Abstract
Hyperleptinemia accompanying obesity affects endothelial nitric oxide (NO) and is a serious factor for vascular disorders. NO, superoxide (O2−), and peroxynitrite (ONOO−) nanosensors were placed near the surface (5 ± 2 μm) of a single human umbilical vein endothelial cell (HUVEC) exposed to leptin or aortic endothelium of obese C57BL/6J mice, and concentrations of calcium ionophore (CaI)-stimulated NO, O2−, ONOO− were recorded. Endothelial NO synthase (eNOS) expression and l-arginine concentrations in HUVEC and aortic endothelium were measured. Leptin did not directly stimulate NO, O2−, or ONOO− release from HUVEC. However, a 12-h exposure of HUVEC to leptin increased eNOS expression and CaI-stimulated NO (625 ± 30 vs. 500 ± 24 nmol/l control) and dramatically increased cytotoxic O2− and ONOO− levels. The [NO]-to-[ONOO−] ratio ([NO]/[ONOO−]) decreased from 2.0 ± 0.1 in normal to 1.30 ± 0.1 in leptin-induced dysfunctional endothelium. In obese mice, a 2.5-fold increase in leptin concentration coincided with 100% increase in eNOS and about 30% decrease in intracellular l-arginine. The increased eNOS expression and a reduced l-arginine content led to eNOS uncoupling, a reduction in bioavailable NO (250 ± 10 vs. 420 ± 12 nmol/l control), and an elevated concentration of O2− (240%) and ONOO− (70%). l-Arginine and sepiapterin supplementation reversed eNOS uncoupling and partially restored [NO]/[ONOO−] balance in obese mice. In obesity, leptin increases eNOS expression and decreases intracellular l-arginine, resulting in eNOS an uncoupling and depletion of endothelial NO and an increase of cytotoxic ONOO−. Hyperleptinemia triggers an endothelial NO/ONOO− imbalance characteristic of dysfunctional endothelium observed in other vascular disorders, i.e., atherosclerosis and diabetes.
Keywords: nitric oxide, endothelium, leptin, nitroxidative stress
obesity is a serious risk factor for vascular disorders such as hypertension and coronary artery disease (14, 32). Endothelial dysfunction has been associated with several vascular diseases (32). Bioavailable nitric oxide (NO) production is diminished in dysfunctional endothelium, which hinders the l-arginine/NO pathway and increases vasoconstriction (13).
There have been several reports suggesting the potential mechanisms for diminished NO production in obesity. It has been proposed that the elevated free fatty acids levels observed in obesity may inhibit endothelial NO synthase (eNOS) (8). Also, the increased levels of cytokines IL-6 and TNF-α observed in obesity may affect the phosphorylation of tyrosine kinases and eNOS expression (16). It also has been suggested that obesity is associated with an increased production of reactive oxygen species in the cardiovascular system (31).
Recent evidence indicates that adipose tissue-derived hormone leptin can be involved in vascular tone control (1, 7) and that leptin receptors (Ob-R) are expressed in endothelial cells (4). In a vascular ring model, leptin induced vasorelaxation in a dose-dependent manner, and this effect was abolished by eNOS inhibitors (18, 19). The in vitro stimulatory effect of leptin on NO production in cultured endothelial cells was also tested by measuring nitrite and nitrate concentrations (30). The intravenous injection of leptin increased nitrite and nitrate concentrations in blood serum of normotensive Wistar rats but not in obese Zucker rats, which have a mutation in their leptin receptor gene (12). Intraperitoneal leptin administration also increased plasma concentration and urinary excretion of NO metabolites, as well as NO second messenger, guanosine 3′,5′-cyclic monophosphate (cGMP) (3). All this data indirectly indicate that leptin may increase NO production in endothelial cells and cause these hypotensive effects. It has also been shown that stimulation of endothelial cells with leptin leads to an increase in the production of reactive oxygen species, in particular superoxide (O2−), which is known to react rapidly with NO to form peroxynitrite (ONOO−) (5, 35). The leptin level increases in obesity (7), but the long-term effect of elevated leptin on the bioavailability of endothelial NO and cytotoxic ONOO− is not known.
This work was designed to clarify the potential role of NO, O2−, and ONOO− in leptin-associated vascular disorders associated with obesity. A system of nanosensors was used for direct simultaneous measurements of the leptin-induced biosynthesis of NO, O2−, and ONOO− in human umbilical vein endothelial cell (HUVEC) or the endothelium of obese mice.
METHODS
All materials were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted.
Cell culture.
HUVECs (ATCC No. CRL-1730, Manassas, VA) were seeded in collagen-coated flasks and incubated in MCDB-131 complete medium at 37°C under an atmosphere of 5% CO2-95% air. After the formation of a confluent monolayer, the cells were trypsinized and then resuspended in MCDB-131 and seeded in 12-well cell culture clusters. After confluence, MCDB-131 complete medium was replaced with Eagle's minimum essential medium without blood serum, and the confluent cells were incubated for 2 or 12 h with different concentrations of leptin or coincubated with 0.1 μmol/l of leptin and elevated concentrations of l-arginine (3 mmol/l) or l-arginine (3 mmol/l) and sepiapterin (0.1 mmol/l).
