Abstract
ATR kinase activation requires the recruitment of the ATR-ATRIP and RAD9-HUS1-RAD1 (9-1-1) checkpoint complexes to sites of DNA damage or replication stress. Replication protein A (RPA) bound to single-stranded DNA is at least part of the molecular recognition element that recruits these checkpoint complexes. We have found that the basic cleft of the RPA70 N-terminal oligonucleotide-oligosaccharide fold (OB-fold) domain is a key determinant of checkpoint activation. This protein-protein interaction surface is able to bind several checkpoint proteins, including ATRIP, RAD9, and MRE11. RAD9 binding to RPA is mediated by an acidic peptide within the C-terminal RAD9 tail that has sequence similarity to the primary RPA-binding surface in the checkpoint recruitment domain (CRD) of ATRIP. Mutation of the RAD9 CRD impairs its localization to sites of DNA damage or replication stress without perturbing its ability to form the 9-1-1 complex or bind the ATR activator TopBP1. Disruption of the RAD9-RPA interaction also impairs ATR signaling to CHK1 and causes hypersensitivity to both DNA damage and replication stress. Thus, the basic cleft of the RPA70 N-terminal OB-fold domain binds multiple checkpoint proteins, including RAD9, to promote ATR signaling.
The DNA damage response coordinates cell cycle transitions, DNA replication, DNA repair, and apoptosis. The major regulators of the DNA damage response are the phosphoinositide-3 kinase-related protein kinases ataxia-telangiectasia mutated (ATM) and ATM and Rad3 related (ATR). ATR is activated during every S phase to regulate the firing of replication origins and the repair of damaged replication forks and to prevent the premature onset of mitosis (10).
ATR is activated in response to many types of DNA lesions, including double-strand breaks, base adducts, and cross-links, as well as replication stress. In most cases, these lesions activate ATR as a consequence of tracts of single-stranded DNA (ssDNA) that are formed during lesion processing (1, 14, 26, 39) or the uncoupling of helicase and polymerase activities at replication forks that encounter the lesion (9). Most forms of ssDNA in the cell, including the ssDNA formed during DNA replication and DNA repair, are rapidly coated by replication protein A (RPA) (19). Depletion of RPA from Xenopus laevis egg extracts reduces the association of ATR with chromatin (13), and RPA-coated ssDNA (hereinafter RPA-ssDNA) is important for localizing ATR to sites of DNA damage in both human and Saccharomyces cerevisiae systems (49).
Although RPA-ssDNA may be sufficient for localizing the ATR-ATR-interacting protein (ATRIP) complex, it is not sufficient for ATR activation (35, 37, 44). ATR signaling is dependent on colocalization of the ATR-ATRIP complex with the RAD9-HUS1-RAD1 (9-1-1) complex, a heterotrimeric ring-shaped molecule related in structure and sequence to the replicative sliding clamp PCNA (42).
Like PCNA, the 9-1-1 complex is loaded onto primer-template junctions in an ATP-dependent reaction that involves the RAD17 damage-specific clamp loader (6, 18, 50). Loading of the 9-1-1 complex occurs at a DNA end that is adjacent to a stretch of RPA-ssDNA. The presence of RPA is critical for this reaction and imparts specificity in loading, creating a preference for a 5′ rather than a 3′ primer end (18, 36). The 9-1-1 complex concentrates an ATR activator, TopBP1, at sites of DNA damage or replication stress. TopBP1 stimulates ATR kinase activity (31) by interacting with both a phosphoinositide 3 kinase-related kinase regulatory domain in ATR and ATRIP (12, 38).
ATR recognition of RPA-ssDNA depends upon ATRIP (49). Biochemical studies indicate that ATRIP binds RPA directly via evolutionarily conserved binding surfaces (2). The primary interaction involves an acidic alpha helix within a checkpoint recruitment domain (CRD) of ATRIP that binds in the basic cleft of the N-terminal oligonucleotide-oligosaccharide fold (OB-fold) domain of the large RPA subunit, RPA70 (RPA70N) (2). Deleting or mutating the ATRIP CRD abolishes its interaction with RPA70 and prevents ATR-ATRIP complexes from being efficiently retained at sites of DNA damage or stalled replication forks (3). Remarkably, mutations within the ATRIP CRD or even deletion of the entire CRD do not cause a large ATR checkpoint signaling defect in human, Xenopus, or budding yeast systems (2, 3, 30). This result is surprising given that the current model of ATR activation postulates that the ATRIP-RPA interaction should be essential for ATR signaling.
In this report, we further investigated the role of RPA in checkpoint signaling. First, the consequences of mutating the ATRIP binding surface on RPA70N were analyzed. As expected, these mutations impair ATRIP binding. However, in contrast to the ATRIP CRD mutations, the RPA binding surface mutations cause a significant defect in ATR-dependent signaling to CHK1. To reconcile these data, we hypothesized that additional ATR-regulatory proteins may bind to RPA using the same binding surface. We found that at least three checkpoint proteins bind to RPA using the same binding cleft within the RPA70N OB-fold domain. Notably, the C-terminal tail of RAD9 binds in the cleft. We show that this interaction is important for RAD9 recruitment to sites of DNA damage and stalled replication forks and is important for ATR signaling. Thus, RPA-ssDNA is a common signal within the DNA damage response for regulating multiple checkpoint complexes.
MATERIALS AND METHODS
NMR analysis.
Nuclear magnetic resonance (NMR) experiments were performed at 25°C using Bruker Avance 500-MHz or 600-MHz NMR spectrometers equipped with a 5-mm single-axis z gradient Cryoprobe. Two-dimensional, gradient-enhanced 15N-1H heteronuclear single-quantum coherence (HSQC) spectra were recorded with 1,024 complex points in the 1H dimension and 96 complex points in the 15N dimension. 1H and 15N backbone NMR assignments for RPA70N were kindly provided by Cheryl Arrowsmith (Ontario Cancer Institute, Toronto, Canada). 15N-enriched RPA70N NMR samples were prepared in a buffer containing 5 mM dithiothreitol, 50 mM NaCl, 20 mM Tris-d11, and 5% D2O at pH 7.4 at a protein concentration of ∼100 μM. ATRIP (DFTADDLEELDTLAD), MRE11 (AFSADDLMSIDLAEQ), and RAD9 (DFANDDIDSYMIAME) peptides were purchased (Sigma) and purified by high-performance liquid chromatography. Peptides were added at a four- to sixfold molar excess to maximize the population of peptide-bound RPA70N molecules. All spectra were processed with TOPSPIN v1.3 (Bruker, Billerica, MA) and analyzed with Sparky v3.1 (University of California, San Francisco, CA).
