Abstract
Human Type 2 diabetes is associated with increased incidence of hypertension and disrupted blood pressure (BP) circadian rhythm. Db/db mice have been used extensively as a model of Type 2 diabetes, but their BP is not well characterized. In this study, we used radiotelemetry to define BP and the circadian rhythm in db/db mice. We found that the systolic, diastolic, and mean arterial pressures were each significantly increased by 11, 8, and 9 mmHg in db/db mice compared with controls. In contrast, no difference was observed in pulse pressure or heart rate. Interestingly, both the length of time db/db mice were active (locomotor) and the intensity of locomotor activity were significantly decreased in db/db mice. In contrast to controls, the 12-h light period average BP in db/db mice did not dip significantly from the 12-h dark period. A partial Fourier analysis of the continuous 72-h BP data revealed that the power and the amplitude of the 24-h period length rhythm were significantly decreased in db/db mice compared with the controls. The acrophase was centered at 0141 in control mice, but became scattered from 1805 to 0236 in db/db mice. In addition to BP, the circadian rhythms of heart rate and locomotor activity were also disrupted in db/db mice. The mean arterial pressure during the light period correlates with plasma glucose, insulin, and body weight. Moreover, the oscillations of the clock genes DBP and Bmal1 but not Per1 were significantly dampened in db/db mouse aorta compared with controls. In summary, our data show that db/db mice are hypertensive with a disrupted BP, heart rate, and locomotor circadian rhythm. Such changes are associated with dampened oscillations of clock genes DBP and Bmal1 in vasculature.
Keywords: radiotelemetry, obesity, blood pressure nondipper, clock gene
more than 170 million people worldwide have diabetes. Hypertension occurs more frequently in these diabetic patients than in those without diabetes. Moreover, when hypertension is superimposed on diabetes, the progression of diabetic complications becomes significantly more severe (40). However, the mechanisms by which diabetes causes an increase in the incidence of hypertension are not well understood. An animal model that has both Type 2 diabetes and hypertension is essential for deciphering the mechanistic links between diabetes and hypertension. Db/db mice have been used extensively in the study of obesity/Type 2 diabetes. These mice manifest a cluster of symptoms similar to those present in Type 2 diabetic patients, including insulin resistance, compensatory hyperinsulinemia, obesity, and hyperglycemia (3). However, conflicting results have been reported regarding blood pressure (BP) levels in db/db mice, depending on the method for BP measurement (radiotelemetry vs. tail cuff), age of mice, and background strain (2, 4, 11, 20, 28). Consequently, it is unclear whether the db/db mice are an appropriate model for studying mechanisms underlying diabetes associated hypertension.
BP and heart rate have a circadian pattern characterized by a low period during sleep; an early morning, postawakening rise; and a high plateau period while the subject is awake (46). Individuals whose average nighttime BP fails to “dip” relative to their average daytime BP by at least 10% are termed “nondippers.” Nondippers have a significantly greater frequency of left ventricular hypertrophy, microalbuminuria, and stroke (15, 30, 37) than do individuals whose BP drops while sleeping by 10% or more. Diabetic patients have been reported to have a diminished nighttime decrease in BP (8, 32). However, it is unknown whether the BP circadian rhythm is altered in db/db mice.
Recent studies have revealed that the endogenous rhythms of various physiological and behavioral processes are generated at the cellular level by circadian core oscillators, which are composed of transcriptional/translational feedback loops involving a set of clock genes (23, 35). These clock genes are expressed not only in the neurons of the suprachiasmatic nucleus but also in peripheral cells including those of the cardiovascular system (34). Dramatic oscillations in circadian clock components have been observed in mouse aortas isolated at different times throughout the 24-h period (36). Among multiple clock genes, DBP and Bmal1 have been shown to be involved in BP regulation (7, 27). However, it is unknown whether the oscillations of these clock genes are altered in db/db mouse vasculature.
The radiotelemetry technology provides an invaluable method that allows for continuous and accurate monitoring of BP in conscious free-moving mice (26). Moreover, an advantage of radiotelemetry technology includes the ability to define locomotor activity as a determinant of BP and through continuous recording to obtain information related to circadian BP rhythms. Therefore, the purpose of this study was to use radiotelemetry in db/db diabetic mice for definition of BP, heart rate, and circadian rhythms in these cardiovascular parameters. In addition, we investigated the clock gene expression in db/db mouse vasculature.
METHODS
Animals.
All experiments were performed using 14- to 16 wk-old male db/db (−/−; n = 12) or age-/gender-matched nondiabetic (+/−; n = 12) C57BL/KsJ control mice. The mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and were housed at an animal care facility at the Medical Center of the University of Kentucky, which is accredited by the American Association for Accreditation of Laboratory Animal Care. All animal protocols were approved by the Institutional Animal Care and Use Committee, and studies conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996). The mice were fed a standard diet and reverse osmosis ultra-filtered water (RO water) ad libitum. Other chemicals and reagents were purchased from Sigma (St. Louis, MO) or Fisher (Pittsburgh, PA).
