed by the addition of yeast SUMO isopeptidase, Ulp1 (Fig. 1b). As shown in cellular studies, sumoylation of Lys194 was extremely efficient and more robust than sumoylation of Lys119 (31, 33). Neither the DBD nor the LBD was required for efficient sumoylation of either Lys119 or Lys194; both sites were sumoylated in constructs lacking SF-1 DBD (Fig. 1b) or SF-1 LBD (data not shown).
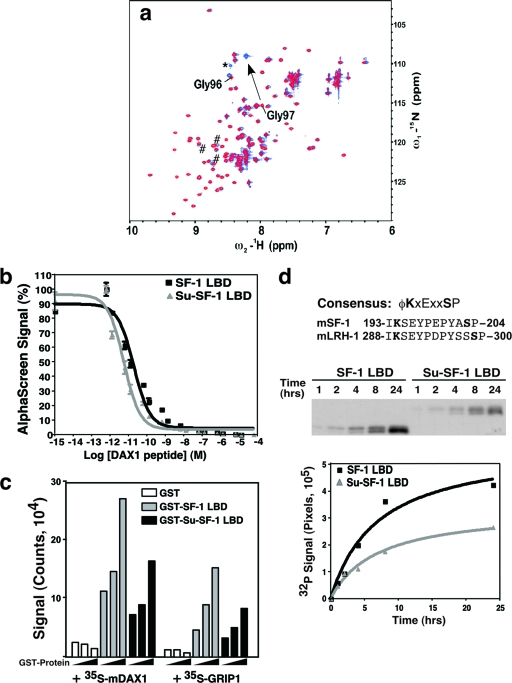
The ability to sumoylate large amounts of SF-1 LBD allowed us to investigate how sumoylation at Lys194 might alter the conformation of the LBD domain. To our surprise, no noncovalent interactions between SUMO1 and SF-1 LBD were detected by nuclear NMR. Indeed, a nearly complete overlap of HSQC spectra was observed for free 15N-SUMO1 and 15N-SUMO1 conjugated to unlabeled SF-1 LBD (Fig. 2a). The only observed changes in SUMO1 were in Gly97, the site of isopeptide bond formation with Lys194 in SF-1, and in the neighboring residue Gly96. These data are similar to the NMR structure reported for sumoylated Ets-1 and RanGAP1 (37, 38). The complementary experiment using 13C ILV methyl-labeled SF-1 LBD also revealed no change in the HSQC spectrum of 13C ILV methyl-SF-1 LBD after conjugation to SUMO1 (see Fig. S1a in the supplemental material). In addition, no changes in the HSQC spectrum of 15N-SUMO1 were observed after the addition of excess SF-1 LBD in trans (data not shown).
FIG. 2.
Lys194 sumoylation does not change SF-1 LBD structure. (a) NMR analysis revealed no changes in the structure of SUMO1 after conjugation to SF-1 LBD, except in the residue involved in the isopeptide bond (Gly97) and the adjacent residue (Gly96). An overlay of the 1H-15N HSQC spectrum of 15N-SUMO1 (red) and 15N-SUMO1-SF-1 LBD (blue) is shown (800 MHz 1H frequency). Gly97 and Gly96 are labeled. The arrow indicates the chemical shift of Gly97. The asterisk marks a resonance that was visible in 15N-SUMO1 spectrum at a lower contour and, most likely, represents a residue in the His6 tag on SUMO1. Resonances marked with a “#” symbol are located at the C terminus, close in space, and experienced a slight perturbation after conjugation. (b) DAX1 peptide binding to purified unmodified (SF-1) or sumoylated SF-1 LBD (Su-SF-1) in the AlphaScreen assay. Increasing concentrations (0 to 500 μM) of nonbiotinylated DAX1 peptide were added to SF-1 LBD protein, preincubated with biotinylated DAX1 peptide. The data are shown as the percent AlphaScreen signal with the no-competitor peptide reaction taken to be 100%. Error bars show the standard error of the mean of triplicates from a single experiment. A representative experiment is shown. (c) The amount of bound coregulator is shown as a bar graph and obtained using GST-pulldown assays with increasing amounts of GST-proteins including the GST control (GST, □), GST-SF-1 LBD ( ), and sumoylated GST-Su-SF-1 LBD (▪) proteins. Signals from bound radiolabeled full-length corepressor mDax-1 and coactivator Grip are shown (the methods are described in the legend to Fig. S1 in the supplemental material). (d) Sequences from putative phospho-sumoyl switch sites in mouse SF-1 and mouse LRH-1 are shown and compared to a reported consensus sequence (58). Sumoylated lysine (K) and phosphorylated serine (S) are shown in boldface. Equimolar quantities (22 μM) of purified unmodified or sumoylated SF-1 LBD protein were phosphorylated by Erk2 in the presence of radiolabeled [γ-32P]ATP for the times indicated. Quantified data are represented as a graph below. SF-1 LBD, unmodified SF-1 LBD; Su-SF-1 LBD, sumoylated SF-1 LBD.
), and sumoylated GST-Su-SF-1 LBD (▪) proteins. Signals from bound radiolabeled full-length corepressor mDax-1 and coactivator Grip are shown (the methods are described in the legend to Fig. S1 in the supplemental material). (d) Sequences from putative phospho-sumoyl switch sites in mouse SF-1 and mouse LRH-1 are shown and compared to a reported consensus sequence (58). Sumoylated lysine (K) and phosphorylated serine (S) are shown in boldface. Equimolar quantities (22 μM) of purified unmodified or sumoylated SF-1 LBD protein were phosphorylated by Erk2 in the presence of radiolabeled [γ-32P]ATP for the times indicated. Quantified data are represented as a graph below. SF-1 LBD, unmodified SF-1 LBD; Su-SF-1 LBD, sumoylated SF-1 LBD.
