Abstract
The phosphatidylinositol 3-kinase (PI3K) signaling pathway regulates multiple cellular processes including cell survival/apoptosis and growth. In the cardiac context, PI3Kα plays important roles in cardiac growth. We have shown that cardiac PI3K activity is highly regulated during development, with the highest levels found during the fetal-neonatal transition period and the lowest levels in the adult. There is a close relationship between cardiomyocyte proliferation and cardiac PI3K activity. In adult transgenic mice, however, the prolonged constitutive activation of PI3Kα in the heart results in hypertrophy. To develop a strategy to allow temporally controlled overexpression of cardiac PI3Kα, we engineered a tetracycline (tet) transactivator tet-off controlled transgenic mouse line with a conditional overexpression of a cardiac-specific fusion protein of the SH2 domain of p85 and p110α. Cardiac PI3K activity and Akt phosphorylation were significantly increased in adult mice after transgene induction following the removal of doxycycline for 2 wk. The heart weight-to-body weight ratio was not changed, and there were no signs of cardiomyopathy. The overexpression of PI3Kα resulted in increased left ventricular (LV) developed pressure and the maximal and minimal positive values of the first derivative of LV pressure, but not heart rate, as assessed in Langendorff hearts. Mice overexpressing PI3Kα also had increases in the levels of Ca2+-regulating proteins, including the L-type Ca2+ channels, ryanodine receptors, and sarco(endo)plasmic reticulum Ca2+-ATPase 2a. Thus the temporally controlled overexpression of cardiac PI3Kα does not induce hypertrophy or cardiomyopathy but results in increased contractility, probably via the increased expression of multiple Ca2+-regulating proteins. These distinct phenotypes suggest a fundamental difference between transgenic mice with temporal or prolonged activation of cardiac PI3Kα.
Keywords: Akt, Ca2+-regulating proteins, Langendorff, tet-off, phosphatidylinositol 3-kinase
the class i phosphatidylinositol 3-kinases (PI3Ks) are lipid kinases regulating many important and diverse cellular processes including cell proliferation, differentiation, survival, adhesion, and motility. The mammalian class IA PI3Ks are heterodimers of a 110 KDa catalytic subunit (p110α, p110β, or p110δ) and a regulatory subunit of 85 or 55 KDa (p85/p55), whereas the class IB PI3K (PI3Kγ) is composed of a p110γ catalytic subunit and a p101 regulatory subunit (21). These kinases phosphorylate the 3′-OH on the inositol ring of phosphatidylinositol 4,5-bisphosphate, the main substrate in vivo, and convert it to phosphatidylinositol 3,4,5,-trisphosphate (PIP3). In most systems, the basal level of PIP3 in cells is low and only rises sharply upon cellular stimulation, which in turn activates downstream signaling proteins including Akt and p70S6K. Past results suggest the class IA PI3Ks are activated by receptor tyrosine kinase pathways, whereas the class IB PI3K (PI3Kγ) is coupled to G protein-coupled receptors (GPCRs). This has been studied most extensively in leukocytes (5, 14). In cardiomyocytes prepared from mutant mice with deletion of cardiac PI3Kγ, there is an increase in basal cAMP (2). The notion that classes IA and IB PI3Ks can only be activated by receptor tyrosine kinase pathways and GPCRs, respectively, is not definite since PI3Kβ is sensitive to Gβγ (9, 12). In addition, we have recently shown that PI3Kα is functionally linked to β-adrenergic receptor stimulation in vivo (22).