Implantation of telemetry transmitter.
Each animal was chronically instrumented in the left common carotid artery with telemetry probe (TA11PA-C10, Data Sciences International, St. Paul, MN) for monitoring arterial pressure, heart rate, and locomotor activity. Mice were anesthetized with isoflurane. The left common carotid artery was isolated, and the catheter inserted and tied securely. The body of the unit was slipped under the skin and down into a dissected free “pocket” along the flank as close to the right hindlimb as possible. The neck incision was closed using silk suture, then further sealed with tissue adhesive. The mice were kept warm on a heating pad and monitored closely until fully recovered from anesthesia. After surgery, mice were singly caged under normal environmental conditions in 12-h light/dark cycles with lights on at 0600. All mice were allowed a 7- to 10-day recovery from surgery before baseline BP, heart rate, and locomotor activity were assessed. Locomotor activity counts reflect the frequency of movement of the animal across a grid. Each count reflects one movement of the animal.
The data from the TA 11PA-C10 device were transmitted via radio frequency signals to a receiver below the home cage (model RPC-1) and thereafter collected using the Dataquest A.R.T. system, version 4.0 (Data Sciences International, St. Paul, MN). The data were sampled continuously day and night for a total of 72 h with a sampling rate of 500 Hz. For calculation of the basal systolic, diastolic, and mean arterial pressure, data collected over 24 h were averaged.
Data analysis and circadian rhythm evaluation.
Circadian variations in BP, heart rate, and locomotor activity were obtained by two methods. First, mean values from the dark period (1800-0600) were compared with mean light period (0600-1800) values. Second, the rhythmicities of BP, heart rate, and locomotor activity were analyzed by means of the nonlinear least-squares fitting program PHARMFIT (25). The 5-min mean BPs over the entire 72 h were calculated for each mouse using the Dataquest A.R.T. system. Twenty-four-hour cosine and all partial Fourier curves, including up to the sixth harmonic, were fitted as models to the data. The following equation was used: y = MESOR+ ∑{amplitude (i) × cos[(x-acrophase (i)) × 2π/period (i)]}, with i being the number of overlapping cosine functions. The program calculates estimates of the MESOR (midline estimating statistic of rhythm, i.e, the rhythm-adjusted 24-h mean), the amplitude (half of peak to trough of rhythmic change), and the acrophase (peak time of each component cosine function) of the harmonics together with the percentage of rhythm. The F test was used to test for zero amplitude.
Real-time PCR determination of clock gene mRNA.
Six pairs of db/db and control mice were killed at Zeitgeber time 23 and another six pairs were killed at Zeitgeber time 11 (ZT 23 or ZT 11, ZT0: light on, ZT 12 light off). Aortas were removed immediately and placed in RNAlater solution. After the adventitia and endothelium was carefully removed, RNA was extracted using an RNeasy mini-kit (Qiagen, Valencia, CA). The aortic smooth muscle tissue was used because vascular clock is best characterized in aortic smooth muscle (34, 36), and db/db mouse aortic smooth muscle showed dramatic enhanced contractile responses similar to small arteries (12). In addition, the aortic smooth muscle is readily available in sufficient quantities. The RNA extraction, cDNA synthesis, and real-time PCR were carried out as previously described (12, 45). The PCR primers used were DBP: 5′-ACCGTGGAGGTGCTAATG-3′ (forward) and 5′-ATGGCCTGGAATGCTTGA-3′ (reverse); Bmal1: 5′-CACTGTCCCAGGCATTCCA-3′ (forward) and 5′-TTCCTCCGCGATCATTCG-3′ (reverse); Per1: 5′-TCGAAACCAGGACACCTTCTCT-3′ (forward) and 5′-GGGCACCCCGAAACACA-3′ (reverse); 36b4: 5′-CCCTGAAGTGCTCGACATCA-3′ (forward) and 5′-TGCGGACACCCTCCAGAA-3′ (reverse). These primers have been demonstrated to be specific for the respective clock genes (33). The mRNAs of clock genes DBP, Bmal1, and Per1 were normalized to 36b4 mRNA and quantified by the standard curve analysis.
All data are expressed as means ± SE. Significant statistical difference was set at P < 0.05. Data were compared by t-tests unless otherwise noted.
RESULTS
Body weight, plasma glucose and insulin in db/db and control mice.
