Abstract
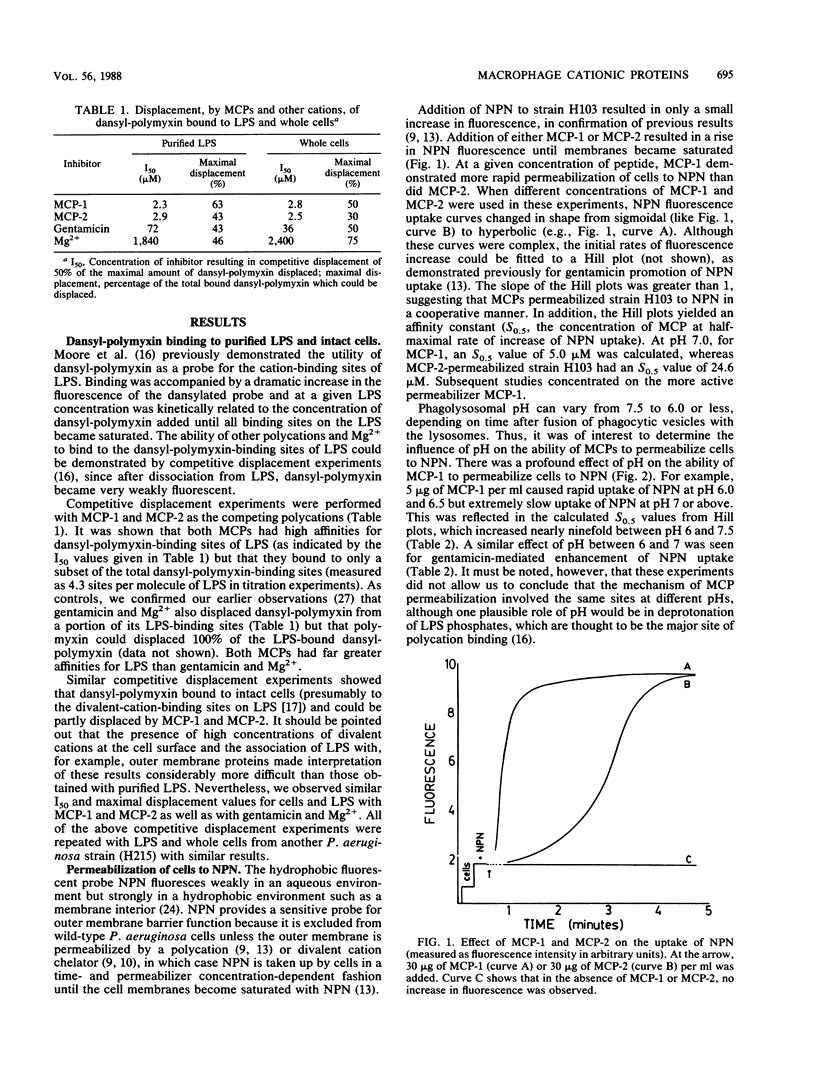
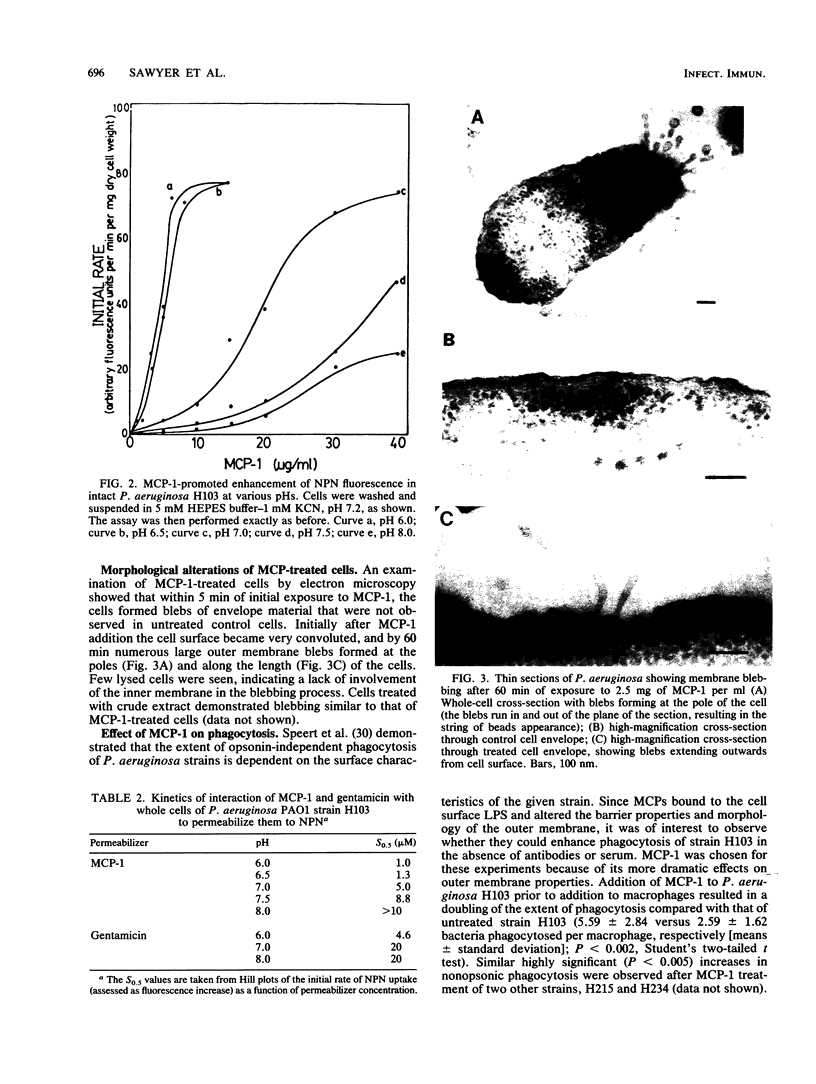
The interaction of the polycationic rabbit alveolar macrophage cationic proteins MCP-1 and MCP-2 (or their identical neutrophil equivalents NP-1 and NP-2) with the surface of Pseudomonas aeruginosa was investigated. Both proteins bound avidly to purified lipopolysaccharide, as judged by their ability to competitively displace the probe dansyl polymyxin with 50% inhibition (I50) values of 2 to 3 microM. Similar I50 were measured with dansyl polymyxin as a probe for cell surface binding, suggesting that the initial binding site for MCP-1 and MCP-2 on the surface of cells was lipopolysaccharide. Both MCP-1 and MCP-2 permeabilized outer membranes to the hydrophobic fluorescent probe 1-N-phenylnaphthylamine (NPN). The initial rate of NPN uptake plotted against the concentration of MCP-1 or MCP-2 gave sigmoidal curves, suggesting cooperative permeabilization of the outer membrane. Replotting the data as a Hill plot gave an affinity parameter, S0.5, the concentration of MCP giving a half-maximal increase in the rate of NPN uptake, of 5 and 25 microM for MCP-1 and MCP-2, respectively, and thus subsequent studies concentrated on the more active permeabilizer MCP-1. Permeabilization of outer membranes to NPN was a function of buffer pH, with lower pH considerably favoring the permeabilizing effects of MCP-1. Thin-section electron microscopic visualization of MCP-1-treated cells showed production of extended blebs. Further evidence of an altered cell surface after MCP-1 treatment was obtained by demonstrating that treated unopsonized cells were more efficiently phagocytosed by unelicited rabbit alveolar macrophages. The data overall suggest that macrophage cationic proteins interact with the P. aeruginosa outer membrane in a manner typical of other polycations and suggest that one of their major functions may be to permeabilize the outer membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cech P., Lehrer R. I. Phagolysosomal pH of human neutrophils. Blood. 1984 Jan;63(1):88–95. [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect Immun. 1987 Mar;55(3):568–571. doi: 10.1128/iai.55.3.568-571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Murray R. G. Ultrastructural study of polymyxin-resistant isolates of Pseudomonas aeruginosa. J Bacteriol. 1976 Jan;125(1):267–281. doi: 10.1128/jb.125.1.267-281.