Abstract
In addition to high pulmonary vascular resistance (PVR) and low pulmonary blood flow, the fetal pulmonary circulation is characterized by mechanisms that oppose vasodilation. Past work suggests that high myogenic tone contributes to high PVR and may contribute to autoregulation of blood flow in the fetal lung. Rho-kinase (ROCK) can mediate the myogenic response in the adult systemic circulation, but whether high ROCK activity contributes to the myogenic response and modulates time-dependent vasodilation in the developing lung circulation are unknown. We studied the effects of fasudil, a ROCK inhibitor, on the hemodynamic response during acute compression of the ductus arteriosus (DA) in chronically prepared, late-gestation fetal sheep. Acute DA compression simultaneously induces two opposing responses: 1) blood flow-induced vasodilation through increased shear stress that is mediated by NO release and 2) stretch-induced vasoconstriction (i.e., the myogenic response). The myogenic response was assessed during acute DA compression after treatment with Nω-nitro-l-arginine, an inhibitor of nitric oxide synthase, to block flow-induced vasodilation and unmask the myogenic response. Intrapulmonary fasudil infusion (100 μg over 10 min) did not enhance flow-induced vasodilation during brief DA compression but reduced the myogenic response by 90% (P < 0.05). During prolonged DA compression, fasudil prevented the time-dependent decline in left pulmonary artery blood flow at 2 h (183 ± 29 vs. 110 ± 11 ml/min with and without fasudil, respectively; P < 0.001). We conclude that high ROCK activity opposes pulmonary vasodilation in utero and that the myogenic response maintains high PVR in the normal fetal lung through ROCK activation.
Keywords: calcium sensitization, persistent pulmonary hypertension of the newborn, pulmonary vascular resistances, lung development
the fetal pulmonary circulation, compared with the normal postnatal lung, is characterized by high pulmonary vascular resistance (PVR) and low pulmonary blood flow. Successful adaptation and survival at birth require a marked decrease in PVR, resulting in a dramatic rise in pulmonary blood flow, thereby allowing the lung to perform gas exchange during postnatal life (7, 12, 14, 48). Some infants fail to achieve or sustain this decrease in PVR at birth, leading to extrapulmonary right-to-left shunting of blood across the ductus arteriosus (DA) and foramen ovale. This syndrome, referred as persistent pulmonary hypertension of the newborn (PPHN), is associated with severe hypoxemia and high morbidity and mortality (4, 27, 29). Mechanisms that maintain high PVR in utero are incompletely understood but include low fetal arterial Po2, the lack of a gas-liquid interface, production of vasoconstricting mediators, and low basal production of vasodilator products (3, 14, 43, 48).
In addition to its high basal PVR, the fetal pulmonary circulation is uniquely characterized by its ability to oppose vasodilation in response to diverse stimuli, including increased oxygen tension, acidosis, infusions of pharmacological vasodilators, and shear stress (5, 6, 8, 11, 12, 47). Although these stimuli cause fetal pulmonary vasodilation, this response is often transient; that is, the rise in fetal pulmonary blood flow and the fall in PVR are followed by a marked decrease in flow, despite continued exposure to dilator stimuli (6, 22). Time-dependent changes in pulmonary vascular tone also have been demonstrated in response to changes in hemodynamic forces induced by partial compression of the DA. Abrupt DA compression in the normal fetus initially increases fetal pulmonary artery pressure and flow, causing a rapid decline in PVR (5, 12, 47). However, during prolonged DA compression, pulmonary blood flow progressively decreases toward baseline values (5). These findings suggest that autoregulatory mechanisms exist in the fetal lung that oppose vasodilation and contribute to high PVR.
Mechanisms that oppose vasodilation in the fetal lung are incompletely understood, but past work suggests that high myogenic tone may contribute to this response. Physiologically, the myogenic response is defined by increased vascular tone induced by elevated intraluminal pressure (26, 47). Although lacking in the normal postnatal pulmonary circulation, the contribution of the myogenic response to high PVR in the normal fetus has previously been reported (9, 40, 46, 47). DA compression simultaneously induces two opposing responses: 1) blood flow-induced vasodilation through increased shear stress and 2) pressure-induced vasoconstriction (i.e., the myogenic response) (12, 47). Whether the myogenic response contributes to the physiological response to diverse dilator stimuli, including hemodynamic forces, in the developing lung is uncertain.
Studies of the systemic circulation have shown that the myogenic response may be mediated by depolarization-induced Ca2+ influx (28), activation of phospholipase C (24, 39), and calcium sensitization (32, 45). Calcium sensitization refers to the inhibition of myosin light chain phosphatase (MLCP), which prevents dephosphorylation of myosin light chains and allows for sustained contraction even if cytosolic Ca2+ levels fall (see review in Ref. 45). Rho-kinase (ROCK) has been shown to mediate the myogenic response in the adult systemic circulation and accounts for calcium sensitization in vascular smooth muscle (19, 31). ROCK inhibits MLCP by phosphorylating the myosin-binding subunit of MLCP, MYPT-1, and/or the MLCP inhibitor protein CPI-17 and thus augments smooth muscle cell contraction at any given intracellular concentration (19, 45). ROCK activity has been involved in vascular tone in various organs, such as coronary arteries (33), mesenteric artery (44), and cerebral arteries (23). Previous studies have shown that ROCK inhibitors (ROCKi) cause pulmonary vasodilation in the adult (2, 15, 17, 37, 38) and the fetal lung (41), but whether high ROCK activity contributes to the myogenic response and modulates time-dependent vasodilation in the developing lung circulation is unknown.
Therefore, we hypothesized that high ROCK activity modulates the myogenic response and regulates pulmonary blood flow during fetal life. To test this hypothesis, we performed a series of experiments in chronically prepared, late-gestation fetal lambs. We report that treatment with a ROCKi does not enhance flow-induced vasodilation but inhibits the myogenic response in fetal sheep. We also found that ROCKi sustains pulmonary vasodilation during prolonged DA compression. These findings suggest that high ROCK activity opposes pulmonary vasodilation in vivo and that the myogenic response maintains high PVR in the normal fetal lung.