Twelve control and 12 db/db male mice were used at age of 14 to 16 wk. Body weights of the db/db mice were significantly heavier than those of the control mice: 47.4 ± 1.20 vs. 27.7 ± 0.48 g (P < 0.0001). Nonfasting plasma glucose levels in db/db mice were significantly higher than in control mice: 537.3 ± 33.3 vs. 232.4 ± 18.61 mg/dl (P < 0.001). Plasma insulin levels were 2.9 ± 0.66 ng/ml in db/db mice and 1.2 ± 0.21 ng/ml in control mice (P < 0.05).
Systolic, diastolic, and mean arterial pressures are increased in db/db mice.
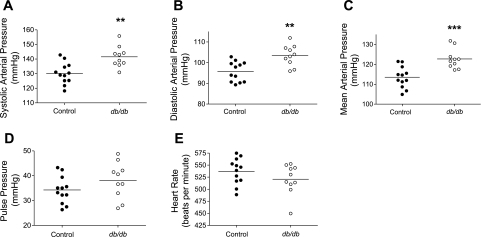
As shown in Fig. 1, when averaged over a 24-h period, the systolic, diastolic, and mean arterial pressures were significantly increased in db/db mice compared with control mice: systolic, 141.5 ± 2.25 vs.130.2 ± 2.10 mmHg (P < 0.01); diastolic, 103.4 ± 1.59 vs. 95.7 ± 1.39 mmHg (P < 0.01); and mean, 122.7 ± 1.58 vs. 113.5 ± 1.54 mmHg (P < 0.001). In contrast, no significant differences were detected in the pulse pressure and heart rate between db/db and control mice: pulse pressure, 34.3 ± 1.57 vs. 38.1 ± 2.30 mmHg (P = 0.74); heart rate, 537.1 ± 7.84 vs. 520.8 ± 9.84 beats/min (P = 0.20).
Fig. 1.
The 24-h average systolic (A), diastolic (B), and mean arterial blood pressure (C), but not pulse pressure (D) and heart rate (E), were increased in db/db mice. Male 14- to 16-wk-old db/db and age- and gender-matched control mice were chronically instrumented in the left common carotid arteries with telemetry probes. Data of the blood pressure and heart rate were collected continuously and are individual values for each mouse with the average value for each group (horizontal line) over a 24-h period. **P < 0.01; ***P < 0.001.
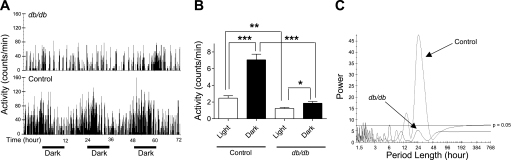
Locomotor activity is dramatically decreased in db/db mice.
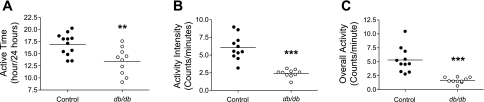
Locomotor activity is an important factor influencing the BP. Therefore, we compared locomotor activity between control and db/db mice. The telemetry system produces an activity “count” that represents a rough index of the animal's locomotor activity. We found that the amount of time the mice were active (i.e., have a count of >0) was significantly decreased in db/db compared with control mice: 13.5 ± 0.88 vs. 16.9 ± 0.66 h/24-h period, respectively (P < 0.01; Fig. 2A). Moreover, when the mice were active, the average intensity of the activity was significantly decreased in db/db mice compared with control mice: 2.4 ± 0.18 vs. 6.0 ± 0.52 counts/min, respectively (P < 0.001; Fig. 2B). Combining these parameters to define total locomotor activity, db/db mice exhibited a decrease in activity compared with control: 1.7 ± 0.16 vs. 5.3 ± 0.68 (P < 0.001; Fig. 2C).
Fig. 2.
db/db Mice exhibit decreased locomotor activity. Locomotor activity was recorded in 14- to 16-wk-old db/db and control mice. A: time the mice were active over a 24-h period. B: intensity of activity when the mice are active. C: overall activity. Individual values for each mouse are depicted, with the group average (horizontal line). **P < 0.01; ***P < 0.001.
BP circadian rhythm is disrupted in db/db mice.
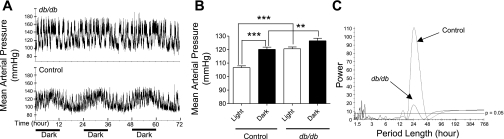
Control mice exhibited a diurnal pattern in mean arterial pressure during 72 h of recording (Fig. 3A, bottom). The average arterial pressure in the 12-h light period significantly “dipped” when compared with that during the 12-h dark period (Fig. 3B). Partial Fourier analysis showed that 24 h was the dominant period length (Fig. 3C) in control mice, and the only other minor rhythm that was detectable was one with a period length of ∼14 h.
Fig. 3.
Disrupted circadian blood pressure rhythm in db/db mice. A: continuous 48-h mean arterial pressure recording obtained via radiotelemetry in control and db/db mice. B: average mean arterial pressure in the 12-h light and dark period. Data are means ± SE. C: representative mean arterial pressure periodograms from a db/db and a control mouse. **P < 0.01; ***P < 0.001.
