Abstract
Human T lymphotropic virus (HTLV)-I is known to cause HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) and other pronounced disease in less than 4% of those infected. However, evidence is accumulating that a proportion of HTLV-I carriers have neurological and urological symptoms without fulfilling criteria for HAM/TSP. Brain white matter (WM) lesions on magnetic resonance imaging (MRI) are frequently seen in HAM/TSP. HTLV-I carriers with MRI scans for other neurological diagnoses have WM lesions more frequently than expected. We studied 10 patients with HAM/TSP and 20 HTLV-I carriers without overt neurological disease and evaluated clinical characteristics, viral load, total, small, large, confluent WM lesion number, and lesion volume on MRI. Cerebral WM lesions were found in of 85% of HTLV-I carriers and 80% of HAM/TSP patients. Lesion number, size or location was no different between carriers and HAM/TSP. Cognitive function was lower in HAM/TSP (p = 0.045) but did not correlate with WM lesion number. Viral load and peripheral blood mononuclear cell interferon-γ production correlated positively (p = 0.001) but did not correlate with lesion number or volume. Conventional brain MRI frequently shows WM lesions in HTLV-I-infected individuals suggesting potential early central nervous system inflammation with rare development of progressive disease.
INTRODUCTION
Human T lymphotrophic virus (HTLV)-I is a retrovirus that infects 10–20 million people worldwide.1 The majority of those infected are carriers, with fewer than 4% developing HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP),2 acute T cell lymphoma leukemia (ATLL),3 or infective dermatitis.4 Recent reports have documented urinary, neurological, and inflammatory symptoms, similar to those seen in HAM/TSP but milder in nature, among the 95% of HTLV-I-infected individuals traditionally considered carriers.5–7 Laboratory findings that generally distinguish between populations of HTLV-I carriers and those with HAM/TSP also overlap among a subset of carriers. HAM/TSP has been associated in general with a higher proviral load than carriers and increased production of inflammatory cytokines in peripheral blood mononuclear cells (PBMC).8,9 High proviral load and interferon-γ (IFN-γ) production, however, are found in a small number of HTLV-I carriers at levels similar to those seen in HAM/TSP.9
Magnetic resonance imaging (MRI) has been proposed as a tool to aid in the diagnosis of HAM/TSP,10 to follow the response to therapy,11 or to distinguish HAM/TSP from multiple sclerosis (MS).12,13 Although the typical clinical presentation of HAM/TSP involves lower extremity paralysis and urological symptoms, which localize to a lesion in the thoracic spine, corresponding MRI lesions are seen in a minority of patients.10,13,14 The incidence of spinal cord atrophy varies from 6% to 74%15,16 as no validated radiological criteria exist. In contrast, small white matter (WM) lesions are frequently seen in subcortical and periventricular areas in patients with HAM/TSP10–21 at a higher frequency than seen in noninflammatory neurological diseases.11,21 The quantity and distribution of small WM lesions do not correspond to symptoms.10,11,15 The duration or grade of HAM/TSP has been shown to correlate with MRI changes by some11 but not others.10
The limited number of series that included HTLV-I carriers who underwent MRI for other neurological diseases has shown a frequency of WM lesions in HTLV-I carriers similar to that seen in patients with HAM/TSP.11,13 No studies have examined HTLV-I carriers without indications for MRI. Moreover, no studies have tried to correlate immunological measures with WM lesions. We performed brain MRIs on 10 patients with HAM/TSP and 20 age/sex-matched HTLV-I carriers from an endemic area without other neurological diseases.
MATERIALS AND METHODS
The study was performed in Salvador, Brazil, a city of 2.5 million inhabitants in Northeastern Brazil. The population is primarily of African/Portuguese descent and is characterized by marked socioeconomic disparities.22
HAM/TSP and HTLV-I carriers were recruited from the HTLV-I multidisciplinary clinic at the Hospital Universitário Prof. Edgard Santos in Salvador. Patients are referred to the clinic from blood banks as well as dermatology or neurology clinics. Diagnosis of HTLV-I was made by enzyme-linked immunosorbent assay (ELISA) (Cambridge Biotech Corp., Worcester, MA) with confirmation by Western blot (HTLV blot 2.4, Genelab, Singapore). HAM/TSP was diagnosed by neurological examination using Kurtzke’s Expanded Disability Status Scale (EDSS)23 and Osame’s Motor Disability Score (OMDS) in accordance with WHO criteria.24 Anti-HTLV-I antibodies were documented in cerebrospinal fluid (CSF) and based on clinical and radiological findings patients did not have other diseases to explain their neurological symptoms.