When we cross-check the response of our sensors using enzyme inhibitors, the cells were preincubated for 2 h before and during measurements with eNOS inhibitor, 0.3 mmol/l NG-nitro-l-arginine methyl ester (l-NAME), 2 mmol/l of membrane permeable superoxide dismutase conjugated with polyethylene glycol (PEG-SOD), or 0.01 mmol/l of peroxynitrite decomposition catalyst/scavenger, Mn(III) tetakis, (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP), or two oxidase inhibitors 2.0 mmol/l apocynin or (0.07 mmol/l) 6,8 diallyl 5,7-dihydroxy 2-(2-allyl 3-hydroxy 4-methoxyphenyl)1-H benzo(b)pryan-4-one (S17834) (Institute de Recherches Services, Suresnes, France).
Animals and diets.
Four-week-old male C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). Animals were housed two per cage, kept in a temperature-controlled room (23°C) with a 12-h:12-h light-dark cycle, and were provided food and water ad libitum. All mice were randomly assigned to one of two diets for 105 days: low-calorie (D12450B, 10 kcal fats) and high-calorie (D12492, 60 kcal fats) diet (Research Diets, New Brunswick, NJ). Mice fed with high-calorie diets were randomly divided into three groups: group 1, received regular water; group 2, received l-arginine (100 mg·kg−1·day−1) in their water; and group 3, received l-arginine (100 mg·kg−1·day−1) in their water and sepiapterin (10 mg·kg−1·day−1) in their powder chow. The 30-day treatment with l-arginine and/or sepiapterin started on the 75th day of the experimental period. In accordance with the protocol approved by the Ohio University Animal Care and Use Committee, the mice were anesthetized and then euthanized with an overdose of Penthotal. Aortas were then dissected to carry out the studies described below.
Serum leptin concentration measurement.
Blood (200 μl) was taken from a tail vein of mice on days 15, 45, 75, and 105 of the experimental period. Serum was isolated and leptin concentrations were measured using an radioimmunoassay kit for mouse leptin (Linco, St. Louis, MO).
Thoracic aorta preparation.
Following euthanasia, the thoracic aorta was excised from each animal and placed in modified Hanks’ balanced salt solution (HBSS) at 4°C and pH 7.4. Under the dissection microscope (Wild M3G; Leica), the aorta was carefully separated from adhering fat and connective tissue. Then small segments from the thoracic aorta were cut into rings 3 to 4 mm in length. Rings were opened longitudinally and pinned on the bottom of a thermostated organ chamber filled with fresh HBSS buffer.
NO, O2−, and ONOO− measurement.
Concurrent measurement of NO, O2−, and ONOO− were performed with electrochemical nanosensors combined into 1 working unit with a total diameter of 2 to 3 μm. Their design was based on previously developed and well-characterized chemically modified carbon-fiber technology (22, 25, 34).
A HUVEC culture cluster was placed in a well on the stage of an inverted research microscope (Olympus IX71). The platinum wire auxiliary electrode counter and Ag|AgCl reference electrodes were then positioned in the well adjacent to the culture cluster. A module of NO, O2−, and ONOO− nanosensors was lowered near the surface (5 ± 2 μm) of a single cell membrane with the aid of a computer-controlled micromanipulator. In a similar manner, during the experiments with aorta, the auxiliary electrodes of the three-electrode system were positioned in an oxygenated organ chamber near an endothelial cell in an aortic strip. To stimulate the release of NO, O2−, and ONOO− from HUVEC or aortic endothelium, eNOS agonist calcium ionophore (CaI) A-23187 (Cal) was injected with a microinjector to reach a final concentration of 1 μmol CaI/l in the medium. To estimate the ability of leptin to directly stimulate eNOS, leptin (10 μg/ml) was injected to reach a final concentration of 0.1 μmol/l in the medium.
The changes in NO, O2−, and ONOO− concentration from the background level with time were measured with a computer-based Gamry VFP600 multichannel potentiostat (detection limit 1 nmol/l and resolution time <50 ms for each sensor). Changes in NO, O2−, and ONOO− concentrations were calculated by means of calibration curves constructed for each sensor before and after measurements with standard solutions (22, 25, 34). The electrochemical sensors measure the net concentrations of diffusible NO, O2−, and ONOO−, which are not consumed by fast chemical reactions.
eNOS expression measurement.
Samples of homogenated cells or tissue, equalized for protein content, were separated by SDS-PAGE (10% gels) and transferred to polyvinylidene difluoride membranes. eNOS was detected with mouse anti-eNOS monoclonal antibody. To compare eNOS expression with the expression of the reference protein, we analyzed the expression of β-actin by Western blot analysis using a monoclonal anti-β-actin antibody (Santa Cruz Biotechnology). Bands were detected with the horseradish peroxidase-conjugated secondary antibodies and visualized by chemiluminescence (21).
l-Arginine and cGMP determination.
l-Arginine determination was made using HPLC with fluorescence detection. HUVEC, blood serum, and aortic endothelial tissue were homogenized in sulfosalicylic acid. After centrifugation and dilution of the supernatant portions, an internal standard (S-carboxymethyl-l-cysteine) was added and a 1-min derivatization with fluorescent reagent o-phthalaldehyde was performed. The 10-μl samples were then injected into an HP1090 liquid chromatograph using a Zorbax Eclipse AAA column, and the separation of the amino acids was obtained with a two-buffer-system gradient elution. l-Arginine was detected using a Shimadzu RF 551 Fluorescence HPLC monitor, and the calculations were made using a standard calibration curve. The concentrations of cGMP were measured in thoracic aortas using an ELISA kit. The protein content of samples was measured by the bicinchoninic acid protein assay kit (Pierce, Rockford, IL).