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Eagon R. G. Ultrastructural and chemical alteration of the cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974 Jan;117(1):302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Raffle V. J., Nicas T. I. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Wong P. G. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother. 1984 Jul;26(1):48–52. doi: 10.1128/aac.26.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovde C. J., Gray B. H. Characterization of a protein from normal human polymorphonuclear leukocytes with bactericidal activity against Pseudomonas aeruginosa. Infect Immun. 1986 Oct;54(1):142–148. doi: 10.1128/iai.54.1.142-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Selsted M. E., Szklarek D., Fleischmann J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect Immun. 1983 Oct;42(1):10–14. doi: 10.1128/iai.42.1.10-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh B., Grant C., Hancock R. E. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Oct;26(4):546–551. doi: 10.1128/aac.26.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N. L., Beveridge T. J. Gentamicin interaction with Pseudomonas aeruginosa cell envelope. Antimicrob Agents Chemother. 1986 Jun;29(6):1079–1087. doi: 10.1128/aac.29.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrzakowski M. C., Spitznagel J. K. Bactericidal activity of fractionated granule contents from human polymorphonuclear leukocytes: antagonism of granule cationic proteins by lipopolysaccharide. Infect Immun. 1979 Aug;25(2):597–602. doi: 10.1128/iai.25.2.597-602.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. A., Hancock R. E. Involvement of outer membrane of Pseudomonas cepacia in aminoglycoside and polymyxin resistance. Antimicrob Agents Chemother. 1986 Dec;30(6):923–926. doi: 10.1128/aac.30.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Mechanisms of macrophage antimicrobial activity. Trans R Soc Trop Med Hyg. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun. 1980 Oct;30(1):180–192. doi: 10.1128/iai.30.1.180-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Szklarek D., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins of rabbit alveolar macrophages: amino acid composition and functional attributes. Infect Immun. 1981 Feb;31(2):723–731. doi: 10.1128/iai.31.2.723-731.