In striking contrast, db/db mice did not exhibit an apparent diurnal pattern in mean arterial pressure during a 72-h recording period (Fig. 3A, top). The average arterial pressure in the 12-h light period did not “dip” when compared with the arterial pressure during the 12-h dark period (Fig. 3B). Consequently, the mean arterial pressure difference between db/db and control mice during the light period was bigger than that during the dark period: 14 vs. 6 mmHg. Partial Fourier analysis indicated that there were several rhythms with variable period lengths in the db/db mice. The period lengths detected include short ones of 1 to 3 h and a much longer one of 24 h (Fig. 3C).
Since 24 h is the one period length that was detected in both control and db/db mice, we further compared the 24-h rhythm attributes between the two strains of mice. The results showed significantly decreased amplitude (half of peak to trough of rhythmic change; Table 1) in db/db mice compared with control mice: 5.67 ± 1.38 vs.11.74 ± 0.53 mmHg, respectively (P < 0.001). The acrophase (peak time of each component cosine function) shifted from1:41 ± 0:09 in control mice to 5:58 ± 2:34 in db/db mice (P = 0.078). Although the time shift in the acrophase was obvious in db/db mice, the difference did not reach statistical significance, which was likely due to the large variation (18:05 to 2:36) in db/db mice. The peak and nadir of the fitted arterial pressures were both significantly increased in db/db mice compared with control mice (Table 1).
Table 1.
The circadian rhythm of mean arterial pressure (MAP) in db/db and control mice
| 24-h Rhythm Attribute | Control | db/db | P Value |
|---|---|---|---|
| Amplitude, mmHg | 11.74±0.53 | 5.67±1.38 | 0.00003 |
| Acrophase, h | 1.69±0.16 | 5.97±2.53 | 0.0775 |
| Peak, mmHg | 126.30±1.98 | 137.10±2.72 | 0.0038 |
| Nadir, mmHg | 101.40±1.43 | 112.50±2.28 | 0.0004 |
| Ratio of dark/light period | 1.12±0.0069 | 1.05±0.0171 | 0.001 |
Values are means ± SE.
The circadian rhythms of heart rate and locomotor activity are disrupted in db/db mice.
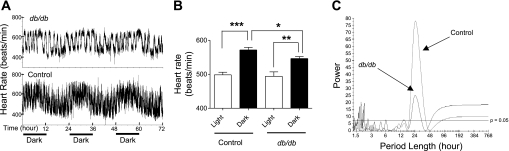
We next characterized the heart rate and locomotor activity circadian rhythms to investigate whether, in addition to BP circadian rhythm, other circadian rhythms were also disrupted in db/db mice. As shown in Fig. 4, the apparent heart rate diurnal pattern present in control mice (Fig. 4A, bottom) was dramatically altered in db/db mice (Fig. 4A, top). However, in both the control and db/db mice, the mean heart rate during the dark cycle was significantly dipped compared with those during the light cycle (Fig. 4B). Periodogram shows that, in control mice, 24 h was the dominant period length (Fig. 4C). In contrast, in db/db mice, there were several additional short period lengths of 1–3 h in addition to the 24-h period length (Fig. 4C). Interestingly, although the amplitude was significantly decreased in db/db mice (39.1 ± 6.94 vs. 59.2 ± 3.07; P = 0.01), the other 24-h rhythm attributes including acrophase, peak, nadir, and the ratio of dark/light period values did not show a statistically significant difference between db/db and control mice (Table 2).
Fig. 4.
Disrupted circadian heart rate rhythm in db/db mice. A: continuous 48-h heart rate recording obtained via radiotelemetry in control and db/db mice. B: average heart rate in the 12-h light and dark periods. Data are means ± SE. C: representative heart rate periodograms from a db/db and a control mouse. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 2.
The circadian rhythm of heart rate in db/db and control mice
| 24-h Rhythm Attribute | Control | db/db | P Value |
|---|---|---|---|
| Amplitude, beats/min | 59.23±3.07 | 39.14±6.94 | 0.0108 |
| Acrophase, h | 6.75±2.97 | 9.92±3.52 | 0.4971 |
| Peak, beats/min | 607.4±8.80 | 592.9±9.37 | 0.2742 |
| Nadir, beats/min | 470.6±8.33 | 450.9±19.53 | 0.3355 |
| Ratio of dark/light period | 1.15±0.010 | 1.11±0.0268 | 0.1873 |
Values are means ± SE.
The locomotor activity rhythmicity showed striking differences between the control and db/db mice. As shown in Fig. 5, the apparent locomotor activity diurnal pattern present in control mice (Fig. 5, A, bottom, and B) was almost absent in db/db mice (Fig. 5, A, top, and B). Periodogram shows that a dominant period length of 24 h is present in the control mice (Fig. 5C), but no significant rhythmicity was detected in the db/db mice (Fig. 5C). Except the nadir, all other 24-h rhythm attributes, including amplitude, acrophase, peak counts, and ratio of dark/light period, showed significant difference between the control and db/db mice (Table 3).