Among the class IA PI3Ks, the PI3Kα isoform has been shown to play important roles in the regulation of cell growth. Mutations in PIK3CA, the gene coding for PI3Kα, are associated with oncogenic transformation (7). In the mutant-transformed cells, there is a constitutive phosphorylation of Akt, p70S6K, and 4E-binding protein 1 (7). Moreover, a common phenotype seen in transgenic mice with an overexpression of constitutively active PI3Kα is cardiac hypertrophy (16). Several Akt transgenic mouse models have been reported. These include the cardiac-specific overexpression of constitutively active Akt, E40K (1) and T308D/S473D (17), membrane-targeted Akt (myr-Akt) (10), and nuclear-targeted Akt (19). Except for the nuclear-targeted model, the overexpression of Akt results in cardiac hypertrophy. On the other hand, these models display distinct phenotypes. For example, an increase in contractility is observed in Akt (E40K) but not in myr-Akt mice (8). Extensive interstitial fibrosis was found in animals overexpressing constitutively active Akt (T308D/S473/D) (17). In an inducible model, short-term (2 wk) myr-Akt overexpression induced reversible hypertrophy without causing interstitial fibrosis (18). In contrast, the sustained overexpression (6 wk) of the same transgene induced extensive hypertrophy, interstitial fibrosis, and contractile dysfunction (18).
Indirect evidence suggests that PI3Kα induces physiological hypertrophy, as the cardiac expression of a dominant negative PI3Kα blunts exercise-induced hypertrophy but not pressure overload-induced hypertrophy (11). This transgenic model, however, cannot be used to examine the immediate short-term effects in the heart because the expression of transgene is manifest throughout fetal, postnatal, and adult life. We have shown that the expression of cardiac PI3Kα is tightly regulated with the highest level seen during the fetal-neonatal transition period and low levels in the adult (20). The goal of the current study was to explore the effects of the temporal activation of PI3Kα in the heart. To achieve this, we engineered a conditional mouse line, which allows the temporally controlled overexpression of PI3Kα. Using this inducible system, we were able to define the immediate short-term effects of PI3Kα activation in the heart before the induction of cardiac hypertrophy.
MATERIALS AND METHODS
DNA constructs.
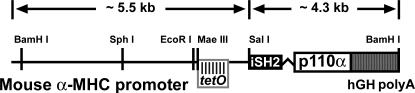
The tetracycline (tet)-controlled (tet-off) conditional transgenic mouse line required two separate transgenic mouse lines: the effector line expressing tet transactivator (tTA)-virion protein 16 (13) and the responder line expressing the target gene iSH2-p110α (3). Both constructs are under the control of the mouse α-myosin heavy chain (MHC) promoter, a cardiomyocyte-specific promoter. The empty tet-responsive α-MHC promoter construct had three GATA sites and two thyroid response elements removed, leaving other cis-acting elements critical for cardiac-specific expression left intact (13). There are seven repeats of the tetO sequences adjacent to the TATA box to ensure tet responsiveness, and the human growth hormone polyadenylation signal follows the unique cloning site. This construct has been shown to be functional in prior studies (13). A pCMV6-iSH2-p110α-MT vector containing the target gene (a generous gift from Drs. Seigo Izumo and Thomas Franke) was digested with SalI (5′ end) and BamHI (3′ end) to release the transgene. Because there is an internal SalI site within the transgene sequence, we performed partial digest of the vector and were able to isolate the full-length 4.4 kb iSH2-p110α transgene fragment with the internal SalI site intact. To insert the transgene into the tet-responsive α-MHC promoter containing vector, we first opened up the vector in the multiple cloning region with SalI (5′ end) and HindIII (3′ end) double digestion. A linker DNA with a BamHI overhang was then ligated to the HindIII end, thus creating matching ligation sites (SalI-BamHI) within the tet-responsive α-MHC promoter containing vector for the iSH2-p110α insert (Fig. 1). After ligation, the final construct was purified, sequenced, and verified by restriction enzyme digestion and sequencing.
Fig. 1.
Engineering of a responder transgene construct. A schematic of the tetracycline (tet)-responsive α-myosin heavy chain (MHC) promoter construct expressing iSH2-p110α is shown. The full-length α-MHC promoter has 3 GATA sites and 2 thyroid response elements deleted with other cardiac-specific cis-acting elements intact. There are 7 repeats of the tet operator (tetO) sequences adjacent to the TATA box. The human growth hormone (hGH) poly adenylation (polyA) signal was immediately downstream of iSH2-p110α.