A convenience sample of patients with confirmed HAM/TSP between the ages of 18 and 65 years was identified. HAM/TSP patients were matched by age and sex with control seropositive HTLV-I carriers from the clinic population selected without regard to symptoms or comorbidities beyond exclusion for diabetes, known cerebrovascular disease, HIV, or active syphilis. Every HAM/TSP patient was matched with two HTLV-I carriers. HTLV-I carriers were selected from 103 carriers who had completed PBMC cultures with IFN-γ determination in a descriptive study of the clinic population. Our cross-sectional study was originally designed, in part, to compare carriers based on IFN-γ production. Consequently, carriers were stratified by IFN-γ production into two groups of >1500 pg/ml or <500 pg/ml. Within these strata, one high IFN-γ and one low IFN-γ control per HAM/TSP subject matched on sex and age ±5 years was selected. Since preliminary analyses revealed no differences in MRI findings in the two strata, the groups were combined to form a group of 20 HTLV-I carriers. All HAM/TSP patients and HTLV-I carriers were submitted to a full general physical and neurological examination as well as completing an extensive questionnaire of neurological, urological, rheumatological, and odontological symptoms as described previously.5
The study was reviewed and approved by the Institutional Review Boards of the Oswaldo Cruz Foundation and the Weill Medical College of Cornell University. Informed consent was obtained from all subjects enrolled in the study.
Mononuclear cell separation and determination of IFN-γ
PBMCs were obtained from heparinized venous blood by density gradient centrifugation using lymphocyte separation media (LSM; Organon Tecnika Corporation, Durham, NC). After washing in saline, the cells were adjusted to 3 × 106 ml in RPMI-1640 (GIBCO, Grand Island, NY) containing 100 U penicillin G and 10 μ/ml of streptomycin and supplemented with 10% AB serum. All cultures were incubated without stimulus at 37°C in 5% CO2 for 72 h. Supernatants were collected and stored at −20°C. IFN-γ was measured by commercial ELISA following the manufacturer’s instructions (PharMingen).
Proviral load
The HTLV-I proviral load in PBMC was quantified using a real-time TaqMan polymerase chain reaction (PCR) method.25 Briefly, SK110/SK111 primers were used to amplify a 186 fragment of the pol gene and the dual-labeled TaqMan probe (5′-FAM and 3′-TAMRA) located at 4829–4858 bp of the HTLV-I reference sequence (HTLVATK). Albumin DNA was quantified in parallel to determine the input cell number as an endogenous reference. Both HTLV-I and albumin gene amplifications were performed on 10 μl of DNA extract using Taq-MAn PCR core reagent kit (Applied Biosystems). Amplification and data acquisition were carried out using the ABI Prism 7700 Sequence detector system (Applied Biosystems). Standard curves were generated using 10-fold serial dilution of a double standard plasmid (pcHTLV-ALB). The HTLV-I-infected human lymphocyte line MT2 was used as a control for quantification. All standard dilution, controls, and patients samples were run in duplicate. The normalized value of the HTLV-I proviral load was calculated as the ratio of (HTLV-I DNA average copy number/albumin DNA average copy number) × 2 × 106 and expressed as the number of HTLV-I copies per 106 PBMCs.
Magnetic resonance imaging
MR imaging was performed on a 1.5-T whole body scanner (Siemens, Erlangen, Germany).