One of the best known functions of nuclear receptor LBDs is the recruitment of coregulator molecules. We tested whether sumoylation of Lys194 would modify SF-1 LBD recruitment of GRIP1 or DAX1 by AlphaScreen peptide binding assay (Fig. 2b) or by GST pull-down assays (Fig. 2c). Although no significant difference was measured between sumoylated or unmodified SF-1 LBD by AlphaScreen, a modest but consistent reduction of the full-length DAX1 or GRIP1 interaction with sumoylated SF-1 was observed in GST pull-down assays. Quantification of these results is shown in Fig. 2c. Taken together, these data suggest that SF-1 repression by sumoylation is unlikely to involve a major conformational change in SF-1 LBD but may alter coregulator recruitment in the context of full-length proteins.
As with other transcription factors, the juxtaposition of phosphorylation and sumoylation sites in the distal hinge region of SF-1 suggests a potential regulatory interplay between these two distinct posttranslational modifications (23, 51, 58). Indeed, in SF-1, the major conserved Erk1/2 phosphorylation site at Ser203 resides very near Lys194 (Fig. 2d), prompting us to examine whether Lys194 and Ser203 are part of a phospho-sumoyl switch. Although phosphorylation of SF-1 on Ser203 enhances coactivator recruitment and increases transcriptional activity (13, 22), our previous data showed that sumoylation at Lys194 was not affected by a nonphosphorylatable mutant S203A (33). Here, under our assay conditions, we found that the extent of sumoylation at Lys194 was unchanged with phosphomimic mutant proteins (S203D and S203E [see Fig. S1c in the supplemental material]) or with in vitro-phosphorylated SF-1 (data not shown). Conversely, we observed reduced levels of in vitro phosphorylation at Ser203 on fully sumoylated SF-1 LBD protein compared to unmodified LBD protein; the magnitude of this reduction is especially apparent at later time points (Fig. 2d and see Fig. S1d in the supplemental material). These data taken together with the modest decrease in coregulator recruitment suggest that sumoylation of SF-1 can attenuate the positive effects of SF-1 phosphorylation on transcriptional activity.
Sumoylation at Lys119 inhibits DNA binding to noncanonical sites.
Having determined that sumoylation at Lys194 has minimal impact on SF-1 LBD functions, we next sought to determine whether SF-1 binding to its extended consensus binding site 5′-YCAAGGYCR-3′ (Y = T/C, R = G/A) (53) is compromised by sumoylation. Although earlier studies suggested that SUMO-modified SF-1 retains the ability to bind DNA, these studies were unable to separately assess the binding of the sumoylated SF-1 from unmodified SF-1 (8, 31). Using a fluorescence-based EMSA with purified recombinant proteins, we were able to detect two distinct DNA-protein complexes using a mixture of unmodified and Lys119 sumoylated SF-1 DBD (Fig. 1a). We confirmed that the slower-migrating SF-1-DNA complex represented a sumoylated protein-DNA complex, as shown by the disappearance of this species after treatment with the Ulp1 isopeptidase (Fig. 3b and c). Moreover, sumoylated SF-1 DBD bound efficiently to the Mullerian inhibiting substance (MIS) high-affinity element but not to a mutant site even at saturating protein concentrations (Fig. 3c and data not shown). No components of the in vitro sumoylation reaction could bind DNA by themselves (Fig. 3c, MIS MUT). Collectively, these data show that sumoylation of Lys119 does not abolish DNA binding on canonical high-affinity sites.
FIG. 3.
Sumoylated SF-1 DBD selectively binds DNA. (a) Core SF-1 binding sites from SF-1-responsive promoters used in EMSA are shown with the entire oligonucleotide sequences provided in Table S1 in the supplemental material. WT, wild type. (b) The corresponding protein used in EMSA reactions (10 μl) was detected by Sypro Red staining and include unmodified SF-1 DBD (−E1, −Ulp1), sumoylated SF-1 DBD (+E1, −Ulp1), or sumoylated SF-1 DBD treated with Ulp1 (+E1, +Ulp1) proteins. (c) EMSAs using unmodified SF-1 DBD (−E1), sumoylated SF-1 DBD (+E1), or sumoylated SF-1 DBD treated with Ulp1 (+E1, +Ulp1) proteins (2-μl in vitro reaction) and double-stranded oligonucleotides (1 μM) from SF-1 target gene promoters are shown. DNA signal was detected by Sybr green staining. (d) EMSA results obtained with various concentrations of sumoylated protein using the CYP7A site (1.5 and 2.5 μl) and the rat inhibinα site (1 to 5.5 μl in 0.5-μl increments) sumoylated SF-1 DBD protein to double-stranded oligonucleotides (1 μM) from human CYP7A and promoters are shown. A protein gel of EMSA input (input, 10 μl) containing unmodified and sumoylated SF-1 DBD protein is shown. For panels c and d, protein-DNA complexes (10 μl) were resolved on a native polyacrylamide gel (EMSA). SF-1 DBD, unmodified SF-1 DBD; Su-SF-1 DBD, sumoylated SF-1 DBD.
We next examined the binding of sumoylated SF-1 protein on natural sites that deviate from the defined consensus. Previous structural studies have shown that NR5A receptors bind similarly to a canonical and a noncanonical binding site, such as that found in rat inhibinα promoter (35, 48). Binding of sumoylated SF-1 was compared between the rat inhibinα site and sites found in human CYP11A1 and human CYP7A (Fig. 3a) (48). In contrast to the robust binding of sumoylated SF-1 DBD to CYP11A and CYP7A sites, no binding was detected to the rat inhibinα site (Fig. 3c and d), despite adding increasing amounts of sumoylated SF-1 (Fig. 3d). Binding of the sumoylated SF-1 DBD was partially restored in the case of the human inhibinα, where a T resides in the +1 position (see Fig. S2a in the supplemental material). These data suggest that sumoylation modifies SF-1 DNA binding at noncanonical sites, which we refer to as SUMO-sensitive sites.
Selective promoter occupancy and enhanced expression of inhibinα by the K119R mutant.
