Abstract
Purpose of review
To identify recent papers showing how human and parasite genetics influence leishmaniasis, and how understanding of the immunopathology may be utilized in immunotherapy for these diseases.
Recent findings
Progress has been made in recent years showing the complexity within populations of Leishmania spp. and indicating that different strains lead to diverse clinical pictures and responses to treatment. Thus detection of parasite genetic tags for the precise identification of infecting strains, and for predictive diagnosis of clinical and therapeutic fates seems now possible. Host genetic loci involved in disease outcome have been detected, which may also be explored for better case management. These developments in diagnosis will demand expanding the therapeutic arsenal to take their expected effect. This is starting to be fulfilled by immunotherapies successfully employed to treat cases refractory to standard first line drugs, as the result of a more profound comprehension of the immunopathology of the leishmaniases.
Summary
The knowledge mounting has already helped explain why different patients present different forms of leishmaniasis and respond differently to treatment, and may be on the verge of catalyzing a major change in the already over a century old paradigm of diagnosing and managing these patients.
Keywords: immune response, leishmania, leishmaniasis, polymorphism, treatment
Introduction
Leishmaniasis refers to a widely divergent constellation of disease syndromes caused by the Leishmania spp. protozoa. The extracellular promastigote form of leishmania is inoculated into humans by a sandfly, after which it undergoes phagocytosis by a mammalian macrophage and transforms to an intracellular amastigote. Major forms of leishmaniasis include cutaneous, mucosal and visceral leishmaniasis although many variations occur. Differences in clinical manifestations of leishmaniasis are explained only in part by the species of organism causing the infection [1]. Many publications delineate the murine and human immune responses during leishmaniasis. However, an understanding of host immunity alone is unlikely to unveil how a single genus is able to cause such a heterogeneous group of diseases. Understanding of the contributions of host and parasite genetic polymorphisms to the spectrum of the leishmaniases may provide insight into disease immunopathogenesis, providing the information needed to make immunotherapy a feasible choice within the therapeutic arsenal against these microorganisms. This review summarizes recent developments in immunity, host genetics and parasite variability that could translate into future approaches to disease management.
The type one response is key to immunity and pathology in leishmaniasis
A type 1 immune response is necessary to control leishmania multiplication and dissemination in humans. In contrast, interleukin (IL)-10 and transforming growth factor (TGF)-β facilitate leishmania proliferation. Promastigotes stimulate interferon (IFN)-γ production by natural killer (NK) cells [2], and IFN-γ in turn activates macrophages to kill leishmania. Even so, progression of leishmaniasis with extensive pathology occurs even in the presence of high levels of IFN-γ, especially in mucosal leishmaniasis [3,4]. Peripheral blood mononuclear cells (PBMCs) from individuals with visceral leishmaniasis and diffuse cutaneous leishmaniasis (DCL) produce low IFN-γ levels when stimulated with soluble leishmania antigen (SLA), allowing parasite multiplication and progressive disease [5]. Nevertheless, IFN-γ is detectable in sera of visceral leishmaniasis patients, suggesting the cytokine is produced by T cells in some body sites [6] but does not control leishmania growth. IL-10 and TGF-β play critical roles in downmodulating type 1 responses during visceral leishmaniasis and DCL, and IFN-γ production by PBMCs is restored in vitro by neutralization of IL-10 [5,7]. Interestingly, splenic T cells producing IL-10 are not CD4+ CD25+ (Foxp3) regulatory cells in Indian visceral leishmaniasis patients [6]. It was recently shown that IL-10 and TGF-β play important roles in the development of post-kala-azar dermal leishmaniasis (PKDL) [8]. Treatment of Indian visceral leishmaniasis patients with amphotericin B enhances IFN-γ and lowers IL-10 and TGF-β levels, and these treated patients rarely progress to PKDL. However, there are some patients in whom IL-10 and TGF-β levels remain high despite treatment with antimony; these individuals often experience reactivation in the form of PKDL [8].
During the earliest phase of localized cutaneous leishmaniasis due to Leishmania braziliensis, low levels of IFN-γ are observed whereas IL-10 is prominent. IL-10 plays a role in parasite persistence [9]. As the infection progresses, all clinical forms of L. braziliensis infection demonstrate antigen specific production of IFN-γ that predominates over IL-10 levels [10]. Leishmania-induced type 2 cytokines are usually low in concentration during both cutaneous leishmaniasis and mucosal leishmaniasis [9]. Even patients coinfected with L. braziliensis and helminths develop a predominant and exaggerated type 1 immune response to leishmania antigens, although these individuals take longer to heal than those without worms [11]. This delay to healing may be in part due to higher levels of IL-10 produced by these individuals.