Cell culture.
HEK293, HeLa, and U2OS cells were cultured in Dulbecco modified Eagle medium (Invitrogen) supplemented with 7.5% fetal bovine serum at 37°C in 5% CO2. RAD9+/+ and RAD9−/− mouse embryonic stem (ES) cells were kindly provided by Howard Lieberman and cultured as described previously (23). RAD9−/− ES cells were transfected with Lipofectamine 2000 (Invitrogen), and stable clones were selected in medium containing G418 (0.2 mg/ml). U2OS cells expressing RAD9 or RAD9-crd (RAD9 with mutations in its putative CRD) were established by retroviral infection and puromycin selection. HeLa cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Nuclear extracts, in vitro translation, chromatin lysate, and cell lysate preparation.
Nuclear extracts were prepared as described previously (38). RAD9 and RAD9-crd proteins were in vitro translated with a TNT quick coupled transcription/translation system (Promega). Chromatin lysates were prepared by sonicating nuclei resuspended in immunoprecipitation buffer (0.3% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 20 mM HEPES, pH 7.9, and 150 mM NaCl supplemented with protease and phosphatase inhibitors [5 μg/ml aprotinin, 5 μg/ml leupeptin, 1 mM NaF, 20 mM β-glycerol phosphate, 1 mM sodium vanadate, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride]). Cell lysates were prepared using Igepal lysis buffer (1% Igepal CA-630, 50 mM Tris, pH 8.0, and 200 mM NaCl supplemented with protease and phosphatase inhibitors).
Antibodies.
Antibodies were purchased from Covance (HA.11), Oncogene Research Products (RPA70), Cell Signaling Technology (CHK1-P-S345 and CHK1-P-S317), Santa Cruz Biotechnology (CHK1 and RAD1), Bethyl Laboratories (KAP1 P-S824, RAD17, and TopBP1), Chemicon/Millipore (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]), and Abgent (RAD9 P-S272 and RAD9 P-S387).
GST pull-down assays.
Recombinant glutathione S-transferase (GST)-tagged RPA70N proteins were purified from Escherichia coli with glutathione-Sepharose 4B beads according to the manufacturer's instructions (GE Healthcare). ATRIP1-217 and ATRIP-crd (D58K D59K)1-217 proteins were also purified with glutathione-Sepharose 4B beads, followed by cleavage with PreScission protease (GE Healthcare). Nuclear extracts were incubated with the GST-tagged proteins bound to beads overnight at 4°C. Beads were washed two times in wash buffer 1 (25 mM Tris, pH 8.0, 25 mM NaCl, 0.1 mM EDTA, 10% glycerol, and 0.25% Trion X-100 supplemented with protease and phosphatase inhibitors) and once in wash buffer 2 (25 mM Tris, pH 8.0, 50 mM NaCl, 0.1 mM EDTA, 5% glycerol, and 0.5% Trion X-100 supplemented with protease and phosphatase inhibitors). Proteins were eluted with sodium dodecyl sulfate (SDS) sample buffer and separated by SDS-polyacrylamide gel electrophoresis (PAGE) prior to immunoblotting.
DNA constructs and siRNA.
Site-directed mutagenesis was performed using QuickChange (Stratagene). The sequences of all constructs generated using PCR were confirmed by sequencing. Small interfering RNA (siRNA) targeting RPA70 (5′-AACACUCUAUCCUCUUUCAUG) was purchased from Dharmacon, Inc.
DNA content analysis.
Harvested cells were fixed in ethanol, stained with propidium iodide, and analyzed on a BD Biosciences FACSCalibur.
Immunofluorescence.
Cells were cultured on a glass coverslip, fixed with paraformaldehyde, and processed as described previously (34), except with antihemagglutinin (anti-HA) primary antibody and fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibody. Cells were imaged with a Zeiss Axioplan microscope equipped with a Zeiss camera and software.
Immunoprecipitation.
Nuclear extracts or chromatin lysates expressing HA-RAD9 or HA-RAD9-crd were incubated with anti-HA agarose beads (Sigma). Immunoprecipitates were washed three times in TGN buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 10% glycerol, and 1% Tween 20 supplemented with protease inhibitors).
Cell survival assays.
ES cells were plated on gelatinized six-well dishes and treated with 10 mM hydroxyurea (HU) for 12 h or UV irradiated (10 J/m2). Surviving cells were stained with methylene blue 7 days later. Colonies were counted, and survival was calculated as the percentage of colonies in the treated dishes relative to the number in the untreated dishes.
RESULTS
The RPA70N domain is a checkpoint signaling module.
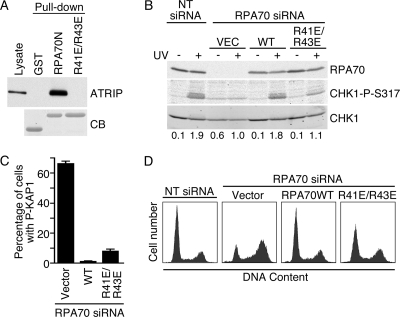
Previous studies did not identify an ATR signaling defect when the primary RPA binding surface within ATRIP is mutated (3). Yet, multiple lines of investigation indicate that RPA is a critical regulator of ATR signaling (10). In an attempt to understand this discrepancy, we mutated the primary ATRIP binding surface within RPA70N. Based on a computational model of the interacting surfaces (2), we predicted that a double charge reversal mutation (R41E R43E) within the basic cleft of the OB-fold domain would abolish the ATRIP interaction. As expected, the RPA70 R41E R43E mutant no longer bound ATRIP (Fig. 1A). Biophysical characterization of the mutant RPA70N was performed to verify that the structural integrity and stability of the domain was retained and therefore that any observed effect in functional assays was due only to alterations in the binding surface.
FIG. 1.
