Abstract
Cardiac-specific overexpression of the catalytic subunit of protein phosphatase type 1 (PP1) in mice results in hypertrophy, depressed contractility, propensity to heart failure, and premature death. To further address the role of PP1 in heart function, PP1 mice were crossed with mice that overexpress a functional COOH-terminally truncated form of PP1 inhibitor-2 (I-2140). Protein phosphatase activity was increased in PP1 mice but was normalized in double transgenic (DT) mice. The maximal rates of contraction (+dP/dt) and of relaxation (−dP/dt) were reduced in catheterized PP1 mice but normalized in DT mice. Similar contractile abnormalities were observed in isolated, perfused work-performing hearts and in whole animals by means of echocardiography. The increased absolute and relative heart weights observed in PP1 mice were normalized in DT mice. Histological analyses indicated that PP1 mice had significant cardiac fibrosis, which was absent in DT mice. Furthermore, PP1 mice exhibited an age-dependent increase in mortality, which was abrogated in DT mice. These results indicate that I-2 overexpression prevents the detrimental effects of PP1 overexpression in the heart and further underscore the fundamental role of PP1 in cardiac function. Therefore, PP1 inhibitors such as I-2 could offer new therapeutic options to ameliorate the deleterious effects of heart failure.
Keywords: transgenic mice, contractility, heart failure, drug target
phosphorylation is a widely recognized mechanism for control of many cellular functions. The phosphorylation state of a protein in the cell is determined by the balance between the activities of protein kinases and protein phosphatases (PP). The main serine/threonine phosphatases present in the heart are PP1, PP2A, and PP2B (calcineurin). These all belong to the same gene family and are highly homologous enzymes that play a critical role in the control of cardiac contractility and hypertrophy. Numerous proteins in the heart are regulated by phosphorylation. For instance, stimulation of β-adrenergic receptors leads via cAMP-dependent phosphorylation to altered function of the L-type calcium channel, probably the ryanodine receptor (RyR), the inhibitory subunit of troponin C protein in the myofilaments, and phospholamban (PLB). The phosphorylation of PLB at Ser16 and Thr17 is reversed by PP1 and PP2A (7, 30). PP1 enzymes are heteromeric complexes consisting of a catalytic subunit (PP1c) associated with regulatory/targeting proteins (8, 9, 11). The activity of PP1, but not PP2A, is inhibited by the heat-stable protein inhibitor-1 (I-1) and inhibitor-2 (I-2) (5, 19, 20, 33). Both inhibitors are expressed in heart. Whereas unphosphorylated I-2 inhibits PP1, I-1 requires phosphorylation by PKA on Thr35 for its inhibitory activity and is rapidly phosphorylated after β-adrenergic stimulation (14, 18, 29).
In both human disease and experimental animal models of heart failure, the activity of PP1 is significantly increased (16, 28). Elevated PP1c mRNA levels and an increased PP1 activity have been observed in end-stage human failing hearts (25, 28). It has been suggested that this increase may be a contributing factor to depressed cardiac function, cardiomyopathy, and death (7, 31). Consistent with this idea, a reduced phosphorylation state of cardiac regulatory proteins such as PLB and troponin I (3, 7, 25, 36) was reported in samples from patients with terminal heart failure. In an animal model, chronic β-adrenergic stimulation by infusion of isoproterenol led to cardiac hypertrophy, increased PP1 activity, and PLB dephosphorylation (4). Cardiac-specific overexpression of PP1c in mice at levels similar to those observed in human heart failure results in dephosphorylation of PLB, depressed basal cardiac function, and depressed β-adrenergic-stimulated cardiac function, dilated cardiomyopathy, and premature mortality consistent with heart failure (7, 31). Increased activity could be due to dephosphorylation of I-1 (7, 12) or reduced expression of I-1 (13) and/or I-2. Ablation of I-1 led to an increase in PP1 activity and an impaired contractile response to β-adrenergic stimulation (7). Cardiac hypertrophy and reduced life span were not observed in this model. Conversely, overexpression of truncated functionally active forms of I-2 or I-1 in the heart improved cardiac function (21, 32). Furthermore, PP2A or PP2B (calcineurin) overexpression led to decreased function and pathological hypertrophy (15, 26).
In the present work, we tested the hypothesis that increased expression of I-2 would abrogate the cardiac hypertrophy and the increased mortality due to transgenic PP1 overexpression. To address this question, we crossbred mice with cardiac-specific overexpression of a COOH-terminally truncated form of I-2 (I-2140 mice) and PP1 (PP1c mice) and analyzed the functional and biochemical consequences of the overexpression of both proteins. The results support the notion that increased activity of PP1 is detrimental to cardiac function and that inhibition of the phosphatase activity ameliorates the cardiac phenotype of PP1c mice.
MATERIALS AND METHODS
Transgenic mice.
Generation of transgenic mice overexpressing the catalytic subunit of PP1, the catalytic subunit of PP2A, or the truncated I-2 (I-2140) has been described previously (7, 15, 21). I-2140 and PP1c mice were generated by injection of DNA into C3H embryos, and the offspring were backcrossed for at least six generations in the DBA background. Littermates of the various genotypes were utilized in the studies. Animals were handled and maintained according to protocols approved by the animal welfare committees of the Universities of Münster and Halle and Indiana University School of Medicine, which conform to the NIH Guide for the Care and Use of Laboratory Animals. Male mice were used in this study.
PP2A transgenic mice.