Although a type 1 response is critical to control the leishmaniases, a loss of appropriate modulation of this response underlies the immunopathology of both visceral and tegumentary leishmaniases. During visceral leishmaniasis, the host loses or suppresses an appropriate type 1 immune response. In the case of tegumentary leishmaniasis, a strong type 1 immune response results in an intense inflammatory response and high expression of nitric oxide synthase (NOS) type 2 in active lesion sites. Late lesions progress toward cure and show only focal inflammation, with a decrease in CD8+ and increase in CD4+ T cells associated with the onset of fibrosis [12]. Leishmania-specific CD8+ T cells are generated during infection and are important for IFN-γ production and cytotoxicity against leishmania-infected macrophages [13], but they may also contribute to the immunopathology of leishmaniasis. Antigen-responsive CD8+ cells are in part responsible for the exaggerated type 1 immune response detected during cutaneous leishmaniasis and mucosal leishmaniasis [14]. These cells do not adequately respond to immunomodulation [3,5], possibly due to a decrease in the expression of IL-10 receptor on their surfaces [14]. Moreover, upregulation of IFN-γ production may stimulate the FAS/FASL pathway and induce apoptosis of keratinocytes with resultant ulcer formation [15]. Overall, these findings underscore the need for different strategies to control the type 1 immune response as a goal of treatment of the different clinical forms of leishmaniasis. During visceral leishmaniasis the goal would be a boost, whereas during tegumentary leishmaniasis the goal would be a partial downmodulation of type 1 cellular immunity.
Host genetic factors contribute to outcome in leishmaniasis
An expanding literature is documenting associations between human genetic loci influencing immunity and the different clinical forms and outcomes of leishmaniasis. Recent studies of the different disease phenotypes and outcomes suggest that genetic factors indeed contribute significantly to the outcome of leishmaniasis. Familial aggregation of visceral leishmaniasis in Brazilian populations suggested that genetic factors contribute to both disease and the development of a delayed-type hypersensitivity (DTH) skin test, a marker of cured symptomatic or asymptomatic infection [16–19]. Candidate gene studies indicated associations between polymorphic alleles of SLC11A1 (formerly called NRAMP1), IL4, IFNGR1, and TNFA (encoding TNF-α) and development of visceral leishmaniasis, asymptomatic infection, or PKDL [20–23]. In addition, several genome-wide scans have been performed using cohorts exposed to the Leishmania species causing visceral leishmaniasis. Bucheton et al. [21,24] reported linkage between a major locus on chromosome 22q12 and visceral leishmaniasis in one ethnic group in a village in the Sudan. However, a separate study documented linkage between visceral leishmaniasis and major loci on chromosomes 1p22 and 6q27 but not the chromosome 22 locus, in two Sudanese villages geographically near the Bucheton cohort, but representing different ethnic backgrounds [25]. Cohorts in these villages could be stratified by Y-chromosome markers indicating there were extended families originating from two male founders in this patriarchal society. Refined linkage analysis stratified according to the Y chromosome haplotype revealed the peaks of linkage originated from one male founder of one village population. These data suggest that analysis of extended pedigrees originating from a single founder, in this case a male founder marked by Y-chromosome haplotype, can reveal susceptibility loci influencing a subset of the entire population [25]. Although the Sudanese populations studied above were infected with Leishmania donovani, visceral leishmaniasis in Brazil is caused by the species Leishmania chagasi infantum [26]. A genome-wide scan of a population in northeast Brazil suggested regions on chromosomes 15 and 19 were possibly linked to the asymptomatic infection (i.e. protection against visceral leishmaniasis), whereas a distinct region on chromosome 9 was possibly linked to visceral leishmaniasis [27].