Statistical analysis.
The data are presented as the means 1 μmol/l ±SE from 4 to 6 experiments. Multiple comparisons were evaluated using ANOVA followed by the Student's t-test. A P < 0.05 was considered as statistically significant.
RESULTS
NO, O2−, and ONOO− release in HUVEC.
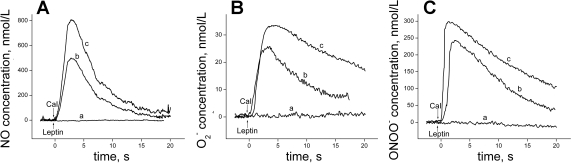
Typical amperometric curves (current proportional to concentration vs. time) obtained for NO, O2−, and ONOO− release from HUVEC are shown in Fig. 1. Leptin did not directly stimulate NO, O2−, and ONOO− release from HUVEC. After CaI administration, a rapid release of NO, O2−, and ONOO− was observed from both control and leptin-treated cells. The production of all three of these species was significantly higher in HUVEC incubated with leptin when compared with control cells.
Fig. 1.
Typical amperometric curves showing nitric oxide (NO; A), superoxide (O2−; B), and peroxynitrite (ONOO−; C) release from a single human umbilical vein endothelial cell (HUVEC). Leptin (0.1 μg/ml; a) or calcium ionophore (CaI; 1 μmol/l) was used to stimulate NO, O2−, and ONOO− generation from a HUVEC incubated for 2 h without leptin (b) or with leptin (0.1 μg/ml; c).
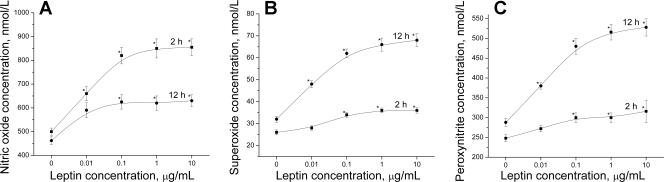
To investigate the relationship between the eNOS functional state and leptin concentration, as well as the leptin exposure time, we measured the CaI-stimulated NO, O2−, and ONOO− release from HUVEC treated with different leptin concentrations during different times. The incubation of cells for both 2 and 12 h with increasing leptin concentrations resulted in a dose-dependent increase of the peak NO, O2−, and ONOO− generation (Fig. 2). The curves reflecting the production of these species reached a semiplateau at about 0.1 μg/ml leptin. It is interesting to report that the incubation of HUVEC with leptin for 2 h stimulated the NO production to a higher degree than treatment with leptin for 12 h. A 2-h exposure of cells to leptin (0.1–10 μg/ml) led to a 1.6-fold increase of NO production compared with basal conditions, whereas the same concentrations of leptin exposure for 12 h caused only a 1.3-fold increase of peak NO release. In contrast to NO, the CaI stimulated peak production of O2− and ONOO− in the HUVECs incubated with leptin (0.1–10 μg/ml) for 12 h was about 2.0 and 1.7 times higher, respectively, than the control HUVEC. Whereas there was only a 1.3- and 1.2-fold increase, respectively, of the O2− and ONOO− peak productions in the HUVECs incubated with 0.1–10 μg/ml leptin for 2 h.
Fig. 2.
The effect of leptin on CaI (1 μmol/l)-stimulated peak concentration of NO (A), O2− (B), and ONOO− (C) released from HUVEC. Cells were incubated with various concentrations of leptin for 2 h (▪) and 12 h (•). *P < 0.05 vs. control (without leptin); n = 5 experiments.
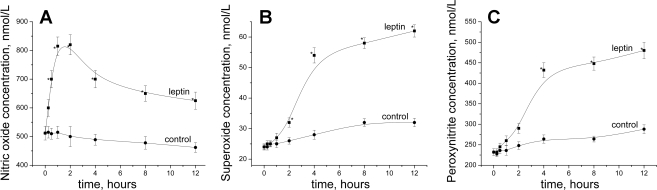
Analysis of the time-dependent effect of constant leptin concentration on stimulated peak NO release showed the rapid rise of NO production for 1 h after the beginning of cell exposure to the hormone (Fig. 3A). After reaching the maximum (820 ± 35 nmol/l at 2 h), a significant linear decrease in NO release was observed. In contrast to NO, the O2− and ONOO− productions sharply increased at 4 h and steadily increased thereafter. As expected, in the presence of membrane-permeable PEG-SOD (O2− scavenger), the concentration of O2− as well as the concentration of ONOO− sharply decreased, whereas NO concentration increased in leptin-treated cells (Fig. 4A). However, in the presence of MnTMPyP (ONOO− scavenger), only a significant decrease in ONOO− was observed.
Fig. 3.
CaI (1 μmol/l)-stimulated peak concentration of NO (A), O2− (B), and ONOO− (C) as a function of incubation time. HUVECs were incubated with leptin (0.1 μg/ml) (▪) or without leptin (•). *P < 0.05 vs. control (without leptin); n = 4 to 5 experiments.
Fig. 4.