We confirmed that Lys119 sumoylation was the cause of the selective attenuation of DNA binding, as shown by a K119A SUMO-deficient mutant SF-1 DBD. As with K119R (33), in vitro sumoylation of K119A does not yield an upshifted band (Fig. 4a, lower panel). As observed previously only unmodified wild-type SF-1 is able to bind the noncanonical inhibinα site (Fig. 4a). Indeed, the loss of bound SF-1 on the inhibinα site correlates well with the extent of SF-1 DBD sumoylation (compare Fig. 3b and 4a, with sumoylation far greater for the protein shown in Fig. 4, lane 5). In contrast, the K119A SUMO-deficient protein is bound to both the CYP11A1 and inhibinα sites under all conditions. We noted a faint residual upshifted DNA-protein complex with the K119A mutant and wild-type proteins (asterisk in Fig. 4a) that likely represents a degenerate weak sumoylation site present in the Ftz-Box; it should be noted that this minor sumoylation event is not evident in SDS-PAGE (Fig. 4a, lower panel). Taken together, these data demonstrate that Lys119 is the primary site of SF-1 DBD sumoylation, and this modification functions to selectively reduce interaction between SF-1 DBD and low-affinity SF-1 binding sites.
FIG. 4.
K119 sumoylation regulates SF-1 binding to and activation of inhibinα transcription. (a) EMSA reactions with CYP11A1 or rat inhibinα oligonucleotides with sumoylated or unmodified wild-type (WT) or K119A mutant SF-1-DBD proteins (the methods are described in the legend to Fig. 3). The DNA signal is shown in upper panel, and sumoylated protein input is shown in lower panel. Residual sumoylation of the K119R SF-1 DBD is noted by an asterisk. (b) Wild-type (WT) and K119R SF-1 protein expression in stable HEK 293 Flp-In T-Rex cells with 0.1 nM tetracycline (+) or with 70% ethanol vehicle control (−). The generation of stable cell lines is described in Materials and Methods. Immunoprecipitated (IP) SF-1 protein with anti-Flag antibody under cross-linking ChIP conditions is shown in the upper panel with the mouse immunoglobulin G control (IgG). The input (2% input) and protein loading control (actin) are shown in the lower panels. (c) ChIP-qPCR results with vehicle control (□) or Tet induction (▪) are shown as a percentage of input (plus the standard deviation), where % input = 2ΔCT × 2.5 and ΔCT = CT(input) − CT(FlagIP) (17). Precipitated DNA was amplified by real-time qPCR with primers flanking the SF-1 binding sites of the inhibinα or CYP11A1 promoters. (d) The relative expression of inhibinα and CYP11A1 transcripts determined by real-time RT-qPCR after vehicle (□) or Tet induction (▪) of either wild-type (WT) or K119R SF-1 is shown. The fold change in transcript expression (plus the standard deviation) was determined by using the ΔΔCT method with cyclophilin A as the reference gene. P values were determined by using an unpaired Student t test.
To extend the findings of our in vitro studies, inducible HEK-293 stable cell lines were generated expressing either wild-type SF-1 or the SUMO-deficient K119R mutant; HEK-293 cells do not endogenously express SF-1. Both wild-type and K119R SF-1 proteins were induced to similar levels with sumoylated species of both detected (Fig. 4b, upper panel, slower-migrating band). The levels of sumoylated K119R appeared to be modestly reduced compared to the wild type. These cell lines were then used to compare the occupancy of wild-type and SUMO-deficient SF-1 on either a SUMO-sensitive site, such as that found in inhibinα, or on a canonical site, such as that found in the CYP11A1 promoter. Indeed, ChIP analyses reveal a dramatic difference in the occupancy of the wild type and K119R on inhibinα but not the CYP11A1 promoter, with much greater recruitment observed for the K119R to inhibinα (Fig. 4c). Consistent with this observation, induction of the SUMO-deficient mutant K119R increased endogenous inhibinα transcripts significantly more than the levels obtained after induction of wild-type protein (Fig. 4d). In contrast, no differences were observed for CYP11A1 transcripts when the wild type and the K119R mutant were compared (Fig. 4d). Similar results were also observed in the human placental JEG3 cells (data not shown). Taken together, these data strongly support the hypothesis that sumoylation selectively modifies how SF-1 recognizes and activates SUMO-sensitive sites within the genome.
DNA binding inhibits SF-1 sumoylation at Lys119.
Given that sumoylation of SF-1 restricts DNA binding on some recognition sites, we also sought to determine whether DNA-bound protein could be sumoylated. Indeed, after SF-1 DBD was allowed to bind a high-affinity binding site (MIS), significant inhibition of in vitro sumoylation at Lys119 was observed (Fig. 5a). Single-stranded control DNA or a mutated binding site failed to inhibit Lys119 sumoylation (Fig. 5). The residual sumoylation observed in the presence of high concentrations of DNA most likely represents the small fraction of misfolded or oxidized SF-1 DBD that is unable to bind DNA. Lys194 sumoylation was not affected by the addition of an SF-1 binding site (see Fig. S2b in the supplemental material).
FIG. 5.

DNA binding inhibits SF-1 DBD sumoylation. (a) In the top panel the sequences of the wild-type (dsWT) and mutant (dsMUT) SF-1 binding sites from the mouse MIS promoter are shown. Complete oligonucleotide sequences are listed in Table S1 in the supplemental material. In the bottom panel, the sumoylation of SF-1 DBD in the presence of DNA was assessed. A Coomassie blue-stained protein gel of in vitro sumoylation reactions containing SF-1 DBD (5 μM) and 50 μM wild-type single-stranded (ssWT), wild-type double-stranded (dsWT), or mutant double-stranded (dsMUT) SF-1 binding sites is shown. (b) The graph shows quantified SF-1 DBD (2.8 μM) sumoylation in the presence of increasing concentration (0, 1.4, 2.8, 5.6, 11.1, and 22.2 μM) of wild-type (WT) or mutant (MUT) MIS SF-1 binding sites from three independent experiments. Error bars show the standard error of the mean. The percent SF-1 DBD sumoylated was determined relative to a control reaction without DNA. Signal was detected by Sypro Red staining. SF-1 DBD, unmodified SF-1 DBD; Su-SF-1 DBD, sumoylated SF-1 DBD.