Fig. 5.
Disrupted circadian locomotor activity rhythm in db/db mice. A: 48-h locomotor activity recording obtained via radiotelemetry in control and db/db mice. B: average locomotor activity in the 12-h light and dark period. Data are means ± SE. C: representative locomotor activity periodograms from a db/db and a control mouse. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 3.
The circadian rhythm of locomotor activity in db/db and control mice
| Rhythm Attribute | Control | db/db | P Value |
|---|---|---|---|
| Amplitude, counts/min | 4.17±0.43 | 0.69±0.12 | 0.0001 |
| Acrophase, h | 1.58±0.20 | 13.14±3.69 | 0.0015 |
| Peak, counts/min | 10.67±1.03 | 3.03±0.24 | <0.0001 |
| Nadir, counts/min | 0.61±0.20 | 0.56±0.12 | 0.8539 |
| Ratio of dark/light period | 3.22±0.234 | 1.52±0.19 | <0.0001 |
Values are means ± SE.
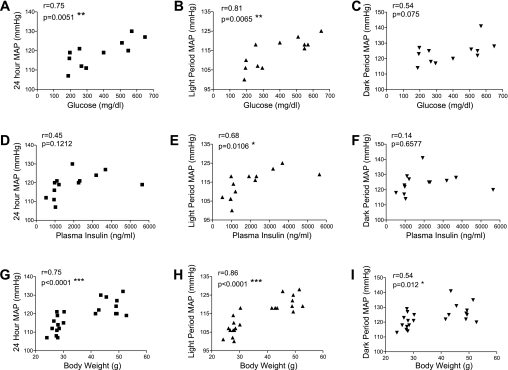
Correlation between BP and plasma glucose, insulin, and body weight.
To start to explore whether BP correlates with some of the metabolic abnormalities in db/db mice, we tested whether plasma glucose, insulin and body weight correlate with 24-h, light period, and dark period mean arterial pressure. Interestingly, the mean arterial pressure during the light period correlates with all three metabolic parameters, including plasma glucose (Fig. 6B), insulin (Fig. 6E), and body weight (Fig. 6H). However, no significant correlation was detected between the mean arterial pressure during the dark period with the plasma glucose (Fig. 6C) and insulin (Fig. 6F).
Fig. 6.
Blood pressure correlates with plasma glucose, insulin, and body weight. Blood pressures were measured continuously in conscious free-moving normal control and diabetic db/db mice by radiotelemetry. Mouse blood was collected at the end of blood pressure measurement. Plasma glucose and insulin levels were determined as described in the text. The correlation between mean arterial pressure over 24 h (A, D, and G), 12-h light period (B, E, and H), and 12-h dark period (C, F, and I) with plasma glucose, insulin, and body weight were plotted, and statistic significance was tested by Pearson's test. *P < 0.05; **P < 0.01; ***P < 0.001.
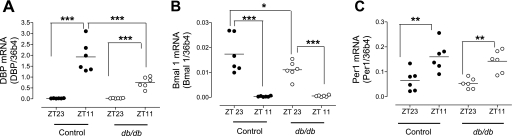
The oscillations of clock genes DBP and bmal1 but not per1 mRNA are dampened in db/db mouse aorta.
To begin to explore the molecular mechanisms underlying the perturbed BP circadian rhythm in db/db mice, we determined the rhythmic expression of several clock genes in the vasculature. DBP, Bmal1, and Per1 were chosen because they have been shown to be involved in BP regulation, and their expressions oscillate in mouse aorta (7, 27, 34). Consistent with literature, DBP and Per1 mRNA level increased, whereas Bmal1 mRNA level decreased from Zeitgeber time 23 to 11 (ZT 23 to ZT 11) in control mice (Fig. 7). Such time-dependent changes in DBP and Bmal 1 were significantly suppressed in db/db mice (Fig. 7, A and B). But the changes of Per1 from ZT23 to ZT11 were not different between the control and db/db mice (Fig. 7C).
Fig. 7.
The oscillations of clock genes DBP and Bmal1 but not Per 1 are suppressed in db/db mice aorta. Male 16-wk-old db/db and control mice were taken down at Zeitgeber time 23 or 11 (ZT 23 or ZT 11, ZT0: light on, ZT 12: light off). Aortas were immediately removed, and mRNA levels of the clock genes DBP (A), Bmal1 (B), and Per1 (C) were determined by real-time PCR. *P < 0.05; **P < 0.01; and ***P < 0.001.