Genetic studies of tegumentary leishmaniasis have focused on association studies of candidate genes, in part because the more sporadic epidemiology of these infections is not conducive to large family studies, and because mucosal leishmaniasis is rare making family-based studies difficult. Alleles of the TNFA locus are associated with both mucosal leishmaniasis and cutaneous leishmaniasis [28]. Analysis of the IL-10 819 polymorphism showed that the CC allele is associated with higher levels of IL-10 than the CT or TT genotypes, and with an increased risk of cutaneous leishmaniasis [9]. In a Brazilian population at high risk for L. braziliensis infection, an IL-6 174 G/C promoter polymorphism was found to be strongly associated with susceptibility to mucosal leishmaniasis but not to cutaneous leishmaniasis [29]. A more thorough understanding of polymorphic host loci that contribute to the subtle determinants of the immune response to this genus of parasites could help explain the individual variability in disease outcome.
Parasite strains as determinants of clinical and therapeutic outcomes of leishmaniasis
Treatment failure is often caused by a lack of appropriate patient’s adherence to first and second line drugs, but there is also a steadily growing body of literature indicating that Leishmania spp. is developing resistance to the most common drugs used to treat the disease, the antimonials [30,31]. Drug resistance is a well recognized problem in Indian visceral leishmaniasis, inspiring treatment trials of new medications [31–34]. Failure of antimony treatment of tegumentary leishmaniasis, the only current measure of resistant parasite strains, has come from highly affected countries such as Iran [35], Peru [36•] and Brazil [37,38]. Current population shifts due to military activities in the Middle East have greatly changed the prevalence of disease, and it is likely that other regions of resistance will emerge in the near future.
Drug resistant strains are being reported amongst evolutionarily distant species (e.g. Leishmania tropica, L. donovani and L. braziliensis) [35,36•,37,38,39•], suggesting that parasites are capable of adapting to drug pressure through parallel and diverse mechanisms. Recent data show that, at least among L. tropica strains, resistance to one antimony based drug confers crossresistance to antimonials but not other drugs [40•]. Furthermore, reports have emphasized that susceptibility to antimonials varies markedly among species and even between geographically distant strains of a same species of Leishmania, emphasizing the role of parasite polymorphisms on antimony resistance/susceptibility [35,36•,37,38,39•]. A recent report showed that the glycoproteins collectively called proteophosphoglycans (PPGs) are overexpressed on the surfaces of promastigotes and amastigotes of stibogluconate resistant clinical isolates of L. donovani from India [39•]. It was also observed that the redox active molecule trypanothione, which serves as the major low molecular weight thiol of the Leishmania spp., underlies antimony resistance of Leishmania tarentolae isolates [41]. As there are currently no markers of drug resistance amongst parasite isolates, it would be of great value to explore the use of PPGs and thiol levels for this purpose.
Several recent studies address molecules that confer parasite resistance to oxidant stress. Arginine metabolism is a determinant of parasite killing or survival in macrophages. Macrophages are stimulated to undergo classical activation and express iNOS by the type 1 cytokines IFN-γ and TNF-α, or stimulated toward alternative activation by the type 2 cytokine IL-4 promoting expression of macrophage arginase [42]. Arginine metabolized by iNOS produces the potent leishmanicidal molecule nitric oxide, whereas metabolism of arginine by arginase results in production of L-ornithine and other polyamines that are essential for intracellular leishmania growth [43]. Recently, it was recognized that leishmania parasite-encoded arginase also contributes to the local macrophage arginine concentration, influencing the macrophage response. Infections in mice inoculated with L. mexicana genetically lacking both alleles of the gene encoding arginase were significantly attenuated in comparison to infections with both wildtype and add-back control parasites. Arginase knock out parasites were defective in their ability to survive intracellularly in macrophages. Importantly, however, this intracellular growth defect was corrected when arginase knockout parasites were grown in macrophages from mice lacking the iNOS gene. This suggests that parasite arginase utilizes and depletes host cell arginine pools, diminishing the amount of substrate available to iNOS for generating NO•. Parasites lacking arginase, therefore, allow arginine concentrations to accumulate, enhancing the activity of iNOS and increasing the generation of the potent leishmanicidal molecule NO• exclusively in host macrophage that expresses iNOS [44•].
Consistent with the previous report, a recent report showed that clinical isolates of Leishmania amazonensis and L. braziliensis may exhibit resistance or susceptibility to NO•-mediated microbicidal activity. Isolates that were resistant to NO• in vitro were derived from human cases of disease with poorer outcomes [45]. These data are also consistent with previous and recent reports showing that the insulin-like growth factor I (IGF-I) promotes in-vivo and in-vitro growth among different species of Leishmania, acting directly on the parasites, at least in part, via activation of their arginase [46•].