Transgenic mice.
All animal handling and procedures, adhering to the APS Guiding Principles in the Care and Use of Animals, were approved by the Institutional Animal Care and Use Committee. The responder transgene construct was delivered by pronuclear microinjection into FVB/n mouse eggs (the transgenic mouse laboratory at Children's Hospital Research Foundation of Cincinnati). Oocytes were then transferred to pseudopregnant mice. The effector transgenic mouse line has been well established and does not induce cardiomyopathy (13). The crossing of the responder and effector transgenic mice resulted in the final double transgenic mice. Tail snips were collected from all newborns for genotyping of tTA and PI3Kα transgenes as well as an essential myosin light chain as an internal control (ELC1v) by PCR. The following primer pairs were used: tTA, forward, 5′-AGCGCATTAGAGCTGCTTAATGAGGTC-3′, and reverse, 5′-GTCGTAATAATGGCGGCATACTATC-3′; PI3Kα, forward, 5′-GATCCTTTAGCGGCCGCATATA-3′, and reverse, 5′-CCAAGCAAGCAACTCAAATG-3′; and ELC1V, forward, 5′-ATCGAGTTCACACCTGAACAGATTG-3′, and reverse, 5′-CCAGGACACGGAGCACCTCTG-3′. Double transgenic mice were maintained with doxycycline (Dox; 0.5 mg/ml) water to repress transgene expression. This relatively low dosing regimen of Dox has been used in the inducible Akt transgenic model with no cardiovascular side effects (18). Removal of Dox for 2 to 3 wk resulted in the overexpression of cardiac-specific PI3Kα.
PI3K activity and Western blotting.
Cardiac tissue lysates were prepared as described (22). PI3K activity was determined by immunoprecipitation (IP) in vitro lipid kinase assay. An antibody (1 μg) specific for phospho-tyrosine (pY; clone 4G10; Millipore) or PI3Kα (sc-7174; Santa Cruz Biotechnology) was added to 20 μl of packed protein G-sepharose (17–0618; GE Healthcare) in 0.5 ml IP buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 5 mM EDTA (pH 8.0), 10 mM Na4P2O7, 2 mM Na3VO4, 100 mM NaF, 1% (vol/vol) nonyl phenoxylpolyethoxylethanol-40, 1 μM peptostatin A, 1 μM leupeptin, 1 μM aprotinin, and 1 μM PMSF and incubated with rotation at room temperature for 1 h. After centrifuge (14,000 g) at room temperature for 1 min, the pellet was washed three times in IP buffer. Excess buffer was removed, and cardiac tissue lysates (0.5 mg) were added to the beads and rotated overnight at 4°C before subjected to lipid kinase assay as described (22). The expression of Akt was assessed with Western blotting using phospho-specific antibodies against phospho-Akt (Ser-473 and Thr-308; Cell Signaling Technology) and an antibody against total Akt as described (20). The expression of several Ca2+-regulating proteins, including sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a; Biomol SA-209), ryanodine receptor (RyR) type 2 (SC-8170; Santa Cruz Biotechnology), and the L-type voltage-gated Ca2+ channel (Cav1.2; ACC-003; Alomone Labs), was also examined.
Histological analysis.
Mouse hearts were collected after removing Dox for 2 wk. After briefly perfused on a Langendorff apparatus, the hearts were quickly embedded in embedding carrier filled with Triangle Biomedical Sciences tissue freezing medium (Fisher Scientific). Hearts were then dipped into isopentane, which had been cooled with liquid N2 to form a thick slurry, frozen for 20 s, and stored at −80°C. Cross sections (15 μm), obtained using a cryotome, were fixed in 3.65% (vol/vol) buffered formaldehyde for 15 min at room temperature and stained with hematoxylin and eosin or Masson trichrome. Sections were visualized with a Nikon ECLIPSE 80i microscope equipped with a SPOT Insight camera.
Measurement of myocardial function.