METHODS
Twenty-four pregnant, mixed-breed (Colombia-Rambouillet) ewes were used in this study. All procedures and protocols were reviewed and approved by the Animal Care and Use Committee of the University of Colorado Health Sciences Center and followed the Guide for the Care and Use of Laboratory Animals established by the National Research Council.
Fetal Surgical Preparation
Surgery was performed at 126 ± 2 days gestation (term = 147 days) in fetuses from 11 singleton and 13 twin pregnancies after ewes had fasted for 24 h. Animals were given intramuscular penicillin G (600,000 units) and gentamicin (80 mg) immediately before surgery. Ewes were sedated with intravenous ketamine (800 mg) and diazepam (10 mg) and were intubated and ventilated with 1–2% isoflurane for the duration of the surgery. Under sterile conditions, a midline abdominal incision was made, and the uterus was externalized. A hysterotomy was made, and the left fetal forelimb was exposed. Polyvinyl catheters (20-gauge) were placed in the left axillary artery and vein and advanced in the ascending aorta (Ao) and superior vena cava (SVC), respectively. A left thoracotomy and pericardial incisions were made, and the heart and great vessels were exposed. With the use of a 16-gauge intravenous placement unit (Angiocath; Travenol, Deerfield, IL), a 22-gauge catheter was placed through purse-string sutures in the left pulmonary artery (LPA) to allow for selective drug infusions. With a 14-gauge intravenous placement unit (Angiocath; Travenol), 20-gauge catheters were placed in the main pulmonary artery (MPA) and left atrium. After gentle, blunt dissection of the bifurcation of MPA, a flow transducer (Transonic Systems, Ithaca, NY) was placed around the LPA to measure blood flow to the left lung (QLPA). The DA was exposed using gentle, blunt dissection. An inflatable vascular occluder (In Vivo Metric, Healdsburg, CA) was placed loosely around the DA. A catheter was placed in the amniotic cavity to serve as a pressure referent. The uterus was sutured, and a dose of ampicillin (500 mg) was given in the amniotic cavity. The catheters and flow transducer cable were externalized to a flank pouch on the ewe after the abdominal wall was closed. Postoperatively, ewes were allowed to eat and drink ad libitum and were generally standing within 1 h. All animals were treated with scheduled buprenorphine (0.6 mg every 8 h on day 1, 12 h on day 2, and 24 h on day 3). Further buprenorphine treatment was based on veterinary assessment of pain. All catheters were gently flushed daily with 1–2 ml of heparinized normal (0.9%) saline to maintain catheter patency.
Drug Preparation
A solution of Nω-nitro-l-arginine (l-NNA; Sigma, St. Louis, MO; 30 mg/ml) was prepared immediately before each study by first dissolving the drug in 1 M HCl and then neutralizing the solution to pH 7.4 with NaHCO3 (41). The infusion rate was 0.1 ml/min over 10 min. Fasudil (HA-1077, catalog no. H-2330; LC Laboratories; 100 μg/ml) was dissolved in normal saline and infused in 1-ml aliquots. The infusion rate was 0.1 ml/min over 10 min.
General Study Design
Ewes were allowed to recover from surgery for a minimum of 24 h before the initiation of physiological studies. During physiological studies, pulmonary artery (PAP), aortic (AoP), and left atrium pressures (LAP) were measured by connecting the externalized catheters to computer-driven pressure transducers (MP100A; Biopac Systems, Santa Barbara, CA). Pressure transducers were calibrated using a mercury column manometer before each study. Pressure measurements were referenced to simultaneously recorded amniotic pressure. The flow transducer was connected to an internally calibrated flowmeter (Transonics Systems, Ithaca, NY) to measure QLPA. Before infusion of any drugs, a 20-min period of stable baseline hemodynamics was established during infusion of normal saline. Because normal saline was used as the vehicle for ROCKi, hemodynamic responses were compared with baseline variables. Hemodynamic variables, including PAP, AoP, and QLPA, were measured continuously for the duration of each study protocol. LAP was measured in two animals (3.0 ± 0.3 mmHg) to ensure the values were constant through all protocols and consistent with previous studies using fasudil in sheep (41). Left lung PVR was calculated using the formula (PAP − LAP)/QLPA, where LAP = 3 mmHg. Heart rate (HR) was determined from phasic pressure tracings. Arterial blood gas measurements included pH, Pco2, Po2, oxygen saturation (SaO2), and Hct (ABL 800; Radiometer, Copenhagen, Denmark).
Experimental Design
Protocol 1: effects of fasudil, a ROCKi, on the hemodynamic response during partial acute compression of the DA.
The purpose of this protocol was to investigate the effects of acute infusions of fasudil on flow-induced pulmonary vasodilation due to increased shear stress. Saline (0.1 ml/min) was initially infused in the LPA catheter, and baseline measurements of QLPA, mean PAP (mPAP), mean AoP (mAoP), and HR were recorded every 10 min. After baseline measurements were recorded for a 30-min period, DA compression was performed for 10 min with or without infusion of fasudil (100 μg) in the LPA for 10 min before acute DA compression. From preliminary studies, this dose of fasudil was selected to cause minimal changes in basal PVR and to provide a stable baseline for short DA compression protocols. The degree of inflation of the DA occluder was set to increase mPAP by 15 mmHg from its baseline value (as described in Ref. 47). Mean PAP was kept constant through the compression period by readjusting the degree of inflation of the occluder as needed. During the compression period, data were recorded at 1, 5, and 10 min. These data were analyzed by comparing these responses with infusions of fasudil (100 μg over 10 min) without DA compression.
Protocol 2: hemodynamic effects of fasudil on the myogenic response in the ovine fetal lung during brief DA compression.
The purpose of this protocol was to investigate the effects of an acute fasudil treatment during acute DA compression after l-NNA treatment. l-NNA (30 mg over 10 min) was used to inhibit endogenous NO production, to directly unmask the myogenic response during acute DA compression, as previously described (12, 47). l-NNA was infused over 10 min in the LPA, beginning 30 min before DA compression (12, 47). After a 30-min recovery period following the first DA compression, fasudil (100 μg over 10 min) was infused into the LPA, followed by a second DA compression.
