Abstract
Decreased cerebral blood flow (CBF) has been observed following the resuscitation from neonatal hypoxic-ischemic injury, but its mechanism is not known. We address the hypothesis that reduced CBF is due to a change in nitric oxide (NO) and superoxide anion O2− balance secondary to endothelial NO synthase (eNOS) uncoupling with vascular injury. Wistar rats (7 day old) were subjected to cerebral hypoxia-ischemia by unilateral carotid occlusion under isoflurane anesthesia followed by hypoxia with hyperoxic or normoxic resuscitation. Expired CO2 was determined during the period of hyperoxic or normoxic resuscitation. Laser-Doppler flowmetry was used with isoflurane anesthesia to monitor CBF, and cerebral perivascular NO and O2− were determined using fluorescent dyes with fluorescence microscopy. The effect of tetrahydrobiopterin supplementation on each of these measurements and the effect of apocynin and Nω-nitro-l-arginine methyl ester (l-NAME) administration on NO and O2− were determined. As a result, CBF in the ischemic cortex declined following the onset of resuscitation with 100% O2 (hyperoxic resuscitation) but not room air (normoxic resuscitation). Expired CO2 was decreased at the onset of resuscitation, but recovery was the same in normoxic and hyperoxic resuscitated groups. Perivascular NO-induced fluorescence intensity declined, and O2−-induced fluorescence increased in the ischemic cortex after hyperoxic resuscitation up to 24 h postischemia. l-NAME treatment reduced O2− relative to the nonischemic cortex. Apocynin treatment increased NO and reduced O2− relative to the nonischemic cortex. The administration of tetrahydrobiopterin following the injury increased perivascular NO, reduced perivascular O2−, and increased CBF during hyperoxic resuscitation. These results demonstrate that reduced CBF follows hyperoxic resuscitation but not normoxic resuscitation after neonatal hypoxic-ischemic injury, accompanied by a reduction in perivascular production of NO and an increase in O2−. The finding that tetrahydrobiopterin, apocynin, and l-NAME normalized radical production suggests that the uncoupling of perivascular NOS, probably eNOS, due to acquired relative tetrahydrobiopterin deficiency occurs after neonatal hypoxic-ischemic brain injury. It appears that both NOS uncoupling and the activation of NADPH oxidase participate in the changes of reactive oxygen concentrations seen in cerebral hypoxic-ischemic injury.
Keywords: apocynin, neonatal rat, vasculature, Nω-nitro-l-arginine methyl ester
hypoxic and ischemic injury in the brains of neonatal infants leads to morbidity, much of which appears in the form of developmental cognitive and motor defects. Considerable effort has been devoted to the goal of understanding the pathophysiology of this type of injury to develop possible therapeutic interventions (40). In this regard, the study of the mechanisms that lead to delayed tissue is of great interest.
A compromise of cerebral vascular function due to a loss of vascular reactivity may be an underlying cause of cerebral injury due to hypoxia-ischemia (H/I) (29). One important determinant of vascular tone and function is nitric oxide (NO), produced by endothelial NO synthase (eNOS, NOS3).
A phenomenon that has gained increasing attention for its influence on blood flow and an underlying cause of vascular injury under pathological conditions is the uncoupling of eNOS, which reduces NO production and results in the production of superoxide anion (O2−) by eNOS. Although eNOS coupling appears to be developmentally regulated in the neonate in other vascular beds (24), there have been few assessments of this phenomenon in the central nervous system.
Although not invariably seen, oxidative stress has been shown to be capable of altering eNOS cofactors such as tetrahydrobiopterin (BH4) that lead to the uncoupling of eNOS (15, 42). The production of O2− by NADPH oxidase in endothelial cells is also thought to be important in establishing vascular tone in response the various stimuli. O2− may react with NO and reduce its concentration and react with BH4 or eNOS to change its activity or uncouple it, resulting in a further increase in O2− production (26).
Here we used an in vivo method to examine the hypothesis that neonatal H/I will impair perivascular production of NO and increase O2− and that these effects can be reversed by a replenishment of the NOS cofactor BH4. We also examined the effect of apocynin, an inhibitor of NADPH oxidase, and Nω-nitro-l-arginine methyl ester (l-NAME), an inhibitor of NOS. We also determined whether cerebral blood flow (CBF) paralleled some of these effects in this model.