Baseline ventricular function in adult (3 to 4 mo old) mice was measured by using the Langendorff isovolumetrically isolated perfused mouse heart preparation (a nonworking heart preparation) as previously described (23). We recorded left ventricular (LV) developed pressure (LVDP), heart rate, coronary flow, and the maximal and minimal positive values of the first derivative of LV pressure (LVdP/dtmax and LVdP/dtmin, respectively).
Statistical analysis.
Results are presented as means ± SE. The difference between PI3Kα overexpressing (Dox-off for 2 to 3 wk) and littermate control (Dox-on) groups was analyzed using Student's t-test. A probability of P < 0.05 was considered to represent a significant difference.
RESULTS AND DISCUSSION
Establishing the conditional cardiac-specific PI3Kα transgenic mouse line.
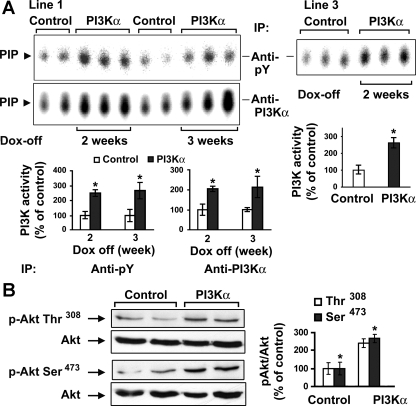
For generation of the responder mouse line, we identified one male and two female mice carrying the iSH2-p110α transgene. Breeding of each of the three responder transgenic mice (PI3Kα overexpressing) with the effector transgenic mice (tTA overexpressing) resulted in some offspring with both transgenes. The final double transgenic mice were confirmed by PCR and genotyping. Of the three double transgenic lines generated, lines 1 and 3 showed robust transgene expression upon the removal of Dox and, except in Fig. 2A, line 1 mice were used exclusively for all subsequent experiments. All mice were maintained with Dox water throughout development to suppress the overexpression of cardiac-specific PI3Kα. Adult mice (2–6 mo old, depending on the scope of the experiment) were then switched to regular drinking water for 2 to 3 wk to initiate transgene overexpression (PI3Kα group). Littermates of the same gender continued to receive Dox drinking water (control group). After 2 wk of transgenic expression, there was a significant increase in cardiac PI3K activity in the PI3Kα group compared with control littermates (Fig. 2A). These assays were performed using an antibody against either pY or PI3Kα in the IP step. This increase in PI3K activity was cardiac specific since assays performed on hepatic and pulmonary tissue lysates did not show any differences between the control and PI3Kα littermates (data not shown). We next measured Akt phosphorylation, an indicator of signaling activity downstream of PI3K. As expected, after Dox was removed for 2 wk, there was a significant increase in Akt phosphorylation in PI3Kα-overexpressing mice compared with control littermates (Fig. 2B). These data indicate that the conditional transgenic mouse displays the appropriate temporal activation via Dox treatment and that elevated levels of cardiac-specific PI3Kα in the adult, which normally has very low measurable activity (20), can be achieved.
Fig. 2.
Activation of cardiac phosphatidylinositol 3-kinase (PI3K) and Akt in the conditional transgenic mice. A: PI3K activity in 2 double transgenic mouse lines. Three-month-old male line 1 double transgenic littermates were maintained with doxycycline (Dox) water (control; n = 4) or regular drinking water (PI3Kα; n = 6) for 2 or 3 wk (left). Similarly, line 3 mice were maintained with Dox water (control; n = 3) or regular drinking water (PI3Kα; n = 3) for 2 wk (right). Cardiac PI3K activity was assessed with in vitro lipid kinase assay. Phosphoinositide 3-phosphate (PIP) is the phosphorylated end-product. Each bar graph shows the densitometric scanning results from 2 individual experiments. Data are expressed as means ± SE of percent change in PI3K activity relative to that of control. *P < 0.05 vs. control. B: Western blotting was performed on cardiac tissue lysates from male control (n = 2) and PI3Kα (2 wk off Dox; n = 2) line 1 mice using phospho (p)-specific antibodies against Akt (Ser-473 and Thr-308) and an antibody against total Akt. The bar graphs show results of densitometric measurement of each phospho-specific antibody normalized with total antibody from 3 separate experiments. *P < 0.05 vs. control. pY, phospho-tyrosine; IP, immunoprecipitation.