The basic cleft of RPA70N is required for ATR-Chk1 activation. (A) Nuclear extracts from HEK293 cells expressing HA-ATRIP were incubated with GST, GST-RPA70N, or GST-RPA70N R41E R43E proteins bound to glutathione beads. Proteins bound to the beads were eluted, separated by SDS-PAGE, and immunoblotted with anti-HA antibody to detect ATRIP or stained with Coomassie blue (CB) to detect GST proteins. (B to D) HeLa cells were transfected with RPA70 siRNA or nontargeting (NT) siRNA as indicated. In addition, cells were cotransfected with expression vectors encoding siRNA-resistant wild-type RPA70 or RPA70 R41E R43E or with an empty vector control (VEC). (B) Transfected cells were left untreated (−) or UV irradiated (50 J/m2). Two hours after irradiation, cell lysates were separated by SDS-PAGE and immunoblotted with RPA70, CHK1-P-S317, and total CHK1 antibodies. Quantitations of phospho-CHK1 and CHK1 were measured by an infrared imaging system (Odyssey), and the ratios of phospho-CHK1 to CHK1 were normalized to those for the sample of cells transfected with the empty vector and RPA70 siRNA after DNA damage. (C) The percentage of successfully transfected cells in the population with an activated DNA damage response (in the absence of an added genotoxic agent) was determined by staining with the phosphopeptide-specific antibodies to the ATM substrate KAP1. At least 300 cells were scored. Error bars indicate standard errors (n = 3). (D) Transfected cells were stained with propidium iodide and analyzed by flow cytometry.
To test the functional consequences of this RPA mutation, HeLa cells were cotransfected with siRNA targeting RPA70 and vectors encoding siRNA-resistant wild-type or mutant RPA70 proteins. Silencing RPA70 causes a reduction in the phosphorylation of the ATR substrate CHK1 in response to UV radiation (Fig. 1B, compare lanes 2 and 4) and also causes an increase in CHK1 phosphorylation in untreated cells compared to that in cells transfected with nontargeting siRNA (compare lanes 1 and 3). This increased CHK1 phosphorylation in untreated cells following RPA70 silencing is presumably due to the stalling and collapse of replication forks, which activate the DNA damage response. Indeed, RPA70 silencing causes activation of ATM (17) and phosphorylation of its substrates, including KAP1 (Fig. 1C). It also causes the activation of a G2 DNA damage checkpoint and accumulation of cells with 4N DNA content (Fig. 1D). Cotransfection of a vector encoding wild-type RPA complements all of these phenotypes, including UV-induced CHK1 phosphorylation. Cotransfection of the RPA R41E R43E mutant also largely complements the spontaneous DNA damage defect, although there is evidence of a continued DNA damage response activation in a small percentage of successfully transfected cells (Fig. 1C). However, the RPA70 R41E R43E protein does not complement the defect in UV-induced CHK1 phosphorylation in the endogenous RPA70-silenced cells (Fig. 1B, compare lanes 4, 6, and 8).
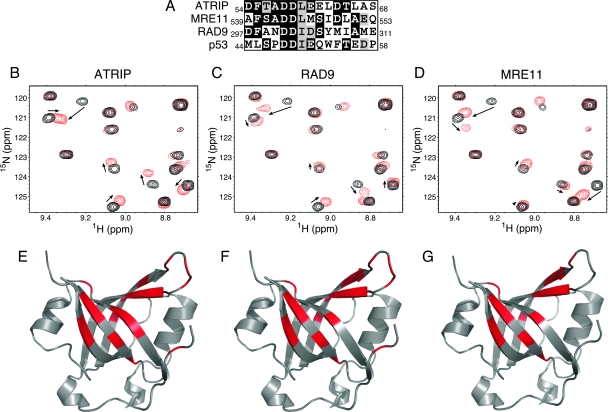
These data, combined with previously published results, indicate that mutations in ATRIP or RPA that abolish their primary binding surfaces do not yield identical phenotypes. The mutation in RPA caused a significant defect in CHK1 phosphorylation following UV radiation (Fig. 1B), whereas the mutation in ATRIP did not (3). A possible explanation for this result is that the RPA mutation may interfere with binding to proteins other than ATRIP that are important for ATR signaling to CHK1. Indeed, this surface of RPA is known to interact with p53 (8), suggesting that it may mediate interactions with multiple proteins. We searched other checkpoint proteins for sequence similarity to the RPA binding surfaces in ATRIP and p53 and found homologous regions in both MRE11 and RAD9 (Fig. 2A). To test whether these peptides were capable of binding to RPA, we employed the NMR-based chemical shift perturbation approach applied previously to investigate the interaction with ATRIP (2). A series of 15N-1H-HSQC NMR spectra were acquired for uniformly 15N-labeled RPA70N protein as unlabeled ATRIP, RAD9, and MRE11 peptides were added into the solution. Significant chemical shift perturbations were observed upon the addition of increasing concentrations of all three peptides to RPA70N (Fig. 2B to D). The chemical shift perturbations observed for the RAD9 and MRE11 peptides were remarkably similar to those of the ATRIP peptide (Fig. 2B to D). Indeed, mapping of the perturbed residues onto the crystal structure of RPA70N reveals that all three peptides bind to the basic cleft of the OB-fold domain in similar manners (Fig. 2E to G). The small differences observed between the spectra of the three complexes are consistent with minor differences in the positioning of each peptide within the binding site, which reflect the differences in the sequence of each protein.
FIG. 2.
ATRIP, MRE11, and RAD9 interact with the same binding surface on RPA70N. (A) Sequence alignment of acidic peptides in RAD9, MRE11, ATRIP, and p53. (B to D) NMR 15N-1H HSQC spectra of 15N-labeled RPA70N obtained in the absence (black) and presence (red) of ATRIP (B), RAD9 (C), or MRE11 (D) peptides. (E to G) RPA70N residues perturbed upon the addition of ATRIP (E), RAD9 (F), and MRE11 peptide (G) were mapped (in red) onto the crystal structure of RPA70N (Protein Data Bank accession no. 2B3G).
The RAD9 C-terminal tail binds to RPA70N.
While MRE11 is known to function upstream of ATR at sites of double-strand breaks, this does not appear to be the case after UV radiation or replication stress (39). RAD9, in contrast, is critical for ATR activation because of its role in recruiting the ATR activator TopBP1 (16, 32). The NMR data suggested that RAD9 uses a similar CRD to bind to RPA70N. Therefore, we tested whether full-length RAD9 indeed binds RPA70N and whether this interaction could explain the CHK1 phosphorylation defect we observed in RPA70 R41E R43E-expressing cells.