METHODS
Neonatal H/I injury.
All procedures involving animals were conducted according to criteria approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Timed pregnant Wistar rats were purchased from a commercial breeder (Charles River), housed in individual cages, and fed a standard laboratory chow ad libitum. Offspring were delivered spontaneously and maintained with their dam; litters were culled to 10 on postnatal day 1 or 2. On postnatal day 7 (P7), H/I was induced as described by Rice et al. (32) with some modifications. The rats were given isoflurane in 100% oxygen in a chamber for induction (2%) and via a face mask for maintenance of anesthesia throughout surgery (2%). The left common carotid artery was exposed through a midline incision in the neck and thoroughly cauterized over a length of 5 mm with a bipolar cauterizing device. After recovery from anesthesia, the pups were returned to the dams, and 2 to 4 h after surgery, the rats were exposed to 7.8% oxygen at 37°C for 120 min. The pups subjected to hyperoxic resuscitation were resuscitated with 100% oxygen at 37°C for 2 h. Pups subjected to normoxic resuscitation were exposed to 21% oxygen at 37°C for 2 h. Sham-operated pups underwent anesthesia and surgery without carotid occlusion, and they were subjected to hypoxia and hyperoxia as above. The pups were then returned to the dam.
Treatment groups.
Hyperoxic resuscitation was used for all pharmacological treatment groups. Groups of pups prepared as above consisting of equal numbers of males and females were injected intraperitoneally with 20 mg/kg of BH4 [(6R)-5,6,7,8-tetrahydrobiopterin dihydrochloride; Sigma-Aldrich], in 80 μl 1% acetic acid and 4 mg/kg apocynin (4-hydroxy-3-methoxyacetophenone, Sigma-Aldrich) in 80 μl PBS, or 12.5 mg/kg l-NAME (Sigma-Aldrich) in 80 μl PBS, sterile filtered, with the first dose following hyperoxia and again the following morning. Control pups received 80 μl of sterile-filtered carrier on the same schedule. The doses for BH4 (12, 16), l-NAME (30), and apocynin (7, 25) were based on prior literature reports that described their effects and specificity. In general, at the doses used, these agents have been shown to be specific with effects relevant to these experiments.
Fluorescent analysis of NO and superoxide production in H/I injury.
Following a survival of 30 min, 2 h, or 24 h, the pups were anesthetized by isoflurane inhalation and injected intraperitoneally with 4,5-diaminofluorescein diacetate (DAF-2 AC), 10 μl of a 5 mmol/l stock solution in dimethylsulfoxide, for visualization of NO production or with dihydroethidium (DHE, Sigma-Aldrich), and 0.5 mg in 10 μl of dimethylsulfoxide, for visualization of O2−. The external jugular vein was exposed via incision and blunt dissection for free hand injection of acetylcholine. In some cases the pups were kept anesthetized, and an equal amount of either dye was injected intracardially 1 to 3 min before transcardial perfusion and fixation. Some pups were left uninjected as controls for endogenous fluorescence.
DAF-2 AC is a derivative of fluorescein, developed as a means of localizing NO production in tissue. It readily diffuses through cellular membranes and becomes deacetylated to produce diaminofluorescein in smooth muscle cells of vascular walls where a reaction with NO produces a highly fluorescent product in a concentration-dependent manner. It is specific for NO and does not react with other reactive oxygen species under physiological conditions (17). These methods were an adaptation for the neonatal model of a method published by Gerzanich et al. (10).
DHE (3,8-diamino-5,6-dihydro-5-ethyl-6-phenylphenanthridine) is a fluorescent indicator for the production of O2− in vivo (3). Whereas there is evidence that DHE reacts with other reactive oxygen species under certain conditions, it does not react with NO (2).
Twenty minutes after an intraperitoneal injection of the dye, acetylcholine (10 μl of a 10 mM solution in normal saline) was injected intravenously via the external jugular vein to stimulate eNOS activity (9). After an additional 5 min, the animals were euthanized by pentobarbital sodium injection (130 mg/kg ip) and perfused transcardially with ice-cold normal saline followed by an excess of 2% gluteraldehyde in a 0.1 phosphate buffer (pH 7).