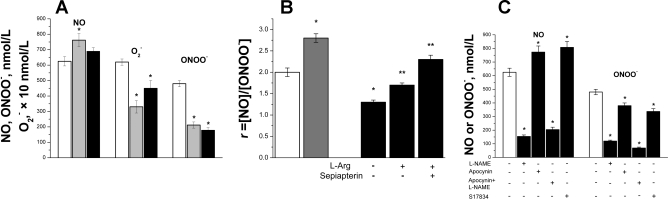
A: maximal NO, O2−, and ONOO− concentration produced by HUVEC in the presence of O2− or ONOO− scavengers. NO, O2−, and ONOO− releases were stimulated by CaI (1 μmol/l) from a HUVEC incubated for 12 h with leptin (0.1 μmol/l) or with polyethylene glycol (PEG)-SOD (100 U/ml) (gray bar) or MnTMPyP (0.02 mmol/l) (black bar); control (white bar), *P < 0.0001 vs. control; n = 4 to 5 experiments. B: the ratio (R) of peak NO concentration to the peak concentration of ONOO− (R = [NO]/[ONOO−]). NO and ONOO− releases were stimulated by CaI (1 μmol/l) from HUVEC incubated for 2 h without leptin (control, white bar) or incubated with leptin (0.1 μg/ml) for 2 h (gray bar) or 12 h (black bar) at normal and elevated l-arginine (l-Arg; 3 mmol/l) or l-arginine (3 mmol/l) and sepiapterin (0.1 mmol/l). *P < 0.05 vs. control and **P < 0.01 vs. 12-h incubation without l-arginine, sepiapterin; n = 4 to 5 experiments. C: CaI-stimulated maximal NO and ONOO− concentration released from HUVEC in the absence (control, white bars) or in the presence (black bars) of NG-nitro-l-arginine methyl ester (l-NAME; 0.3 mmol/l) and apocynin (3 mmol/l) or S-17834 (0.07 mmol/l). The concentration of NO and ONOO− was measured after 12 h incubation with leptin (0.1 μg/ml). *P < 0.001 vs. control, n = 4 to 5 experiments.
We used the ratio of NO to ONOO− concentration (R = [NO]/[ONOO−]) to quantify the relation between bioavailable NO and cytotoxic ONOO− in the endothelium. A high R value indicates strong eNOS coupling and high bioavailable NO and/or low nitroxidative stress (low level of ONOO−).
Intriguingly, the R value was 2.8 ± 0.1 in HUVEC incubated for 2 h with 0.1 μg/ml leptin when compared with control cells (2.0 ± 0.1) (Fig. 4B). In contrast, HUVEC treated with the same leptin concentration for 12 h showed a decrease of the R value to 1.3 ± 0.1. This indicated that there was a gradual increase in eNOS uncoupling on longer exposure to leptin. The eNOS uncoupling was partially reversed in the presence of elevated l-arginine concentration (3 mmol/l) and sepiapterin (0.1 mmol/l), a precursor of tetrahydrobiopterin (a cofactor of eNOS) (Fig. 4B). In the presence of l-arginine and sepiapterin, the R value increased to 2.3 ± 0.1.
In addition, we confirmed that the release of NO and ONOO− was related to eNOS activation because eNOS inhibition of the enzyme by NG-nitro-l-arginine methyl ester (0.3 mmol/l) significantly blocked (about 75%) CaI-stimulated release of both NO and ONOO− (Fig. 4C). We tested the potential contribution of the NAD(P)H oxidase-generated O2− to the overall formation of ONOO− and the diminished bioavailable NO. First, we measured NO and ONOO− in the presence of apocynin, a nonspecific NAD(P)H oxidase inhibitor. We then measured NO and ONOO− in the presence of S-17834, a more selective NAD(P)H oxidase inhibitor. Treatment of endothelial cells with apocynin or S-17834 increased NO release by about 20–30% and decreased ONOO− level by 20–30%. This indicates that about a 20–30% concentration of O2− is generated by membrane-bound NAD(P)H oxidase and about 70–80% of O2− is generated by membrane-bound eNOS for the production of ONOO− (after CaI stimulation). Another potentially major source of NO, O2−, and ONOO− in HUVEC is mitochondria. Nevertheless, under experimental conditions used in this study (sensors located 5 ± 2 μm from the outer surface of the cell membrane), it is rather unlikely that mitochondrial O2− or ONOO− can diffuse to, and be measured by, the sensors.
eNOS expression and l-arginine concentration in HUVEC.
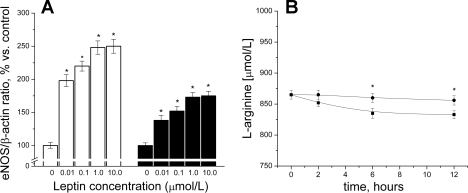
As shown in Fig. 5A, eNOS expression increased after cell incubation with leptin in a dose-dependent manner. HUVEC treated with leptin for 2 h showed a higher increase of eNOS expression compared with the cells incubated for 12 h. Figure 5B shows that during incubation, leptin-treated HUVEC had significantly lower intracellular l-arginine concentration levels than untreated cells.
Fig. 5.
A: endothelial NO synthase (eNOS) expression in HUVEC incubated with various concentrations of leptin for 2 h (white bars) and 12 h (black bars). *P < 0.05 vs. control (without leptin); n = 4 to 5 experiments. B: time-dependent effect of constant leptin concentration (0.1 μg/ml) on l-arginine level in HUVEC. Cells were incubated with leptin (▪) or without leptin (•) leptin for 12 h. *P < 0.05, n = 4 to 5 experiments.
Body weight, leptin concentration, and eNOS expression.