Temporally controlled overexpression of cardiac-specific PI3Kα does not induce hypertrophy or cardiomyopathic changes.
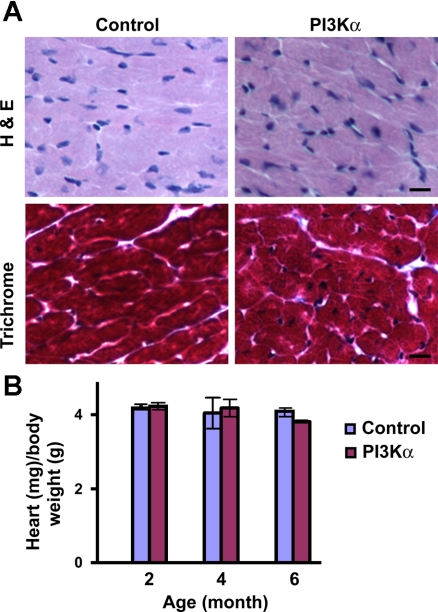
To examine whether the temporal cardiac-specific overexpression of PI3Kα induces hypertrophy or cardiomyopathic changes, adult male (3 to 4 mo old) double transgenic mice were maintained with regular drinking water for 2 wk. The microscopic examination of cross sections revealed no evidence of necrosis or myocyte disarray in PI3Kα mice compared with littermate controls (Fig. 3A, top). Masson trichrome stain also showed no difference in interstitial fibrosis between these animals (Fig. 3A, bottom). To further examine whether the temporal overexpression of PI3Kα resulted in cardiac hypertrophy, mice aged 2, 4, or 6 mo old were maintained with or without Dox water for 2 wk. This overexpression regimen did not induce cardiac hypertrophy, since the ratio of heart (ventricle) weight (milligrams) to body weight (grams) was not significantly different between control and PI3Kα littermates (Fig. 3B). In the inducible (tet-off) myr-Akt transgenic model, the removal of Dox for 2 wk induces Akt activation as well as cardiac hypertrophy, which can be reversed to normal size 2 wk after restarting Dox treatment (18). When the overexpression is extended to 6 wk, however, the resulting hypertrophy became only partially reversible, and there were extensive interstitial fibrosis and contractile dysfunction (18). By comparison, we show that 2 wk overexpression of PI3Kα does not induce hypertrophy. It appears that although PI3K and Akt belong to the same signaling pathway, transgenic animals overexpressing Akt may have a more direct impact on cardiac growth than do transgenic animals overexpressing PI3Kα.
Fig. 3.
Absence of cardiomyopathy and hypertrophy following temporal (2 wk) overexpression of cardiac-specific PI3Kα. A: histological analysis of heart sections from 3-mo-old male PI3Kα and control littermate. A, top: hematoxylin and eosin (H & E) staining. A, bottom: Masson trichrome staining. Bars represent 10 μm. B: heart weight-to-body weight ratio in PI3Kα and control littermates. Mice were 2 (n = 8), 4 (n = 10), or 6 (n = 8) mo old before initiation of cardiac PI3Kα overexpression.
The phenotypic characteristics of the transgenic mice expressing constitutively active PI3Kα include cardiac hypertrophy (16). In essence, both the constitutively active transgenic model and our model result in the induction of the same transgene: iSH2-p110α. The main difference is that our model allows the temporally controlled expression at select developmental stages. We chose to induce cardiac PI3Kα only in the adult and only for 2 wk, thus effectively eliminating the chance of developing hypertrophy. We have noted no sign of hypertrophy in our transgenic mice if the induction periods were less than 2 mo. Hence the new conditional transgenic model can provide a unique window of opportunity to study the immediate phenotypic changes in vivo following the activation of cardiac PI3Kα but before hypertrophy is present.