DISCUSSION
db/db Mice have been used extensively as a Type 2 diabetes and obesity model and have provided important insights into the pathogenesis of diabetes and obesity. However, the few studies that have characterized BP in db/db mice have produced inconsistent results, potentially related to methods used for BP monitoring. Using radiotelemetry, results from the current study show that db/db mice have increased 24-h systolic, diastolic, and mean arterial pressures under conscious, free-moving conditions. In addition, our results demonstrate that db/db mice have severely disrupted circadian rhythms for BP, heart rate, and locomotor activity.
The differences between the control and db/db mouse 24-h average systolic pressure was 11 mmHg, diastolic pressure was 8 mmHg, and mean arterial pressure was 9 mmHg (Fig. 1). Although these are not dramatic net increases in BP in db/db mice, such differences can have a very significant impact in cardiovascular outcomes in diabetic patients because BP values clearly bear a continuous linear relation with the incidence of cardiac and cerebrovascular events (6, 24). Moreover, it has been demonstrated that for each 10-mmHg decrease in mean systolic BP, there is a reduced (by 11%) risk of myocardial infarction and microvascular complications (by 13%), resulting in a 15% decreased risk of death from diabetes (1, 21).
Our finding that db/db mice have increased BP is consistent with a previous report (2) but differs from other reports (11, 20, 28). Although the precise mechanism(s) accountable for the inconsistency of these studies is unclear, it may relate to the fact that the tail-cuff method was used in these previous studies defining BP in db/db mice. Tail-cuff method may artificially enlarge or reduce the BP difference between the db/db and control mice. BP is variable throughout a 24-h period. Measuring BP at one time point using the tail-cuff method may have hindered the ability to detect a mean of 9-mmHg difference between the control and db/db mice in some studies (11, 20, 28). On the other hand, the tail-cuff method may amplify the BP difference between the two strains of mice. For example, although both our data (present study) and those by Baji et al. (2) showed increased BP in db/db mice, the BP difference detected by us using radiotelemetry is smaller than that reported by Baji et al. using tail-cuff method (9 vs. 35 mmHg). Restraint of mice during the tail-cuff measurement procedure may have contributed to this discrepancy. Restraint is a known stress that increases mouse BP (7, 42). The BP response to restraint stress may be bigger in the db/db mice than in normal control mice since we and others have reported that vascular reactivity to contractile stimuli are enhanced in db/db mice (12, 17, 18, 31). An additional factor related to variable findings depending on the method of BP measurement is that it is difficult to detect an arterial pulse in the tail of extremely obese db/db mice (Su W, Gong M, unpublished observation).
The current study used radiotelemetry to continually monitor and compare arterial pressure in db/db and control mice. This method facilitates an accurate direct assessment of arterial pressure that is not confounded by the stress of tethering or a limited number of sampling times. The results of the current study demonstrate that the 24-h average systolic, diastolic, and mean arterial pressures are elevated in db/db mice. A recent study used radiotelemetry to measure BP in db/db mice and found no significant differences in BP between db/db and control mice (4). Differences in findings between our results and those by Bodary et al. include the age of mice and the genetic background of db/db mice. Results showing no difference in BP between db/db and control mice were obtained in 8- to 10-wk-old mice. Our results, obtained in older mice (14–16 wk) may suggest that longer durations of diabetes are needed to cause diabetes-associated hypertension. In addition, it is well recognized that the phenotype of the leptin receptor mutation is dramatically influenced by the mouse genetic background (13). The mice used in the current study were on a C57BL/KsJ background with plasma glucose of 537.3 ± 33.3 mg/dl, whereas the mice used by Bodary et al. (4) were on a C57BL/6J background with plasma glucose of 340 mg/dl. The phenotype difference due to the background strain variation may have contributed to the differences in BP.