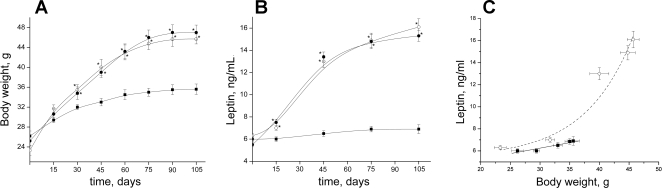
The D-12492 mice had statistically significant increased mean body weight versus D-12450B group as early as 1 mo after diet initiation (Fig. 6A). Later the high-calorie-fed mice continued to gain body weight much faster than mice maintained on the low-calorie diet. After 105 days, the mean body weight of D-12492 mice was about 1.4 times higher than that of the control group. No influence of treatment with l-arginine on body weight was detected.
Fig. 6.
A: body weight of C57BL/6J mice maintained on low-calorie diet (▪) or high-calorie (60 kcal fat) diet with (○) or without (•) supplementation of l-arginine. B: leptin concentration changes in blood plasma of C57BL/6J mice maintained on low-calorie diet (▪) or high-calorie D-12492 (60 kcal fat) diet with (○) or without supplementation (•) of l-arginine (•). *P < 0.05 vs. control mice (low-calorie diet); n = 4 to 5 mice. C: leptin vs. body weight linear plot (r = 0.93, P < 0.05) for the low-calorie diet (▪, solid line) or exponential plot (r2 = 0.95, P < 0.0001) for high-calorie diet D-12492 (60 kcal fat) diet (○, dashed line).
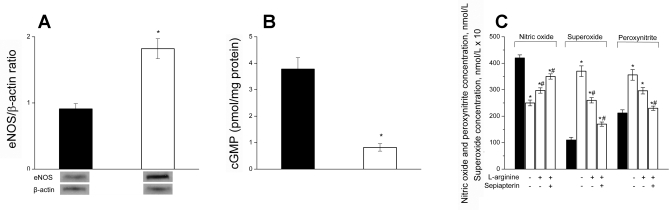
The shape of curves reflecting the increase of leptin concentration in serum of mice fed with high- and low-calorie diets (Fig. 6B) were very similar to the curves reflecting the gain of body weight in corresponding animal groups. Whereas the leptin concentration at the end of the experimental period (105 days) was 6.9 ± 0.8 ng/ml in the control group, the maximal leptin concentration at the same time was 2.5 times higher (16.1 ± 1.5 ng/ml) in the mice provided with the high-calorie diet and untreated water. As in the case with body weight, l-arginine did not significantly affect the leptin concentration in obese mice. A slight linear increase (about 8% maximal) in leptin concentration with the body weight was observed in mice on a low-calorie diet (Fig. 6C). Nonetheless, the increase in leptin concentration with the body weight was exponential for obese mice. It is interesting to note that the most remarkable increase in leptin concentration was observed in obese mice at a weight exceeding the maximal weight (about 35 g) of the low-calorie diet D-12450B mice. In addition, the 2–5 fold increase in leptin concentration correlated with the increase in eNOS in obese mice expression (about a 2-fold increase compared with control). The expression of inducible NO synthase in the endothelium did increase significantly in obese mice (data not shown). The cGMP level (aortic wall) correlated inversely with eNOS expression (Fig. 7, A and B) and directly reflected the diminished NO concentration observed in obese mice (Fig. 2A).
Fig. 7.
A: eNOS expression in aortic endothelial cells of C57BL/6J mice maintained on a low-calorie diet (control, white bars) and high-calorie diet (60 kcal fat; black bars) for 105 Days. B: cGMP content in aortic wall of C57BL/6J mice maintained on a low-calorie diet (control, black bars) and a high-calorie diet (60 kcal fat; white bars) *P < 0.005, n = 4 to 5 mice. C: CaI (1 μmol/l)-stimulated NO, O2−, and ONOO− release from aortic endothelial cells of C57BL/6J mice maintained for 105 days on low-calorie diet (black bars) or high-calorie (60 kcal fat, white bars) with or without supplementation with l-arginine (100 mg·kg−1·day−1) or l-arginine (100 mg·kg−1·day−1) and sepiapterin (10 mg·kg−1·day−1). *P < 0.01 vs. control (low-calorie diet); #P < 0.05 vs. obese mice without l-arginine supplementation; n = 4 to 5 mice.
NO, O2−, and ONOO− release in aortic endothelium.
To deeper investigate the ability of leptin to affect the functional state of endothelial cells, the CaI-stimulated NO, O2−, and ONOO− production in aortic endothelium of normal (control) and obese C57BL/6J mice was measured (Fig. 7C). NO concentration released from the aortic endothelium of control mice was 420 ± 12 nmol/l, about 1.7 times higher than from aortic endothelium of mice with obesity (250 ± 11 nmol/l). Obese mice treated with l-arginine showed higher NO production (297 ± 10 nmol/l, P < 0.05) compared with untreated obese mice. A further increase (∼25%) in NO levels was observed after l-arginine plus sepiapterin treatment.
The CaI-stimulated peaks of O2− and ONOO− release were significantly elevated in obese mice. The maximal O2− production in aortic endothelium of mice maintained on a high-calorie diet and untreated water was about 3.4 times higher than in control mice. The peak ONOO− release was 1.7 times higher in obese mice than in control mice. A high-caloric diet supplementation with l-arginine or l-arginine plus sepiapterin resulted in the decrease of O2− and ONOO− and an increased NO production in the aortas of obese mice by 30% and 17%, respectively. The high-calorie diet l-arginine supplementation resulted in the decrease of O2− and ONOO−. This effect was even more pronounced after supplementation with l-arginine plus sepiapterin (Fig. 7C).