Generation of the PP2A transgenic mice has been described previously by our group (15). In brief, cDNA coding for the catalytic subunit of protein phosphatase 2A (PP2Ac, from mouse) was cloned into the multiple cloning site of a construct that drives expression under the control of the α-myosin heavy chain promoter. This allowed for heart-specific overexpression of the catalytic subunit of PP2A.
Western immunoblotting analyses.
Hearts were homogenized at 4°C for 1 min in buffer containing (in mM) 20 Tris·HCl (pH 7.4), 5 MgCl2, 1 EDTA, 1 dithiothreitol, and 1 PMSF with 5 μg/ml leupeptin with the use of a Polytron PT-10 (Kinematica, Lucerne, Switzerland). Homogenates were centrifuged at 4°C for 20 min at 10,000 g. Supernatants were treated with equal volumes of loading buffer (7.5% SDS, 62.5 mM Tris·HCl, pH 6.8, 20% glycerol, 40 mM dithiothreitol). For immunoblot analysis, supernatant proteins were separated on SDS-PAGE and transferred to nitrocellulose membranes, which were probed with antibodies to PP1c (Santa Cruz Biotechnology, Santa Cruz, CA), PP2Ac (Upstate Biotechnology, Lake Placid, NY), or I-2 (21) that detect both the endogenous and the overexpressed proteins. For quantitative detection of I-2, 13 μg of protein was loaded on the gel for wild-type and PP1c samples, whereas 2.6 μg of protein was loaded for I-2140 and double transgenic (DT) samples. This was necessary in order to be in the linear range for detection of the high level of expression of I-2. The membrane was then blotted with affinity-purified polyclonal antibody raised against recombinant rabbit I-2, which recognizes both mouse (endogenous) and human (transgenic) I-2. For detection of PP1c, 13 μg of protein was loaded for all samples. To confirm protein loading, membranes were stained with Ponceau S before blotting. Membranes were also probed with rabbit polyclonal antibodies against RyR and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a, both kindly provided by Dr. L. R. Jones (Krannert Institute of Cardiology, Indianapolis, IN) and with monoclonal anti-PLB (A-1, Upstate Biotechnology).
Protein phosphatase assays.
Protein phosphatase activity was assayed with [32P]phosphorylase a as substrate as previously described (27). Mouse ventricular tissue was homogenized at 4°C for 1 min in buffer containing 4 mM EDTA (pH 7.4) and 15 mM 2-mercaptoethanol with a Polytron PT-10 homogenizer. Homogenates were centrifuged at 14,000 g for 20 min at 4°C, and the supernatants were used for determination of phosphorylase phosphatase activity. The reaction mixture contained (in mM) 20 Tris·HCl (pH 7.4), 5 caffeine, 0.1 EDTA, and 15 2-mercaptoethanol and aliquots of the supernatants. The dephosphorylation reactions were initiated by adding [32P]phosphorylase a and carried out at 30°C for 10 min. The reaction was terminated by addition of 50% trichloroacetic acid. The precipitated proteins were sedimented by centrifugation at 14,000 g for 5 min, and an aliquot of the supernatants was counted in a liquid scintillation counter.
Immunohistochemistry.
Immunostaining was carried out on 5-μm-thick tissue sections. Before application to the sections, a monoclonal antibody raised against residues 1-144 of human I-2 (Transduction Laboratories, Lexington, KY) was directly labeled with biotin and a commercially available kit (animal research kit peroxidase, K3954, DAKO, Carpinteria, CA). After blocking of endogenous peroxidase, the labeled I-2 antibody was incubated overnight at 4°C. The antibody binding was visualized with streptavidin-peroxidase and reacted with diaminobenzidine-hydrogen peroxidase as a chromogenic substrate (DAKO). Sections were counterstained with hematoxylin. Additional sections were stained with Sirius red in order to assess fibrosis (1).
Echocardiography and Doppler studies.
Transthoracic echocardiographic measurements were performed on mice anesthetized intraperitoneally with a mixture of ketamine S (25 mg/kg) and xylazine (10 mg/kg), allowing spontaneous breathing, as previously described (15). All measurements were made with a commercially available echocardiographic system (Hewlett-Packard Sonos 5500) equipped with a 15-MHz linear transducer for two-dimensional and M-mode imaging and a 12-MHz transducer for Doppler measurements. The parasternal long and short axes were obtained. Five heartbeats for every parameter were analyzed. The fractional shortening of the heart was calculated from the M-mode left ventricular (LV) diameters as (LVEDD − LVESD)/LVEDD × 100, where LVEDD is LV end-diastolic diameter and LVESD is LV end-systolic diameter. In addition, Doppler flow measurements of aortic and mitral flow were performed. The analyses were performed by two observers who were blinded to the mouse lineage.
Hemodynamic performance.
LV catheterization was performed in closed-chest mice as described previously (21).
Action potential measurements in isolated hearts.
To determine action potential duration, hearts were isolated and retrogradely perfused on a modified Langendorff apparatus equipped with three monophasic action potential catheters by published methods (22).
Mortality.
Animals were monitored in the cages daily for the occurrence of death.
Statistical analysis.
Data are reported as means ± SE. Statistical significance was assessed by ANOVA analyses followed by Bonferroni's or Student's t-test as appropriate. P < 0.05 was considered significant.
RESULTS
Expression of PP1 and I-2 in wild-type and transgenic mice.
