Abstract
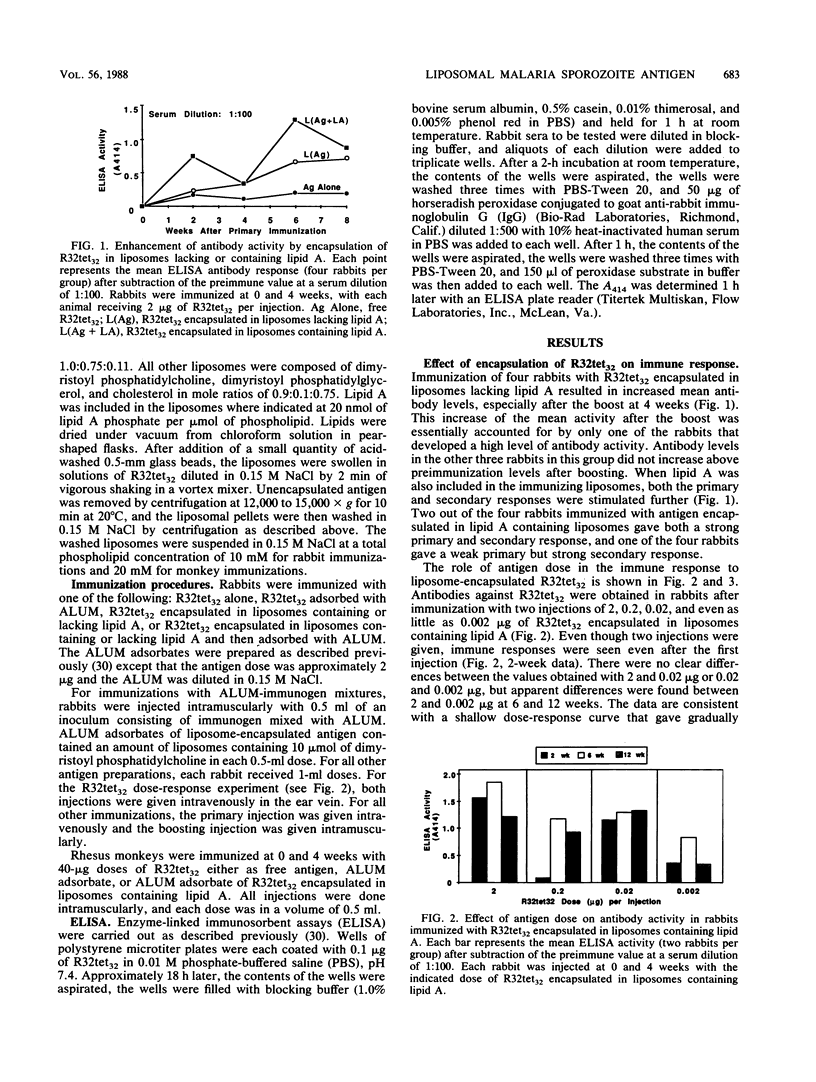
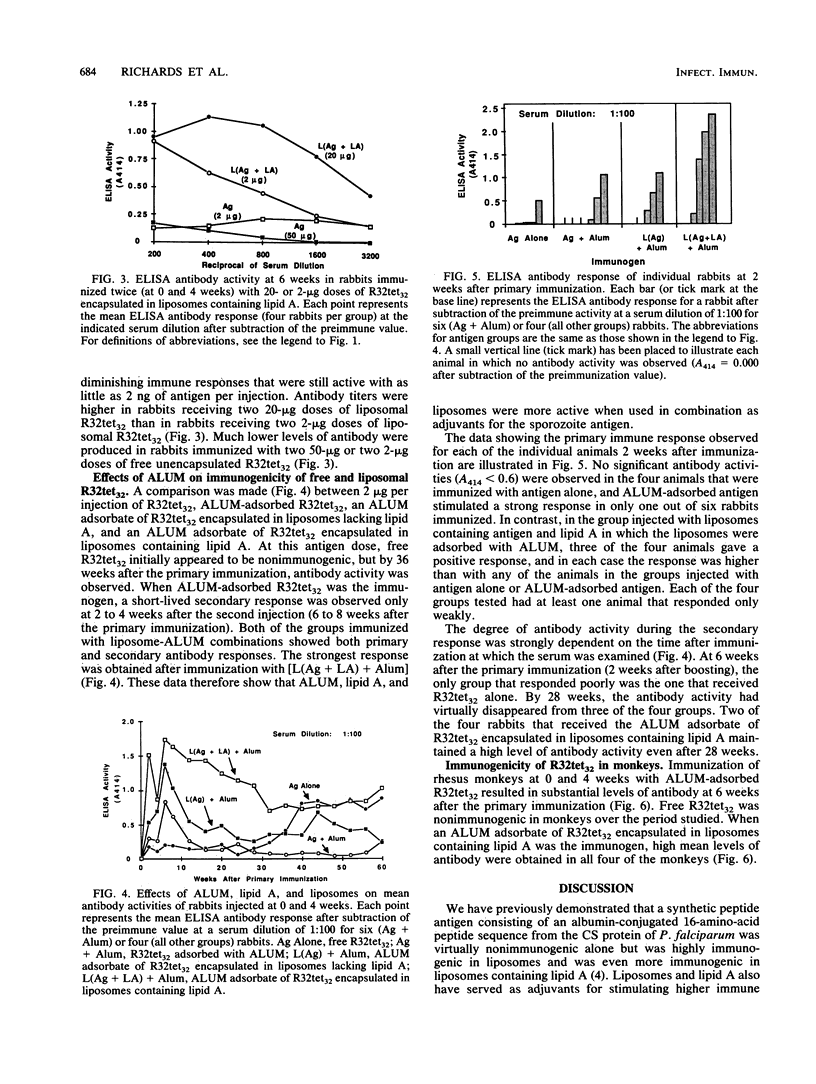
A liposome-encapsulated cloned protein (R32tet32) containing sequences from the tetrapeptide repeat region of the circumsporozoite protein of Plasmodium falciparum sporozoites was examined for immunogenicity with rabbits and monkeys. Effects of adjuvants were tested by encapsulation of the antigen in liposomes either lacking or containing lipid A and adsorption with aluminum hydroxide (ALUM). When rabbits were immunized with R32tet32 alone, a primary antibody response was not seen and a secondary response did not appear until 32 to 36 weeks after boosting. Immunization with ALUM-adsorbed R32tet32 resulted in a minimal primary antibody response. A moderate secondary antibody response was detected within 2 weeks after boosting, but antibody levels decreased to preimmunization levels 8 weeks after boosting. When R32tet32 was encapsulated in liposomes containing lipid A, strong primary and secondary antibody responses were observed. Strong primary and secondary responses also were obtained when R32tet32 was encapsulated in liposomes either containing or lacking lipid A and the liposomes were adsorbed with ALUM. The strongest antibody response was obtained by immunization with ALUM-adsorbed liposomes containing lipid A and R32tet32, suggesting that the adjuvant effects of liposomes, lipid A, and ALUM were additive or synergistic.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Coune A., Sculier J. P., Frühling J., Stryckmans P., Brassinne C., Ghanem G., Laduron C., Atassi G., Ruysschaert J. M., Hildebrand J. Iv administration of a water-insoluble antimitotic compound entrapped in liposomes. Preliminary report on infusion of large volumes of liposomes to man. Cancer Treat Rep. 1983 Nov;67(11):1031–1033. [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Danforth H. D., Aikawa M., Cochrane A. H., Nussenzweig R. S. Sporozoites of mammalian malaria: attachment to, interiorization and fate within macrophages. J Protozool. 1980 May;27(2):193–202. doi: 10.1111/j.1550-7408.1980.tb04680.x. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., Mellors J. W., Ryan J. L., Szoka F. C. Modulation of the biological activity of bacterial endotoxin by incorporation into liposomes. J Immunol. 1987 Apr 15;138(8):2663–2670. [PubMed] [Google Scholar]