Resistance to intracellular hydrogen peroxide is another phenotype implicated in progressive leishmaniasis [47]. It was recently reported that clones of Leishmania guyanensis capable of metastasis in golden hamsters contain cytoplasmic peroxiredoxin and peroxidase activities that differ from those of nonmetastatic parasites [48]. Also, laboratory strains of L. guyanensis that are capable of metastasis contain different isoforms of the molecules tryparedoxin peroxidase and elongation factor-1β compared with those of nonmetastatic L. guyanensis strains [49]. The tryparedoxin/tryparedoxin peroxidase system is a critical counterpart of the trypanothione–trypanothione reductase system, allowing parasites to both reduce and oxidize the low molecular weight thiol trypanothione and in this manner evade oxidative killing [41,50].
Several previous reports underscore the major role of parasite polymorphism on infection outcome. One goal of such research is to identify markers that would allow us to track different strains and improve epidemiologic detection of outbreaks as well as case management. Epidemiological studies have also indicated that parasite genotype influences disease even at the intraspecies level. Two reports from Colombia [51,52] described an increased frequency of mucosal involvement among humans infected with particular L. braziliensis zymodemes or strains. Another more recent report described a multiclonal population of L. braziliensis from a region endemic for tegumentary leishmaniasis in northeastern Brazil, and also reported a statistically significant association between distinct parasite genotypes (clades) and the clinical outcome (i.e. cutaneous leishmaniasis, mucosal leishmaniasis or disseminated leishmaniasis) [53].
Studies of Old World L. donovani infections have shown that development of PKDL is, at least in part, strain dependent. Such parasite strains may be differentiated by the use of genetic tags [54,55]. Recent developments have even indicated that some genes are significantly overexpressed among isolates from patients with PKDL compared with those obtained from cases of visceral leishmaniasis [56].
Immunotherapy as an emerging option for treating cutaneous leishmaniasis and mucosal leishmaniasis
There is still no effective vaccine against human leishmaniasis. Human trials using killed parasites and recombinant proteins for vaccination have resulted in only short-term protective immunity [5,10]. Only a few advances have been made in the treatment of these disorders over recent decades. The pentavalent antimonials are no longer practical as a standard treatment for Indian kala-azar due to high failure rates, and concerns have also arisen about resistance in cutaneous leishmaniasis and mucosal leishmaniasis [36•].
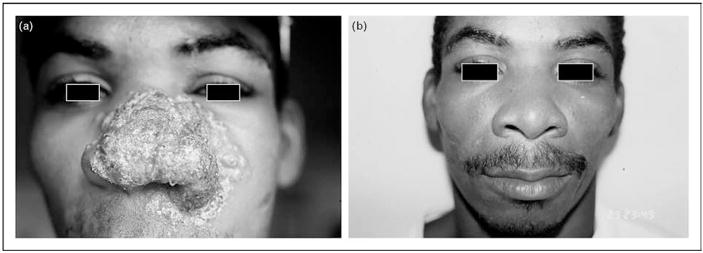
Miltefosine and aminosidine (paromomycin) are promising treatment options being pursued [57,58]. Furthermore, immunotherapy combined with standard antimony treatment has been successfully tested in cutaneous leishmaniasis and mucosal leishmaniasis. The immuno-modulatory options are based upon the rationale that a T helper type 1 (Th1)-mediated inflammatory response causes the pathology of tegumentary leishmaniasis. In a double blind controlled trial, addition of the TNF-α inhibitor pentoxifylline to standard antimony treatment was more effective than antimony and placebo, reducing the healing time of mucosal lesions (Fig. 1) [59]. Similar findings were reported for patients infected with Leishmania major [5]. Several studies provide evidence that granulocyte macrophage colony-stimulating factor (GM-CSF) given in conjunction with antimony is more effective and leads to shorter healing time of the cutaneous ulcers than antimony and placebo [60,61]. Many studies are needed to test the efficacy of these already-tried immunotherapeutic approaches as well as other new and promising therapies to determine whether they are effective against infection with the different forms and species of Leishmania.
Figure 1. Immunotherapy is a promising new tool in the arsenal to fight leishmaniasis.
(a) Facial lesion of a disseminated leishmaniasis patient after the fourth consecutive course of standard treatment with intravenous Glucantime at 20 mg/kg of body weight for 30 days. (b) The same patient after a single course of Glucantime (20 mg/kg of body weight) as in (a), combined with oral pentoxifylline at 400 mg, three times a day for 30 days.