The acquisition MR imaging protocol included a sagittal localizer followed by axial T1 [spin-echo (SE) echo time (TE) = 16 msec, repetition time (TR) = 65 msec], T2 [fast spin-echo time (FSE) = 102 msec, TR = 42 msec], and fluid-attenuated inversion recovery (FLAIR) (TE = 141 msec, TR = 10,002 sec, TI = 2200 msec) sequences, all with 20 contiguous 5-mm-thick sections with 256 × 192 pixels across a 24-cm field of view (FOV). Examinations were evaluated by a neurologist (J.O.F.) and a neuroradiologist (C.A.) independently without knowledge of the disease state of an individual with consensus achieved on number of lesions when initial disagreement occurred. A lesion was considered present if its size was >3 mm. Small lesions were 3–6 mm, large lesions >6 mm in largest dimension, and confluent lesions were irregular in shape and >6 mm. Brain atrophy was quantified by visual inspection as described by Victoroff et al. 26 Briefly, three brain regions (frontal, parietal, and temporal lobes) were rated from zero (no atrophy) to three (severe atrophy) and the total score was the sum of the three regions (possible score range zero to nine). Total lesion volume was calculated from the summed cross-sectional area of FLAIR hyperintense lesions using ImageJ software.
Statistical analysis
Differences between carriers and HAM/TSP patients were calculated using the Mann–Whitney U-test (Wilcoxon rank sum test) or the Fishers exact test. Correlations were performed with Spearman’s rank test. All calculations were performed using Stata 7 software (Stata Corporation, College Station, TX). A p value <0.05 was considered significant.
RESULTS
Twelve patients with HAM/TSP were contacted from the HTLV-I clinic. One was excluded for diabetes and one for stroke. Twenty-one matched carriers were identified, one of whom was excluded for possible latent syphilis. Patients were 70% female and their median age was 47 years (HAM/TSP) and 49 years (controls). The median duration of HAM/TSP was 3 years (range 2–20 years). The median EDSS was 4 (range 2–8) in HAM/TSP and 0 (range 0–1) in controls. The median OMDS score was 4 (range 3–11) in HAM/TSP and 0 in controls (by definition). Hyperreflexia was present in 100% of HAM/TSP and 15% (3/20) of carriers. Hypertension was present in 3/10 (30%) of HAM/TSP and 5/20 (25%) of HTLV-I carriers. No HAM/TSP patients and 1/20 (5%) of HTLV-I carriers reported smoking tobacco. No patients in either group had known cardiovascular disease.
The median log of proviral load was 4.69 log10 copies/106 PBMCs (range 0–5.52) in HAM/TSP and 4.81 log10 copies/106 PBMCs (range 1.07–5.52) in carriers, which was not statistically different (p = 0.89).
Urinary incontinence was reported by 50% (5/10) of HAM/TSP and 20% (4/20) of HTLV-I carriers. Difficulty walking was reported by 90% (9/10) of HAM/TSP and 5% (1/20) of HTLV-I carriers. Leg weakness was reported by 80% (8/10) of HAM/TSP and 20% (4/20) of HTLV-I carriers. Dry eyes and dry mouth were reported by 40% (4/10) of HAM/TSP and 20% (4/20) of HTLV-I carriers. Overall, 55% (11/20) of HTLV-I carriers had at least one of the aforementioned symptoms.
Table 1 displays MRI findings by group. The median number of lesions was 5 (Interquartile range (IQR) 2–16) in HAM/TSP and 4.5 (IQR 2–8) in HTLV-I carriers (p = 0.64). The median lesion volume was 3.0 ml (IQR 1.0–12.7 ml) in HAM/TSP and 1.0 ml (IQR 0.2–8.4 ml) in carriers (p = 0.49). No statistical difference between HTLV-I carriers and HAM/TSP was seen with respect to any variable presented in Table 1. An example of a WM lesion is presented in Fig. 1.
Table 1.
MRI Findings in HTLV-I Carriers and HAM/TSP Patients, Shown as Percentage of Total or as Median (Interquartile Range)
| Findings on MRI | Carriers (n = 20) | HAM/TSP (n = 10) |
|---|---|---|
| One or more lesions | 85% (17) | 80% (8) |
| Lesions 3–6 mm | 80% (16) | 70% (7) |
| Lesions > 6 mm | 15% (3) | 20% (2) |
| Confluent lesions | 35% (7) | 20% (2) |
| Median number of lesions | 4.5 (2–8) | 5 (2–16) |
| Median number of subcortical lesions | 3 (1–7) | 3.5 (1–13) |
| Median number of paraventricular lesions | 1 (0.5–2) | 0.5 (0–2) |
| Posterior fossa lesions | 0 (0–0)a | 0 |
| Median lesion volume (ml) | 1.1 (0.2–8.4) | 3.0 (0.1–12.7) |
| Atrophy | 0.3 (0–0.9) | 0.7 (0–1.0) |
One carrier had a single brainstem lesion.