The brains were then quickly removed and flash frozen in 2-methylbutane and dry ice. Frozen sections were prepared and examined using a Nikon Eclipse 80i epifluorescence microscope. Some sections were treated with 50 mM glycine and 0.1% Tween 20 in PBS for 30 min to suppress gluteraldehyde-induced fluorescence. An image analysis of digitized photomicrographs using ImageJ software (freely available from the National Institutes of Health) was done to measure fluorescence intensity in cortical vascular tissue affected by H/I compared with nonischemic cortex. Fluorescence intensity measurements were made of the region of the vessel wall, and background fluorescence was subtracted to obtain fluorescence intensity of the perivascular region. The localization of the hypoxic ischemic areas was based on the distribution of injury as determined by hematoxylin and eosin-stained sections from animals subjected to the same H/I injury after 24 h survival, which corresponded closely to previously published results for the same animal model (33). For such comparisons, regions of the parietal cortex were examined ipsilateral and contralateral to the carotid occlusion. The pia and arachnoid mater were excluded from the analysis. The ratio of the mean fluorescence intensity of vessels in the ipsilateral and contralateral cortex on the same section was determined for each animal, thus these measurements were semiquantitative in nature.
CBF changes immediately following H/I injury.
Rat pups (7 day old) were subjected to cerebral H/I as described in Neonatal H/I injury, except that immediately following hypoxia, the pups were anesthetized by inhalation with isoflurane and 100% or 21% oxygen and the calvarium exposed by incision. Measurements of CBF were made using a fiberoptic laser-Doppler device with dual probe tips oriented perpendicular to the calvarium bilaterally over the frontoparietal cortex. Laser-Doppler determination of CBF correlates well with other methods of measuring CBF in hypoxia and ischemia in rats, and the thin, translucent calvarium of most of the P7 rats enables monitoring of CBF in real time without opening the skull (6). The monitoring of CBF was continued in this manner for at least 2 h. CBF measurements were normalized to baseline in each subject. These groups were not injected with acetylcholine.
Measurement of expired CO2.
The differences in recovery from hypocapnea that develop during hypoxia may account for the differences in results in the hyperoxic and normoxic resuscitation groups. Thus expired CO2 was determined during resuscitation using a microcapnographic instrument with response times of <1 s (model CI240, Columbus Instruments, Columbus, OH). The instrument was calibrated according to manufacturer's recommendations. Groups of P7 pups were subjected to hypoxia with normoxic or hyperoxic resuscitation as described in Neonatal H/I injury. Measurements of expired CO2 were done on unanesthetized pups using a nose cone before the onset of hypoxia and at 5, 30, 60, 90, and 120 min of resuscitation after hypoxia.
Statistical analyses.
Comparisons between group means were done using independent group analysis of variance with post hoc tests of group difference significance done using the freely available statistical programming package from the R Project (http://cran.r-project.org). Statistical significance was assumed if P < 0.05.
RESULTS
Injury produced by H/I.
Hematoxylin and eosin-stained coronal sections examined after 24 and 72 h showed some changes after 24 h with the appearance of pyknotic neuronal cell bodies in cortical and hippocampal cell layers. After 72 h, much more extensive changes were seen on the hypoxic ischemic side of the brain with the loss of neurons of most cell layers in the cortex, hippocampus, and striatum (Fig. 1). In general, pups tolerate the period of hypoxia well, becoming inactive and sedate, and return quickly to normal activities upon resuscitation.
Fig. 1.