In NR5A DBDs, the unique Ftz-F1 helix enhances DNA binding by stabilizing noncovalent interactions between the core DBD and the DNA phosphate backbone (35, 48). Thus, Ftz-F1 helix mutants, which are known to reduce DNA binding, might conversely enhance sumoylation at Lys119. Based on the SF-1 DBD structure, Lys100 and Arg103 create a positively charged surface in the Ftz-F1 helix that lies proximal to the phosphate backbone of the DNA (Fig. 6a) (35). As predicted, mutating these residues to aspartic acid (K100D, R103D) severely compromised DNA binding (Fig. 6b) but enhanced sumoylation at Lys119 (Fig. 6c and d). These data imply that conformational changes accompanying DBD binding (24) directly interfere with the sumoylation machinery and provide a mechanism for resisting sumoylation when the receptor is actively bound to DNA.
FIG. 6.
DNA binding is necessary to inhibit SF-1 Lys119 sumoylation. (a) A ribbon diagram of Lys100 and Arg103 (sticks) in the Ftz-F1 helix of SF-1 in relation to DNA (gray sticks). The diagram was made by using the mouse SF-1 DBD structure (PDB 2FF0) (12, 35). (b) EMSA results showing the binding of wild-type SF-1 DBD (WT, 4 and 5 μl) or mutant SF-1 DBD (K100D/R103D, 4 and 5 μl) protein to double-stranded oligonucleotide (1 μM) from mouse MIS promoter. A protein gel of EMSA input (input, 10 μl) containing wild-type or K100D/R103D mutant SF-1 DBD is shown. Protein signal was detected by Sypro Red staining. Protein-DNA complexes (10 μl) were resolved on a native polyacrylamide gel (EMSA). DNA signal was detected by Sybr green staining. (c) Coomassie blue-stained protein gels of sumoylation reaction containing wild-type or K100D/R103D mutant SF-1 DBD (2.8 μM, +E1) and wild-type double-stranded (dsWT, 28 μM) or mutant double-stranded (dsMUT, 28 μM) mouse MIS SF-1 binding sites. No sumoylation control (−DNA, −E1) and a control reaction without DNA (−DNA, +E1) are included. (d) The graph shows the quantified sumoylation of wild-type (WT) or K100D/R103D mutant SF-1 DBD (2.8 μM) in the presence of increasing concentrations (0, 1.4, 2.8, 5.6, 11.1, and 22.2 μM) of the wild-type mMIS SF-1 binding site. The percent SF-1 DBD sumoylated was determined relative to a control reaction without DNA. Protein signal was detected by Sypro Red staining. SF-1 DBD, unmodified SF-1 DBD; Su-SF-1 DBD, sumoylated SF-1 DBD.
Sumoylated SF-1 human mutant does not bind DNA.
For SF-1 and other monomeric nuclear hormone receptors, DNA binding specificity is partially conferred by the A-box loop region that resides C-terminal to the zinc fingers (18, 56). Intriguingly, a homozygous missense human mutation is found in the A-box region of SF-1, resulting in an arginine-to-glutamine substitution (R92Q) (1). Although this individual exhibited a phenotype that is identical to SF-1 loss-of-function nonsense mutations and presented at birth as an XY female with marked adrenal dysfunction, in vitro studies showed that the R92Q protein retains lowered, but appreciable DNA binding and transcriptional activity (1, 26). Based on the LRH-1 DBD structure of the analogous residue (Arg165), the R92Q mutation reduces the number of hydrogen bonds that would normally contact the minor groove of DNA (Fig. 7a) (12, 48). As shown here, R92Q exhibits substantial binding to a canonical SF-1 site (Fig. 7c). Consistent with these findings, sumoylation of the SF-1 R92Q mutant protein is inhibited in the presence of DNA, although less robustly than for wild-type protein (Fig. 7b, lower panel). Surprisingly, despite the diminished, but clear DNA binding exhibited by the R92Q mutant, DNA recognition of either a canonical or atypical site was completely absent after sumoylation of this mutant protein (Fig. 7c). This is in sharp contrast to wild-type sumoylated protein, which was still able to bind canonical sites. Taken together, we show that the ability of SF-1 to recognize DNA is finely modulated by sumoylation, whereby lower affinity sites or a slightly impaired SF-1 protein amplify the repressive effects of receptor sumoylation.
FIG. 7.
Sumoylated R92Q SF-1 human mutant does not bind DNA. (a) Model depicting possible amino acid-DNA contacts for wild-type (WT) and R165Q mutant LRH-1 proteins. The model was created by using human LRH-1 DBD structure and the mutagenesis function in PyMol (PDB 2A66) (12, 48). (b) Coomassie blue-stained gels of sumoylation assay containing wild-type (WT) or R92Q mutant SF-1 DBD (2.8 μM, +E1) and wild-type double-stranded (dsWT, 28 μM) or mutant double-stranded (dsMUT, 28 μM) mMIS SF-1 binding sites. No sumoylation control (−DNA, −E1) and a reaction without DNA (−DNA, +E1) are included. (c) EMSA using unmodified wild-type (WT) or R92Q mutant SF-1 DBD (−E1), sumoylated wild-type or R92Q mutant SF-1 DBD (+E1), or sumoylated R92Q mutant SF-1 DBD treated with Ulp1 (+E1, +Ulp1) proteins (2-μl in vitro reaction) and double-stranded oligonucleotides (1 μM) from SF-1 target gene promoters. Protein-DNA complexes (10 μl) were resolved on a native polyacrylamide gel (EMSA). DNA was detected by Sybr Green staining. Protein gels of EMSA input (input, 10 μl) containing wild-type or R92Q mutant SF-1 DBD protein, either unmodified or as a mixture of unmodified and sumoylated proteins, are shown. The protein signal was detected by Sypro Red staining. SF-1 DBD, unmodified SF-1 DBD; Su-SF-1 DBD, sumoylated SF-1 DBD.