- Edelman R. Vaccine adjuvants. Rev Infect Dis. 1980 May-Jun;2(3):370–383. doi: 10.1093/clinids/2.3.370. [DOI] [PubMed] [Google Scholar]
- Egan J. E., Weber J. L., Ballou W. R., Hollingdale M. R., Majarian W. R., Gordon D. M., Maloy W. L., Hoffman S. L., Wirtz R. A., Schneider I. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science. 1987 Apr 24;236(4800):453–456. doi: 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- Epstein M. A., Morgan A. J., Finerty S., Randle B. J., Kirkwood J. K. Protection of cottontop tamarins against Epstein-Barr virus-induced malignant lymphoma by a prototype subunit vaccine. Nature. 1985 Nov 21;318(6043):287–289. doi: 10.1038/318287a0. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Fainstein V., Hopfer R., Mehta K., Sullivan M. P., Keating M., Rosenblum M. G., Mehta R., Luna M., Hersh E. M. Liposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: a preliminary study. J Infect Dis. 1985 Apr;151(4):704–710. doi: 10.1093/infdis/151.4.704. [DOI] [PubMed] [Google Scholar]
- Mazier D., Mellouk S., Beaudoin R. L., Texier B., Druilhe P., Hockmeyer W., Trosper J., Paul C., Charoenvit Y., Young J. Effect of antibodies to recombinant and synthetic peptides on P. falciparum sporozoites in vitro. Science. 1986 Jan 10;231(4734):156–159. doi: 10.1126/science.3510455. [DOI] [PubMed] [Google Scholar]
- Morgan A. J., Epstein M. A., North J. R. Comparative immunogenicity studies on Epstein-Barr virus membrane antigen (MA) gp340 with novel adjuvants in mice, rabbits, and cotton-top tamarins. J Med Virol. 1984;13(3):281–292. doi: 10.1002/jmv.1890130310. [DOI] [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig V., Nussenzweig R. S., Collins W. E., Harinasuta K. T., Tapchaisri P., Chomcharn Y. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982 Jul 1;156(1):20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor P. T., Larsen H. S., Huang L., Rouse B. T. In vivo induction of anti-herpes simplex virus immune response by type 1 antigens and lipid A incorporated into liposomes. Infect Immun. 1982 Jun;36(3):1209–1216. doi: 10.1128/iai.36.3.1209-1216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey R. B., Hamner M. B., Alving B. M., Finlayson J. S., Alving C. R., Evatt B. L. Effects of lipid A and liposomes containing lipid A on platelet and fibrinogen production in rabbits. Blood. 1980 Aug;56(2):307–310. [PubMed] [Google Scholar]