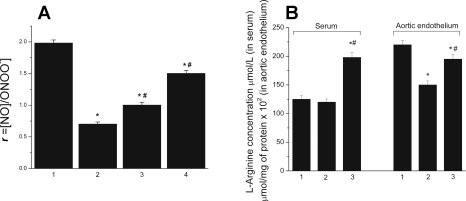
The R value decreased about 64% in obese mice compared with control mice (Fig. 8A). l-Arginine treatment increased the R value about 30% over the untreated obese mice, and treatments with l-arginine plus sepiapterin doubled the R value over the untreated obese mice.
Fig. 8.
A: the ratio of NO concentration to the concentration of ONOO− (R = [NO]/[ONOO−]). NO and ONOO− releases were stimulated by CaI (1 μmol/l) from endothelial cells of the aortas of C57BL/6J mice maintained for 105 days on low-calorie diet (1) or on high-calorie (60 kcal fat) diet (2) with supplementation of l-arginine (100 mg·kg−1·day−1) (3) or with supplementation with l-arginine (100 mg·kg−1·day−1) and sepiapterin (10 mg/kg/day) (4). *P < 0.01 vs. control (low-calorie diet); #P < 0.05 vs. obese mice without supplementation with l-arginine or l-arginine and sepiapterin; n = 5 mice. B: l-arginine concentration in serum and aortic endothelium of C57BL/6J mice maintained for 105 days on low-calorie diet (1) or on high-calorie (60 kcal fat) without (2) or with supplementation with l-arginine (100 mg·kg−1·day−1) (3). *P < 0.01 vs. control (low-calorie diet); #P < 0.05 vs. obese mice without supplementation with l-arginine; n = 5 mice.
l-Arginine concentration in blood and aortic endothelium.
The level of l-arginine in the blood of obese mice did not change significantly compared with the control mice (Fig. 8B). In contrast, obesity resulted in about a 30% decrease of l-arginine level in aortic endothelium of obese mice compared with control mice. l-Arginine supplementation caused a significant increase of l-arginine level both in blood (70%) and in aortic endothelium (30%) of the obese mice.
DISCUSSION
It is generally accepted that obesity is closely associated with an increased risk of hypertension and heart failure, but the mechanisms involved are still not fully understood (14, 32). It has been reported that the adipocyte-derived hormone leptin is involved in the regulation of blood vessel tonus (21). However, the effect of leptin on NO production in endothelial cells has been assessed only with the use of indirect methods (3, 12, 18, 19). In contrast, this study used highly sensitive, selective, millisecond response-time electrochemical nanosensors (22, 25, 34) to directly measure the concentration of CaI-stimulated NO, O2−, and ONOO− release from HUVEC treated with leptin as well as from the aortic endothelium of normal and obese mice with hyperleptinemia.
Our results clearly demonstrated that leptin does not directly stimulate NO, O2−, or ONOO− release from the endothelium. However, the exposure of endothelial cells to leptin resulted in the dose-dependent upregulation of eNOS expression and an enhanced NO production that lasted for about 2 h.
Leptin is known to increase cell proliferation, which profoundly influences the abundance of the eNOS transcript. Thus any factors that influence endothelial cell growth rate may confound the interpretation of the primary effects on eNOS gene expression (30). Therefore, the leptin-enhanced expression of eNOS in endothelial cells can be explained, at least in part, by a transcriptional and/or posttranscriptional mechanism. We acknowledge, however, that further work beyond the limits of the present study is required to verify the mechanism of leptin-enhanced eNOS expression in the endothelium.
A short-time exposure of HUVEC to leptin did not increase O2− and ONOO− levels significantly. This indicates that an acute exposure of endothelial cells to leptin may have beneficial effects on the cardiovascular system by increasing the potential for the generation of bioavailable NO.
In contrast, long-term (12 h) exposure of endothelial cells to leptin decreased CaI-stimulated bioavailable NO despite a twofold increase in eNOS expression in the endothelium. This apparent paradox can be explained by the enhanced generation of O2− and ONOO− after long-term exposure of the HUVEC to leptin. Indeed, a wide variety of proatherogenic risk factors like obesity and other cardiovascular disease states can be associated with increased oxidative stress and diminished NO bioavailability (10).
To better specify the relationship between leptin, eNOS expression, and obesity in animals, another portion of our experiments involved an inbred C57BL/6J strain of obese mice. These mice were not obese on a standard chow diet but became obese and developed hyperleptinemia when fed a high-fat diet (28). We clearly documented the exponential increase in serum leptin concentration with body weight of obese mice. The increased leptin was especially dramatic when the weight of obese mice exceeded the maximal weight of low-calorie diet mice.
The present study provides direct evidence that obesity causes an increase in leptin level that inversely correlates with NO bioavailability in the aortic endothelium and with cGMP in the aortic wall. Simultaneously, with the suppression of NO production in obese mice, we registered the considerable increase in O2− and ONOO− generation. The most probable mechanism for the NO release diminishment in this case, like in the experiments with cell culture, is the overproduction of O2−, resulting in the fast reaction with NO to form ONOO−. This direct quantitative data are concordant with previous indirect studies that indicate the role of oxidative stress in obesity (11, 27). Dobrian et al. (9) recently reported that there was a ∼1.8-fold decrease of the plasma and urine nitrate and nitrite levels and the same increase in O2− production from the aortas from obese Sprague-Dawley rats compared with lean ones. At the same time, the eNOS expression in aorta of obese animals was increased ∼8-fold compared with control. This apparent discrepancy can be clearly explained with the data presented in this study.