FIG. 1.
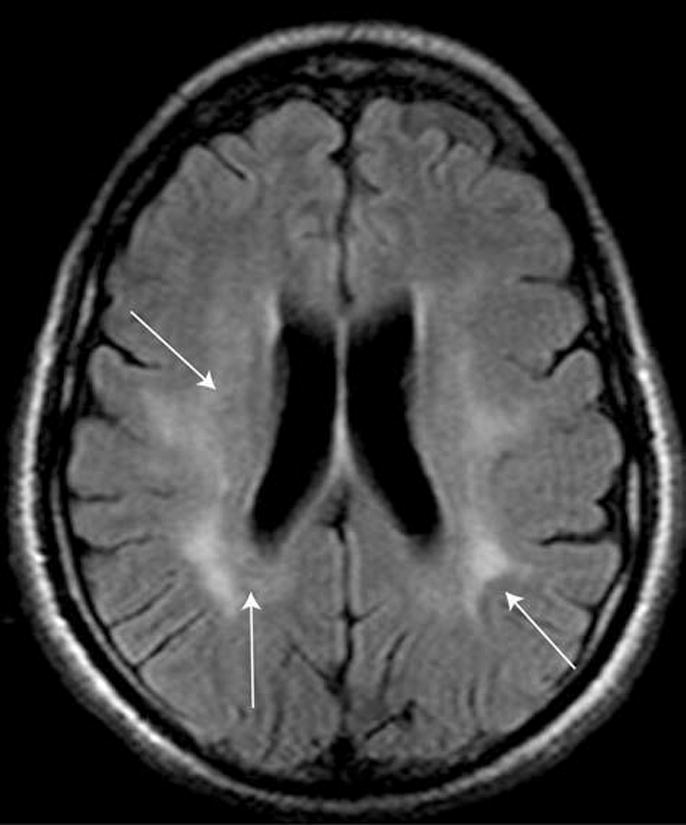
FLAIR-enhanced image showing confluent lesions seen in some HTLV-I-infected patients (arrows indicate lesions, which extend from periventricular to subcortical locations).
The number of WM lesions seen in carriers and HAM/TSP patients is shown in Fig. 2. Lesion number and volume did not correlate with EDSS or OMDS or duration of HAM/TSP. The median IFN-γ was 27 pg/ml (range 0–435) in 10 low IFN-γ carriers, 2971 pg/ml (range 1936–4980) in 10 high IFN-γ carriers, and 2329 pg/ml (range 306–4075) in HAM/TSP. No difference in lesion number was seen between high and low IFN-γ groups (p = 0.88). No correlation between IFN-γ and WM lesion number or volume was observed. No association was found between the presence of any symptoms and IFN-γ level (p = 0.19). No correlation between the presence of any symptoms and number of WM lesions was found. Proviral load did not correlate with EDSS (p = 0.77), OMDS (p = 0.91), total lesion number (p = 0.96), or lesion volume (p = 0.48). Proviral load significantly correlated with PBMC IFN-γ production (p = 0.001, r2 = 0.42). The Mini-Mental State Exam score was significantly lower in HAM/TSP, 25, than in HTLV-I carriers, 28 (p = 0.045) and did not correlate with lesion number or volume (p = 0.71 and p = 0.92). Lesion number was positively correlated with age for all HTLV-I-infected patients (R2 = 0.25, p = 0.005).
FIG. 2.