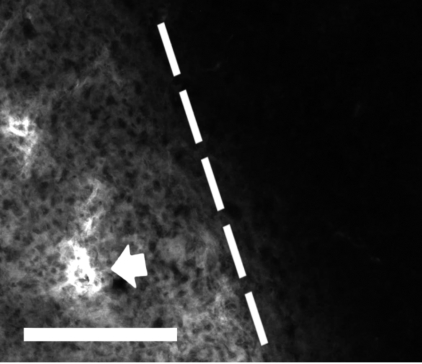
Coronal sections from brains of postnatal day 7 rat pups subjected to hypoxia-ischemia (H/I) and hyperoxia (see text). Injury after 24 h (left) is subtle with pyknotic neuronal cells in cortical layers III and V being most noticeable on the ischemic side (black arrow). Injury after 72 h (right) shows more extensive changes with loss of neurons throughout the cortex, striatum, and hippocampus on the ischemic side (black arrow). Black arrows indicate the cortex within which fluorescence of vessel walls in tissue affected by H/I were made. White arrows indicate the region of the cortex within which measurements of fluorescence of vessel walls nonischemic tissue were made (hematoxylin and eosin).
Relative regional production of NO (by DAF-2 AC fluorescence) and O2− (by DHE fluorescence).
Fluorescent product was apparent in vessel walls of the brains of animals injected with DAF-2 AC. This fluorescence was visible in pia matter and the choroid but reduced in vessels in hypoxic-ischemic cortex at 30 min, 2 h, and 24 h. (Fig. 2). In animals injected with DHE, there was fluorescent product seen in the pia mater and the choroid, but little was apparent in the parenchyma of tissue not affected by H/I (Fig. 3). Within the region of the hypoxic-ischemic effect, there was a general increase in fluorescence, and visual inspection and intensity quantitation demonstrated that this fluorescence was more intense in blood vessel walls at all survival intervals (Fig. 4). Measurements of fluorescence intensity of perivascular tissue confirmed that there was a significant increase in the DHE fluorescence ratio present in ischemic versus nonischemic tissues at 2 and 24 h and a significant decrease in DAF-2 AC fluorescence ratio when ischemic and nonischemic tissues were compared after 24 h (Fig. 4). In animals that were not injected with dye, glutaraldehyde-induced fluorescence was seen in the pia arachnoid and choroid plexus but only faint fluorescence was noted in the parenchyma and associated vasculature (data not shown).
Fig. 2.
4,5-Diaminofluorescein diacetate (DAF-2 AC) fluorescence in cortical tissue as an indication of nitric oxide (NO) production. The line represents the border of the hypoxic ischemic region, which is on the left, and nonischemic tissue is on the right. There is noticeably less vascular fluorescence intensity in the vascular walls within the hypoxic-ischemic tissue. Long arrows indicate fluorescence in blood vessel walls in the ischemic territory. Short arrows indicate vascular fluorescence in the nonischemic peri-ischemic territory. Bar = 200 μm.
Fig. 3.
Dihydroethidium (DHE) fluorescence in a tissue at the margin of the region affected by H/I. The hypoxic-ischemic region is on the left, and nonischemic region is on the right. The border between these regions is indicated by the line. The arrow indicates vascular fluorescence in the ischemic territory. There is no fluorescence detectable in the nonischemic peri-ischemic territory. Bar = 200 μm.
Fig. 4.
Ratio of intensity of vascular fluorescence of cortical tissue within the hypoxic-ischemic region and intensity of nonischemic tissue as measured from digitized images of the same sections. There is an overall reduction of fluorescence intensity of DAF-2 AC, an increase in DHE fluorescence ratio, and a significant increase at 24 h compared with that at 30 min. *P < 0.05 by ANOVA with Dunnett post hoc test. Error bars are means ± SD.
Hyperoxic resuscitation versus normoxic resuscitation.
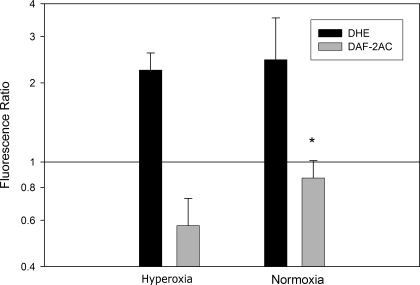
DHE staining in tissue affected by H/I was not significantly different in animals resuscitated with 2 h of 100% oxygen compared with those resuscitated with room air. DAF-2 AC staining in normoxic resuscitated pups was significantly higher than it was in pups resuscitated with 100% oxygen in tissue subjected to H/I (Fig. 5).
Fig. 5.