To study the relationship between pulmonary artery pressure and blood flow after ROCKi treatment, we performed successive DA compressions at four different levels of mPAP, as previously described (5, 47). After baseline measurements were stable for a 30-min period, the DA occluder was partially inflated for successive 3-min periods to progressively increase PAP by 10, 20, 30, and 40% above baseline (47). Changes in QLPA, mPAP, mAoP, and HR were recorded during this 3-min period. This protocol was repeated in random order over 3 consecutive days that included DA compression after 1) saline infusion in the LPA (0.1 ml/min), 2) l-NNA treatment, and 3) combined l-NNA and fasudil treatments.
Protocol 3: hemodynamic effects of fasudil during prolonged DA compression in the ovine fetal lung.
The first step of this protocol was to investigate the hemodynamic effects of prolonged fasudil infusions (for 2 h) on pulmonary blood flow, PVR, and pulmonary and systemic pressures. Saline (0.1 ml/min) was first infused in the LPA catheter, and baseline measurements were recorded for QLPA, mPAP, mAoP, and HR every 10 min. After baseline measurements were stable for a 30-min period, fasudil (10 μg/min for 2 h) was infused into the LPA. Hemodynamic measurements were continued for a 30-min recovery period.
The second step of this protocol was to investigate the effects of prolonged fasudil infusions during DA compressions for 2 h. After baseline measurements were obtained for 30-min, a 2-h DA compression was performed with or without concomitant infusion of fasudil (50 μg/min into the SVC for 2 h). The degree of inflation was set to increase mPAP by 15 mmHg from its baseline value (47). Mean PAP was kept constant through the compression period by readjusting the degree of inflation of the occluder. During the compression period, data were recorded every 5 min during the first 30 min, then every 10 min, and then until 30 min after the DA compression was released. These data were analyzed to compare to effects of DA compression with or without fasudil treatment and the response to fasudil without DA compression.
Statistical Analysis
Statistical analysis was performed with the StatView software package (SAS Institute, Cary, NC). Statistical comparisons were performed using repeated-measures analysis of variance and Fisher's protected least significant differences test. Results are means ± SE. The significance level was set at P < 0.05.
RESULTS
Protocol 1: Effects of Fasudil on the Hemodynamic Response to Partial Acute DA Compression
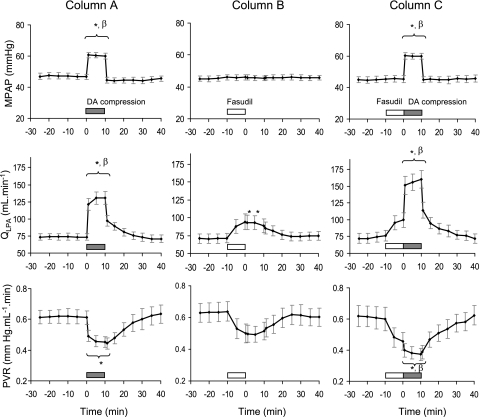
We found that intrapulmonary infusion of low doses of fasudil (100 μg over 10 min) did not enhance flow-induced vasodilation during brief (10 min) DA compression. Acute DA compression without fasudil increased mPAP above baseline by 30.2 ± 2.0% (from 46.6 ± 1.9 to 60.5 ± 1.7 mmHg; P < 0.001), increased QLPA by 80.9 ± 12.8% (from 73.4 ± 4.9 to 130.7 ± 9.0 ml/min; P < 0.001), and reduced PVR from 0.61 ± 0.04 to 0.45 ± 0.03 mmHg·ml−1·min (P < 0.05) (Fig. 1, column A). During acute DA compression, HR increased from 159.8 ± 4.7 to 189.0 ± 6.0 beats/min (P < 0.01). Mean AoP (baseline value: 42.4 ± 1.2 mmHg) and blood gas parameters (baseline values: pH, 7.38 ± 0.01; Pco2, 41.4 ± 1.3 mmHg; Po2, 23.2 ± 1.0 mmHg; SaO2, 57.7 ± 3.6%; and Hct, 33.3 ± 1.4%) did not change during acute DA compression.
Fig. 1.
Changes in mean pulmonary artery pressure (mPAP), left pulmonary artery flow (QLPA), and pulmonary vascular resistance (PVR) during partial compression of the ductus arteriosus (DA; column A, n = 9), fasudil infusion (100 μg over 10 min; column B, n = 8), and combined DA compression after fasudil infusion (column C, n = 7). Low-dose intrapulmonary infusion of fasudil, a Rho-kinase (ROCK) inhibitor, did not enhance flow-induced vasodilation during brief DA compression. Values are means ± SE. *P < 0.05 compared with baseline. βP < 0.05 compared with fasudil infusion alone.
Fasudil infusion alone (100 μg over 10 min) did not change mPAP but increased QLPA by 32.5 ± 3.8% (from 71.3 ± 6.5 to 94.3 ± 10.9 ml/min; P < 0.05) and tended to reduce PVR (from 0.64 ± 0.06 to 0.49 ± 0.05 mmHg·ml−1·min; P = 0.08) (Fig. 1, column B). HR, mAoP, and blood gas parameters did not change during brief, low-dose fasudil infusion. Brief compression of the DA after treatment with fasudil increased mPAP above baseline by 32.7 ± 2.7% (from 45.5 ± 2.2 to 60.0 ± 1.8 mmHg; P < 0.001), increased QLPA by 119.8 ± 23.7% (from 76.3 ± 7.9 to 160.1 ± 13.1 ml/min; P < 0.001), and reduced PVR (from 0.60 ± 0.08 to 0.37 ± 0.04 mmHg·ml−1·min; P < 0.01) (Fig. 1, column C). During brief DA compression after fasudil infusion, HR increased from 161.7 ± 4.3 to 188.7 ± 8.3 beats/min (P < 0.01). Mean AoP and blood gas parameters did not change during DA compression following fasudil infusion.