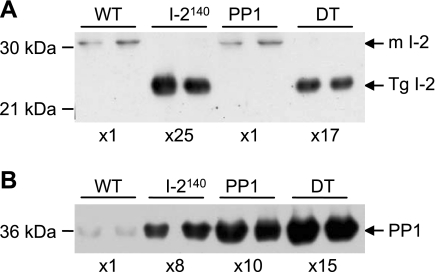
Analysis of protein expression showed that I-2140 is overexpressed by ∼25-fold (Fig. 1A) in I-2140 transgenic mice compared with the endogenous protein in wild-type animals as previously reported (21) and a similar level (∼17-fold) of expression was observed in the DT mice. Consistent with previous findings (7, 21), the catalytic subunit of PP1 was increased by ∼10-fold over the endogenous catalytic subunit of PP1 in PP1 mice as well as in I-2140 mice (8-fold), and the levels were close to additive (15-fold) in the DT animals (Fig. 1B). The increased catalytic subunit of PP1 in the I-2140 mice is most likely due to protein stabilization, whereby overexpression of the regulatory subunit stabilizes the catalytic subunit of PP1. The converse, however, does not occur, and overexpression of the catalytic subunit of PP1 does not result in elevated levels of I-2 or the PP1 muscle-specific glycogen/sarcoplasmic reticulum-associating subunit RGL (7). Furthermore, determination of expression of PLB and other Ca2+ cycling proteins in the heart (Table 1) showed that the level of PLB was not different between the groups; SERCA2a and RyR were increased in the I-2 hearts and decreased in the PP1 hearts. However, whereas SERCA2a levels were normalized in the DT mice, although there was a tendency of RyR to increase in the DT it did not reach the level of the wild type.
Fig. 1.
Expression of protein phosphatase (PP)1 and inhibitor-2 (I-2) in wild-type (WT) and transgenic mice. WT, COOH-terminally truncated I-2-overexpressing (I-2140), PP1, and double transgenic (DT) heart extract samples were immunoblotted with I-2 (A)- and PP1 catalytic subunit (PP1c; B)-specific antibodies as described in materials and methods. For Western blots of I-2, 13 μg of protein was loaded for samples from WT and PP1c hearts, whereas only 2.6 μg of protein was loaded for I-2140 and DT hearts. Therefore, the endogenous I-2 [mouse (m)I-2] cannot be detected in the lanes where less protein was loaded. For Western blotting of PP1 13 μg of protein was loaded for all samples. Fold overexpression is calculated with respect to the level in the WT samples and taking into account the protein loaded, and is given at bottom. Molecular mass markers are indicated on left. Tg, transgenic.
Table 1.
SR proteins in WT, I-2140, PP1, and DT mouse hearts
| WT | I-2140 | PP1 | DT | |
|---|---|---|---|---|
| PLB | 100±1.8 | 103±4.9 | 93.0±5.7 | 108±4.4 |
| SERCA2a | 100±4.8 | 121±5.0* | 83.6±5.7* | 92.6±9.5 |
| Ryanodine receptor | 100±7.1 | 133±1.7* | 68.7±4.7* | 75.5±3* |
Data are expressed as means ± SE % of wild-type (WT) value; n = 9–12 hearts. Expression of cardiac regulatory sarcoplasmic reticulum (SR) proteins in homogenates of WT, protein phosphatase 1-overexpressing (PP1), COOH-terminally truncated PP1 inhibitor-2-overexpressing (I-2140), and double transgenic (DT) mouse hearts was determined by Western blotting. PLB, phospholamban; SERCA2a, sarco(endo)plasmic reticulum Ca2+-ATPase 2a.
P < 0.05 vs. WT.
Protein phosphatase activity in wild type and transgenic mice.
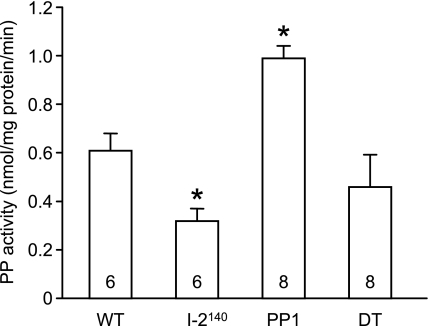
Phosphatase activity was increased in PP1 mice, decreased in I-2140 mice, and returned to wild-type levels in DT mice (Fig. 2). The altered phosphatase activity shown in Fig. 2 was determined in 30-wk-old mice, at which time PP1 mice exhibited hypertrophy (Table 2). However, similar effects on phosphatase activity were observed at an earlier time point, before hypertrophy had developed in the PP1 mice (12 wk old; data not shown). At 12 wk of age, hypertrophy had not yet developed (Table 2). These findings are consistent with the notion that prolonged presence of enhanced PP1 activity precedes and might cause hypertrophy and these biochemical alterations are blocked by additional overexpression of I-2140. Moreover, in agreement with previous observations, the main remaining PP activity in I-2140 mice and DT mice is contributed by the catalytic subunit of PP2A, because it is blocked by low concentrations (10 nM) of okadaic acid (data not shown). It should be noted that although the levels of the catalytic subunit of PP1 are increased more than the measured activity, it is important to consider that PP1c in the cell associates with a number of regulatory/targeting subunits to form complexes that are not necessarily spontaneously active and may have distinct substrate specificity. In addition, although the level of the catalytic subunit of PP1 is higher in I-2140 and DT mice, the presence of the inhibitor dampens the phosphatase activity.