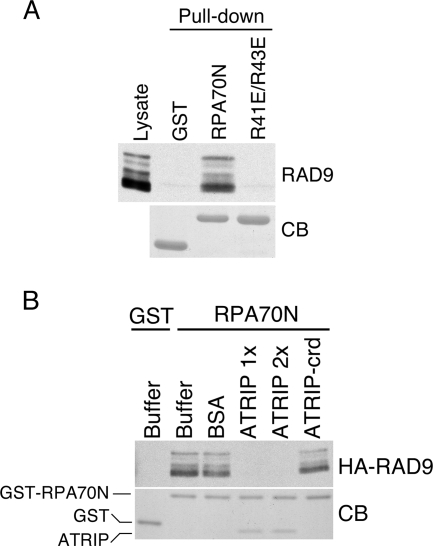
Purified GST-RPA70N binds RAD9 (Fig. 3A). In contrast, neither GST nor the GST-RPA70N R41E R43E mutant interacts with RAD9. We confirmed that RAD9 and ATRIP compete for the same binding surface on RPA70N by doing a competition binding assay. When purified ATRIP1-217 containing its CRD was added to the pull-down experiment, it bound to RPA70N and blocked the ability of RPA70N to bind HA-RAD9 (Fig. 3B).
FIG. 3.
RAD9 and ATRIP compete for the same binding surface on RPA70N. (A) Nuclear extracts from HEK293 cells expressing HA-RAD9 were incubated with GST, GST-RPA70N, or GST-RPA70N R41E R43E protein bound to glutathione beads. Proteins bound to the beads were eluted, separated by SDS-PAGE, and immunoblotted with anti-HA antibody to detect RAD9 or stained with Coomassie blue (CB) to detect GST proteins. (B) Bovine serum albumin (1.6 nmol), purified ATRIP1-217 (0.8 nmol [1×] or 1.6 nmol [2×]), or purified ATRIP-crd (D58K D59K)1-217 (1.6 nmol) was added as indicated to nuclear extracts from HEK293 cells expressing HA-RAD9. The nuclear extracts were incubated with 0.8 nmol of GST-RPA70N or GST proteins bound to glutathione beads. Proteins bound to the beads were eluted, separated by SDS-PAGE, and immunoblotted with anti-HA antibody to detect RAD9 or stained with Coomassie blue to detect GST proteins.
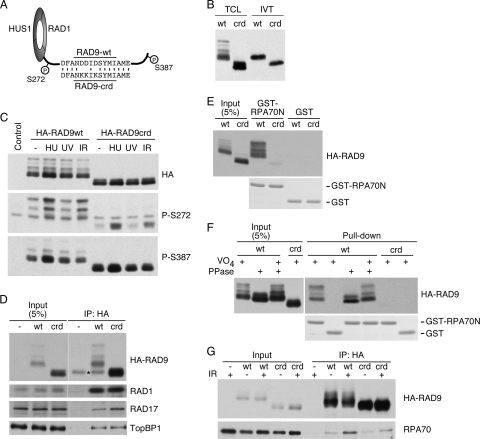
To determine whether the RPA binding surface on RAD9 contains the acidic C-terminal peptide, mutations were engineered into the putative RAD9 CRD (Fig. 4A). This RAD9 mutant (RAD9-crd) was epitope tagged and expressed either in HEK293 cells or in rabbit reticulocyte lysates. The RAD9-crd mutant made in both systems migrates significantly faster than wild-type RAD9 on SDS-PAGE gels (Fig. 4B). Wild-type RAD9 migrates as multiple bands due to its extensive phosphorylation (16, 45). We initially considered whether the CRD mutation might perturb RAD9 phosphorylation. However, both DNA damage-induced S272 phosphorylation and damage-independent S387 phosphorylation were retained (Fig. 4C). Furthermore, both the RAD9 and RAD9-crd proteins form complexes with RAD1, RAD17, and TopBP1, as expected (Fig. 4D). The interaction with TopBP1 is mediated by S387 phosphorylation (16), while the interaction with RAD1 is mediated by the PCNA homology region within the rest of the protein (42). Thus, the RAD9-crd protein is stable, is phosphorylated, and retains its known protein-protein interactions. Its aberrant migration on SDS-PAGE gels is probably due to an increase in its ability to bind SDS because of the replacement of three negative charges with three positively charged residues. We observed a similar change in the SDS-PAGE mobility of ATRIP when we made mutations in the ATRIP CRD (data not shown), further supporting this interpretation.
FIG. 4.
The RAD9 CRD interacts with RPA70N. (A) Diagram of the RAD9-crd protein illustrating the positions of the engineered mutations and two of the phosphorylated residues. (B) HA-wild-type RAD9 (wt) or HA-mutant RAD9-crd (crd) was expressed in HEK293 cells or rabbit reticulocyte lysates. Total cell lysates (TCL) or the in vitro transcription/translation reaction mixtures (IVT) were resolved by SDS-PAGE and immunoblotted with HA antibodies. (C) HA-wild-type RAD9 or the RAD9-crd mutant was expressed in HEK293 cells. Cells were left untreated (−) or treated with HU (10 mM), UV (50 J/m2), or IR (20 Gy). One hour after treatment, cells were harvested, lysates were resolved by SDS-PAGE, and immunoblots were probed with antibodies to RAD9 P-S272, RAD9 P-S387, or HA. The control was the lysate from untransfected cells. (D) Nuclear extracts from HEK293 cells expressing HA-RAD9 or HA-RAD9-crd (crd) and untransfected (−) cells were incubated with anti-HA agarose beads. The immunoprecipitated (IP) proteins were separated by SDS-PAGE, followed by immunoblotting with antibodies to RAD1, RAD17, TopBP1, and HA. The asterisk marks the position of an immunoglobulin G heavy-chain background band. (E and F) Nuclear extracts from HEK293 cells expressing HA-RAD9 (wt) or HA-RAD9-crd were incubated with GST-RPA70N or GST. Proteins bound to the beads were eluted, separated by SDS-PAGE, and detected by immunoblotting with anti-HA antibody or staining with Coomassie blue to detect GST-RPA70N and GST. (F) The extracts were treated with lambda phosphatase (PPase) in the absence or presence of sodium vanadate (VO4), as indicated, prior to incubation with GST proteins. (G) HEK293 cells expressing HA-RAD9 (wt) or HA-RAD9-crd (crd) were treated with IR (20 Gy), as indicated, followed by 2 h of incubation. Chromatin lysates were prepared and incubated with anti-HA agarose beads. The immunoprecipitated proteins were separated by SDS-PAGE, followed by immunoblotting with antibodies to HA and RPA70.