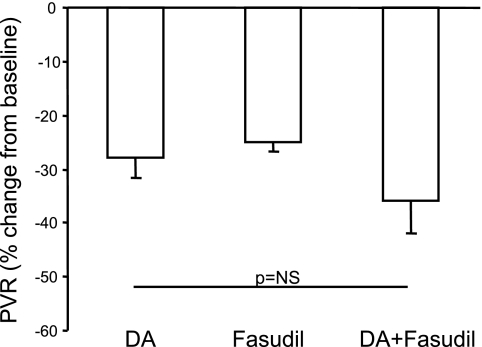
Overall, the maximal increase in flow during acute DA compression alone was not different from values achieved during DA compression after infusion of low doses of fasudil (Fig. 1, columns A and C). Similarly, there was no statistical difference in the maximal decrease in PVR between acute DA compression with or without fasudil at the doses used for this study (Fig. 2).
Fig. 2.
Response to brief (10 min) DA compressions, expressed as the percent change in PVR from baseline for the 3 study conditions (DA, n = 9; fasudil, n = 8; DA+fasudil, n = 7). As shown, fasudil did not augment the vasodilator effects during brief DA compression. Values are means ± SE. *P < 0.05. NS, not significant.
Protocol 2: Hemodynamic Effects of Fasudil on the Myogenic Response in the Ovine Fetal Lung
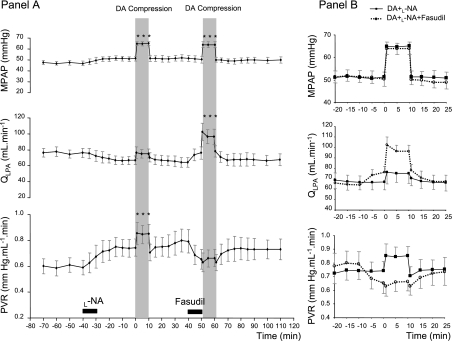
These studies were performed after treatment with the nitric oxide synthase inhibitor l-NNA, to unmask the myogenic response by blocking flow-induced vasodilation, as previously described (47). Forty minutes after l-NNA treatment, pulmonary hemodynamics were not significantly different from baseline values, although there were trends for increased mPAP and PVR and reduced QLPA (Fig. 3). Similarly, HR (baseline value: 153 ± 11 beats/min) and blood gas parameters (baseline values: pH, 7.36 ± 0.01; Pco2, 37.7 ± 2.7 mmHg; Po2, 24.4 ± 1.5 mmHg; SaO2, 66.8 ± 5.3%; and Hct, 29.8 ± 0.5%) did not significantly change after l-NNA infusion. The first DA compression after l-NNA treatment increased mPAP from 51.3 ± 2.2 to 65.1 ± 1.8 mmHg (P < 0.001) without altering QLPA, reflecting an increase in PVR from 0.74 ± 0.07 to 0.85 ± 0.07 mmHg·ml−1·min (P < 0.05) (Fig. 3). DA compression after l-NNA infusion did not change HR, mAoP, or blood gas parameters.
Fig. 3.
Hemodynamic responses to brief (10 min) DA compression (shaded bars) after Nω-nitro-l-arginine (l-NA) infusion and with and without fasudil treatment (A, n = 6). After l-NA administration, mPAP was increased by +28% above baseline during DA compression, resulting in a marked increase in PVR. After fasudil infusion, for a similar increase in mPAP, QLPA increased by 50% and PVR did not increase. Mean PAP, QLPA, and PVR changes are represented as superimposed plots on the same time scale in B. Values are means ± SE. ***P < 0.001 compared with baseline.
To assess the effects of ROCKi on the myogenic response, we performed a second DA compression after l-NNA and fasudil treatment. Mean PAP was increased to levels similar to those achieved after the first DA compression (from 50.4 ± 3.1 to 64.0 ± 2.8 mmHg; P < 0.001). In contrast with the initial DA compression, QLPA increased from 64.2 ± 6.1 to 96.3 ± 9.6 ml/min during DA compression after fasudil treatment (P < 0.05). Fasudil treatment completely inhibited the rise in PVR during DA compression without fasudil (0.65 ± 0.08 vs. 0.66 ± 0.07 mmHg·ml−1·min, baseline vs. DA compression after fasudil, respectively; P = 0.51) (Fig. 3). DA compression after combined l-NNA and fasudil treatment did not change HR, mAoP, or blood gas parameters.
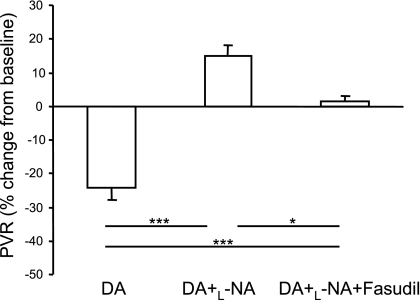
When expressed as the percent change of PVR from baseline, DA compression after l-NNA infusion increased by 15 ± 3%, which is strikingly different from the fall in PVR measured during DA compression alone (−27 ± 4%; P < 0.01 for comparison of treatment groups). Fasudil treatment before DA compression completely blocked the increase in PVR (+2 ± 2%; P < 0.05) (Fig. 4).
Fig. 4.
Hemodynamic responses to a brief (10 min) DA compression alone, after l-NA administration, and with and without fasudil treatment, expressed as the percent change in PVR from baseline (n = 6). Compared with DA compression alone, DA compression after l-NA injection increased PVR. Fasudil treatment completely blocked the increase in PVR (P < 0.05 for all comparisons). Values are means ± SE. *P < 0.05; ***P < 0.001.