DISCUSSION
We sought to determine whether sumoylation serves as an active or passive mark to restrain the transcriptional activity of the nuclear receptor SF-1. Using purified recombinant SF-1 proteins, we found that sumoylation at Lys119 and Lys194 impairs the normal functions of the DBD and LBD, respectively. Although no changes in the LBD structure were observed after Lys194 sumoylation, phosphorylation at Ser203 and coregulator recruitment were modestly decreased. More importantly, sumoylation modulates DNA-binding activity in a sequence-specific manner, where noncanonical, atypical SF-1 sites are found to be especially sensitive and are no longer bound by sumoylated SF-1. In addition, DNA-bound SF-1 is refractory to sumoylation at the DBD site, suggesting that the sumoylation machinery is unable to modify this transcription factor while it interacts directly with the genome. Based on this regulatory relationship between sumoylation and DNA binding recognition as illustrated in Fig. 8, we propose that the extent of SF-1 sumoylation is an important mechanism for differentially activating and fine-tuning gene expression.
FIG. 8.

Model depicting the regulation of SF-1 DNA binding by sumoylation. SF-1 exists in two mutually exclusive states, either bound to DNA or sumoylated at Lys119 but unbound to DNA. Our findings show that DNA-bound SF-1 is resistant to sumoylation at Lys119, and Lys119 can only be sumoylated when SF-1 is unbound from DNA. Once sumoylated at Lys119, SF-1 does not bind low-affinity noncanonical sites, which are referred to as SUMO-sensitive sites. In our model an inverse relationship between SF-1 binding site affinity and the inhibition of DNA-binding by sumoylation is predicted. Noncanonical or “SUMO-sensitive” targets will be differentially affected by sumoylation, whereas canonical or “SUMO-resistant” sites are not.
The most dramatic effect of SF-1 sumoylation was the selective loss of all DNA binding at noncanonical, atypical binding sites. Although a previous study suggested that sumoylated LRH-1 exhibited lowered promoter occupancy, other reports showed that sumoylation of SF-1 does not compromise DNA binding (7, 8, 31). Our data clearly show that sumoylated SF-1 DBD is incapable of interacting with certain SF-1 binding sites, even at very high protein concentrations. Thus, SUMO-sensitive targets, such as inhibinα, would remain silent when pools of sumoylated SF-1 are elevated (Fig. 8) or activated when sumoylation is decreased (as shown in Fig. 4d). To date, few of these atypical SF-1 binding sites have been discovered. However, interestingly, a global genome-wide analyses identified a sizable fraction of sites bound by another monomeric nuclear hormone receptor, ERRα (NR3B1), that do deviate from the high-affinity consensus previously defined by biochemical analyses (15).
While others have suggested that sumoylation compromises DNA binding, most of these studies were unable to directly compare binding of sumoylated protein to nonsumoylated protein (LRH-1, HSF) (2, 7). Our ability to show a distinctly bound species of SF-1 that is susceptible to Ulp1 protease treatment allowed us to assess the differences in DNA recognition following sumoylation. We speculate that the selective loss of DNA binding observed after SF-1 sumoylation involves an interaction between SUMO and SF-1 DBD residues that mediate binding to atypical sites. Thus, this interaction must substantially weaken DNA recognition. Structural studies show that residues in the A-box loop of NR5A DBDs are critical for recognizing the 5′ region of the binding site (35, 48). Once DNA binding is compromised further by A-box mutations, as shown for the R92Q mutant, sumoylation eliminates all binding to both canonical and noncanonical sites. Our findings are similar to those reported for the TGD enzyme, where SUMO1 conjugation affects its interaction with DNA (3, 49). There, sumoylation induces a conformational change in TGD that results in dissociation of the enzyme from substrate DNA (3). Further studies will be needed to determine the structural consequences of SUMO modification to both the A-box loop and the Ftz-F1 helix of NR5A receptors. This promises to be challenging given the antagonistic relationship between Lys119 sumoylation and SF-1 DNA binding, which would preclude refolding SF-1 DBD in the presence of DNA prior to sumoylation.
The conserved location of the sumoylation sites in all other NR5A members, as well as other nuclear receptors, suggests that this posttranslational modification has been preserved during evolution. Indeed, the founding member of the NR5A subfamily, Drosophila Ftz-F1, has a single sumoylation site located next to the DBD, and Ftz-F1 is sumoylated in vitro (M. D. Show, unpublished data); no sumoylation site is found near the LBD. Similarly, nonsteroidal receptors ERR (NR3B) and PPAR (NR1C) have sumoylation sites in close proximity to their DBDs (43, 54). We propose that receptor sumoylation can “fine-tune” gene expression in multiple ways. First, our data suggest that varying the pools of sumoylated receptor in space and time provides an added layer of gene regulation. Although little is known about the regulation of sumoylation, it is worth noting that key players in the sumoylation machinery are critical for embryonic development, as observed in mouse knockouts of Ubc9 and the SUMO isopeptidase, SENP1 (9, 40, 41). Second, sumoylation could contribute to different transcriptional outcomes observed for factors with overlapping expression and similar DNA recognition. Indeed, SF-1 and NGFI-B are both expressed in the adrenal cortex and bind identical response elements, but when they are knocked out dramatically different phenotypes are observed (56). The relative occupancy of either NGFI-B or SF-1 on SUMO-sensitive sites would directly reflect the degree of receptor sumoylation, as suggested by our ChIP analysis of inhibinα (Fig. 4c). Finally, the ability of sumoylated SF-1 to discriminate between canonical and atypical sites implies that sumoylation can amplify subtle changes in the SF-1 binding site. For example, sumoylated SF-1 recognizes the human inhibinα site much better than the rodent site, which vary by only 1 bp. Thus, a single genomic base pair change during evolution would allow sumoylation to regulate overlapping but distinct sets of target genes in different organisms.