Fig. 2.
Total phosphatase activity. Phosphatase (PP) activity was determined in homogenates prepared from WT, PP1, I-2140, and DT mouse hearts (30 wk of age) with 32P-labeled phosphorylase a as substrate. Values are means ± SE. The elevated PP activity in PP1 mice is normalized in DT mice. *P < 0.05 vs. WT.
Table 2.
Morphometric parameters of WT and transgenic mice
| n | Body Weight, g | Heart Weight, mg | Heart-to-Body Weight Ratio, mg/g | |
|---|---|---|---|---|
| 30 wk of age | ||||
| WT | 20 | 31.9±0.7 | 190±7.9 | 6.0±0.2 |
| I-2140 | 16 | 28.7±1.1 | 167±8.6 | 5.9±0.3 |
| PP1 | 20 | 29.3±0.9 | 255±31.0* | 9.1±1.4* |
| DT | 21 | 28.8±1.0 | 166±8.1 | 5.8±0.3 |
| 12 wk of age | ||||
| WT | 7 | 24±1.4 | 144±7.7 | 5.9±0.2 |
| I-2140 | 6 | 27.2±1.2 | 143±6.6 | 5.3±0.1 |
| PP1 | 6 | 25.8±1.0 | 143±8.4 | 5.5±0.1 |
| DT | 6 | 27.4±0.9 | 152±7.2 | 5.6±0.5 |
| 12 wk of age | ||||
| WT | 12 | 26±1.2 | 139±6.9 | 5.4±0.1 |
| I-2140 | 8 | 28.4±1.3 | 144±5.6 | 5.1±0.2 |
| PP2A | 7 | 26.9±1.4 | 200±19.1* | 7.7±0.4* |
| PP2A-I-2140 | 12 | 28.5±1.1 | 192±8.3* | 6.8±0.3* |
Values are means ± SE for n hearts. Body weight and heart weight were determined in WT, I-2140, PP1, DT, PP2A, and PP2A-I-2140 double transgenic mice.
P < 0.05 vs. WT.
Histological analyses.
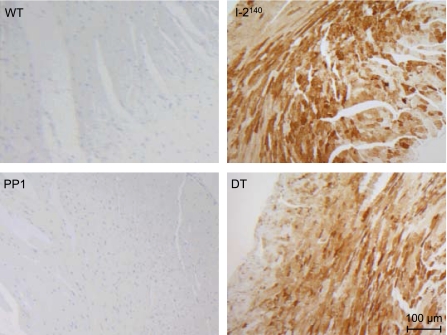
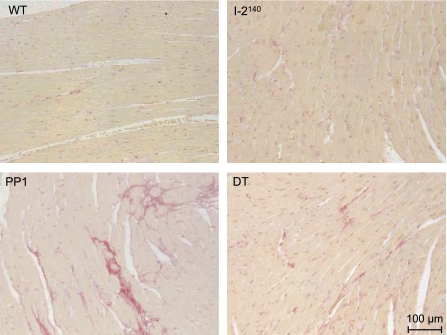
Overexpression of I-2140 was localized to myocytes as determined by immunohistological analyses. With an antibody that recognizes only the transgenic I-2, high levels of I-2 expression were detected in myocytes, but not in nonmyocytes, of I-2140 and DT mice (Fig. 3). Overexpression of PP1 led to fibrosis as indicated by staining of cells with Sirius red, whereas as reported previously (21) I-2140 overexpression did not (Fig. 4). Most interestingly, in DT mice fibrosis was greatly reduced if not completely absent (Fig. 4); more specifically, the percentage of collagen in the PP1 mice, 3.21 ± 0.34%, was reduced to 1.78 ± 0.16% in the DT animals (n = 4 each; P < 0.05).
Fig. 3.
Immunohistochemical analysis of I-2. Longitudinal sections from paraffin-embedded hearts of WT, I-2140, PP1, and DT mice were immunoreacted with mouse monoclonal antibody raised against human I-2 as described in materials and methods. Note that the monoclonal antibody used here does not cross-react with the endogenous mouse I-2. A representative set of sections is shown. Magnification, ×100. Note detection of I-2 in cardiomyocytes of I-2140 and DT mice.
Fig. 4.
Histochemical detection of fibrosis. Longitudinal sections from paraffin-embedded hearts of WT, I-2140, PP1, and DT mice at 30 wk of age were stained with Sirius red. Representative sections are shown. Note fibrosis in PP1 and great attenuation of fibrosis in DT.
Cardiac hypertrophy.
Heart weight, body weight, and relative heart weight, directly measured with scales (Table 2) at 12 wk of age, were not significantly different among WT, I-2140, PP1, and DT mice. However, at 30 wk of age PP1 but not I-2140 or DT mice exhibited an increased heart weight. This indicates that the development of PP1-induced cardiac hypertrophy is age dependent and is rescued by the simultaneous overexpression of I-2140 (Table 2). Cardiac weight as calculated from echocardiographic parameters was increased in PP1 mice at 16 and 24 wk of age (Fig. 5, LV mass, LV/body wt). This increase was not observed in DT mice (Fig. 5). The effect of I-2140 to inhibit development of cardiac hypertrophy is specific, as I-2140 did not prevent the hypertrophy of mice overexpressing in the heart the catalytic subunit of PP2A (Table 2). It should be noted that PP2A mice develop cardiac hypertrophy at 12 wk of age, in contrast to the PP1 animals (Table 2).