To explain the differences in the effect of leptin exposure on NO production versus O2− and ONOO− production, it may help to realize that the NO sensor used in this study detects only the net concentration of NO (i.e., NO that is not consumed in fast chemical reactions and can freely diffuse to a target cell and trigger cGMP production). This net concentration depends not only on the activity of eNOS but also on the production of O2−. The rapid CaI stimulation of eNOS produces a large NO release accompanied by O2− production, suggesting that some of the production of O2− is calcium dependent, similar to the production of NO by eNOS.
Also, it is helpful to realize that both membrane-bound eNOS and NAD(P)H oxidase may contribute to the overall generation of ONOO− (23, 33). At low to moderate activity of eNOS, the main generator of O2− in the vascular wall is NAD(P)H (23). In this study, after rapid CaI-stimulation of eNOS, the contribution of eNOS generated O2− to the ONOO− production was about three times higher than the contribution of NAD(P)H oxidase generated O2−. Furthermore, endothelial mitochondria can be a generator of O2− and subsequently ONOO−. But, the nanotechnological set-up used in this study is not suitable for measuring O2− or ONOO− directly in mitochondria without disturbing cell membranes and stimulating NO and O2− release. These studies elucidate mainly NO, O2− generation by membrane-bound eNOS.
One of the most probable mechanisms underlying the leptin-stimulated endothelial dysfunction may be the imbalance between eNOS expression and intracellular l-arginine and/or tetrahydrobiopterin level. According to our results, leptin significantly increases the amount of eNOS protein in HUVEC and endothelium of obese mice. This enhanced eNOS expression in sustained hyperleptinemia was also recently observed by other investigators (9, 26, 36). Coincident with the increase in eNOS expression, we observed a tendency toward diminishment of the l-arginine concentration in leptin-incubated HUVEC and a significantly diminished l-arginine level in obese animals with hyperleptinemia. l-Arginine is required not only as a substrate for NO synthesis but also as a stabilizer of eNOS, preventing it from uncoupling. The relative l-arginine and/or eNOS cofactor tetrahydrobiopterin insufficiencies result in the uncoupling of the oxidative and reductive domains of eNOS, which results in O2− generation (6, 17). The net effect of the reaction between NO and increased O2− caused the diminishment of NO bioavailability. The [NO]/[ONOO−] ratios were significantly decreased in both HUVEC treated with leptin for 12 h and aortic endothelium of obese mice. Furthermore, in our experiments, the obese mice treated with l-arginine or l-arginine and sepiapterin (a precursor of tetrahydrobiopterin) showed significantly higher NO production and lower O2− and ONOO− release from the aortic endothelium compared with the untreated obese mice.
The most potentially damaging property of the eNOS dimer (called anticooperative bonding) occurs because the two eNOS monomers can act independently (24). During moderate l-arginine or tetrahydrobiopterin deficiencies, after activation, it is very probable that one monomer of an eNOS dimer could be coupled and rapidly making NO, while the adjacent monomer of the same eNOS dimer is uncoupled and rapidly making O2−. The near diffusion-limited reaction of O2− with NO to form ONOO− is even faster than the dismutation of O2− by superoxide dismutase. Unlike NO and O2−, which are not strong oxidants, ONOO− is a potent oxidant. ONOO− is relatively unstable in its anionic form. However, when protonated, the peroxynitrous acid (HONOO) at high concentrations may freely diffuse several cell diameters before it rearranges to form nitric acid (HNO3) or undergoes homolytic or heterolytic cleavage to generate highly toxic oxidative species, including .NO2, NO2+, and .OH. These products are among the most reactive and damaging species in biological systems. They may initiate a cascade of events leading to the oxidation of proteins, DNA, and lipids and finally resulting in cytotoxicity and cellular dysfunction (2). The sum of unfavorable events developed in cells due to ONOO− hyperproduction is collectively called “nitroxidative stress,” which is a major component of oxidative stress.
There is another contributing mechanism that may partially help explain our results. It has been suggested that increased free fatty acid concentration in obesity may be related to increased levels of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of NO synthase (2). l-Arginine competes with ADMA for the eNOS enzyme (29). Therefore, an increase in NO production after supplementation with l-arginine to obese mice may be due in part to an increase of the arginine-to-ADMA ratio in endothelial cells and the reduction of the ADMA inhibitory effect.