We next tested whether the RAD9-crd mutation impairs RPA binding. In contrast to the interaction between wild-type RAD9 and RPA70N, that between RAD9-crd and RPA70N was reduced although not abolished (Fig. 4E and F). The lack of interaction is not due to a change in RAD9-crd phosphorylation since the wild-type-RAD9-RPA70N interaction is insensitive to phosphatase treatment (Fig. 4F). Moreover, the RAD9-crd mutant has significantly reduced binding to endogenous RPA70 compared to that of wild-type RAD9 when measured by coimmunoprecipitation from solubilized chromatin fractions (Fig. 4G). The residual coimmunoprecipitation may be due to an interaction between RAD9 and RPA32 (47). Combined with the NMR data, these results indicate that RAD9 binds to RPA70 through an acidic peptide within its C-terminal tail and the basic cleft of the RPA70N OB-fold domain, largely in the same way that ATRIP binds RPA70 (2).
The RAD9-RPA interaction regulates RAD9 localization and ATR checkpoint signaling.
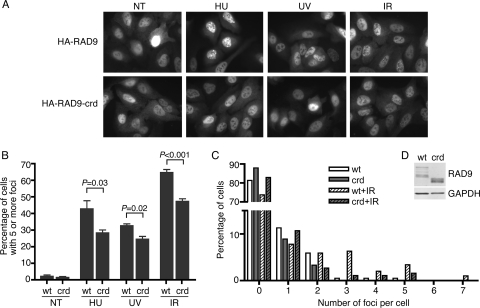
Mutation of the ATRIP CRD prevents ATRIP from efficiently localizing to sites of DNA damage or replication stress (3). To determine whether the RAD9 CRD performs a similar function, we created U2OS cells that express HA-RAD9 or HA-RAD9-crd. Both proteins were localized throughout the nuclei of untreated cells. Treatment with HU, UV, or ionizing radiation (IR) causes the relocalization of both proteins to intranuclear foci; however, the RAD9-crd mutant consistently relocalized less efficiently (Fig. 5A and B). To examine this more closely, the numbers of foci in each cell were counted. This analysis revealed that following a low dose of IR, there were significantly fewer RAD9-crd nuclear foci than wild-type RAD9 foci (Fig. 5C). The RAD9 wild type and the crd mutant are expressed at equal levels (Fig. 5D). Thus, although the RAD9 CRD-RPA70N interaction is not absolutely required to recruit RAD9 to sites of DNA damage, it does contribute to the efficiency of its localization.
FIG. 5.
The RAD9-RPA interaction promotes RAD9 localization to sites of DNA damage. (A and B) U2OS cells stably expressing HA-RAD9 or HA-RAD9-crd were not treated (NT) or treated with HU (10 mM for 4 h), UV (50 J/m2, followed by 1 h of recovery), or IR (10 Gy, followed by 6 h recovery). Fixed cells were stained with anti-HA antibody and fluorescein isothiocyanate-conjugated secondary antibody. (A) Representative images are shown. (B) Cells were scored for RAD9 localization to DNA damage foci. Three hundred cells were scored per experiment. Error bars indicate standard errors (n = 3). P values were calculated using an unpaired, two-tailed t test. wt, wild type. (C) HA-RAD9- or HA-RAD9-crd-expressing U2OS cells were treated with low-dose IR (1 Gy) or left untreated, followed by 6 h of incubation. The number of RAD9 foci in each cell was scored. (D) The expression level of HA-RAD9 and HA-RAD9-crd in U2OS cells was analyzed by immunoblotting with anti-HA or anti-GAPDH antibody.
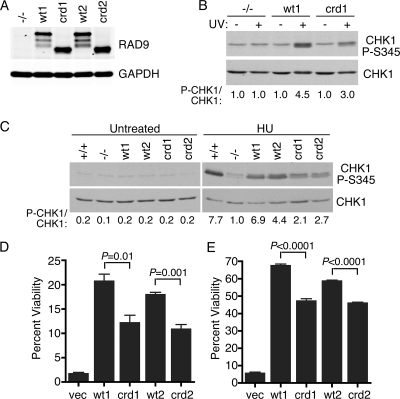
Finally, we examined whether the RAD9-crd mutant could functionally complement RAD9−/− ES cells. CHK1 is not phosphorylated in response to DNA damage in RAD9−/− ES cells because of a defect in activating ATR (23). We created RAD9−/− ES cell clones stably expressing either wild-type RAD9 or the RAD9-crd mutant. Two cell clones expressing each protein were analyzed to ensure that the results were not due to clonal selection. These cell clones expressed similar amounts of RAD9 protein (Fig. 6A). Immunofluorescence analysis indicated detectable expression of exogenous RAD9 proteins in approximately 90% of all cells in all four cell lines. While the wild-type RAD9 protein efficiently complemented the checkpoint signaling defect in the RAD9−/− cells, the RAD9-crd mutant was consistently less capable of supporting checkpoint signaling (Fig. 6B and C). Following UV radiation, CHK1 phosphorylation in the RAD9-crd-expressing cells was reduced by 33% compared to the level in wild-type-RAD9-expressing cells (Fig. 6B, compare lanes 4 and 6). Following HU exposure, the difference was approximately 60% (Fig. 6C, compare lanes 9 and 10 and lanes 11 and 12).
FIG. 6.
The RAD9-RPA interaction regulates DNA damage and replication stress responses. (A) Protein expression levels in two independent clones of RAD9−/− ES cells stably expressing HA-RAD9 or HA-RAD9-crd were analyzed by immunoblotting total cell lysates. (B and C) RAD9+/+ and RAD9−/− cells complemented with the vector (−/−), HA-RAD9 (wt1 and wt2), or HA-RAD9-crd (crd1 and crd2) were treated with UV (50 J/m2), HU (10 mM), or left untreated as indicated. Following incubation for 1 h, lysates were prepared, resolved by SDS-PAGE, and immunoblotted with antibodies to CHK1-P-S345 or total CHK1. The phospho-CHK1 (P-CHK1) and CHK1 levels were quantitated with NIH Image software. The numbers are the ratios of phospho-CHK1 to CHK1 normalized to the ratio for the sample from cells complemented with the vector (−/−) after damage. (D and E) ES cell clones were treated with HU (10 mM for 12 h) (D) or UV (10 J/m2) (E) and grown for 7 days prior to the staining and scoring of surviving colonies. Percent viability was calculated relative to the viability of untreated cell populations. Error bars indicate standard error (n = 3). P values were calculated using an unpaired, two-tailed t test. vec, vector.