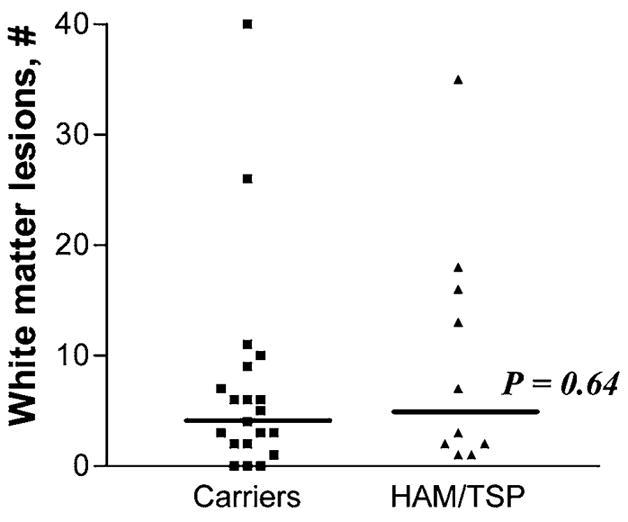
Total number of cerebral T2 white matter lesions >3 mm in HTLV-I carriers and HAM/TSP patients.
DISCUSSION
In the first study to compare HAM/TSP to HTLV-I carriers who did not undergo MRI for other neurological reasons, we found a high rate of WM lesions in carriers. The distribution and quantity of WM lesions were not different between groups. No correlations with clinical findings, including EDSS or OMDS scores, were found.
WM lesions in HTLV-I have been postulated as evidence of a small vessel vasculitis.11 Small WM lesions have been observed more frequently in HTLV-I infection than in seronegative patients with other noninflammatory neurological diseases.11,21 As others have described,11,13 our results confirm that a large number of both HAM/TSP and HTLV-I carriers have WM lesions. The frequency appears higher than that reported in healthy adult volunteers.27 In one study by Fazekas, WM lesions were found in 37% of healthy volunteers <70 years of age,27 which would suggest that the rate of WM lesions in HTLV-infected patients in our study was higher than normal. Some have found more lesions in HAM/TSP than carriers. In one study, when >10 WM lesions was used as a definition, 38% of HAM/TSP patients as compared to 10% of HTLV-I carriers were identified.11 We found no difference with this cutoff in our population. Our study was limited by not including HTLV-I-negative controls although few WM lesions are expected in nonelderly subjects without neurological complaints. We found a correlation between age and number of WM lesions, which have been described in a HTLV-I-seronegative population.27 Prior studies of HTLV-I have not found this correlation.11,21
The clinical significance of cerebral WM lesions has also been debated. Cerebral WM lesions are the most common changes in HAM/TSP, occurring in 33–100% of patients,10–21 although WM lesion number does not correlate with symptoms. The high frequency of WM lesions observed in our study is likely due to highly sensitive modern MRI techniques as well as a possible bias to identify lesions that may be overlooked in retrospective reviews of MRIs obtained for clinical purposes, as ours was a prospective research protocol. Although a segment of HTLV-I carriers reported symptoms such as incontinence (20%) and lower extremity weakness (20%) or were found to have hyperreflexia (15%), these findings did not correlate with the presence or number of WM lesions. There was also no correlation between IFN-γ levels and WM lesions. There is a known association between proviral load and HAM/TSP.8 In this study we found no correlation between WM lesion number or volume and proviral load. We did, however, find a significant correlation between IFN-γ level and proviral load. The possibility exists that there is a less robust association between these and WM lesions that was not seen because of the small sample in this study.
Among our patients, two with advanced HAM/TSP (both EDSS 8) had diffuse WM changes that were more severe than MRIs of any HTLV-I carriers. Others have found large lesions to be predictive of MS rather than HAM/TSP in the appropriate clinical setting.12,13 Both of these patients had definite clinical HAM/TSP, with no evidence of cognitive or other cerebral dysfunction.
In summary, our prospective, cross-sectional study using HTLV-I carriers without other neurological disease demonstrates that cerebral WM lesions occur in a large percentage of all HTLV-I-infected individuals. A large proportion of HTLV-I carriers has neurological and urological manifestations similar to those observed in HAM/TSP.5,6,7,28 Recently proposed criteria for the diagnosis of HAM/TSP add urological symptoms and neurological symptoms to OMDS and EDSS measures.29 It is apparent that morbidity associated with HTLV-I infection is higher than has been previously reported. Our findings, that cerebral WM lesions occur in the majority of HTLV-I carriers, suggest that neurological damage with or without translation to symptoms is documented in the majority of HTLV-I carriers. Longitudinal studies are needed to delineate the natural history of cerebral MRI abnormalities in this population.