Fluorescent markers of NO and superoxide production in cortical tissue. There is a small but significant increase in NO in normoxic resuscitated pups compared with hyperoxic resuscitation pups (*P < 0.05 by t-test, n = 5 in each group, DAF-2 AC intensity ratio, normoxia vs. hyperoxia). DHE staining for superoxide shows no difference in normoxia and hyperoxia resuscitation (n = 4 in each group). Error bars are means ± SD.
Effects of BH4, apocynin, and l-NAME treatment.
Treatment with tetrahydrobiopterin increased CBF at 2 h posthypoxia compared with that in controls (Fig. 6). The ratio of DHE reaction product in the hypoxic-ischemic tissue to that in nonischemic tissue was reduced in animals treated with BH4, apocynin, and l-NAME compared with that in controls after 24 h survival. BH4 and apocynin treatment significantly increased the ratio of DAF-2 AC reaction product fluorescence in hypoxic-ischemic tissue compared with fluorescence in nonischemic tissue with 24 h of survival (Fig. 6).
Fig. 6.
Fluorescent markers of NO and superoxide anion (O2−) production in cortical tissue sections. Effects of pharmacological agents on O2− (DHE) and NO (DAF-2 AC) levels in hyperoxically resuscitated groups. Control, carrier only (n = 4 in each group); Apo, apocynin (n = 4 in each group); l-NAME, Nω-nitro-l-arginine methyl ester (n = 4 in each group); BH4, tetrahydrobiopterin (n = 5 in each group). *P < 0.05 for significant difference from respective control group, ANOVA with Dunnett post hoc test. Error bars are means ± SD.
CBF following hypoxic-ischemic injury.
Laser-Doppler measurements of CBF showed a significant reduction in CBF over the 2 h following hypoxic-ischemic injury in animals subjected to H/I with hyperoxic resuscitation but not with normoxic resuscitation. Treatment with BH4 resulted in a significant increase in CBF during hyperoxic resuscitation. CBF in the sham-operated hypoxic-ischemic group was not significantly different from normoxically resuscitated subjects or subjects treated with BH4 and hyperoxic resuscitation (Fig. 7).
Fig. 7.
Cerebral blood flow (CBF) measured by laser-Doppler (CBFLD) flowmetry following H/I injury. All measurements are normalized to CBF at the beginning of the monitoring period. Hyperoxia indicates CBF measurements in hypoxic-ischemic cortex in animals treated with carrier only and subjected to hyperoxic resuscitation. BH4 plus hyperoxia indicates CBF measurements during hyperoxic resuscitation in hypoxic-ischemic cortex in animals treated with BH4 immediately after H/I. Normoxia indicates CBF measurements in hypoxic ischemic cortex in animals treated with carrier only subjected to normoxic resuscitation. Sham plus hyperoxia indicates CBF measurements in animals that did not undergo carotid occlusion and were exposed to hyperoxic resuscitation. The CBF in the hyperoxic group on the hypoxic-ischemic side is significantly lower than the Sham, normoxic, and BH4-treated groups (P < 0.05 by ANOVA with post hoc testing of group differences). Error bars are means ± SD (n = 4 in each group).
Expired CO2 during resuscitation.
Rat pups had lower than normal expired CO2 following hypoxia. Hypocapnea recovered similarly in normoxia and hyperoxia resuscitation groups (Fig. 8).
Fig. 8.
Measurements of expired carbon dioxide during resuscitation with 100% oxygen (hyperoxia) or room air (normoxia.) Significant differences were seen between baseline measurements done before 2 h of hypoxia (−120 min) and 5-, 30-, 60-, and 90-min groups during resuscitation (P < 0.05 by ANOVA with post hoc testing of group differences, n = 5 for both resuscitation groups or 10 for the baseline group). When comparing group measurements of expired CO2 done at the same time intervals following the onset of resuscitation, no significant differences were seen between hypoxia and normoxia resuscitation groups. Error bars are means ± SD.