Conclusion
The diagnostic approach to leishmaniasis has remained unchanged over the last century. Studies of parasite and host genotypes are revealing several tiers to the complexity of leishmaniasis. Data summarized in this review suggest that the experimental basis is emerging that could lead to new paradigms in the approach to diagnosis and management of these diseases. Precise identification of parasite strains and correlation with clinical outcome could allow more meaningful approaches to treatment regimens. Identification of host loci that influence immune responses to infection might prove helpful in identifying new immunotherapeutic approaches and causes of treatment failure. New drugs and new approaches to immunotherapy could provide the greater array of therapeutic options that is critically needed to control this polymorphic group of diseases.
Acknowledgments
We thank Luiz Henrique Guimarães and Olívia Bacellar for helping prepare this review. This work was supported by NIH grant # AI-30639.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• • of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 556–558).
- 1.WHO. Leishmaniasis: burden of disease. 2007. http://www.who.int/leishmaniasis/burden/en.
- 2.Nylen S, Masho K, Soderstrom K, et al. Live Leishmania promastigotes can directly activate primary human natural killer cells to produce interferon-gamma. Clin Exp Immunol. 2003;131:457–467. doi: 10.1046/j.1365-2249.2003.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho LP, Passos S, Bacellar O, et al. Differential immune regulation of activated T cell between cutaneous and mucosal leishmaniasis as a model for pathogenesis. Par Immunol. 2007;29:251–258. doi: 10.1111/j.1365-3024.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaze ST, Dutra WO, Lessa M, et al. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol. 2006;63:70–78. doi: 10.1111/j.1365-3083.2005.01707.x. [DOI] [PubMed] [Google Scholar]
- 5.Almeida RP, De Jesus AR, Rosa MEA, et al. Immunopathogenesis and immunotherapy in tegumentary leishmaniasis. Curr Res Immunol. 2007;1:91–126. [Google Scholar]
- 6.Nylen S, Maurya R, Eidsmo L, et al. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaiasis. J Exp Med. 2007;204:805–817. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28:378–384. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Saha S, Mondal S, Ravindran R, et al. IL-10 and TGF-beta-mediated susceptibility in kala-azar and postkala-azar dermal leishmaniasis: the significance of amphoterecin B in the control of Leishmania donovani infection in India. J Immunol. 2007;179:5592–5603. doi: 10.4049/jimmunol.179.8.5592. [DOI] [PubMed] [Google Scholar]
- 9.Salhi A, Rodrigues V, Jr, Santoro F, et al. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J Immunol. 2008;180:6139–6148. doi: 10.4049/jimmunol.180.9.6139. [DOI] [PubMed] [Google Scholar]
- 10.Gomes-Silva A, Bittar RC, Nogueira RS, et al. Can interferon-γ and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin Exp Immunol. 2007;149:440–444. doi: 10.1111/j.1365-2249.2007.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Neal S, Guimarães LH, Machado PR, et al. Influence of helminth infections on the clinical course of and immune response to Leishmania braziliensis cutaneous leishmaniasis. J Infect Dis. 2007;195:142–148. doi: 10.1086/509808. [DOI] [PubMed] [Google Scholar]
- 12.Morgado FN, Schubach A, Rosalino CMV, et al. Is the in situ inflammatory reaction an important tool to understand the cellular immune response in American tegumentary leishmaniasis? Br J Dermatol. 2007;158:50–58. doi: 10.1111/j.1365-2133.2007.08255.x. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz JH, Becker I. CD8 cytotoxic T cells in cutaneous leishmaniasis. Par Immunol. 2007;29:671–678. doi: 10.1111/j.1365-3024.2007.00991.x. [DOI] [PubMed] [Google Scholar]
