Abstract
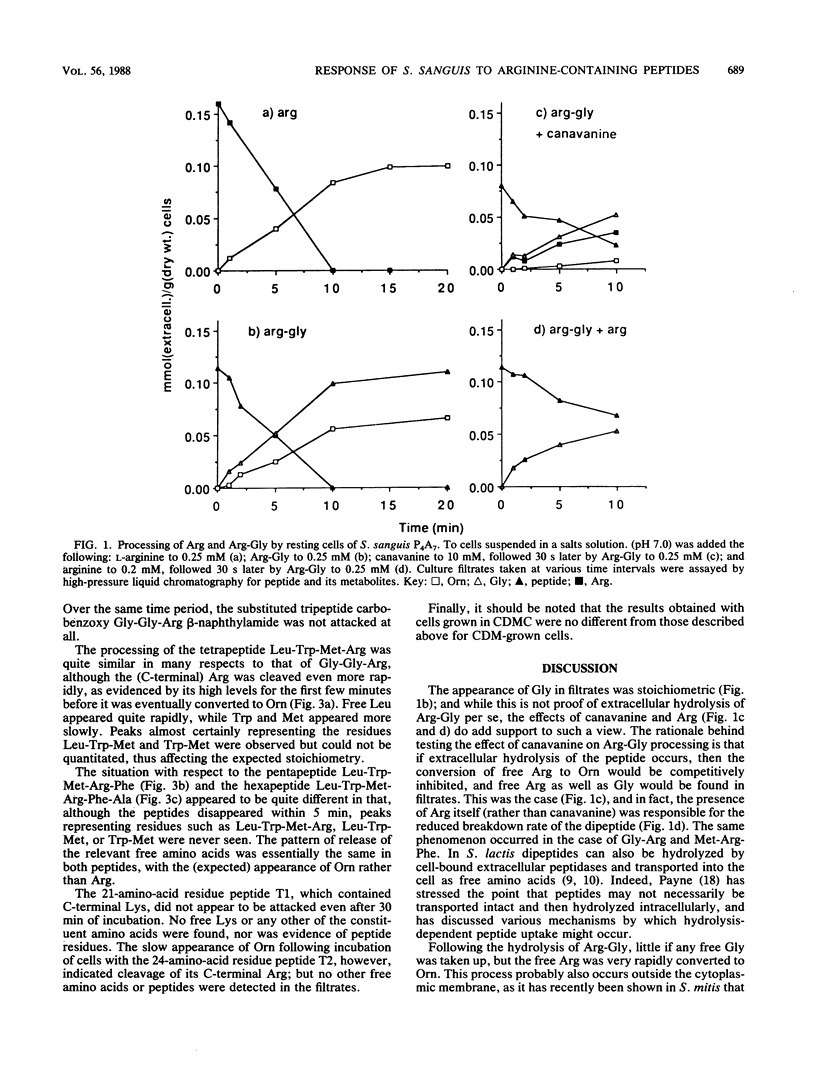
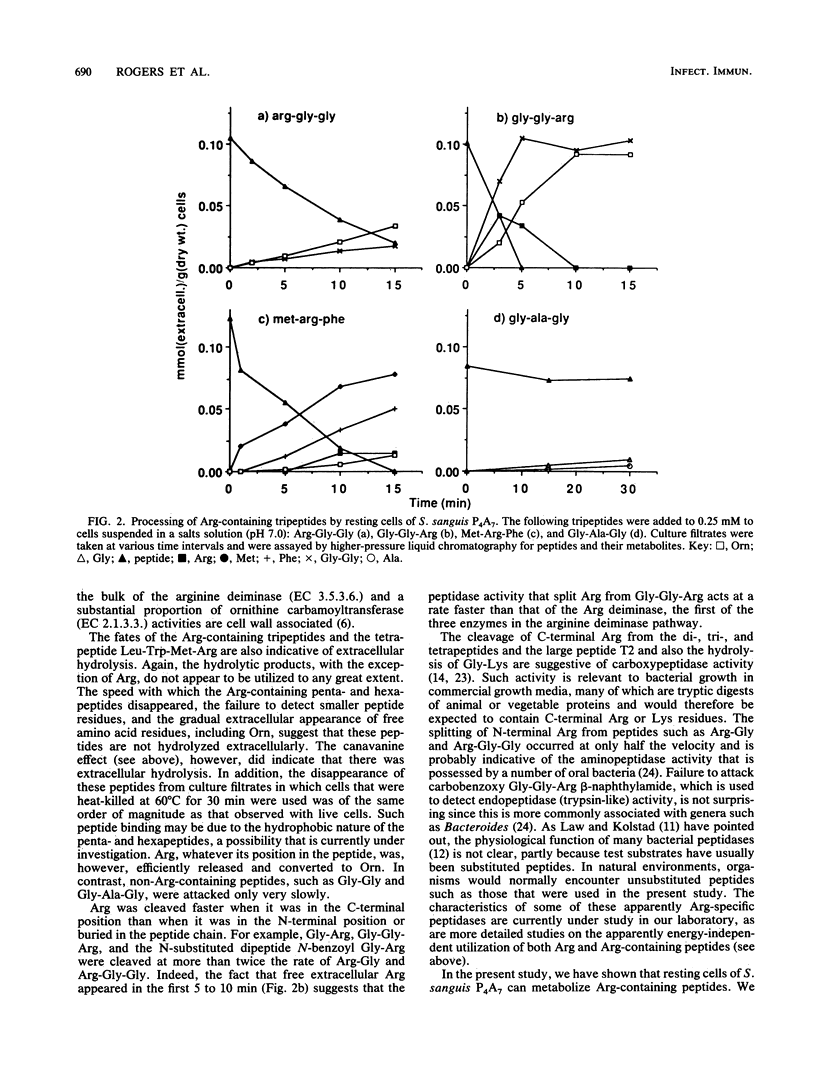
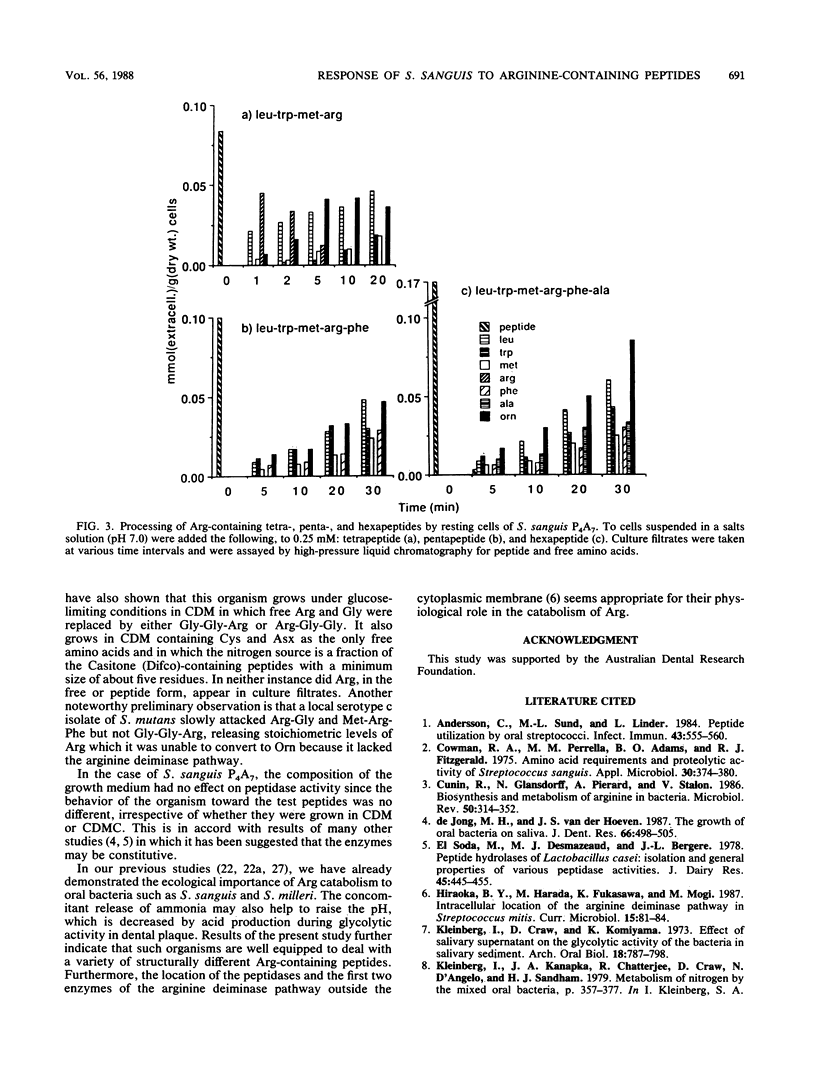
For dental plaque organisms such as Streptococcus sanguis, the ecological importance of the ability to utilize arginine as an energy source has been established in previous studies. The present investigation was undertaken to determine the ability of a strain of S. sanguis to process unsubstituted arginine-containing peptides. The organism was grown under glucose-limited conditions in a chemically defined medium, and peptide was added to washed, resting cells in a pH-stat at pH 7.0. Filtrates taken at appropriate time intervals were assayed for peptide, free amino acids, and metabolites. Irrespective of the position of the arginine residue, all peptides tested were attacked, although those that possessed a C-terminal arginine (including a tetrapeptide) were processed at a faster rate than were those in which arginine was N terminal. However, C-terminal arginine was cleaved only slowly from a peptide containing 24 residues. In each case, most of the released arginine was converted to ornithine via the arginine deiminase pathway. Such peptidase activities appeared to occur at or near the cell surface and were probably constitutive. It was found that the organism grew in chemically defined medium containing arginine that was present solely in the form of a tripeptide, and also that a strain of S. mutans possessed only a limited ability to attack arginine-containing peptides and was unable to utilize the released arginine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson C., Sund M. L., Linder L. Peptide utilization by oral streptococci. Infect Immun. 1984 Feb;43(2):555–560. doi: 10.1128/iai.43.2.555-560.