In summary, we have provided direct evidence that, despite leptin-induced increased eNOS expression, bioavailable NO is reduced, whereas O2− and ONOO− levels are increased in obesity. Increased O2− and ONOO− production that contributes to high oxidative/nitroxidative stress in endothelial cells is most likely due to a discrepancy between enhanced eNOS expression and diminished intracellular l-arginine concentration in obesity. Thus long-term exposure of endothelium to leptin may lead to enzymatic uncoupling of the oxidative and reductive domains of eNOS. l-Arginine and sepiapterin treatment during the long term hyperleptinemia accompanying obesity increases l-arginine (and most likely tetrahydrobiopterin) concentration in aortic endothelium and significantly reduces eNOS uncoupling (decreasing O2− and ONOO− production and preserving bioavailable NO). Leptin-induced [NO]/[ONOO−] imbalance in the vascular endothelium observed in obesity is similar to the redox state that has been reported in other endothelium-impaired function disorders, including hypertension, atherosclerosis, diabetes, and others (9, 32, 33).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-55397, the Marvin White Endowment, and the Biomedical Nanoscience Nanotechnology Program (Ohio University).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agata J, Masuda A, Takada M, Higashiura K, Murakami H, Miyazaki Y, Shimamoto K. High plasma immunoreactive leptin level in essential hypertension. Am J Hypertens 10: 1171–1174, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. In: Methods in Enzymology, edited by Packer L. San Diego, CA: Academic, 1994, p. 229–240. [DOI] [PubMed]
- 3.Beltowski J, Wojcicka G, Borkowska E. Human leptin stimulates systemic nitric oxide production in the rat. Obes Res 10: 939–946, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bouloumié A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res 83: 1059–1066, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bouloumié A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J 13: 1231–1238, 1999. [PubMed] [Google Scholar]
- 6.Cosentino F, Patton S, d'Uscio LV, Werner ER, Werner-Felmayer G, Moreau P, Malinski T, Lüscher TF. Tetrahydrobiopterin alters superoxide and nitric oxide release in prehypertensive rats. J Clin Invest 101: 1530–1537, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Davda RK, Stepniakowski KT, Lu G, Ullian ME, Goodfriend TL, Egan BM. Oleic acid inhibits endothelial nitric oxide synthase by a protein kinase C-independent mechanism. Hypertension 26: 764–770, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension 375: 554–560, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Drexler H, Hornig B. Endothelial dysfunction in human disease. J Mol Cell Cardiol 3: 51–60, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Fenster CP, Weinsier RL, Darley-Usmar VM, Patel RP. Obesity, aerobic exercise, and vascular disease: the role of oxidant stress. Obes Res 10: 964–968, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Frühbeck G Pivotal role of nitric oxide in the control of blood pressure after leptin administration. Diabetes 48: 903–908, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Harrison DG Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 100: 2153–2157, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes WG, Morgan DA, Walsh SA, Sivitz WI, Mark AL. Cardiovascular consequences of obesity: role of leptin. Clin Exp Pharmacol Physiol 25: 65–69, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100: 270–278, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271: 665–668, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Huk I, Nanobashvili J, Neumayer Ch Punz A, Mueller M, Afkhampour K, Mittlboeck M, Losert U, Polterauer P, Roth E, Patton S, Malinski T. l-Arginine treatment alters the kinetics of nitric oxide and superoxide release and reduces ischemia-reperfusion injury in skeletal muscle. Circulation 96: 667–675, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K, Tsuda K, Baba A, Kawabe T, Boh-oka S, Ibata M, Moriwaki C, Hano T, Nishio I. Involvement of nitric oxide in endothelium-dependent arterial relaxation by leptin. Biochem Biophys Res Commun 273: 745–749, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d'Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes 49: 293–297, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Lundman P, Eriksson MJ, Stühlinger M, Cooke JP, Hamsten A, Tornvall P. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J Am Coll Cardiol 38: 111–116, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Madajka M, Korda M, White J, Malinski T. Effect of aspirin on constitutive nitric oxide synthase and the bioavailability of NO. Thromb Res 110: 317–321, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Malinski T, Taha Z. Nitric oxide release from a single cell measured in situ by a porphyrinic based microsensor. Nature 358: 676–678, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Mason RP, Kalinowski L, Jacob RF, Jacoby AM, Malinski T. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black Americans. Circulation 112: 3795–3801, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Mayer B, Andrew P. Nitric oxide synthases: catalytic function and progress toward selective inhibition. Naunyn Schmiedebergs Arch Pharmacol 358: 127–133, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Mesáros S, Vankova Z, Grunfeld S, Mesárosová A, Malinski T. Preparation and optimization of superoxide microbiosensor. Anal Chim Acta 358: 27–33, 1998. [Google Scholar]
- 26.Nishimatsu H, Suzuki E, Satonaka H, Takeda R, Omata M, Fujita T, Nagai R, Kitamura T, Hirata Y. Endothelial dysfunction and hypercontractility of vascular myocytes are ameliorated by fluvastatin in obese Zucker rats. Am J Physiol Heart Circ Physiol 288: H1770–H1776, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes 50: 159–165, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet induced type II diabetes in C57BL/6J mice. Diabetes 37: 1163–1167, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Toth J, Racz A, Kaminski PM, Wolin MS, Bagi Z, Koller A. Asymetric dimethylarginine inhibits shear stress-induced nitric oxide release and dilation and elicits superoxide-mediated increase in arterioral tone. Hypertension 49: 563–568, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B, Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes 51: 168–173, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Vincent HK, Powers SK, Dirks AJ, Scarpace PJ. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord 25: 378–388, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Williams IL, Wheatcroft SB, Shah AM, Kearney MT. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord 26: 754–764, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Wolin MS, Ahmed M, Gupte SA. The sources of oxidative stress in the vessel wall. Kidney Int 67: 1659–1661, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Xue J, Ying X, Chen J, Xian Y, Jin L. Amperometric ultramicrosensors for peroxynitrite detection and its application toward single myocardial cells. Anal Chem 72: 5313–5321, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem 276: 25096–25100, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Zanetti M, Barazzoni R, Vadori M, Stebel M, Biolo G, Guarnieri G. Lack of direct effect of moderate hyperleptinemia to improve endothelial function in lean rat aorta: role of calorie restriction. Atherosclerosis 175: 253–259, 2004. [DOI] [PubMed] [Google Scholar]