- 14.Faria DR, Gollob KJ, Barbosa J, Jr, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–7859. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eidsmol L, Nylen S, Khamesipour A, et al. The contribution of the Fas/Fasl apoptotic pathway in ulcer formation during leishmania major-induced cutaneous leishmaniasis. Am J Pathol. 2005;166:1099–1108. doi: 10.1016/S0002-9440(10)62330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabello PH, Lima AMVMD, Azevedo ES, Krieger H. Familial aggregation of Leishmania chagasi infection in northeastern Brazil. Am J Trop Med Hyg. 1995;52:364–365. doi: 10.4269/ajtmh.1995.52.364. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim ME, Lambson B, Yousif AO, et al. Kala-azar in a high transmission foci: an ethnic and geographical dimension. Am J Trop Med Hyg. 1999;61:941–944. doi: 10.4269/ajtmh.1999.61.941. [DOI] [PubMed] [Google Scholar]
- 18.Zijlstra EE, El-Hassan AM, Ismael A, Ghalib HW. Endemic kala-azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post-kala-azar dermal leishmaniasis. Am J Trop Med Hyg. 1994;51:826–836. doi: 10.4269/ajtmh.1994.51.826. [DOI] [PubMed] [Google Scholar]
- 19.Peacock CS, Collins A, Shaw MA, et al. Genetic epidemiology of visceral leishmaniasis in northeastern Brazil. Gen Epidemiol. 2001;20:383–396. doi: 10.1002/gepi.8. [DOI] [PubMed] [Google Scholar]
- 20.Karplus TM, Jeronimo SMB, Chang H, et al. An association between the TNF locus and the clinical outcome of Leishmania chagasi infection. Infect Immun. 2002;70:6919–6925. doi: 10.1128/IAI.70.12.6919-6925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucheton B, Abel L, Kheir MM, et al. Genetic control of visceral leishmaniasis in a Sudanese population: candidate gene testing indicates a linkage to the NRAMP1 region. Genes Immun. 2003;4:104–109. doi: 10.1038/sj.gene.6363927. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed HS, Ibrahim ME, Miller EN, et al. Genetic susceptibilty to visceral leishmaniasis in the Sudan: linkage and association with IL4 and IFNGR1. Genes Immun. 2003;4:351–355. doi: 10.1038/sj.gene.6363977. [DOI] [PubMed] [Google Scholar]
- 23.Mohamed HS, Ibrahim ME, Miller EN, et al. SLC11A1 (formerly NRAMP1) and susceptibility to visceral leishmaniasis in the Sudan. Eur J Hum Genet. 2004;12:66–74. doi: 10.1038/sj.ejhg.5201089. [DOI] [PubMed] [Google Scholar]
- 24.Bucheton B, Abel L, El-Safi S, et al. A major susceptibility locus on chromosome 22q12 plays a critical role in the control of kala-azar. Am J Hum Genet. 2003;73:1052–1060. doi: 10.1086/379084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller EN, Fadl M, Mohamed HS, et al. Y chromosome lineage- and village-specific genes on chromosomes 1p22 and 6q27 control visceral leishmaniasis in Sudan. PLoS Genet. 2007;3:e71. doi: 10.1371/journal. pgen.0030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauricio IL, Stothard JR, Miles MA. The strange case of Leishmania chagasi. Parasitol Today. 2000;16:188–189. doi: 10.1016/s0169-4758(00)01637-9. [DOI] [PubMed] [Google Scholar]
- 27.Jeronimo SMB, Duggal P, Ettinger NA, et al. Genetic predisposition to self-curing infection with the protozoan leishmania chagasi: a genomewide scan. J Infect Dis. 2007;196:1261–1269. doi: 10.1086/521682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabrera M, Shaw MA, Sharples C, et al. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med. 1995;182:1259–1264. doi: 10.1084/jem.182.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castelluci L, Menezes E, Oliveira J, et al. IL6-174 G/C promoter polymorphism influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. JID. 2006;194:519–527. doi: 10.1086/505504. [DOI] [PubMed] [Google Scholar]
- 30.Berman J. Current treatment approaches to leishmaniasis. Curr Opin Infect Dis. 2003;16:397–401. doi: 10.1097/00001432-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Chappius F, Sundar S, Hailu A. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nature Rev Microbiol. 2007;5:S7–S16. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 32.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundar S, Mehta H, Suresh AV, et al. Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clin Infect Dis. 2004;38:377–383. doi: 10.1086/380971. [DOI] [PubMed] [Google Scholar]
- 34.Sundar S, Jha TK, Thakur CP, et al. Oral miltefosine for Indial visceral leishmaniasis. N Engl J Med. 2002;347:1739–1746. doi: 10.1056/NEJMoa021556. [DOI] [PubMed] [Google Scholar]