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman R. A., Perrella M. M., Adams B. O., Fitzgerald R. J. Amino acid requirements and proteolytic activity of Streptococcus sanguis. Appl Microbiol. 1975 Sep;30(3):374–380. doi: 10.1128/am.30.3.374-380.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Glansdorff N., Piérard A., Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986 Sep;50(3):314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong M. H., Van der Hoeven J. S. The growth of oral bacteria on saliva. J Dent Res. 1987 Feb;66(2):498–505. doi: 10.1177/00220345870660021901. [DOI] [PubMed] [Google Scholar]
- El Soda M., Desmazeaud M. J., Bergère J. L. Peptide hydrolases of Lactobacillus casei: isolation and general properties of various peptidase activities. J Dairy Res. 1978 Oct;45(3):445–455. doi: 10.1017/s0022029900016666. [DOI] [PubMed] [Google Scholar]
- Kleinberg I., Craw D., Komiyama K. Effect of salivary supernatant on the glycolytic activity of the bacteria in salivary sediment. Arch Oral Biol. 1973 Jul;18(7):787–798. doi: 10.1016/0003-9969(73)90050-2. [DOI] [PubMed] [Google Scholar]
- Law B. A., Kolstad J. Proteolytic systems in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983 Sep;49(3):225–245. doi: 10.1007/BF00399500. [DOI] [PubMed] [Google Scholar]
- Law B. A. Peptide utilization by group N streptococci. J Gen Microbiol. 1978 Mar;105(1):113–118. doi: 10.1099/00221287-105-1-113. [DOI] [PubMed] [Google Scholar]
- Machuga E. J. Aryl-L-aminoacylamidase activities in extracts of Streptococcus durans. J Bacteriol. 1982 May;150(2):747–754. doi: 10.1128/jb.150.2.747-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay T. J., Phelan A. W., Plummer T. H., Jr Comparative studies on human carboxypeptidases B and N. Arch Biochem Biophys. 1979 Oct 15;197(2):487–492. doi: 10.1016/0003-9861(79)90271-6. [DOI] [PubMed] [Google Scholar]