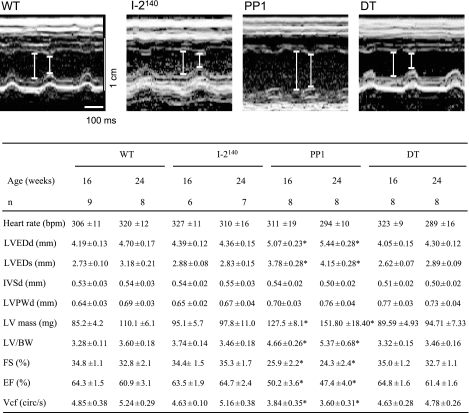
Fig. 5.
Representative 2-dimensional parasternal axis view echocardiographic images from littermates aged 24 wk with left ventricular (LV) diameters indicated in diastole and systole (top) and values from motion mode (M-mode) measurements (bottom). Values are means ± SE for n mice. LVEDd and LVEDs, LV end-diastolic and end-systolic diameters; IVSd, diastolic thickness of the septum; LVPWd, diastolic posterior wall thickness of LV; LV mass, calculated LV mass {[(IVSd + LVEDd + LVPWd)3 − LVEDd] × 1.055}; BW, body weight; FS, % of fractional shortening calculated as (LVEDd − LVESd)/LVEDd × 100; EF, ejection fraction (Teichholz); Vcf, velocity of circumferential fiber shortening {FS/LV ejection time [circumference (circ)/s]}; bpm, beats/min. *P < 0.05 vs. WT.
Cardiac function.
Cardiac function was evaluated by three different methods, all of which led to similar conclusions. Cardiac performance, as assessed by hemodynamic measurements in the left ventricle, supported the notion of a beneficial effect of PP1 inhibition with consequent PLB phosphorylation. While cardiac activity was greatly diminished in PP1 mice, nearly normal cardiac function was observed in DT mice (Table 3). Similar results were obtained by echocardiography in intact animals (Fig. 5). The ejection fraction, a widely used clinical parameter of contractility, was greatly reduced in PP1 animals. This decline in cardiac contractility was not observed in DT animals (Fig. 5). Concordant findings were also obtained in isolated work-performing preparations (Table 4). The reason for the higher heart rate detected in the PP1 mice by the work-performing heart procedure (Table 4) and not by echocardiography (Fig. 5) or catheterization (Table 3) could be explained if in the intact animals (Fig. 5) increased vagal tone overcame the higher intrinsic heart rate in PP1 mice (Table 4).
Table 3.
Cardiac catheterization and action potentials of WT and DT mice
| WT | I-2140 | PP1 | DT | |
|---|---|---|---|---|
| N | 14 | 10 | 8 | 10 |
| Heart rate, beats/min | 371±14.6 | 384±11.4 | 387±14.8 | 406±18.4 |
| LVP, mmHg | 83.4±2.4 | 89.4±1.8 | 75.7±2.5* | 82.8±2.6 |
| dP/dtmax, mmHg/s | 5,111±248 | 6,332±147* | 4,106±303* | 5,308±374 |
| dP/dtmin, mmHg/s | −5,467±252 | −6,037±125 | −4,121±394* | −4,865±289 |
| n | 23/8 | 21/8 | 20/7 | 27/10 |
| APD90, ms | 32.6±1.5 | 29.6±1.0 | 37.2±1.4* | 31.2±1.0 |
| APD70, ms | 19.1±1.6 | 16.7±1.1 | 24.9±1.5* | 19.5±0.9 |
Values are means ± SE for N mice or n action potentials/hearts. Contractile parameters of closed-chest anesthetized WT, I-2140, PP1, and DT mice were studied by cardiac catheterization at 30 wk of age at basal level. LVP, left ventricular pressure; dP/dtmax, maximum rate of LVP development; dP/dtmin, maximum rate of LVP decline. For determination of action potential duration (APD) to 70% and 90% repolarization (APD70 and APD90), hearts were isolated and retrogradely perfused on a modified Langendorff apparatus.
P < 0.05 vs. WT.
Table 4.
Work-performing heart preparations
| WT | I-2140 | PP1 | DT | |
|---|---|---|---|---|
| n | 5 | 5 | 6 | 7 |
| Heart rate, beats/min | 381±18 | 395±27 | 465±11* | 408±21 |
| LVPmax, mmHg | 90±4 | 86±3 | 70±4* | 87±4 |
| LVPmin, mmHg | 4±3 | 1±3 | 16±4* | 3±3 |
| dP/dtmax, mmHg/s | 3,254±206 | 3,033±167 | 1,757±274* | 2,931±168 |
| dP/dtmin, mmHg/s | −2,263±287 | −2,228±200 | −1,487±182* | −2,129±228 |
| t90, ms | 45±1 | 46±3 | 50±1* | 50±1* |
Values are means ± SE for n hearts. Contractile parameters of WT, I-2140, PP1, and DT mouse hearts (30 wk of age) were studied at an afterload of 50 mmHg in the work-performing mode. LVPmax, maximum LVP; LVPmin, minimum LVP; t90, time to 90% relaxation.
P < 0.05 vs. WT.
Moreover, echocardiography revealed an increase in LV volume of PP1 mice, indicative of dilated cardiomyopathy. Consistent with LV dilatation and hypertrophy, action potential durations were prolonged in PP1 mice. DT animals did not show any dilatation (Fig. 5), and the action potential prolongation (Table 3) was completely normalized. This effect was present at both 70% and 90% repolarization, consistent with the reversal of the electrophysiological defects of PP1 by increased expression of I-2140 (Table 3, bottom).