Acknowledgments
This work was supported by the National Institutes of Health (NIH), 1 RO3-AI60830 and T32–AI07613 (training grant to D.J.M. and M.F.C.), Brazilian National Research Council (CNPq), and Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB). E.M.C. is a senior investigator of CNPq.
References
- 1.De The G, Bomford R. An HTLV-I vaccine: Why how, for whom? AIDS Res Hum Retroviruses. 1993;9:381–386. doi: 10.1089/aid.1993.9.381. [DOI] [PubMed] [Google Scholar]
- 2.Orland JR, Engstrom J, Fridey J, et al. Prevalence and clinical features of HTLV neurologic disease in the HTLV outcomes study. Neurology. 2003;61:1588–1594. doi: 10.1212/01.wnl.0000096011.92542.da. [DOI] [PubMed] [Google Scholar]
- 3.Hinuma Y, Nagata K, Hanaoka M, et al. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaGrenade L, Hanchard B, Fletcher V, Cranston B, Blattner W. Infective dermatitis of Jamaican children: A marker for HTLV-I infection. Lancet. 1990;336(8727):1345–1347. doi: 10.1016/0140-6736(90)92896-p. [DOI] [PubMed] [Google Scholar]
- 5.Caskey MF, Morgan DJ, Porto MAF, Giozza SP, Muniz AL, Orge MG, Travassos MJ, Baron Y, Carvalho EM, Glesby MJ. Clinical manifestations associated with HTLV type I infection: A cross-sectional study. AIDS Res Hum Retroviruses. 2007;23(3):365–371. doi: 10.1089/aid.2006.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zunt JR, Alacron JO, Montano S, Longstreth WT, Jr, Price R, Holmes KK. Quantitative assessment of subclinical spasticity in human T-cell lymphotropic virus type I infection. Neurology. 1999;53(2):386–390. doi: 10.1212/wnl.53.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro N, Oliveira P, Junior WR, Muniz A, Carvalho E. Erectile dysfunction and HTLV-I infection: A silent problem. Int J Impot Res. 2005;17:364–369. doi: 10.1038/sj.ijir.3901335. [DOI] [PubMed] [Google Scholar]
- 8.Nagai M, Usuku K, Matsumoto W, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: High proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 9.Santos SB, Porto AF, Muniz AL, et al. Exacerbated inflammatory cellular immune response characteristic of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic carriers. BMC Infect Dis. 2004;2:4–7. doi: 10.1186/1471-2334-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagnato F, Butman JA, Mora CA, et al. Conventional magnetic resonance imaging features in patients with tropical spastic paraparesis. J Neurovirol. 2005;11:525–534. doi: 10.1080/13550280500385039. [DOI] [PubMed] [Google Scholar]
- 11.Kira J, Fujihara K, Itoyama Y, et al. Leukoencephalopathy in HTLV-I associated myelopathy/tropical spastic paraparesis: MRI analysis and a two year follow-up study after corticosteroid therapy. J Neurol Sci. 1991;106:41–49. doi: 10.1016/0022-510x(91)90192-a. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda Y, Matsui M, Yukitake M, et al. Assessment of MRI criteria for MS in Japanese MS and HAM/TSP. Neurology. 1995;45:30–33. doi: 10.1212/wnl.45.1.30. [DOI] [PubMed] [Google Scholar]
- 13.Howard AK, Li DK, Oger J. MRI contributes to the differentiation between MS and HTLV-I associated myelopathy in British Columbian coastal natives. Can J Neurol Sci. 2003;30:41–48. doi: 10.1017/s0317167100002420. [DOI] [PubMed] [Google Scholar]
- 14.Gessain A, Gout O. Chronic myelopathy associated with human T-lymphotropic virus type I (HTLV-I) Ann Intern Med. 1992;117:933–946. doi: 10.7326/0003-4819-117-11-933. [DOI] [PubMed] [Google Scholar]