The defective activation of the DNA damage response in the RAD9-crd-expressing cells correlated with a hypersensitivity of these cells to HU and UV compared to the sensitivity of wild-type-RAD9-expressing cells (Fig. 6D and E). In both cases, the RAD9-crd-expressing cells exhibited an intermediate sensitivity to these agents compared to the sensitivities of RAD9−/− cells expressing wild-type RAD9 or transfected with an empty vector.
DISCUSSION
RPA functions as an ssDNA binding protein in most nucleic acid metabolic processes. Its interactions with other proteins facilitate replication, repair, and checkpoint signaling (7, 19). RPA-ssDNA is thought to be the common intermediate in activating the ATR checkpoint pathway in response to a diversity of genome integrity challenges (10). ATR activation requires colocalization of the ATR-ATRIP and 9-1-1 complexes. RPA-ssDNA and a 5′ primer-template junction are critical ligands for recruiting these protein complexes (3, 6, 18, 36, 49, 50). We now show that a common protein interaction surface on the N-terminal OB-fold domain of RPA70 binds to both ATRIP and RAD9. Both ATRIP and RAD9 contain an acidic patch that is predicted to fold into an alpha-helical structure capable of binding into the basic cleft of RPA70N. Given its similarity to the ATRIP CRD, we have named this region the RAD9 CRD.
Mutations in ATRIP, RAD9, or RPA that disrupt the ATRIP-RPA70 or RAD9-RPA70 interactions cause defects in checkpoint protein localization (this study and references 2 and 3). Both the RAD9-crd and the RPA70 R41E R43E mutant proteins cause similar deficiencies in ATR-dependent signaling to CHK1. The ATRIP-crd mutant does not cause a detectable ATR signaling defect, at least as assayed by RNA interference complementation analysis (3). However, it is possible that there are other contact points between RPA and ATRIP (40), and there are RPA-independent means of localizing the ATR-ATRIP complex (22, 27, 48). RPA frequently binds proteins using multiple subunits (43), and both RPA70 and RPA32 have been implicated in RAD9 interactions (47).
Our data on the role of human RPA in checkpoint signaling are consistent with the function of Saccharomyces cerevisiae RPA (Rfa). The rfa1-t11 mutant in budding yeast supports DNA replication but is compromised in DNA damage responses, including a defect in loading of the yeast 9-1-1 complex (4, 29, 33, 46). This mutation is a charge reversal in Rfa1 at residue K45 (analogous to the R41 site of human RPA70). Our data are also consistent with a recent analysis of a human RPA70 R41E Y42F mutant, which was reported to cause a G2/M checkpoint defect without causing a defect in ssDNA binding or DNA replication (21).
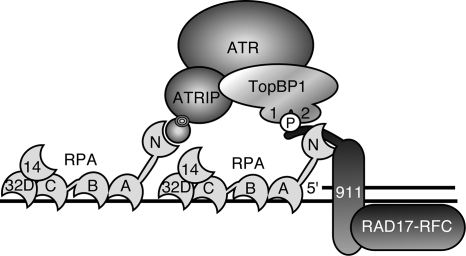
The RPA70N OB-fold domain is attached to the rest of RPA70 through a flexible linker (25). This flexibility may be important in allowing RPA to recruit different checkpoint proteins and position them correctly for ATR kinase activation. In addition, since RPA binds ssDNA with a defined 5′-3′ polarity (15, 24), the RPA70N OB-fold domain is positioned near the 5′ end of a primer-template junction (Fig. 7). This position places it in an ideal location to mediate the 5′-junction specificity of 9-1-1 complex loading (18, 36). The RPA-RAD9 interaction may also help to position the RAD9 tail such that it can present TopBP1 to ATR-ATRIP complexes in an optimal orientation.
FIG. 7.
Simplified model of the RPA protein and DNA interactions that promote ATR signaling. RPA is a heterotrimer of three subunits, RPA70, RPA32, and RPA14, that contain six OB-fold domains. Four of these OB-fold domains can bind ssDNA in a specific orientation such that the amino-terminal RPA70 OB-fold domain (70N) is positioned near the 5′ primer-template junction. The RPA70N OB-fold domain binds checkpoint proteins, including ATRIP and RAD9. These interactions help to concentrate ATR-ATRIP and 9-1-1-TopBP1 complexes to promote TopBP1's activation of ATR. Many additional protein-protein and protein-nucleic acid interactions also participate but are not shown in this simplified diagram.
Since ATRIP and RAD9 bind to the same surface of RPA, they are unlikely to be capable of binding at the same time. Indeed, ATRIP and RAD9 compete for binding to RPA70N (Fig. 3B). This raises an interesting question about the timing of these interactions. Competitive binding to the same RPA surface may indicate that at least two molecules of RPA need to be present to efficiently activate ATR. Such a scenario would ensure that the ATR checkpoint is activated only when longer stretches of ssDNA are available. This hypothesis is consistent with the ssDNA length dependency for ATR activation that was observed using defined DNA templates to activate ATR in X. laevis egg extracts (35). It is also possible that another protein retains the ATR-ATRIP or 9-1-1 complexes at the site of DNA damage or replication stress. Once loaded, the 9-1-1 checkpoint clamp is topologically linked to the DNA. The RPA interaction may serve to decrease its ability to slide away from the ssDNA gap.
While we did not pursue our observation that MRE11 contains a peptide that binds RPA70N (Fig. 2), a recent publication mapped an RPA binding surface on MRE11 to amino acids 521 to 569 (41). Notably, this region contains the acidic peptide that we used in the NMR experiment. Furthermore, double mutation of D543 and D544 in MRE11 abolished the RPA interaction, impaired the localization of MRE11 to replication centers, and caused a defect in the S-phase checkpoint (41). Our data suggest that this mutant disrupts an MRE11 CRD that interacts with the basic cleft of RPA70N. These data further support our conclusions that RPA70N provides a common checkpoint protein binding surface, and RPA-ssDNA is a signal for recruitment of multiple DNA damage response proteins.