There is increasing evidence that not only does the average level of BP, but particularly an abnormal circadian BP rhythm with a decreased fall in night BP (nondipping), determine the development of diabetic complications and cardiovascular outcomes (16). The mechanisms that account for the abnormal BP diurnal pattern in Type 2 diabetes remain largely unknown, although several observations suggest the involvement of decreased urinary albumin excretion rate (9), impaired circadian modulation of sympathovagal activity (14, 38, 39), and impaired circadian modulation of the plasma norepinephrine levels (19). A Type 2 diabetic animal model showing disrupted BP circadian rhythms should be an extremely useful tool for elucidating the precise relationship between diabetes/obesity and disruption of circadian rhythms. Our results demonstrate a severely disrupted BP circadian rhythm with changed period length, amplitude and a shifted acrophase in db/db mice. The nephropathy (5, 10) and neuropathy (29, 41) present in the 14- to 16-wk-old db/db mice may have contributed to the BP increase and BP circadian rhythm disruption. In addition, emerging studies demonstrate clock gene dysregulation as a mechanism that may contribute to hypertension and BP circadian dysregulation (7). To start to explore the role of clock genes, we investigated the expression of several clock genes in vascular wall. Our data demonstrate a selected dampening of the oscillations of two clock genes, DBP and Bmal1, in db/db mice aorta, supporting a role of clock gene in circadian rhythm dysregulation in db/db mice. DBP and Bmal1 have been recently implicated in BP circadian rhythm regulation. Global knocking out Bmal1 completely abolished the diurnal variation in MAP, a phenotype very similar to what we observed in db/db mice (7). In contrast, selective suppression of Bmal1 in endothelium does not affect BP diurnal variation, suggesting Bmal1 in sites other than endothelium is essential for maintaining BP diurnal variation (43). Interestingly, in Bmal1 knockout mouse aorta, the DBP expression is decreased (7). DBP has been shown to be a component of the circadian output pathway (22), and its oscillation is suppressed in Dahl Salt-sensitive rats (27). Importantly, a recent study by Woon et al. first reported a strong association in humans of Bmal1 single nucleotide polymorphisms SNP halotypes with hypertension and Type 2 diabetes (44). However, many questions remained to be addressed to identify a causal role of DBP and Bmal1 in db/db mice BP abnormalities. For example, for clock gene oscillations dampened in small arteries, endothelium, brain, heart, and kidney, which are directly relevant to BP regulation? Does restoring the DBP and/or Bmal1 oscillation in db/db mice recover their BP diurnal rhythm? Is the disrupted circadian rhythm in db/db mice caused directly by the loss of leptin receptor signaling, or alternatively is it a consequence of the disrupted metabolism resulting from the leptin receptor mutation?
In summary, our results demonstrate that db/db mice are hypertensive with a disrupted BP, heart rate, and locomotor activity circadian rhythm. Such changes are associated with suppressed clock gene DBP and Bmal1 oscillation in vasculature.
GRANTS
This work is supported by National Heart, Lung, and Blood Institute Grant HL-082791 and an American Diabetes Association Career Development Award (1-04-CD-04) (M. C. Gong), by NS-39774 (D. C. Randall), and by HL-73085 (L. A. Cassis).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of Type 2 diabetes (UKPDS 36): prospective observational study. BMJ 321: 412–419, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol 25: 1610–1616, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bell RH, Hye RJ. Animal models of diabetes mellitus: physiology and pathology. J Surg Res 35: 433–460, 1983. [DOI] [PubMed] [Google Scholar]
- 4.Bodary PF, Shen Y, Ohman M, Bahrou KL, Vargas FB, Cudney SS, Wickenheiser KJ, Myers MG Jr, Eitzman DT. Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler Thromb Vasc Biol 27: 70–76, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MP, Masson N, Hud E, Ziyadeh F, Han DC, Clements RS. Inhibiting albumin glycation ameliorates diabetic nephropathy in the db/db mouse. Exp Nephrol 8: 135–143, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH. Blood pressure, stroke, and coronary heart disease. Part 2: Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 335: 827–838, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA 104: 3450–3455, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuspidi C, Meani S, Lonati L, Fusi V, Valerio C, Sala C, Magnaghi G, Maisaidi M, Zanchetti A. Short-term reproducibility of a non-dipping pattern in Type 2 diabetic hypertensive patients. J Hypertens 24: 647–653, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Equiluz-Bruck S, Schnack C, Kopp HP, Schernthaner G. Nondipping of nocturnal blood pressure is related to urinary albumin excretion rate in patients with Type 2 diabetes mellitus. Am J Hypertens 9: 1139–1143, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Gartner K Glomerular hyperfiltration during the onset of diabetes mellitus in two strains of diabetic mice (c57bl/6j db/db and c57bl/ksj db/db). Diabetologia 15: 59–63, 1978. [DOI] [PubMed] [Google Scholar]