Our NMR studies with sumoylated SF-1 LBD revealed no detectable interactions between SUMO1 and the SF-1 LBD, suggesting that potent repression of SF-1 activity after sumoylation is unlikely to involve structural changes in the LBD. Similar to the findings with Ets-1 and RanGAP1, SUMO1 must exist as a separable noninteracting domain to be one of two “beads-on-a-string.” In agreement with these results, we found that recruitment of coregulator peptides was not affected by sumoylation of SF-1 LBD, which is consistent with our earlier findings that DAX1 potently represses both wild-type and SUMO mutant SF-1 in cellular assays (33). Thus, aside from the negative effects of sumoylation on Ser203 phosphorylation, as shown here (Fig. 2c), we do not find a clear and robust effect of sumoylation on the properties of the LBD that we evaluated. This result is somewhat surprising given the more dramatic effect of a K194R versus a K119R mutation in a Gal-4 DBD-SF-1 LBD chimera (33) and the fact that Lys194 appears to be a more optimal substrate for sumoylation in most cellular contexts. Subtle changes in the SF-1 LBD conformation are always possible and would require further amino acid backbone assignment of the LBD; currently, the poor spectral dispersion of the hinge-LBD precludes this analysis. However, as shown previously, LBDs of constitutively active receptors do not exhibit the same dynamic and allosteric changes observed for steroid hormone receptors. Indeed, no obvious conformational changes in the LBD are observed with coregulator peptide binding (32), and our results suggest strongly that the LBD of SF-1 is also unchanged after sumoylation. Obtaining full-length SF-1 receptor for structural studies will be needed to fully delineate the dynamic consequences of this posttranslational modification.
Knowing when, and how much, SF-1 is sumoylated in vivo will be required to fully appreciate the biological effects of this posttranslational modification. In this regard, it is interesting that the amount of SF-1 protein made from a single wild-type allele is insufficient to compensate for the partial loss of SF-1 in both human and mouse SF-1 mutants (5, 30). This implies that the pool of transcriptionally active SF-1 protein is limited. Therefore, the observed phenotypes might arise in these mutants if pools of sumoylated SF-1 are large and the amount of active SF-1 protein is small. Our data also predict that an activating SF-1 loss-of-sumoylation mutant would have phenotypic consequences in vivo. To date, overexpressing SF-1 using its own promoter leads to adrenocortical cell proliferation, demonstrating that SF-1 levels and activity need to be tightly regulated (14). Furthermore, attempts to overexpress SF-1 from stronger promoters have failed, suggesting that high levels of active SF-1 protein might potentially be harmful during development (H. A. Ingraham, unpublished results). Thus, removing constraints imposed by sumoylation of SF-1 might result in earlier lethality than observed in the SF-1-null mice or in the dysregulation of normal endocrine signaling, as shown previously in cultured cells (46). Preliminary analyses of mice expressing SF-1 mutated at both Lys119 and Lys194 support this prediction (H. A. Ingraham, unpublished results). In sum, our findings support the notion that sumoylation is an active posttranslational mark that provides another layer to regulate gene expression.
Supplementary Material
Acknowledgments
We thank E. Sablin, C. Campbell, and R. Blind for helpful discussions; Christopher Lima (Sloan-Kettering Memorial Center, New York) for the generous gift of a bacterial strain expressing human SUMO E1; M. Stallcup and E. R. McCabe for providing reagents; and Patrick Escaron and Max Cooper of Perkin-Elmer for help with the AlphaScreen assay and instrumentation.
This study was funded by an NSF predoctoral fellowship to L.A.C., an NRSA fellowship support to E.J.F., a Lalor Foundation fellowship to M.D.S., a Hellman Family Foundation Research Scholar award to J.D.G., and RO1-NIH-NIDDK support to H.A.I.
Footnotes
Published ahead of print on 6 October 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Achermann, J. C., G. Ozisik, M. Ito, U. A. Orun, K. Harmanci, B. Gurakan, and J. L. Jameson. 2002. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J. Clin. Endocrinol. Metab. 871829-1833. [DOI] [PubMed] [Google Scholar]
- 2.Anckar, J., V. Hietakangas, K. Denessiouk, D. J. Thiele, M. S. Johnson, and L. Sistonen. 2006. Inhibition of DNA binding by differential sumoylation of heat shock factors. Mol. Cell. Biol. 26955-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba, D., N. Maita, J. G. Jee, Y. Uchimura, H. Saitoh, K. Sugasawa, F. Hanaoka, H. Tochio, H. Hiroaki, and M. Shirakawa. 2005. Crystal structure of thymine DNA glycosylase conjugated to SUMO-1. Nature 435979-982. [DOI] [PubMed] [Google Scholar]
- 4.Bayer, P., A. Arndt, S. Metzger, R. Mahajan, F. Melchior, R. Jaenicke, and J. Becker. 1998. Structure determination of the small ubiquitin-related modifier SUMO-1. J. Mol. Biol. 280275-286. [DOI] [PubMed] [Google Scholar]
- 5.Bland, M. L., C. A. Jamieson, S. F. Akana, S. R. Bornstein, G. Eisenhofer, M. F. Dallman, and H. A. Ingraham. 2000. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc. Natl. Acad. Sci. USA 9714488-14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capili, A. D., and C. D. Lima. 2007. Taking it step by step: mechanistic insights from structural studies of ubiquitin/ubiquitin-like protein modification pathways. Curr. Opin. Struct. Biol. 17726-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalkiadaki, A., and I. Talianidis. 2005. SUMO-dependent compartmentalization in promyelocytic leukemia protein nuclear bodies prevents the access of LRH-1 to chromatin. Mol. Cell. Biol. 255095-5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, W. Y., W. C. Lee, N. C. Hsu, F. Huang, and B. C. Chung. 2004. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J. Biol. Chem. 27938730-38735. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, J., X. Kang, S. Zhang, and E. T. Yeh. 2007. SUMO-specific protease 1 is essential for stabilization of HIF1α during hypoxia. Cell 131584-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou, C. C., C. Chang, J. H. Liu, L. F. Chen, C. D. Hsiao, and H. Chen. 2007. Small ubiquitin-like modifier modification regulates the DNA binding activity of glial cell missing Drosophila homolog a. J. Biol. Chem. 28227239-27249. [DOI] [PubMed] [Google Scholar]
- 11.Delaglio, F., S. Grzesiek, G. W. Vuister, G. Zhu, J. Pfeifer, and A. Bax. 1995. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6277-293. [DOI] [PubMed] [Google Scholar]
- 12.DeLano, W. L. 2007. MacPyMOL: a PyMOL-based molecular graphics application for MacOS X. DeLano Scientific LLC, Palo Alto, CA.