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Geisow M., Garcia R., Harper A., Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981 Apr 2;290(5805):406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Lehrer R. I. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983 Dec 10;258(23):14485–14489. [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S., Ganz T., Schilling J. W., Lehrer R. I. Primary structures of three human neutrophil defensins. J Clin Invest. 1985 Oct;76(4):1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Casey S. G., Spitznagel J. K. Lipid A and resistance of Salmonella typhimurium to antimicrobial granule proteins of human neutrophil granulocytes. Infect Immun. 1984 Mar;43(3):834–838. doi: 10.1128/iai.43.3.834-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984 Jul;45(1):29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Late intraphagosomal hydrogen ion concentration favors the in vitro antimicrobial capacity of a 37-kilodalton cationic granule protein of human neutrophil granulocytes. Infect Immun. 1986 Sep;53(3):651–655. doi: 10.1128/iai.53.3.651-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Onunka V., Hitchcock P. J. A spontaneous mutant of Neisseria gonorrhoeae with decreased resistance to neutrophil granule proteins. J Infect Dis. 1986 May;153(5):910–917. doi: 10.1093/infdis/153.5.910. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Eftekhar F., Puterman M. L. Nonopsonic phagocytosis of strains of Pseudomonas aeruginosa from cystic fibrosis patients. Infect Immun. 1984 Mar;43(3):1006–1011. doi: 10.1128/iai.43.3.1006-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Franson R. C., Beckerdite S., Schmeidler K., Elsbach P. Partial characterization and purification of a rabbit granulocyte factor that increases permeability of Escherichia coli. J Clin Invest. 1975 Jan;55(1):33–42. doi: 10.1172/JCI107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Hutzler M., Kao L. Environmental modulation of lipopolysaccharide chain length alters the sensitivity of Escherichia coli to the neutrophil bactericidal/permeability-increasing protein. Infect Immun. 1986 Feb;51(2):594–599. doi: 10.1128/iai.51.2.594-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Victor M., Elsbach P. Role of charge and hydrophobic interactions in the action of the bactericidal/permeability-increasing protein of neutrophils on gram-negative bacteria. J Clin Invest. 1983 Mar;71(3):540–549. doi: 10.1172/JCI110798. [DOI] [PMC free article] [PubMed] [Google Scholar]