- 35.Hadighi R, Mohebali M, Boucher P, et al. Unresponsiveness to glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLOS Med. 2006;3:659–667. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Llanos-Cuentas A, Tulliano G, Araujo-Castillo R, et al. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin Infect Dis. 2008;46:223–231. doi: 10.1086/524042. Risk factors for antimony failure in CL included age, short disease duration (less than five weeks), and infection with Leishmania peruviana or L. braziliensis. [DOI] [PubMed] [Google Scholar]
- 37.Arevalo J, Ramirez L, Adaui V, et al. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis. 2007;195:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- 38.Azeredo-Coutinho RB, Mendonça SC, Callahan H, et al. Sensitivity of Leishmania braziliensis promastigotes to meglumine antimoniate (glucantime) is higher than that of other Leishmania species and correlates with response to therapy in American tegumentary leishmaniasis. J Parasitol. 2007;93:688–693. doi: 10.1645/GE-1031R.1. [DOI] [PubMed] [Google Scholar]
- 39•.Samant M, Sahasrabuddhe AA, Singh N, et al. Proteophosphoglycan is differentially expressed in sodium stibogluconate-sensitive and resistant Indian clinical isolates of Leishmania donovani. Parasitology. 2007;134:1175–1184. doi: 10.1017/S0031182007002569. This study compared by FACS and western blot, and found statistically significant differences in the amounts of PPG between three isolates of L. donovani from cases of Bihar (India) that responded to sodium stibogluconate (SSB) and were in-vitro sensitive to SSB, and three isolates from cases that did not respond and were in-vitro resistant to SSB. [DOI] [PubMed] [Google Scholar]
- 40•.Hadighi R, Boucher P, Khamesipour A, et al. Glucantime-resistant Leishmania tropica isolated from Iranian patients with cutaneous leishmaniasis are sensitive to alternative antileishmania drugs. Parasitol Res. 2007;101:1319–1322. doi: 10.1007/s00436-007-0638-0. In this work intracellular amastigotes of L. tropica clinically and in-vitro resistant to Glucantime (N-methylglucamine) were crossresistant to Pentostam (stibogluconate), but sensitive to miltefosine and paromomycin. [DOI] [PubMed] [Google Scholar]
- 41.Wyllie S, Vickers TJ, Fairlamb AH. Roles of trypanothione-S-transferase and tryparedoxin peroxidase in resistance to antimonials. Antimicrob Agents Chemother. 2008;52:1359–1365. doi: 10.1128/AAC.01563-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hölscher C, Arendse B, Schwegmann A, et al. Impairment of alternative macrophage activation delays cutaneous leishmaniasis in nonhealing BALB/c mice. J Immunol. 2006;176:1115–1121. doi: 10.4049/jimmunol.176.2.1115. [DOI] [PubMed] [Google Scholar]
- 43.Iniesta V, Gómez-Nieto LC, Corraliza I. The inhibition of arginase by N-hydroxy-L-arginine controls the growth of leishmania inside macrophages. J Exp Med. 2001;193:777–783. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Gaur U, Roberts SC, Dalvi RP, et al. An effect of parasite-encoded arginase on the outcome of murine cutaneous leishmaniasis. J Immunol. 2007;179:8446–8453. doi: 10.4049/jimmunol.179.12.8446. This is a very elegant work that not only unprecedently implicates the parasite’s own arginase as a parasitism resilience factor, but also indicates that resistance to the host’s clearance mechanisms is mediated, at least in part, through the inhibition of nitric oxide production by the host cells detectable both during in-vitro experiments, and also during in-vivo infections of BALB/c mice with L. mexicana. [DOI] [PubMed] [Google Scholar]
- 45.Giudice A, Camada I, Leopoldo PTG, et al. Resistance of Leishmania (Leishmania) amazonensis and Leishmania (Viannia) braziliensis to nitric oxide correlates with disease severity in tegumentary leishmaniasis. BMC Infect Dis. 2007;7:1–12. doi: 10.1186/1471-2334-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Vendrame CMV, Carvalho MDT, Rios FJO, et al. Effect of insulin-like growth factor-I on Leishmania amazonensis promastigote arginase activation and reciprocal inhibition of NOS2 pathway in macrophage in vitro. Scand J Immunol. 2007;66:287–296. doi: 10.1111/j.1365-3083.2007.01950.x. This study shows that exposure of murine macrophages infected L. amazonensis to IGF-I leads to a significant increase in the expression and activities of both host and parasite arginases, and proliferation of the parasites within a 48h time course with the growth factor. [DOI] [PubMed] [Google Scholar]