- 13.Desclozeaux, M., I. N. Krylova, F. Horn, R. J. Fletterick, and H. A. Ingraham. 2002. Phosphorylation and intramolecular stabilization of the ligand binding domain in the nuclear receptor steroidogenic factor 1. Mol. Cell. Biol. 227193-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doghman, M., T. Karpova, G. A. Rodrigues, M. Arhatte, J. De Moura, L. R. Cavalli, V. Virolle, P. Barbry, G. P. Zambetti, B. C. Figueiredo, L. L. Heckert, and E. Lalli. 2007. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol. Endocrinol. 212968-2987. [DOI] [PubMed] [Google Scholar]
- 15.Dufour, C. R., B. J. Wilson, J. M. Huss, D. P. Kelly, W. A. Alaynick, M. Downes, R. M. Evans, M. Blanchette, and V. Giguere. 2007. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and gamma. Cell Metab. 5345-356. [DOI] [PubMed] [Google Scholar]
- 16.Faus, H., and B. Haendler. 2006. Post-translational modifications of steroid receptors. Biomed Pharmacother. 60520-528. [DOI] [PubMed] [Google Scholar]
- 17.Frank, S. R., M. Schroeder, P. Fernandez, S. Taubert, and B. Amati. 2001. Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev. 152069-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gearhart, M. D., S. M. Holmbeck, R. M. Evans, H. J. Dyson, and P. E. Wright. 2003. Monomeric complex of human orphan estrogen-related receptor-2 with DNA: a pseudo-dimer interface mediates extended half-site recognition. J. Mol. Biol. 327819-832. [DOI] [PubMed] [Google Scholar]
- 19.Ghisletti, S., W. Huang, S. Ogawa, G. Pascual, M. E. Lin, T. M. Willson, M. G. Rosenfeld, and C. K. Glass. 2007. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARγ. Mol. Cell 2557-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goddard, T. D., and D. G. Kneeler. 1999. Sparky 3. University of California, San Francisco.
- 21.Gummow, B. M., J. O. Scheys, V. R. Cancelli, and G. D. Hammer. 2006. Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol. Endocrinol. 202711-2723. [DOI] [PubMed] [Google Scholar]
- 22.Hammer, G. D., I. Krylova, Y. Zhang, B. D. Darimont, K. Simpson, N. L. Weigel, and H. A. Ingraham. 1999. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol. Cell 3521-526. [DOI] [PubMed] [Google Scholar]
- 23.Hietakangas, V., J. Anckar, H. A. Blomster, M. Fujimoto, J. J. Palvimo, A. Nakai, and L. Sistonen. 2006. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc. Natl. Acad. Sci. USA 10345-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmbeck, S. M., H. J. Dyson, and P. E. Wright. 1998. DNA-induced conformational changes are the basis for cooperative dimerization by the DNA binding domain of the retinoid X receptor. J. Mol. Biol. 284533-539. [DOI] [PubMed] [Google Scholar]
- 25.Holmstrom, S. R., S. Chupreta, A. Y. So, and J. A. Iniguez-Lluhi. 2008. Sumo-mediated inhibition of glucocorticoid receptor synergistic activity depends on stable assembly at the promoter but not on Daxx. Mol. Endocrinol. 222061-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito, M., J. C. Achermann, and J. L. Jameson. 2000. A naturally occurring steroidogenic factor-1 mutation exhibits differential binding and activation of target genes. J. Biol. Chem. 27531708-31714. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, E. S. 2004. Protein modification by SUMO. Annu. Rev. Biochem. 73355-382. [DOI] [PubMed] [Google Scholar]
- 28.Kerscher, O. 2007. SUMO junction-what's your function? New insights through SUMO-interacting motifs. EMBO Rep. 8550-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerscher, O., R. Felberbaum, and M. Hochstrasser. 2006. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22159-180. [DOI] [PubMed] [Google Scholar]
- 30.Kohler, B., L. Lin, B. Ferraz-de-Souza, P. Wieacker, P. Heidemann, V. Schroder, H. Biebermann, D. Schnabel, A. Gruters, and J. C. Achermann. 2007. Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum. Mutat. [DOI] [PMC free article] [PubMed]
- 31.Komatsu, T., H. Mizusaki, T. Mukai, H. Ogawa, D. Baba, M. Shirakawa, S. Hatakeyama, K. I. Nakayama, H. Yamamoto, A. Kikuchi, and K. Morohashi. 2004. Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol. Endocrinol. 182451-2462. [DOI] [PubMed] [Google Scholar]
- 32.Krylova, I. N., E. P. Sablin, J. Moore, R. X. Xu, G. M. Waitt, J. A. MacKay, D. Juzumiene, J. M. Bynum, K. Madauss, V. Montana, L. Lebedeva, M. Suzawa, J. D. Williams, S. P. Williams, R. K. Guy, et al. 2005. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120343-355. [DOI] [PubMed] [Google Scholar]
- 33.Lee, M. B., L. A. Lebedeva, M. Suzawa, S. A. Wadekar, M. Desclozeaux, and H. A. Ingraham. 2005. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol. Cell. Biol. 251879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, Y., M. Choi, G. Cavey, J. Daugherty, K. Suino, A. Kovach, N. C. Bingham, S. A. Kliewer, and H. E. Xu. 2005. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol. Cell 17491-502. [DOI] [PubMed] [Google Scholar]
- 35.Little, T. H., Y. Zhang, C. K. Matulis, J. Weck, Z. Zhang, A. Ramachandran, K. E. Mayo, and I. Radhakrishnan. 2006. Sequence-specific deoxyribonucleic acid (DNA) recognition by steroidogenic factor 1: a helix at the carboxy terminus of the DNA binding domain is necessary for complex stability. Mol. Endocrinol. 20831-843. [DOI] [PubMed] [Google Scholar]