- 11.Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V, Adler GK. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of Types 1 and 2 diabetes mellitus. Endocrinology 147: 5363–5373, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, Gong MC. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res 67: 723–735, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hummel KP, Coleman DL, Lane PW. The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL-KsJ and C57BL-6J strains. Biochem Genet 7: 1–13, 1972. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda T, Matsubara T, Sato Y, Sakamoto N. Circadian blood pressure variation in diabetic patients with autonomic neuropathy. J Hypertens 11: 581–587, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Imai Y, Hozawa A, Ohkubo T, Tsuji I, Yamaguchi J, Matsubara M, Michimata M, Hashimoto J, Fujiwara T, Nagai K, Kitaoka H, Satoh H, Hisamichi S. Predictive values of automated blood pressure measurement: what can we learn from the Japanese population: the Ohasama study. Blood Press Monit 6: 335–339, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Izzedine H, Launay-Vacher V, Deray G. Abnormal blood pressure circadian rhythm: a target organ damage? Int J Cardiol 107: 343–349, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kamata K, Kojima S. Characteristics of contractile responses of aorta to norepinephrine in db/db mice. Res Commun Mol Pathol Pharmacol 96: 319–328, 1997. [PubMed] [Google Scholar]
- 18.Kanie N, Kamata K. Contractile responses in spontaneously diabetic mice. II. Effect of cholestyramine on enhanced contractile response of aorta to norepinephrine in C57BL/KsJ (db/db) mice. Gen Pharmacol 35: 319–323, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Kondo K, Matsubara T, Nakamura J, Hotta N. Characteristic patterns of circadian variation in plasma catecholamine levels, blood pressure and heart rate variability in Type 2 diabetic patients. Diabet Med 19: 359–365, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for Type 2 diabetes. FASEB J 14: 439–447, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Lindholm LH The outcome of STOP-hypertension-2 in relation to the 1999 WHO/ISH hypertension guidelines. Blood Press Suppl 2: 21–24, 2000. [PubMed] [Google Scholar]
- 22.Lopez-Molina L, Conquet F, Dubois-Dauphin M, Schibler U. The DBP gene is expressed according to a circadian rhythm in the suprachiasmatic nucleus and influences circadian behavior. EMBO J 16: 6762–6771, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 5: 407–441, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1: Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 335: 765–774, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Mattes A, Witte K, Hohmann W, Lemmer B. PHARMFIT: a nonlinear fitting program for pharmacology. Chronobiol Int 8: 460–476, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, Kramer K. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol 88: 1537–1544, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Mohri T, Emoto N, Nonaka H, Fukuya H, Yagita K, Okamura H, Yokoyama M. Alterations of circadian expressions of clock genes in Dahl salt-sensitive rats fed a high-salt diet. Hypertension 42: 189–194, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Moriyama T, Oka K, Ueda H, Imai E. Nilvadipine attenuates mesangial expansion and glomerular hypertrophy in diabetic db/db mice, a model for Type 2 diabetes. Clin Exp Nephrol 8: 230–236, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Norido F, Canella R, Zanoni R, Gorio A. Development of diabetic neuropathy in the C57BL/Ks (db/db) mouse and its treatment with gangliosides. Exp Neurol 83: 221–232, 1984. [DOI] [PubMed] [Google Scholar]
- 30.Pickering TG, Kario K. Nocturnal non-dipping: what does it augur? Curr Opin Nephrol Hypertens 10: 611–616, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Piercy V, Taylor SG. A comparison of spasmogenic and relaxant responses in aortae from C57/BL/KsJ diabetic mice with those from their non-diabetic litter mates. Pharmacology 56: 267–275, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Pistrosch F, Reissmann E, Wildbrett J, Koehler C, Hanefeld M. Relationship between diurnal blood pressure variation and diurnal blood glucose levels in Type 2 diabetic patients. Am J Hypertens 20: 541–545, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Reilly DF, Curtis AM, Cheng Y, Westgate EJ, Rudic RD, Paschos G, Morris J, Ouyang M, Thomas SA, FitzGerald GA. Peripheral circadian clock rhythmicity is retained in the absence of adrenergic signaling. Arterioscler Thromb Vasc Biol 28: 121–126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reilly DF, Westgate EJ, FitzGerald GA. Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol 27: 1694–1705, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 418: 935–941, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation 112: 2716–2724, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Shimada K, Kario K. Altered circadian rhythm of blood pressure and cerebrovascular damage. Blood Press Monit 2: 333–338, 1997. [PubMed] [Google Scholar]
- 38.Spallone V, Bernardi L, Ricordi L, Solda P, Maiello MR, Calciati A, Gambardella S, Fratino P, Menzinger G. Relationship between the circadian rhythms of blood pressure and sympathovagal balance in diabetic autonomic neuropathy. Diabetes 42: 1745–1752, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Spallone V, Maiello MR, Cicconetti E, Menzinger G. Autonomic neuropathy and cardiovascular risk factors in insulin-dependent and non insulin-dependent diabetes. Diabetes Res Clin Pract 34: 169–179, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Stas SN, El-Atat FA, Sowers JR. Pathogenesis of hypertension in diabetes. Rev Endocr Metab Disord 5: 221–225, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis 28: 276–285, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology 143: 4520–4526, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA, FitzGerald GA. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation 117: 2087–2095, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA 104: 14412–14417, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Z, Gong MC, Su W, Turk J, Guo Z. Group VIA phospholipase A2 (iPLA2beta) participates in angiotensin II-induced transcriptional up-regulation of regulator of g-protein signaling-2 in vascular smooth muscle cells. J Biol Chem 282: 25278–25289, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziegler MG Sleep disorders and the failure to lower nocturnal blood pressure. Curr Opin Nephrol Hypertens 12: 97–102, 2003. [DOI] [PubMed] [Google Scholar]