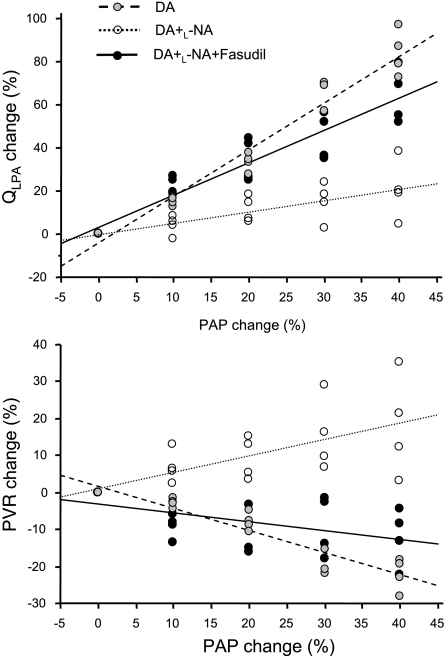
To further evaluate relationships between pulmonary artery blood flow, PVR, and mPAP, we recorded serial measurements during brief progressive compressions of the DA (Fig. 5). QLPA directly increased in linear fashion with increases in mPAP during brief, small incremental DA compressions (QLPA = −4.14 + 2.16 mPAP; r2 = 0.957, P < 0.001), resulting in a linear decrease in PVR (PVR = 1.69 − 0.60 mPAP; r2 = 0.891, P < 0.001). After NO-mediated vasodilation was blocked with l-NNA treatment, QLPA increased only slightly during serial increases in mPAP (QLPA = −0.26 + 0.51 mPAP; r2 = 0.514, P < 0.001), with PVR increasing in parallel with the increase in mPAP (PVR = 1.01 + 0.45 mPAP; r2 = 0.443, P < 0.01). After treatment with l-NNA and fasudil, QLPA progressively increased in linear fashion with the increase in mPAP (QLPA = 3.08 + 1.50 mPAP; r2 = 0.870, P < 0.001), reflecting a progressive decrease in PVR (PVR = −3.13 − 0.24 mPAP; r2 = 0.263, P < 0.001). For each level of mPAP, the mean QLPA was significantly increased after l-NNA and fasudil treatments compared with l-NA treatment alone (P < 0.05). For each level of mPAP, mean PVR was significantly lower after l-NNA and fasudil compared with l-NNA treatment alone and was not statistically different from the values of PVR measured at each level of mPAP without l-NNA and fasudil treatment (P < 0.05).
Fig. 5.
Relationships between mPAP and QLPA (top) and PVR (bottom) after l-NA administration with (n = 4) and without (n = 4) fasudil treatment. As shown, QLPA increased linearly with the increase in mPAP and decreased PVR. After l-NA treatment, QLPA only slightly changed with serial increases in mPAP, and PVR increased linearly with the increase in mPAP. After both l-NA and fasudil treatment, QLPA increased linearly with mPAP, resulting in a linear decrease in PVR. The slope for QLPA was significantly increased after l-NA and fasudil compared with l-NA alone (P < 0.05).
Protocol 3: Hemodynamic Effects of Fasudil During Prolonged DA Compression in the Ovine Fetal Lung
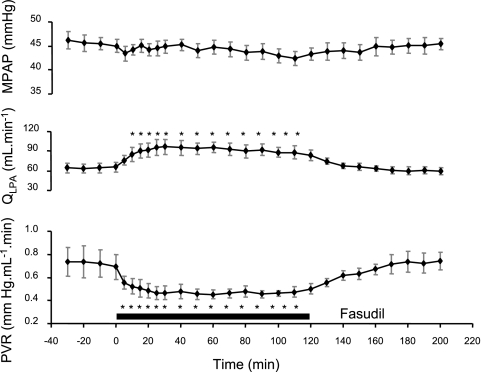
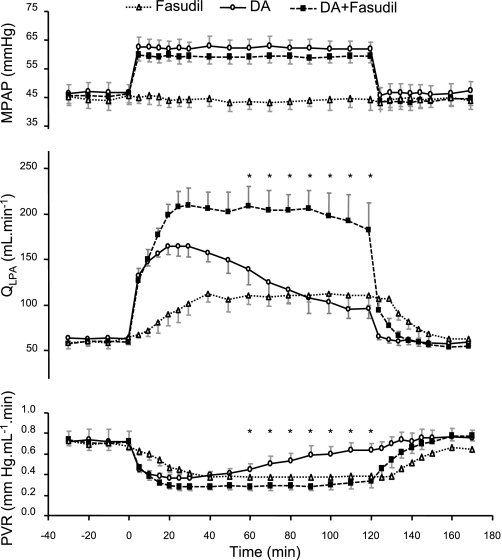
Low-dose fasudil infusion caused a sustained increase in QLPA and a decrease in PVR for the entire 2-h study period. Mean PAP did not change during the study period (Fig. 6). There also were no changes in HR (baseline value: 174 ± 12 beats/min), mAoP (baseline value: 42.9 ± 1.8 mmHg), and blood gas parameters (baseline values: pH, 7.38 ± 0.01; Pco2, 44.1 ± 2.2 mmHg; Po2, 19.2 ± 1.9 mmHg; SaO2, 54.6 ± 4.7%; and Hct, 34.0 ± 2.4%). On an alternate day in these same animals, the hemodynamic effects of DA compression without pretreatment with l-NNA or concurrent infusions of fasudil were studied. As shown in Fig. 7, DA compression caused a progressive rise in QLPA during the first hour of study. However, despite maintaining mPAP constant, QLPA progressively decreased toward baseline values during the second hour of compression. In contrast, DA compression with continuous fasudil infusion led to a sustained elevation of QLPA throughout the entire 2-h study period (Fig. 7). Fasudil infusion during the 2-h DA compression caused a sustained decrease in PVR, whereas a 2-h partial DA compression alone resulted in only a transient decrease in PVR during the first hour (Fig. 7). HR increased to 203 ± 2 beats/min during fasudil infusion with DA compression compared with baseline (169 ± 13 beats/min; P < 0.05) and fasudil infusion alone (179 ± 9 beats/min; P < 0.05). Mean AoP and blood gas parameters did not change during acute DA compression (Table 1).
Fig. 6.
Pulmonary hemodynamic response during prolonged fasudil infusion (10 μg/min for 2 h) into the left pulmonary artery (n = 6). Fasudil caused a sustained increase in QLPA and a decrease in PVR for the entire 120-min study period, which returned to the baseline 10 min after termination of drug infusion. No change was observed for PAP. *P < 0.05 compared with baseline.
Fig. 7.
Changes in mPAP, QLPA, and PVR during prolonged DA compression with (n = 4) or without (n = 4) a concomitant fasudil infusion. Fasudil treatment caused a sustained increase in QLPA and decrease in PVR during DA compression, whereas DA compression alone resulted in only transient changes during the first hour, which were not sustained during the second hour. Values are means ± SE. *P < 0.05 between DA compression alone and DA compression with a concomitant fasudil infusion.
Table 1.