- McKee A. S., McDermid A. S., Baskerville A., Dowsett A. B., Ellwood D. C., Marsh P. D. Effect of hemin on the physiology and virulence of Bacteroides gingivalis W50. Infect Immun. 1986 May;52(2):349–355. doi: 10.1128/iai.52.2.349-355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- Payne J. W. Peptides and micro-organisms. Adv Microb Physiol. 1976;13:55–113. doi: 10.1016/s0065-2911(08)60038-7. [DOI] [PubMed] [Google Scholar]
- Progulske A., Holt S. C. Studies on the growth of Eikenella corrodens strain 23834. Oral Microbiol Immunol. 1987 Mar;2(1):2–9. doi: 10.1111/j.1399-302x.1987.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Rogers A. H., de Jong M. H., Zilm P. S., van der Hoeven J. S. Estimation of growth parameters for some oral bacteria grown in continuous culture under glucose-limiting conditions. Infect Immun. 1986 Jun;52(3):897–901. doi: 10.1128/iai.52.3.897-901.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K., Watanabe T. Carboxypeptidase activity in human mycoplasmas. J Bacteriol. 1986 Nov;168(2):1045–1047. doi: 10.1128/jb.168.2.1045-1047.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suido H., Nakamura M., Mashimo P. A., Zambon J. J., Genco R. J. Arylaminopeptidase activities of oral bacteria. J Dent Res. 1986 Nov;65(11):1335–1340. doi: 10.1177/00220345860650111101. [DOI] [PubMed] [Google Scholar]
- Tavss E. A., Eigen E. Factors affecting pH rise of suspended salivary sediment. Caries Res. 1986;20(3):244–250. doi: 10.1159/000260942. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff A. J., van Steenbergen T. J., Kippuw N., de Graaff J. Enzymatic characterization of oral and non-oral black-pigmented Bacteroides species. Antonie Van Leeuwenhoek. 1986;52(2):163–171. doi: 10.1007/BF00429320. [DOI] [PubMed] [Google Scholar]