DISCUSSION
These results show for the first time the in vivo evidence that neonatal cerebral hypoxic-ischemic injury results in a decrease in vascular wall NO concentration and an increase in perivascular O2− production at a time when CBF is reduced. Treatment with BH4 reversed these fluorescent changes and restored CBF, and l-NAME reduced O2− production relative to nonischemic cortex. In support of an interaction between NADPH and NOS, apocynin increased both perivascular O2− and NO production. Although we have not identified the source of these radicals, these effects are consistent with what is expected if there is significant uncoupling of eNOS due to BH4 depletion (42). In addition, these results support the idea that NADPH oxidase contributes to the increase in O2− and the reduction in NO seen in the injury (4). Regardless of the source of O2−, an increase could result in a loss of BH4 with an uncoupling of NO production from O2− and reduce the endothelial-dependent vasodilatory capacity of the ischemic tissue, rendering it susceptible to further injury (26).
Limitations of the interpretation of our results include the possibility that the systemic administration of these antagonists had other effects, such as a change in blood pressure that may indirectly produce some of the findings seen here. Although our findings are consistent with the concept of uncoupling, other approaches to inhibit these enzymes will be necessary to confirm these results.
We also studied 2 h of hyperoxic resuscitation after an initial hypoxic-ischemic injury. Although many clinicians now strive to restrict exposure to hyperoxic resuscitation, hyperoxic resuscitation of various durations is commonly employed in clinical practice for infants suspected of H/I. There is a large body of evidence indicating that oxidative stress, including O2− production, is increased during reoxygenation and reperfusion following hypoxia (20), consistent with the results seen here. This regimen has raised concerns about the potential hazards of an increase in oxidative stress (37) and has prompted the adoption of clinical precautions to prevent oxidative injury in neonates (1). However, there is little data on the optimal use of oxygen in this period, and experimental work comparing hyperoxic and normoxic resuscitation shows mixed results. Some show no difference (35), and others show increased injury associated with hyperoxic resuscitation (38). Our prior work suggests that the duration of the initial ischemic injury alters subsequent oxygen free radical production and may be a factor in the differences reported in these studies (8). Note also that some studies do show that hyperoxic resuscitation, as performed here, produces a more severe injury, and those deficits in resuscitation CBF may be a factor (22). The main effect of hyperoxia compared with normoxia resuscitation in the present study was a reduction in perivascular NO production with comparable increases in O2−. In addition to the acute effects, endothelial-derived NO has a critical role in vascular remodeling and angiogenesis that may affect recovery from hypoxic-ischemic injury (36).
Although the cause of reduced perivascular NO seen here has not been definitively established, the findings are compatible with eNOS uncoupling based on the perivascular location of the fluorescence and on the effects of the pharmacological agents employed. Oxidative stress may promote eNOS uncoupling through several mechanisms, including the oxidation of cofactors, kinase activation, and phosphorylation of eNOS (19). eNOS is found in cerebral endothelial cells and some neuronal populations (5, 28). Although much of the NO production in brain tissue in response to injury is due to the activity of neuronal NOS (nNOS) and inducible NOS (iNOS), eNOS releases smaller amounts of NO in response to physiological stimuli. The NO released from endothelial cells regulates local blood flow, platelet aggregation, and neutrophil adherence (13, 39). Inhibition of iNOS and nNOS has been found to be protective in a rat model of cerebral H/I (11). In adult cerebral ischemia models loss of eNOS is deleterious, but it has not been studied in neonatal H/I. It is thought that the increased production of NO by iNOS and nNOS is partly responsible for the propagation of cerebral hypoxic-ischemic injury through the toxic effects of NO reaction products, particularly peroxynitrite (34). Increased NO from eNOS may reduce cerebral injury by improving vascular flow in the adult stroke models. However, in hyperglycemic stroke in the adult, in which eNOS may be uncoupled as in the model studied here, the treatment with the nonspecific NOS inhibitor l-NAME reduces injury (31).
Few studies have measured NO production under similar conditions, and none has specifically addressed vascular NO content in the neonate. Studies of the expression of eNOS protein and/or mRNA and NO production in the neonatal H/I model have shown mixed results (14, 21, 41). Our results show that a decrease in net vascular NO in hypoxic-ischemic tissue may result from eNOS dysfunction rather than the level of NOS protein expression.