Arterial blood gas values, aortic pressure, and heart rate for partial DA compressions with and without fasudil infusion or for fasudil infusion alone
| Baseline | 60 Min | 120 Min | 150 Min | |
|---|---|---|---|---|
| pH | ||||
| DA | 7.38±0.01 | 7.37±0.03 | 7.37±0.03 | 7.38±0.01 |
| DA + fasudil | 7.39±0.01 | 7.37±0.01 | 7.35±0.03 | 7.37±0.02 |
| Fasudil | 7.38±0.02 | 7.38±0.00 | 7.36±0.01 | 7.33±0.04 |
| Pco2, mmHg | ||||
| DA | 46.0±1.0 | 46.2±1.1 | 45.1±1.5 | 46.4±2.9 |
| DA + fasudil | 46.0±1.5 | 47.5±2.2 | 50.5±2.0 | 46.7±1.8 |
| Fasudil | 44.8±2.3 | 46.2±1.6 | 49.8±1.7 | 46.7±0.1 |
| Po2, mmHg | ||||
| DA | 21.0±2.2 | 20.0±1.5 | 21.6±1.1 | 22.4±2.0 |
| DA + fasudil | 21.0±1.6 | 19.1±1.2 | 19.2±0.8 | 21.2±1.2 |
| Fasudil | 19.0±2.2 | 21.3±1.4 | 19.1±2.1 | 20.7±1.5 |
| SaO2, % | ||||
| DA | 53.7±6.9 | 50.3±5.6 | 55.9±1.9 | 58.1±4.9 |
| DA + fasudil | 53.9±3.6 | 45.3±3.1 | 45.9±5.8 | 54.7±4.1 |
| Fasudil | 46.5±10.9 | 54.0±1.6 | 45.0±5.1 | 50.3±3.2 |
| Hct, % | ||||
| DA | 38.7±1.3 | 39.7±1.5 | 39.3±0.5 | 39.9±1.2 |
| DA + fasudil | 37.3±1.8 | 39.0±1.5 | 39.3±2.7 | 36.6±2.1 |
| Fasudil | 35.3±1.9 | 35.5±1.3 | 38.5±4.4 | 37.0±3.6 |
| HR, beats/min | ||||
| DA | 186.5±11.4 | 190.3±3.5 | 191.3±6.2 | 184.3±8.0 |
| DA + fasudil | 169.0±13.3 | 198.5±13.8 | 203.5±2.0*β | 165.8±7.3 |
| Fasudil | 181.0±9.8 | 186.7±5.5 | 179.3±8.8 | 189.7±3.5 |
| AoP, mmHg | ||||
| DA | 39.5±0.9 | 39.0±1.8 | 38.0±1.6 | 38.8±1.0 |
| DA + fasudil | 39.1±1.6 | 39.3±2.1 | 39.0±1.8 | 39.3±0.6 |
| Fasudil | 37.3±1.4 | 37.6±1.6 | 38.3±0.9 | 38.5±2.3 |
Data are means ± SE for baseline, early [60 min after the beginning of ductus arteriosus (DA) compression], late (120 min after the beginning of DA compression), and recovery times (150 min; 30 min after ending each protocol).
P < 0.05 compared with baseline. βP < 0.05 between fasudil and DA + fasudil treatments.
The effects of fasudil infusion during DA compression were further examined by expressing changes in QLPA above baseline. QLPA was markedly increased during the first 30 min of compression (+165.4 ± 21.5% from baseline values) but was significantly lower at 2 h (+53.6 ± 9.2%; P < 0.01 vs. 30 min). With fasudil treatment, the increase in QLPA at 30 min (+253.4 ± 38.3%) was preserved throughout the study period (+209.3 ± 54.2%; P = not significant vs. 30 min). Parallel findings were recorded for PVR.
DISCUSSION
Past studies have suggested that increased myogenic tone maintains high PVR in the normal fetal lung (46, 47), but mechanisms that mediate the myogenic response in the fetus are poorly understood. Since ROCK activity contributes to the myogenic response in the postnatal systemic circulation (31), we studied the effects of ROCKi on the myogenic response in the fetal pulmonary circulation. We found that fasudil, a specific ROCKi, did not enhance flow -induced pulmonary vasodilation during brief DA compression in the ovine fetus. However, fasudil completely blocked the myogenic response induced by acute DA compression after inhibition of NO-mediated vasodilation with l-NNA. These findings suggest that high ROCK activity contributes significantly to the myogenic response in the fetal pulmonary circulation.
On the basis of previous work that demonstrated the inability to sustain vasodilation during prolonged exposure to diverse dilator stimuli in the fetal lung, we further hypothesized that a potent myogenic response opposed vasodilation in the fetal lung through increased ROCK activity. To study the role of ROCK activity on the pulmonary hemodynamic effects of prolonged DA compression, we infused low doses of fasudil during 2 h of DA compression. In contrast with the progressive fall in QLPA during DA compression, fasudil treatment led to sustained elevations of QLPA and reduced PVR. Overall, these findings suggest that activation of the myogenic response opposes pulmonary vasodilation during prolonged exposure to hemodynamic forces in the fetal lung.
This study is the first to demonstrate that ROCK activity mediates the myogenic response in the fetal pulmonary circulation and links high ROCK activity as a critical mechanism underlying time-dependent pulmonary vasodilation in the fetus. Past studies have demonstrated that many pulmonary vasodilators often have only a transient effect in the fetal lung despite prolonged treatment with such stimuli as increased oxygen tension, acetylcholine, bradykinin, histamine, tolazoline, prostaglandins, and hemodynamic forces induced during DA compression (6, 22). These past studies have shown that autoregulatory mechanisms exist in the fetal lung that oppose vasodilation and contribute to high PVR. In our study, we showed that fasudil, a ROCKi, can block the myogenic response during brief and prolonged DA compression and can cause sustained elevation of pulmonary blood flow with continuous infusion.