- Richardson E. C., Banerji B., Seid R. C., Jr, Levin J., Alving C. R. Interactions of lipid a and liposome-associated lipid A with Limulus polyphemus amoebocytes. Infect Immun. 1983 Mar;39(3):1385–1391. doi: 10.1128/iai.39.3.1385-1391.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Ionescu-Matiu I., Dreesman G. R., Kramp W., Six H. R., Hollinger F. B., Melnick J. L. Humoral and cellular immunity to hepatitis B virus-derived antigens: comparative activity of Freund complete adjuvant alum, and liposomes. Infect Immun. 1980 Dec;30(3):728–733. doi: 10.1128/iai.30.3.728-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzak E., Wolff S. M., Dinarello C. A., Conlon T., el Kholy A., Bahr G. M., Choay J. P., Morin A., Chedid L. Clinical evaluation of the immunoadjuvant murabutide, a derivative of MDP, administered with a tetanus toxoid vaccine. J Infect Dis. 1986 Mar;153(3):628–633. doi: 10.1093/infdis/153.3.628. [DOI] [PubMed] [Google Scholar]
- Vosika G. J., Barr C., Gilbertson D. Phase-I study of intravenous modified lipid A. Cancer Immunol Immunother. 1984;18(2):107–112. doi: 10.1007/BF00205743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Glezen W. P., Hannoun C., Laver W. G. Potentiation of the immune response to influenza virus subunit vaccines. J Immunol. 1977 Dec;119(6):2073–2077. [PubMed] [Google Scholar]
- Wirtz R. A., Ballou W. R., Schneider I., Chedid L., Gross M. J., Young J. F., Hollingdale M., Diggs C. L., Hockmeyer W. T. Plasmodium falciparum: immunogenicity of circumsporozoite protein constructs produced in Escherichia coli. Exp Parasitol. 1987 Apr;63(2):166–172. doi: 10.1016/0014-4894(87)90158-5. [DOI] [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Endotoxin enhanced adjuvant effect of liposomes, particularly when antigen and endotoxin are incorporated within the same liposome. Immunol Commun. 1980;9(8):747–757. doi: 10.3109/08820138009109684. [DOI] [PubMed] [Google Scholar]