The ATR signaling pathway is a potential target for cancer therapy (11, 28). ATR signaling inhibitors are expected to sensitize cells to DNA-damaging agents. Cancer cells are also known to have higher levels of replicative stress than adjacent normal cells (5, 20). Thus, cancer cells may be more dependent on replication stress responses than normal cells to complete replication and retain viability. Thus, targeting the replication stress response could be a useful therapy. Our results suggest that the basic cleft in RPA70N may be a useful target for the development of a protein-protein interaction inhibitor. Inhibiting RPA from binding multiple checkpoint proteins (at least ATRIP, RAD9, MRE11, and p53) should significantly impair the replication stress response. We are currently investigating whether our understanding of the structural basis for binding specificity can enable the development of a selective DNA damage checkpoint inhibitor that suppresses the DNA damage response without eliminating the essential replication function of RPA.
Acknowledgments
This work was supported by grants from the National Cancer Institute (R01 CA102729 to D.C.) and the National Institute of General Medical Sciences (R01 GM065484 to W.J.C.). Support for research facilities was provided by the Vanderbilt Center for Molecular Toxicology (grant P30 ES000267) and the Vanderbilt-Ingram Cancer Center (grant P30 CA068485).
We thank Howard Lieberman and Larry Karnitz for kindly providing reagents, Cheryl Arrowsmith for providing the NMR chemical shift assignments for RPA70N, and Earl Ruley and Qing Ling for assistance with ES cell culture protocols.
Footnotes
Published ahead of print on 20 October 2008.
REFERENCES
- 1.Adams, K. E., A. L. Medhurst, D. A. Dart, and N. D. Lakin. 2006. Recruitment of ATR to sites of ionising radiation-induced DNA damage requires ATM and components of the MRN protein complex. Oncogene 253894-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball, H. L., M. R. Ehrhardt, D. A. Mordes, G. G. Glick, W. J. Chazin, and D. Cortez. 2007. Function of a conserved checkpoint recruitment domain in ATRIP proteins. Mol. Cell. Biol. 273367-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball, H. L., J. S. Myers, and D. Cortez. 2005. ATRIP binding to RPA-ssDNA promotes ATR-ATRIP localization but is dispensable for Chk1 phosphorylation. Mol. Biol. Cell 162372-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlow, J. H., M. Lisby, and R. Rothstein. 2008. Differential regulation of the cellular response to DNA double-strand breaks in G1. Mol. Cell 3073-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartkova, J., Z. Horejsi, K. Koed, A. Kramer, F. Tort, K. Zieger, P. Guldberg, M. Sehested, J. M. Nesland, C. Lukas, T. Orntoft, J. Lukas, and J. Bartek. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434864-870. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez, V. P., L. A. Lindsey-Boltz, A. J. Cesare, Y. Maniwa, J. D. Griffith, J. Hurwitz, and A. Sancar. 2003. Loading of the human 9-1-1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17-replication factor C complex in vitro. Proc. Natl. Acad. Sci. USA 1001633-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binz, S. K., A. M. Sheehan, and M. S. Wold. 2004. Replication protein A phosphorylation and the cellular response to DNA damage. DNA Repair (Amsterdam) 31015-1024. [DOI] [PubMed] [Google Scholar]
- 8.Bochkareva, E., L. Kaustov, A. Ayed, G. S. Yi, Y. Lu, A. Pineda-Lucena, J. C. Liao, A. L. Okorokov, J. Milner, C. H. Arrowsmith, and A. Bochkarev. 2005. Single-stranded DNA mimicry in the p53 transactivation domain interaction with replication protein A. Proc. Natl. Acad. Sci. USA 10215412-15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byun, T. S., M. Pacek, M. C. Yee, J. C. Walter, and K. A. Cimprich. 2005. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 191040-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimprich, K. A., and D. Cortez. 2008. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9616-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins, I., and M. D. Garrett. 2005. Targeting the cell division cycle in cancer: CDK and cell cycle checkpoint kinase inhibitors. Curr. Opin. Pharmacol. 5366-373. [DOI] [PubMed] [Google Scholar]
- 12.Cortez, D., S. Guntuku, J. Qin, and S. J. Elledge. 2001. ATR and ATRIP: partners in checkpoint signaling. Science 2941713-1716. [DOI] [PubMed] [Google Scholar]
- 13.Costanzo, V., D. Shechter, P. J. Lupardus, K. A. Cimprich, M. Gottesman, and J. Gautier. 2003. An ATR- and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol. Cell 11203-213. [DOI] [PubMed] [Google Scholar]
- 14.Cuadrado, M., B. Martinez-Pastor, M. Murga, L. I. Toledo, P. Gutierrez-Martinez, E. Lopez, and O. Fernandez-Capetillo. 2006. ATM regulates ATR chromatin loading in response to DNA double-strand breaks. J. Exp. Med. 203297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Laat, W. L., E. Appeldoorn, K. Sugasawa, E. Weterings, N. G. Jaspers, and J. H. Hoeijmakers. 1998. DNA-binding polarity of human replication protein A positions nucleases in nucleotide excision repair. Genes Dev. 122598-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delacroix, S., J. M. Wagner, M. Kobayashi, K. Yamamoto, and L. M. Karnitz. 2007. The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev. 211472-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodson, G. E., Y. Shi, and R. S. Tibbetts. 2004. DNA replication defects, spontaneous DNA damage, and ATM-dependent checkpoint activation in replication protein A-deficient cells. J. Biol. Chem. 27934010-34014. [DOI] [PubMed] [Google Scholar]
- 18.Ellison, V., and B. Stillman. 2003. Biochemical characterization of DNA damage checkpoint complexes: clamp loader and clamp complexes with specificity for 5′ recessed DNA. PLoS Biol. 1E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanning, E., V. Klimovich, and A. R. Nager. 2006. A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res. 344126-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorgoulis, V. G., L. V. Vassiliou, P. Karakaidos, P. Zacharatos, A. Kotsinas, T. Liloglou, M. Venere, R. A. Ditullio, Jr., N. G. Kastrinakis, B. Levy, D. Kletsas, A. Yoneta, M. Herlyn, C. Kittas, and T. D. Halazonetis. 2005. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434907-913. [DOI] [PubMed] [Google Scholar]