BH4 serves as a cofactor for eNOS in that it binds to the enzyme in its dimeric state and enables the transfer of a free electron from the heme complex to l-arginine. Without BH4, the electron is diverted to molecular oxygen, producing O2−. BH4 readily reacts with oxidative molecular species, rendering it inactive as an eNOS cofactor. An exposure of cultured endothelial cells to oxidative stress results in a loss of BH4, uncoupling of eNOS, and a decline of NO production that is restored by replenishment of BH4. Clinically, BH4 is capable of improving vascular endothelial cell-dependent vasodilation and NO production in several conditions associated with a dysfunction of endothelial cell-dependent vasodilation (4a). Other studies on the ability of BH4 to improve functional outcome are supported by these results.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant HD-39833.
Acknowledgments
We thank Drs. J. Marc Simard and Volodymyr Gerzanich for advice on the fluorescent methods and Dr. James J. Grady with regard to the statistical analysis. The expert technical assistance of Mohammed Ali and William Dalmeida is appreciated.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American Heart Association. Part 13: Neonatal Resuscitation Guildlines. Circulation 112: 188–195, 2005. [Google Scholar]
- 2.Barbacanne MA, Souchard JP, Darblade B, Iliou JP, Nepveu F, Pipy B, Bayard F, Arnal JF. Detection of superoxide anion released extracellularly by endothelial cells using cytochrome c reduction, ESR, fluorescence and lucigenin-enhanced chemiluminescence techniques. Free Radic Biol Med 29: 388–396, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Carter WO, Narayanan PK, Robinson JP. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol 55: 253–258, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Channon KM, Guzik TJ. Mechanisms of superoxide production in human blood vessels: relationship to endothelial dysfunction, clinical and genetic risk factors. J Physiol Pharmacol 53: 515–524, 2002. [PubMed] [Google Scholar]
- 4a.Channon KM Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med 14: 323–327, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Dinerman JL, Dawson TM, Schell MJ, Snowman A, Snyder SH. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proc Natl Acad Sci USA 91: 4214–4218, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dirnagl U, Kaplan B, Jacewicz M, Pulsinelli W. Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. J Cereb Blood Flow Metab 9: 589–596, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension 45: 283–287, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Fabian RH, Kent TA. Superoxide anion production during reperfusion is reduced by an antineutrophil antibody after prolonged cerebral ischemia. Free Radic Biol Med 26: 355–361, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Fang Q, Sun H, Arrick DM, Mayhan WG. Inhibition of NADPH oxidase improves impaired reactivity of pial arterioles during chronic exposure to nicotine. J Appl Physiol 100: 631–636, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Gerzanich V, Ivanova S, Zhou H, Simard JM. Mislocalization of eNOS and upregulation of cerebral vascular Ca2+ channel activity in angiotensin-hypertension. Hypertension 41: 1124–1130, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Hamada Y, Hayakawa T, Hattori H, Mikawa H. Inhibitor of nitric oxide synthesis reduces hypoxic-ischemic brain damage in the neonatal rat. Pediatr Res 35: 10–14, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Haruna Y, Morita Y, Komai N, Yada T, Sakuta T, Tomita N, Fox DA, Kashihara N. Endothelial dysfunction in rat adjuvant-induced arthritis: vascular superoxide production by NAD(P)H oxidase and uncoupled endothelial nitric oxide synthase. Arthritis Rheum 54: 1847–1855, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377: 239–242, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Ioroi T, Yonetani M, Nakamura H. Effects of hypoxia and reoxygenation on nitric oxide production and cerebral blood flow in developing rat striatum. Pediatr Res 43: 733–737, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Karaa A, Kamoun WS, Clemens MG. Oxidative stress disrupts nitric oxide synthase activation in liver endothelial cells. Free Radic Biol Med 39: 1320–1331, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kase H, Hashikabe Y, Uchida K, Nakanishi N, Hattori Y. Supplementation with tetrahydrobiopterin prevents the cardiovascular effects of angiotensin II-induced oxidative and nitrosative stress. J Hypertens 23: 1375–1382, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70: 2446–2453, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci USA 89: 6348–6352, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 282: C227–C241, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Lubec B, Kozlov AV, Krapfenbauer K, Berger A, Hoeger H, Herrera-Marschitz M, Nohl H, Koeck T, Lubec G. Nitric oxide and nitric oxide synthase in the early phase of perinatal asphyxia of the rat. Neuroscience 93: 1017–1023, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Lundstrom KE, Pryds O, Greisen G. Oxygen at birth and prolonged cerebral vasoconstriction in preterm infants. Arch Dis Child Fetal Neonatal Ed 73: F81–F86, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martini SR, Kent TA. Hyperglycemia in acute cerebral ischemia: a vascular perspective. J Cereb Blood Flow Metab 27: 435–451, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Mata-Greenwood E, Jenkins C, Farrow KN, Konduri GG, Russell JA, Lakshminrusimha S, Black SM, Steinhorn RH. eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol 290: L232–L241, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayhan WG, Arrick DM, Sharpe GM, Patel KP, Sun H. Inhibition of NAD(P)H oxidase alleviates impaired NOS-dependent responses of pial arterioles in type 1 diabetes mellitus. Microcirculation 13: 567–575, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol 25: 274–278, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA 88: 10480–10484, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pourcyrous M, Parfenova H, Bada HS, Korones SB, Leffler CW. Changes in cerebral cyclic nucleotides and cerebral blood flow during prolonged asphyxia and recovery in newborn pigs. Pediatr Res 41: 617–623, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Prickaerts J, De Vente J, Markerink-Van Ittersum M, Steinbusch HW. Behavioural, neurochemical and neuroanatomical effects of chronic postnatal N-nitro-l-arginine methyl ester treatment in neonatal and adult rats. Neuroscience 87: 181–195, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Quast MJ, Wei J, Huang NC. Nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester decreases ischemic damage in reversible focal cerebral ischemia in hyperglycemic rats. Brain Res 677: 204–212, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9: 131–141, 1981. [DOI] [PubMed] [Google Scholar]
- 33.Ringel M, Bryan RM, Vannucci RC. Regional cerebral blood flow during hypoxia-ischemia in the immature rat: comparison of iodoantipyrine and iodoamphetamine as radioactive tracers. Brain Res Dev Brain Res 59: 231–235, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigo J, Alonso D, Fernandez AP, Serrano J, Richart A, Lopez JC, Santacana M, Martinez-Murillo R, Bentura ML, Ghiglione M, Uttenthal LO. Neuronal and inducible nitric oxide synthase expression and protein nitration in rat cerebellum alter oxygen and glucose deprivation. Brain Res 909: 20–45, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Rootwelt T, Loberg EM, Moen A, Oyasaeter S, Saugstad OD. Hypoxemia and reoxygenation with 21% or 100% oxygen in newborn pigs: changes in blood pressure, base deficit, and hypoxanthine and brain morphology. Pediatr Res 32: 107–113, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 15: 731–736, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saugstad OD Oxidative stress in the newborn—a 30-year perspective. Biol Neonate 88: 228–236, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Shimabuku R, Ota A, Pereyra S, Veliz B, Paz E, Nakachi G, More M, Oliveros M. Hyperoxia with 100% oxygen following hypoxia-ischemia increases brain damage in newborn rats. Biol Neonate 88: 168–171, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Stagliano NE, Dietrich WD, Prado R, Green EJ, Busto R. The role of nitric oxide in the pathophysiology of thromboembolic stroke in the rat. Brain Res 759: 32–40, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci 27: 81–86, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Veltkamp R, Rajapakse N, Robins G, Puskar M, Shimizu K, Busija D. Transient focal ischemia increases endothelial nitric oxide synthase in cerebral blood vessels. Stroke 33: 2704–2710, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Wainwright MS, Arteaga E, Fink R, Ravi K, Chace DH, Black SM. Tetrahydrobiopterin and nitric oxide synthase dimer levels are not changed following hypoxia-ischemia in the newborn rat. Brain Res Dev Brain Res 156: 183–192, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Hattori Y, Satoh H, Iwata C, Banba N, Monden T, Uchida K, Kamikawa Y, Kasai K. Tetrahydrobiopterin prevents endothelial dysfunction and restores adiponectin levels in rats. Eur J Pharmacol 555: 48–53, 2007. [DOI] [PubMed] [Google Scholar]