Mortality.
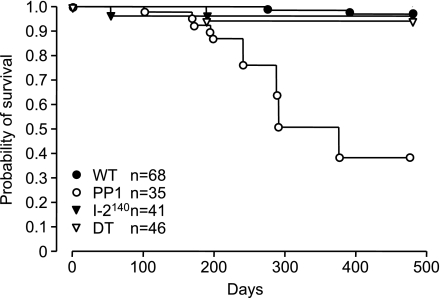
An increased mortality in PP1 mice was previously reported (7). A question was then whether the rescue of the physiological parameters was associated with improved survival. As shown in Fig. 6, over time the PP1 mice showed increased mortality, whereas the DT mice exhibited a life span comparable to WT mice. Therefore, restored cardiac function by overexpression of I-2140 also prevented the early mortality of the PP1 mice.
Fig. 6.
Survival curves of WT, PP1, I-2140, and DT mice. Note the enhanced mortality in PP1 mice, which is abrogated in DT mice, indicating protection against death by the overexpressed I-2140. n = 35–68 mice.
DISCUSSION
The most important finding of the present study is that the detrimental effects of PP1 overexpression in the heart can be blocked by inhibition of PP1 activity through overexpression of I-2140.
Previously, it was shown that overexpression of PP1 resulted in an increase in PP1 activity, which was associated with decreased phosphorylation of PLB at Ser16 and depressed contractility as determined by the blunted response to β-adrenergic stimulation and by reduced fractional shortening velocity measured by echocardiography (7). Genetic deletion (knockout) of I-1, a protein that after phosphorylation on Thr35 by PKA specifically inhibits PP1, increased phosphatase activity in the heart, reduced force of contraction and mechanical relaxation, and attenuated the response to β-adrenergic stimulation in isolated hearts and in vivo (7). In contrast, overexpression of a constitutively active form of I-1 or I-2 resulted in decreased phosphatase activity, increased PLB phosphorylation at Ser16 (in I-1- and I-2140-overexpressing mice), elevated PLB phosphorylation at Thr17 (in I-1- but not in I-2140-overexpressing mice), and enhanced contractility (21, 32). In PKCα-deficient mice, contractility was augmented and PP1 activity reduced because of increased I-1 efficacy, and higher PLB phosphorylation at Ser16 (6) was observed. Interestingly, ablation of PKCα rescued the PP1 phenotype. On the other hand, overexpression of PKCα was associated with a reduced contractility, increased PP1 activity, and lower PLB phosphorylation (6). In a rat model of aortic banding accompanied by signs of heart failure, gene transfer of active I-1 restored cardiac function, at least in part (32). Together these findings suggested that an increased PP1 activity might impair cardiac function.
This report demonstrates that selective PP1 inhibition by I-2140 not only ameliorates cardiac function, hypertrophy, and failure but also rescues mortality. As previously reported (21), the beneficial functional effects of I-2140 could be attributed to the increase in PLB phosphorylation as well as the enhanced expression of SERCA and RyR. This can be mechanistically important, because there is evidence that enhanced SERCA activity is beneficial in experimental and genetic models of heart failure (10, 24). Similarly, the depressed function in the PP1 mice can be accounted for not only by the decreased PLB phosphorylation but also by the reduced expression of SERCA2a and RyR. The restored function in the DT mice, despite the slight decrease in RyR levels, could be explained by a compensatory action of the persistent, increased PLB phosphorylation (data not shown).
A role of PP1 activity in cardiac contractility is also supported by previous work using cell membrane-permeant natural inhibitors of protein phosphatase activity such as okadaic acid and cantharidin. Treatment of isolated heart preparations with these compounds induced a positive inotropic effect, which was accompanied by increased phosphorylation of PLB (27).
As mentioned in the introduction, evidence for a role of PP1 in the control of cardiac function comes from the observations that PP1 activity is increased (28) and the level and phosphorylation state of I-1 are decreased (12) in failing human hearts. In fact, the increased PP1 activity could be due to enhanced expression of PP1, to decreased expression of I-1 (12, 13) or I-2, to reduced phosphorylation and hence diminished inhibitory action of I-1 (7), or to a combination of these. Moreover, whereas it is well known that phosphorylation of I-1 by PKA at Thr35 increases the potency of I-1, more recent evidence suggests that I-1 activity is reduced when I-1 is phosphorylated on Ser67 (34, 35). Interestingly, the same authors also reported that, in samples from failing human hearts, phosphorylation of I-1 at Ser67 was enhanced compared with normal hearts, which may lead to higher PP1 activity (6). Regardless of the underlying mechanism for enhanced PP1 activity, in isolated electrically driven cells from failing human hearts the effect of β-adrenergic stimulation on cell shortening and Ca2+ transients was normalized after infection of cells with an adenovirus that codes for an active mutant of I-1 (7).