- 47.Miller MA, McGowan SE, Gantt KR, et al. Inducible resistance to oxidant stress in the protozoan Leishmania chagasi. J Biol Chem. 2000;275:33883–33889. doi: 10.1074/jbc.M003671200. [DOI] [PubMed] [Google Scholar]
- 48.Acestor N, Masina S, Ives A, et al. Resistance to oxidative stress is associated with metastasis in mucocutaneous leishmaniasis. J Infect Dis. 2006;194:1160–1167. doi: 10.1086/507646. [DOI] [PubMed] [Google Scholar]
- 49.Walker J, Acestor N, Gongora R, et al. Comparative protein profiling identifies elongation factor-1 beta and tryparedoxin peroxidase as factors associated with metastasis in Leishmania guyanensis. Mol Biochem Parasitol. 2006;145:254–264. doi: 10.1016/j.molbiopara.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Dumas C, Ouellette M, Tovar J, et al. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 1997;16:2590–2598. doi: 10.1093/emboj/16.10.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saravia NG, Segura I, Holguin AF, et al. Epidemiologic, genetic, and clinical associations among phenotypically distinct populations of Leishmania (Viannia) in Colombia. Am J Trop Med Hyg. 1998;59:86–94. doi: 10.4269/ajtmh.1998.59.86. [DOI] [PubMed] [Google Scholar]
- 52.Saravia NG, Weigle, Navas C, et al. Heterogeneity, geographic distribution, and pathogenicity of serodemes of Leishmania viannia in Colombia. Am J Trop Med Hyg. 2002;66:738–744. doi: 10.4269/ajtmh.2002.66.738. [DOI] [PubMed] [Google Scholar]
- 53.Schriefer A, Schriefer ALF, Góes-Neto A, et al. Multiclonal Leishmania braziliensis population structure and its clinical implication in a region of endemic American tegumentary leishmaniasis (ATL) Infect Immun. 2004;72:508–514. doi: 10.1128/IAI.72.1.508-514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das Gupta S, Ghosh DK, Majumder HK. A cloned kinetoplast DNA mini-circle fragment from a Leishmania spp. specific for postkala-azar dermal leishmaniasis strains. Parasitology. 1991;102:187–191. doi: 10.1017/s0031182000062478. [DOI] [PubMed] [Google Scholar]
- 55.Sreenivas G, Raju BV, Singh R, et al. DNA polymorphism assay distinguishes isolates of Leishmania donovani that cause kala-azar from those that cause postkala-azar dermal leishmaniasis in humans. J Clin Microbiol. 2004;42:1739–1741. doi: 10.1128/JCM.42.4.1739-1741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salotra P, Duncan RC, Singh R, et al. Upregulation of surface proteins in Leishmania donovani isolated from patients of post kala-azar dermal leishmaniasis. Microbes Infect. 2006;8:637–644. doi: 10.1016/j.micinf.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Sundar S, Rai M. Advances in the treatment of leishmaniasis. Curr Opin Infect Dis. 2002;15:593–598. doi: 10.1097/00001432-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Sotto J, Rea J, Balderrama M, et al. Efficacy of miltefosine for Bolivian cutaneous leishmaniasis. Am J Trop Med Hyg. 2008;78:210–211. [PubMed] [Google Scholar]
- 59.Machado PRL, Lessa H, Lessa M, et al. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis. 2007;44:788–793. doi: 10.1086/511643. [DOI] [PubMed] [Google Scholar]
- 60.Almeida R, D’Oliveira A, Jr, Machado P, et al. Randomized, double-blind study of stibogluconate plus human granulocyte macrophage colony-stimulating factor versus stibogluconate alone in the treatment of cutaneous leishmaniasis. J Infect Dis. 1999;180:1735–1737. doi: 10.1086/315082. [DOI] [PubMed] [Google Scholar]
- 61.Santos JB, Ribeiro de Jesus A, Machado PR, et al. Antimony plus recombinant human granulocyte-macrophage colony-stimulating factor applied topically in low doses enhances healing of cutaneous leishmaniasis ulcer: a randomized, double-blind, placebo-controlled study. J Infect Dis. 2004;190:1793–1796. doi: 10.1086/424848. [DOI] [PubMed] [Google Scholar]