- 36.Luo, X., Y. Ikeda, and K. L. Parker. 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77481-490. [DOI] [PubMed] [Google Scholar]
- 37.Macauley, M. S., W. J. Errington, M. Okon, M. Scharpf, C. D. Mackereth, B. A. Schulman, and L. P. McIntosh. 2004. Structural and dynamic independence of isopeptide-linked RanGAP1 and SUMO-1. J. Biol. Chem. 27949131-49137. [DOI] [PubMed] [Google Scholar]
- 38.Macauley, M. S., W. J. Errington, M. Scharpf, C. D. Mackereth, A. G. Blaszczak, B. J. Graves, and L. P. McIntosh. 2006. Beads-on-a-string, characterization of ETS-1 sumoylated within its flexible N-terminal sequence. J. Biol. Chem. 2814164-4172. [DOI] [PubMed] [Google Scholar]
- 39.Matunis, M. J., X. D. Zhang, and N. A. Ellis. 2006. SUMO: the glue that binds. Dev. Cell 11596-597. [DOI] [PubMed] [Google Scholar]
- 40.Mukhopadhyay, D., and M. Dasso. 2007. Modification in reverse: the SUMO proteases. Trends Biochem. Sci. 32286-295. [DOI] [PubMed] [Google Scholar]
- 41.Nacerddine, K., F. Lehembre, M. Bhaumik, J. Artus, M. Cohen-Tannoudji, C. Babinet, P. P. Pandolfi, and A. Dejean. 2005. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev. Cell 9769-779. [DOI] [PubMed] [Google Scholar]
- 42.Parker, K. L., and B. P. Schimmer. 1997. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocrinol. Rev. 18361-377. [DOI] [PubMed] [Google Scholar]
- 43.Pascual, G., A. L. Fong, S. Ogawa, A. Gamliel, A. C. Li, V. Perissi, D. W. Rose, T. M. Willson, M. G. Rosenfeld, and C. K. Glass. 2005. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reverter, D., and C. D. Lima. 2005. Insights into E3 ligase activity revealed by a SUMO-RanGAP1-Ubc9-Nup358 complex. Nature 435687-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reverter, D., and C. D. Lima. 2006. Structural basis for SENP2 protease interactions with SUMO precursors and conjugated substrates. Nat. Struct. Mol. Biol. 131060-1068. [DOI] [PubMed] [Google Scholar]
- 46.Sadovsky, Y., P. A. Crawford, K. G. Woodson, J. A. Polish, M. A. Clements, L. M. Tourtellotte, K. Simburger, and J. Milbrandt. 1995. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc. Natl. Acad. Sci. USA 9210939-10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharrocks, A. D. 2006. PIAS proteins and transcriptional regulation: more than just SUMO E3 ligases? Genes Dev. 20754-758. [DOI] [PubMed] [Google Scholar]
- 48.Solomon, I. H., J. M. Hager, R. Safi, D. P. McDonnell, M. R. Redinbo, and E. A. Ortlund. 2005. Crystal structure of the human LRH-1 DBD-DNA complex reveals Ftz-F1 domain positioning is required for receptor activity. J. Mol. Biol. 3541091-1102. [DOI] [PubMed] [Google Scholar]
- 49.Steinacher, R., and P. Schar. 2005. Functionality of human thymine DNA glycosylase requires SUMO-regulated changes in protein conformation. Curr. Biol. 15616-623. [DOI] [PubMed] [Google Scholar]
- 50.Suzawa, M., and H. A. Ingraham. 2008. The herbicide atrazine activates endocrine gene networks via non-steroidal NR5A nuclear receptors in fish and mammalian cells. PLoS ONE 3e2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tremblay, A. M., B. J. Wilson, X. J. Yang, and V. Giguere. 2008. Phosphorylation-dependent sumoylation regulates estrogen-related receptor-alpha and -gamma transcriptional activity through a synergy control motif. Mol. Endocrinol. 22570-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuruzoe, S., K. Ishihara, Y. Uchimura, S. Watanabe, Y. Sekita, T. Aoto, H. Saitoh, Y. Yuasa, H. Niwa, M. Kawasuji, H. Baba, and M. Nakao. 2006. Inhibition of DNA binding of Sox2 by the SUMO conjugation. Biochem. Biophys. Res. Commun. 351920-926. [DOI] [PubMed] [Google Scholar]
- 53.Ueda, H., and S. Hirose. 1991. Defining the sequence recognized with BmFTZ-F1, a sequence specific DNA binding factor in the silkworm, Bombyx mori, as revealed by direct sequencing of bound oligonucleotides and gel mobility shift competition analysis. Nucleic Acids Res. 193689-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vu, E. H., R. J. Kraus, and J. E. Mertz. 2007. Phosphorylation-dependent sumoylation of estrogen-related receptor α1. Biochemistry 469795-9804. [DOI] [PubMed] [Google Scholar]
- 55.Wang, W., C. Zhang, A. Marimuthu, H. I. Krupka, M. Tabrizizad, R. Shelloe, U. Mehra, K. Eng, H. Nguyen, C. Settachatgul, B. Powell, M. V. Milburn, and B. L. West. 2005. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc. Natl. Acad. Sci. USA 1027505-7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson, T. E., T. J. Fahrner, and J. Milbrandt. 1993. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol. Cell. Biol. 135794-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamashita, D., T. Yamaguchi, M. Shimizu, N. Nakata, F. Hirose, and T. Osumi. 2004. The transactivating function of peroxisome proliferator-activated receptor gamma is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes Cells 91017-1029. [DOI] [PubMed] [Google Scholar]
- 58.Yang, X. J., and S. Gregoire. 2006. A recurrent phospho-sumoyl switch in transcriptional repression and beyond. Mol. Cell 23779-786. [DOI] [PubMed] [Google Scholar]
- 59.Yunus, A. A., and C. D. Lima. 2005. Purification and activity assays for Ubc9, the ubiquitin-conjugating enzyme for the small ubiquitin-like modifier SUMO. Methods Enzymol. 39874-87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.