The fact that PP1 activity is elevated in the failing human heart and that phosphorylation of proteins that are involved in regulation of contractile function is reduced argues that enhanced PP1 activity may be detrimental for cardiac performance. A negative role of PP1 activity is consistent with the observation that PP1 overexpression in the heart leads to impaired contractility, cardiac hypertrophy, and increased mortality after 6 mo of age (7) and is further supported by the present work, which shows that inhibition of PP1 activity by I-2140 abrogates these detrimental effects. Therefore, pharmacological inhibition of PP1 activity may provide a therapy for alleviation of heart failure. However, more work is required to prove that PP1 inhibition is free of untoward effects. Pharmacological interventions with the currently available compounds are predicted not to be feasible. We have shown that concentrations of PP inhibitors like cantharidin that are required to increase force of contraction also lead to vasoconstriction in human coronary arteries (23). Hence, compounds that inhibit PP1 solely in cardiomyocytes would be necessary for clinical purposes. The same limitations hold true for adenoviral delivery of I-2 to the heart. If the virus were injected into the coronary arteries, it is predicted that the foremost endothelial and smooth muscle cells would be infected before infection of cardiomyocytes could occur. Perhaps engineering of viruses with promoters specific for cardiomyocytes would offer a feasible therapeutic option.
Overexpression of I-2140 exerts beneficial effects on cardiac function and abrogation of cardiac hypertrophy in a specific manner. In fact, whereas PP1-induced hypertrophy was blocked by crossbreeding the PP1 mice with the I-2140 mice, PP2A-induced hypertrophy was not alleviated by overexpression of I-2140 (Table 1). These in vivo findings are consistent with the known specificity of I-2, which inhibits PP1 and not PP2A activity.
The importance of the present studies is that we clearly show that inhibition of genetically enhanced PP1 activity by I-2140 can be beneficial. Whether a deteriorated cardiac function due to enhanced PP1 activity can thereafter be restored by PP1 inhibition has not been thoroughly investigated. However, delivery of I-2 adenovirus to the heart of the cardiomyopathic hamster, a nontransgenic model of heart failure, improved cardiac function and survival up to 26 wk (37). One approach for future research would be to generate mice with cardiac-specific inducible overexpression of I-2 and then crossbreed them with PP1 mice. The result of these kinds of experiments would provide information on whether the detrimental effects of PP1 on heart function could be reversed. Nevertheless, the present results clearly demonstrate that the cardiac dysfunction induced by increased PP1 activity can be prevented by I-2 co-overexpression in the mammalian heart.
In summary, the present work underscores the detrimental role of enhanced PP1 activity in the heart and the potential pathophysiological role of this enzyme in human heart failure. Therefore, inhibition of PP1 activity may represent a potential target for therapeutic interventions.
GRANTS
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to J. Neumann and by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-36569 to A. A. DePaoli-Roach.
Acknowledgments
We thank Dr. med. Vet Lisa Fortmüller, IZKF Münster, ZPG4a for help with echocardiography. We also thank Dr. Peter Roach for critical reading of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Baba HA, Iwai T, Irlbeck M, Schmid KW, Zimmer HG. Differential effects of angiotensin II receptor blockade on pressure-induced left ventricular hypertrophy and fibrosis in rats. J Mol Cell Cardiol 31: 445–455, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Bartel S, Stein B, Eschenhagen T, Mende U, Neumann J, Schmitz W, Krause EG, Karczewski P, Scholz H. Protein phosphorylation in isolated trabeculae from nonfailing and failing human hearts. Mol Cell Biochem 157: 171–179, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation 96: 1495–1500, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Boknik P, Fockenbrock M, Herzig S, Knapp J, Linck B, Lüss H, Müller FU, Müller T, Schmitz W, Schröder F, Neumann J. Protein phosphatase activity is increased in a rat model of long-term β-adrenergic stimulation. Naunyn Schmiedebergs Arch Pharmacol 262: 222–231, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Brandt H, Lee EYC, Killilea SD. A protein inhibitor of rabbit liver phosphorylase phosphatase. Biochem Biophys Res Commun 63: 950–956, 1975. [DOI] [PubMed] [Google Scholar]
- 6.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med 10: 248–254, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing SL, Allen PB, Greengard P, Yatani A, Hoit BD, Grupp IL, Hajjar RJ, DePaoli-Roach AA, Kranias EG. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol 22: 4124–4135, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceulemans H, Stalmans W, Bollen M. Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution. Bioessays 24, 371–381, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Cohen PT Protein phosphatase 1—targeted in many directions. J Cell Sci 115, 241–256, 2002. [DOI] [PubMed] [Google Scholar]
- 10.del Monte F, Hajjar RJ, Harding SE. Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure. Circ Res 88: E66–E67, 2001. [DOI] [PubMed] [Google Scholar]
- 11.DePaoli-Roach AA Protein phosphatase 1 binding proteins. In: Handbook of Cellular Signaling, edited by Bradshaw RA and Dennis ED. San Diego, CA: Academic, 2003, p. 613–619.