- 15.Ferraz AC, Gabbai AA, Abdala N, Nogueira RG. Magnetic resonance in HTLV-1 associated myelopathy [Portuguese] Arq Neuropsiquiatr. 1997;55(4):728–736. doi: 10.1590/s0004-282x1997000500008. [DOI] [PubMed] [Google Scholar]
- 16.Milagres AC, Jorge ML, Marchiori PE, Segurado AA. Human T cell lymphotropic virus type 1-associated myelopathy in Sao Paulo, Brazil. Epidemiologic and clinical features of a university hospital cohort. Neuroepidemiology. 2002;21(3):153–158. doi: 10.1159/000054813. [DOI] [PubMed] [Google Scholar]
- 17.Alcindor F, Valderrama R, Canavaggio M, Lee H, et al. Imaging of human T-lymphotropic virus type I-associated chronic progressive myeloneuropathies. Neuroradiology. 1992;35(1):69–74. doi: 10.1007/BF00588283. [DOI] [PubMed] [Google Scholar]
- 18.Melo A, Gomes I, Mattos K. HTLV-1 associated myelopathies in the city of Salvador, Bahia [Portuguese] Arq Neuropsiquiatr. 1994;52(3):320–325. doi: 10.1590/s0004-282x1994000300006. [DOI] [PubMed] [Google Scholar]
- 19.Rudge P, Ali A, Cruichshank JK. Multiple sclerosis, tropical spastic paraparesis and HTLV-1 infection in Afro-Caribbean patients in the United Kingdom. J Neurol Neurosurg Psychiatry. 1991;54(8):689–694. doi: 10.1136/jnnp.54.8.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godoy AJ, Kira J, Hasou K, Goto I. Characterization of cerebral white matter lesions of HTLV-I-associated myelopathy/tropical spastic paraparesis in comparison with multiple sclerosis and collagen-vasculitis: A semiquantitative MRI study. J Neurol Sci. 1995;133:102–111. doi: 10.1016/0022-510x(95)00161-t. [DOI] [PubMed] [Google Scholar]
- 21.Kira J, Minato S, Itoyama Y, Goto I, Kato M, Hasuo K. Leukoencephalopathy in HTLV-I-associated myelopathy: MRI and EEG data. J Neurol Sci. 1988:221–232. doi: 10.1016/0022-510x(88)90247-x. [DOI] [PubMed] [Google Scholar]
- 22.Azevedo ES, Fortuna CM, Santos MG, et al. Spread and diversity of human populations in Bahia, Brazil. Hum Biol. 1982;54:329–341. [PubMed] [Google Scholar]
- 23.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 24.Osame M. Review of WHO Kagoshima meeting and diagnostic guidelines for HAM/TSP. In: Blattner W, editor. Human Retrovirology: HTLV. Raven Press; New York: 1990. pp. 191–197. [Google Scholar]
- 25.Dehee A, Cesaire R, Desire N, et al. Quantitation of HTLV-I proviral load by a TaqMan real-time PCR assay. J Virol Methods. 2002;102:37–51. doi: 10.1016/s0166-0934(01)00445-1. [DOI] [PubMed] [Google Scholar]
- 26.Victoroff J, Mack WJ, Grafton ST, Schreiber SS, Chui HC. A method to improve interrater reliability of visual inspection of brain MRI. Neurology. 1994;44(12):2267–2276. doi: 10.1212/wnl.44.12.2267. [DOI] [PubMed] [Google Scholar]
- 27.Fazekas F. Magnetic resonance signal abnormalities in asymptomatic individuals: Their incidence and functional correlates. Eur Neurol. 1989;29:164–168. doi: 10.1159/000116401. [DOI] [PubMed] [Google Scholar]
- 28.Castro NM, Freitas DM, Junior WR, Muniz A, Oliveira P, Carvalho E. Urodynamic features of voiding dysfunction in HTLV-I infected individuals. Int Braz J Urol. 2007;33(2):238–244. doi: 10.1590/s1677-55382007000200016. discussion 244–245. [DOI] [PubMed] [Google Scholar]
- 29.Castro-Costa CM, Ara jo AQC, Barreto MM, et al. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I- associated myelopathy (TSP/HAM) AIDS Res Hum Retroviruses. 2006;22(10):931–935. doi: 10.1089/aid.2006.22.931. [DOI] [PubMed] [Google Scholar]