Recent studies have shown that pulmonary hypertension induced by chronic hypoxia is associated with increased ROCK expression and/or activity (20, 36). In vivo, prolonged fasudil treatment reduced the development of pulmonary hypertension and attenuated pulmonary artery remodeling in animal models (1, 17, 30, 36). Studies on the acute effects of ROCKi treatment of animals with pulmonary hypertension have consistently shown a striking decrease in mPAP, which is often greater than the effects of inhaled NO or calcium channel blockade (15, 25, 37, 38). Clinically, brief treatment with fasudil was recently shown to acutely lower PVR in patients with pulmonary artery hypertension, suggesting a potential therapeutic role (18, 21). Thus most studies on the pulmonary circulation have focused on the pathophysiological role of ROCK in the development and maintenance of pulmonary hypertension.
We found that fasudil, a ROCKi, can block the myogenic response induced by a partial DA compression with blockage of the blood flow-induced vasodilation. Past studies have shown that the myogenic response is involved in high PVR in the normal fetus (5, 9, 40, 46, 47). ROCK has been shown to mediate the myogenic response in the adult systemic circulation (23, 33, 44), but the presence of the myogenic response in the normal adult lung circulation has not been reported. However, a recent study suggested that myogenic tone may develop in small pulmonary arteries after chronic hypoxia in vitro, but this has yet to be demonstrated in the intact lung or whole animal (10). In our study, fasudil infusion, at low doses that were not sufficient to acutely decrease pulmonary vascular resistance alone, inhibited the myogenic response induced by DA compression, highlighting the contribution of ROCK to myogenic tone in the normal fetus (Fig. 8). Our results suggest that flow-induced vasodilation may be enhanced by prolonged infusions of fasudil during the initial 20–30 min of DA compression. This is likely due to inhibition of the pressure-induced vasoconstriction that is induced during DA compression (Fig. 7). Since the myogenic response is greater in fetal sheep exposed to chronic intrauterine pulmonary hypertension, an experimental model of PPHN (46), we speculate that ROCKi may have a significant effect on modulating vasoreactivity in the pathological setting as well as in normal fetal sheep.
Fig. 8.
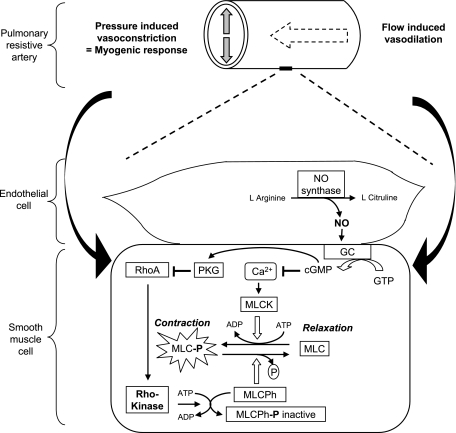
Schematic illustrating proposed mechanisms underlying the net effects of DA compression on fetal pulmonary blood flow during DA compression, which represent an equilibrium between flow-induced vasodilation and pressure-induced vasoconstriction (myogenic tone). Rho-kinase pathway is related to calcium sensitization, high vascular tone, and myogenic response, which are offset through activation of the nitric oxide (NO) pathway, which induces vasodilation. GC, guanylate cyclase; GTP, guanosine 5′-triphosphate; cGMP, guanosine 3′,5′-cyclic monophosphate; Ca2+, intracellular calcium; MLC, myosin light chain; MLC-P, phosphorylated MLC; MLCK, MLC kinase; MLCPh, MLC phosphatase (active); MLCPh-P, phosphorylated MLC phosphatase (inactive).
It is currently reported that myosin light chain kinase (MLCK) activation contributes to initial vasoconstriction, whereas inhibition of MLCP via either ROCK or protein kinase C (PKC) is primarily responsible for the sustained contraction (34, 45). Several studies in smooth muscle cells have shown a partial inhibition of PKC-related protein kinase (PRK) isoform activity with ROCK inhibitors, in addition to Rho kinase inhibition (13, 16, 35). However, the effects of ROCK inhibitors on PKC activity are at least two to three times less potent than the effects of these agents on ROCK activity (13). Apparently, nonselective effects of ROCK inhibitors predominantly occur at high concentrations (42). In addition, others have reported that PKC activity is decreased by fasudil at doses beyond those required to achieve a significant decrease in vascular tone (42). For a small decrease in vascular tone (∼35%), the decrease in PKC activity was only ∼10% (42). At the low doses of fasudil that we used in our study, PVR was reduced only by ∼25% (Fig. 2). Although we cannot rule out a potential role for PKC in contributing to the myogenic response, we speculate that the effect of fasudil on PKC activity was small in our protocols.
Furthermore, other mechanisms may modulate the hemodynamic effects of acute DA compression in the fetus. For example, NO activates PKG, which can inhibit RhoA, suggesting that the loss of endothelium-derived NO production may augment ROCK activity in vascular smooth muscle (34), highlighting the complexity of interactions between these pathways.
Potential limitations of this study include only the use of fasudil as a ROCKi due to the extensive number of experiments in this study. However, we observed the same inhibitory effect on the myogenic response during acute DA compression with Y-27632, another ROCKi, as observed with fasudil treatment (data not shown). A major strength of this work is related to the fact that we performed these experiments in vivo with the chronically prepared, late-gestation fetal sheep model. However, these in vivo studies do not allow for the possibility of directly measuring rapid changes in ROCK activity. Further work is needed to determine specific arterial sites of the myogenic response within the lung vasculature, perhaps with the use of isolated conduit and small pulmonary arteries, as recently suggested by Broughton et al. (10).
We conclude that ROCK activity contributes to high PVR in the normal fetal pulmonary circulation, opposes vasodilation to hemodynamic forces due to DA compression, and mediates the striking myogenic response in the normal fetal pulmonary circulation before birth. We speculate that persistence of high myogenic reactivity may contribute to the pathophysiology of PPHN and that ROCK inhibitors may provide novel therapeutic strategies for treating severe pulmonary hypertension in newborns.
GRANTS
This project was supported by grants from the Société Française de Médecine Périnatale, the Conseil Régional de Picardie, France, and National Heart, Lung, and Blood Institute Grants R01 HL68702 (to S. H. Abman) and K08 HL072916 (to T. Grover).