- 12.El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res 61: 87–93, 2004. [DOI] [PubMed] [Google Scholar]
- 13.El-Armouche A, Rau T, Zolk O, Ditz D, Pamminger T, Zimmermann WH, Jäckel E, Harding SE, Boknik P, Neumann J, Eschenhagen T. Evidence for protein phosphatase inhibitor-1 playing an amplifier role in beta-adrenergic signaling in cardiac myocytes. FASEB J 17: 437–439, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Foulkes JG, Cohen P. The hormonal control of glycogen metabolism. Phosphorylation of protein phosphatase inhibitor-1 in vivo in response to adrenaline. Eur J Biochem 97: 251–256, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Gergs U, Boknik P, Buchwalow I, Fabritz L, Matus M, Justus I, Hanske G, Schmitz W, Neumann J. Overexpression of the catalytic subunit of protein phosphatase 2A impairs cardiac function. J Biol Chem 279: 40827–40834, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gupta RC, Mishra S, Rastogi S, Imai M, Habib O, Sabbah HN. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am J Physiol Heart Circ Physiol 285: H2373–H2381, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Gupta RC, Neumann J, Watanabe AM, Lesch M, Sabbah HN. Evidence for presence and hormonal regulation of protein phosphatase inhibitor-1 in ventricular cardiomyocyte. Am J Physiol Heart Circ Physiol 270: H1159–H1164, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Gupta RC, Neumann J, Watanabe AM, Sabbah HN. Inhibition of type 1 protein phosphatase activity by activation of beta-adrenoceptors in ventricular myocardium. Biochem Pharmacol 63: 1069–1076, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Holmes CFB, Campbell DG, Caudwell FB, Aitken A, Cohen P. The protein phosphatases involved in cellular regulation. Primary structure of inhibitor-2 from rabbit skeletal muscle. Eur J Biochem 155: 173–182, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Huang FL, Glinsmann WH. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur J Biochem 70: 419–426, 1976. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhefer U, Baba HA, Boknik P, Breeden KM, Mavila N, Bruchert N, Justus I, Matus M, Schmitz W, DePaoli-Roach AA, Neumann J. Enhanced cardiac function in mice overexpressing protein phosphatase inhibitor-2. Cardiovasc Res 68: 98–108, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhof P, Fabritz L, Kilic A, Begrow F, Breithardt G, Kuhn M. Ventricular arrhythmias, increased cardiac calmodulin kinase II expression, and altered repolarization kinetics in ANP receptor deficient mice. J Mol Cell Cardiol 36: 691–700, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Knapp J, Boknik P, Deng MC, Huke S, Gombosova I, Klein-Wiele O, Linck B, Lüss H, Müller FU, Nacke P, Scheld HH, Schmitz W, Vahlensieck U, Neumann J. On the contractile function of phosphatases in isolated human coronary arteries. Naunyn Schmiedebergs Arch Pharmacol 360: 464–472, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, Martone M, Wang Y, Ross J Jr, Kranias E. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell 99: 313–322, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Mishra S, Gupta RC, Tiwari N, Sharov V, Sabbah HN. Molecular mechanisms of reduced sarcoplasmic reticulum Ca2+ uptake in human failing left ventricular myocardium. J Heart Lung Transplant 21: 366–373, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann J, Boknik P, Herzig S, Schmitz W, Scholz H, Gupta RC, Watanabe AM. Evidence for physiological functions of protein phosphatases in the heart: evaluation with okadaic acid. Am J Physiol Heart Circ Physiol 265: H257–H266, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Neumann J, Eschenhagen T, Jones LR, Linck B, Schmitz W, Scholz H, Zimmermann N. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J Mol Cell Cardiol 29: 265–272, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Neumann J, Gupta RC, Schmitz W, Scholz H, Nairn AC, Watanabe AM. Evidence for isoproterenol-induced phosphorylation of phosphatase inhibitor-1 in the intact heart. Circ Res 69: 1450–1457, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Neumann J, Maas R, Boknik P, Jones LR, Zimmermann N, Scholz H. Pharmacological characterization of protein phosphatase activities in preparations from failing human hearts. J Pharmacol Exp Ther 289: 188–193, 1999. [PubMed] [Google Scholar]
- 31.Neumann J, Suzuki Y, Field LJ, DePaoli-Roach AA. Targeted overexpression of glycogen/SR-associated protein phosphatase to murine heart (Abstract). Naunyn Schmiedebergs Arch Pharmacol 357, Suppl 1: R109, 1998. [Google Scholar]
- 32.Pathak A, del Monte F, Zhao W, Schultz JE, Lorenz JN, Bodi I, Weiser D, Hahn H, Carr AN, Syed F, Mavila N, Jha L, Qian J, Marreez Y, Chen G, McGraw DW, Heist EK, Guerriero JL, DePaoli-Roach AA, Hajjar RJ, Kranias EG. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res 96: 756–766, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Roach P, Roach PJ, DePaoli-Roach AA. Phosphoprotein phosphatase inhibitor-2. Identification as a species of molecular weight 31,000 in rabbit muscle, liver, and other tissues. J Biol Chem 260: 6314–6317, 1985. [PubMed] [Google Scholar]
- 34.Rodriguez P, Mitton B, Nicolaou P, Chen G, Kranias EG. Phosphorylation of human inhibitor-1 at Ser67 and/or Thr75 attenuates stimulatory effects of protein kinase A signaling in cardiac myocytes. Am J Physiol Heart Circ Physiol 293: H762–H769, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez P, Mitton B, Waggoner JR, Kranias EG. Identification of a novel phosphorylation site in protein phosphatase inhibitor-1 as a negative regulator of cardiac function. J Biol Chem 281: 38599–38608, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Schwinger RH, Münch G, Bolck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca2+-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol 31: 479–491, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Yamada M, Ikeda Y, Yano M, Yoshimura K, Nishino S, Aoyama H, Wang L, Aoki H, Matsuzaki M. Inhibition of protein phosphatase 1 by inhibitor-2 gene delivery ameliorates heart failure progression in genetic cardiomyopathy. FASEB J 20: 1197–1199, 2006. [DOI] [PubMed] [Google Scholar]