- 21.Haring, S. J., A. C. Mason, S. K. Binz, and M. S. Wold. 2008. Cellular functions of human RPA1. Multiple roles of domains in replication, repair, and checkpoints. J. Biol. Chem. 28319095-19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermand, D., and P. Nurse. 2007. Cdc18 enforces long-term maintenance of the S phase checkpoint by anchoring the Rad3-Rad26 complex to chromatin. Mol. Cell 26553-563. [DOI] [PubMed] [Google Scholar]
- 23.Hopkins, K. M., W. Auerbach, X. Y. Wang, M. P. Hande, H. Hang, D. J. Wolgemuth, A. L. Joyner, and H. B. Lieberman. 2004. Deletion of mouse Rad9 causes abnormal cellular responses to DNA damage, genomic instability, and embryonic lethality. Mol. Cell. Biol. 247235-7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iftode, C., and J. A. Borowiec. 2000. 5′→3′ molecular polarity of human replication protein A (hRPA) binding to pseudo-origin DNA substrates. Biochemistry 3911970-11981. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs, D. M., A. S. Lipton, N. G. Isern, G. W. Daughdrill, D. F. Lowry, X. Gomes, and M. S. Wold. 1999. Human replication protein A: global fold of the N-terminal RPA-70 domain reveals a basic cleft and flexible C-terminal linker. J. Biomol. NMR 14321-331. [DOI] [PubMed] [Google Scholar]
- 26.Jazayeri, A., J. Falck, C. Lukas, J. Bartek, G. C. Smith, J. Lukas, and S. P. Jackson. 2006. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 837-45. [DOI] [PubMed] [Google Scholar]
- 27.Jiang, G., and A. Sancar. 2006. Recruitment of DNA damage checkpoint proteins to damage in transcribed and nontranscribed sequences. Mol. Cell. Biol. 2639-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaelin, W. G., Jr. 2005. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer 5689-698. [DOI] [PubMed] [Google Scholar]
- 29.Kanoh, Y., K. Tamai, and K. Shirahige. 2006. Different requirements for the association of ATR-ATRIP and 9-1-1 to the stalled replication forks. Gene 37788-95. [DOI] [PubMed] [Google Scholar]
- 30.Kim, S. M., A. Kumagai, J. Lee, and W. G. Dunphy. 2005. Phosphorylation of Chk1 by ATM- and Rad3-related (ATR) in xenopus egg extracts requires binding of ATRIP to ATR but not the stable DNA-binding or coiled-coil domains of ATRIP. J. Biol. Chem. 28038355-38364. [DOI] [PubMed] [Google Scholar]
- 31.Kumagai, A., J. Lee, H. Y. Yoo, and W. G. Dunphy. 2006. TopBP1 activates the ATR-ATRIP complex. Cell 124943-955. [DOI] [PubMed] [Google Scholar]
- 32.Lee, J., A. Kumagai, and W. G. Dunphy. 2007. The Rad9-Hus1-Rad1 checkpoint clamp regulates interaction of TopBP1 with ATR. J. Biol. Chem. 28228036-28044. [DOI] [PubMed] [Google Scholar]
- 33.Lee, S. E., J. K. Moore, A. Holmes, K. Umezu, R. D. Kolodner, and J. E. Haber. 1998. Saccharomyces Ku70, mre11/rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell 94399-409. [DOI] [PubMed] [Google Scholar]
- 34.Lovejoy, C. A., K. Lock, A. Yenamandra, and D. Cortez. 2006. DDB1 maintains genome integrity through regulation of Cdt1. Mol. Cell. Biol. 267977-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDougall, C. A., T. S. Byun, C. Van, M. C. Yee, and K. A. Cimprich. 2007. The structural determinants of checkpoint activation. Genes Dev. 21898-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majka, J., S. K. Binz, M. S. Wold, and P. M. Burgers. 2006. Replication protein A directs loading of the DNA damage checkpoint clamp to 5′-DNA junctions. J. Biol. Chem. 28127855-27861. [DOI] [PubMed] [Google Scholar]
- 37.Michael, W. M., R. Ott, E. Fanning, and J. Newport. 2000. Activation of the DNA replication checkpoint through RNA synthesis by primase. Science 2892133-2137. [DOI] [PubMed] [Google Scholar]
- 38.Mordes, D. A., G. G. Glick, R. Zhao, and D. Cortez. 2008. TopBP1 activates ATR through ATRIP and a PIKK regulatory domain. Genes Dev. 221478-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers, J. S., and D. Cortez. 2006. Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J. Biol. Chem. 2819346-9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Namiki, Y., and L. Zou. 2006. ATRIP associates with replication protein A-coated ssDNA through multiple interactions. Proc. Natl. Acad. Sci. USA 103580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson, E., C. J. Nievera, E. Liu, A. Y. Lee, L. Chen, and X. Wu. 2007. The Mre11 complex mediates the S-phase checkpoint through an interaction with replication protein A. Mol. Cell. Biol. 276053-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parrilla-Castellar, E. R., S. J. Arlander, and L. Karnitz. 2004. Dial 9-1-1 for DNA damage: the Rad9-Hus1-Rad1 (9-1-1) clamp complex. DNA Repair (Amsterdam) 31009-1014. [DOI] [PubMed] [Google Scholar]
- 43.Stauffer, M. E., and W. J. Chazin. 2004. Structural mechanisms of DNA replication, repair, and recombination. J. Biol. Chem. 27930915-30918. [DOI] [PubMed] [Google Scholar]
- 44.Stokes, M. P., R. Van Hatten, H. D. Lindsay, and W. M. Michael. 2002. DNA replication is required for the checkpoint response to damaged DNA in Xenopus egg extracts. J. Cell Biol. 158863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.St. Onge, R. P., B. D. Besley, J. L. Pelley, and S. Davey. 2003. A role for the phosphorylation of hRad9 in checkpoint signaling. J. Biol. Chem. 27826620-26628. [DOI] [PubMed] [Google Scholar]
- 46.Umezu, K., N. Sugawara, C. Chen, J. E. Haber, and R. D. Kolodner. 1998. Genetic analysis of yeast RPA1 reveals its multiple functions in DNA metabolism. Genetics 148989-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, X., S. M. Shell, and Y. Zou. 2005. Interaction and colocalization of Rad9/Rad1/Hus1 checkpoint complex with replication protein A in human cells. Oncogene 244728-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshioka, K., Y. Yoshioka, and P. Hsieh. 2006. ATR kinase activation mediated by MutSα and MutLα in response to cytotoxic O6-methylguanine adducts. Mol. Cell 22501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou, L., and S. J. Elledge. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 3001542-1548. [DOI] [PubMed] [Google Scholar]
- 50.Zou, L., D. Liu, and S. J. Elledge. 2003. Replication protein A-mediated recruitment and activation of Rad17 complexes. Proc. Natl. Acad. Sci. USA 10013827-13832. [DOI] [PMC free article] [PubMed] [Google Scholar]