Acknowledgments
We thank Gates Roe and Christine Hunt-Peacock for technical help.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res 94: 385–393, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Abe K, Tawara S, Oi K, Hizume T, Uwatoku T, Fukumoto Y, Kaibuchi K, Shimokawa H. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharmacol 48: 280–285, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Abman SH Abnormal vasoreactivity in the pathophysiology of persistent pulmonary hypertension of the newborn. Pediatr Rev 20: e103–e109, 1999. [PubMed] [Google Scholar]
- 4.Abman SH Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology 91: 283–290, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Abman SH, Accurso FJ. Acute effects of partial compression of ductus arteriosus on fetal pulmonary circulation. Am J Physiol Heart Circ Physiol 257: H626–H634, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Abman SH, Accurso FJ. Sustained fetal pulmonary vasodilation with prolonged atrial natriuretic factor and GMP infusions. Am J Physiol Heart Circ Physiol 260: H183–H192, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol Heart Circ Physiol 259: H1921–H1927, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Accurso FJ, Alpert B, Wilkening RB, Petersen RG, Meschia G. Time-dependent response of fetal pulmonary blood flow to an increase in fetal oxygen tension. Respir Physiol 63: 43–52, 1986. [DOI] [PubMed] [Google Scholar]
- 9.Belik J The myogenic response of arterial vessels is increased in fetal pulmonary hypertension. Pediatr Res 37: 196–201, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 294: L797–L806, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Cassin S, Dawes GS, Mott JC, Ross BB, Strang LB. The vascular resistance of the foetal and newly ventilated lung of the lamb. J Physiol 171: 61–79, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornfield DN, Chatfield BA, McQueston JA, McMurtry IF, Abman SH. Effects of birth-related stimuli on l-arginine-dependent pulmonary vasodilation in ovine fetus. Am J Physiol Heart Circ Physiol 262: H1474–H1481, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawes GS, Mott JC. The vascular tone of the foetal lung. J Physiol 164: 465–477, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhaliwal JS, Casey DB, Greco AJ, Badejo AM Jr, Gallen TB, Murthy SN, Nossaman BD, Hyman AL, Kadowitz PJ. Rho kinase and Ca2+ entry mediate increased pulmonary and systemic vascular resistance in l-NAME-treated rats. Am J Physiol Lung Cell Mol Physiol 293: L1306–L1313, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Eto M, Kitazawa T, Yazawa M, Mukai H, Ono Y, Brautigan DL. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C alpha and delta isoforms. J Biol Chem 276: 29072–29078, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287: L656–L664, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, Shimokawa H. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart 91: 391–392, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukumoto Y, Tawara S, Shimokawa H. Recent progress in the treatment of pulmonary arterial hypertension: expectation for rho-kinase inhibitors. Tohoku J Exp Med 211: 309–320, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Guilluy C, Sauzeau V, Rolli-Derkinderen M, Guerin P, Sagan C, Pacaud P, Loirand G. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol 146: 1010–1018, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, Nakano T. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J 70: 174–178, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Ivy DD, Kinsella JP, Abman SH. Endothelin blockade augments pulmonary vasodilation in the ovine fetus. J Appl Physiol 81: 2481–2487, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Jarajapu YP, Knot HJ. Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am J Physiol Heart Circ Physiol 289: H1917–H1922, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Jarajapu YP, Knot HJ. Role of phospholipase C in development of myogenic tone in rat posterior cerebral arteries. Am J Physiol Heart Circ Physiol 283: H2234–H2238, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Jiang BH, Tawara S, Abe K, Takaki A, Fukumoto Y, Shimokawa H. Acute vasodilator effect of fasudil, a Rho-kinase inhibitor, in monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol 49: 85–89, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Johansson B Myogenic tone and reactivity: definitions based on muscle physiology. J Hypertens Suppl 7: S5–S9, 1989. [PubMed] [Google Scholar]
- 27.Kinsella JP, Abman SH. Recent developments in the pathophysiology and treatment of persistent pulmonary hypertension of the newborn. J Pediatr 126: 853–864, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin DL, Heymann MA, Kitterman JA, Gregory GA, Phibbs RH, Rudolph AM. Persistent pulmonary hypertension of the newborn infant. J Pediatr 89: 626–630, 1976. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Xia W, Li A, Zhao C, Sun R. Long-term inhibition of Rho kinase with fasudil attenuates high flow induced pulmonary artery remodeling in rats. Pharmacol Res 55: 64–71, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 98: 322–334, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Massett MP, Ungvari Z, Csiszar A, Kaley G, Koller A. Different roles of PKC and MAP kinases in arteriolar constrictions to pressure and agonists. Am J Physiol Heart Circ Physiol 283: H2282–H2287, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 105: 1545–1547, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Murthy KS Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem J 374: 145–155, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagaoka T, Gebb SA, Karoor V, Homma N, Morris KG, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol 100: 996–1002, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Osol G, Laher I, Kelley M. Myogenic tone is coupled to phospholipase C and G protein activation in small cerebral arteries. Am J Physiol Heart Circ Physiol 265: H415–H420, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Parker TA, Grover TR, Kinsella JP, Falck JR, Abman SH. Inhibition of 20-HETE abolishes the myogenic response during NOS antagonism in the ovine fetal pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 289: L261–L267, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Parker TA, Roe G, Grover TR, Abman SH. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 291: L976–L982, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Rattan S, Patel CA. Selectivity of ROCK inhibitors in the spontaneously tonic smooth muscle. Am J Physiol Gastrointest Liver Physiol 294: G687–G693, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Rudolph AM, Yuan S. Response of the pulmonary vasculature to hypoxia and H+ ion concentration changes. J Clin Invest 45: 399–411, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiga N, Hirano K, Hirano M, Nishimura J, Nawata H, Kanaide H. Long-term inhibition of RhoA attenuates vascular contractility by enhancing endothelial NO production in an intact rabbit mesenteric artery. Circ Res 96: 1014–1021, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Storme L, Parker TA, Kinsella JP, Rairigh RL, Abman SH. Chronic hypertension impairs flow-induced vasodilation and augments the myogenic response in fetal lung. Am J Physiol Lung Cell Mol Physiol 282: L56–L66, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Storme L, Rairigh RL, Parker TA, Kinsella JP, Abman SH. In vivo evidence for a myogenic response in the fetal pulmonary circulation. Pediatr Res 45: 425–431, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Strang LB The lungs at birth. Arch Dis Child 40: 575–582, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]