Temporally controlled overexpression of cardiac-specific PI3Kα increases contractility.
It has been shown that the constitutive overexpression of cardiac PI3Kα results in hypertrophy without affecting systolic function under resting conditions (16). In contrast, deletion of PI3Kγ results in hypercontractility (4). To examine whether the temporal overexpression of cardiac PI3Kα induces alterations in ventricular function, control and PI3Kα (after 2 wk transgene induction) mice were subjected to analysis on a Langendorff apparatus. We found that the temporal overexpression of cardiac-specific PI3Kα induced significant increases in LVDP, LVdP/dtmax, and LVdP/dtmin without altering heart rate and coronary flow (Table 1). Because heart rates were similar between the groups, these results suggest that there is enhanced contractility in the animals with temporal cardiac-specific overexpression of PI3Kα. This observation is reminiscent to that seen in transgenic mice overexpressing a constitutively active Akt (1). In contrast, the prolonged constitutive transgenically induced activation of cardiac PI3Kα is not associated with changes in LV dimensions or systolic function as assessed by M-mode echocardiography (16). Our data suggest there are fundamental phenotypic differences between these distinct transgenic models (constitutive transgenic expression vs. temporally controlled expression).
Table 1.
Basal myocardial function assessed by Langendorff perfusion system
| Control | PI3Kα | P Value | |
|---|---|---|---|
| n | 5 | 6 | |
| LV developed pressure, mmHg | 125.4±4.5 | 148.2±6.5* | 0.022 |
| LVdP/dtmax | 3,066±125 | 3,619±108* | 0.008 |
| LVdP/dtmin | 3,000±142 | 3,500±105* | 0.018 |
| Heart rate, beats/min | 335±25 | 323±20 | 0.716 |
| Coronary flow rate, ml/min | 3.72±0.15 | 3.27±0.30 | 0.234 |
Values are means ± SE; n, no. of littermates/group. Male double transgenic littermates (3 to 4 mo old) were maintained with doxycycline (control) or regular water [phosphatidylinositol 3-kinase (PI3K)α] for 2 wk before measurement.
P < 0.05. LVdP/dtmax and LVdP/dtmin, the maximal and minimal positive values of the first derivative of left ventricular (LV) pressure, respectively.
Two major distinct but related branches of cellular effect can be induced by the class I PI3K: one on cell growth and one on cell cycle (review see Ref. 4). One possible mechanism underlying the different phenotypes between the temporal and constitutively active overexpression of cardiac PI3Kα is that constitutively active activation may push the pathway toward favoring cell growth effect, leading eventually to cardiac hypertrophy. Hence the increase in contractility presents an early phenotype following the temporal activation of cardiac PI3Kα.
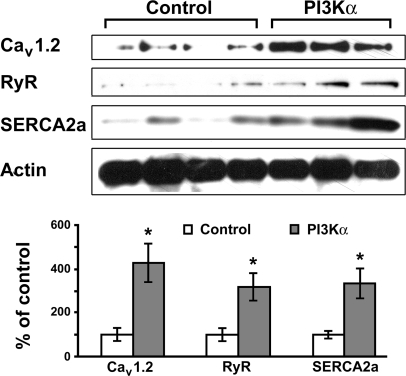
Ca2+ turnover is a well-recognized mechanism for the regulation of contractility, and the dysregulation of Ca2+ turnover often leads to heart failure (review Refs. 6 and 15). The regulation of Ca2+ turnover has been speculated as a possible mechanism underlying the change in contractility seen in transgenic mice with the overexpression of constitutively active Akt (nonnuclear targeted), but this has not been confirmed (1). To explore the mechanism(s) underlying the increase in contractility following the temporally controlled overexpression of PI3Kα, we examined the expression of several Ca2+-regulating proteins that are critical for Ca2+ homeostasis and myocardial function/adaptation (6, 15). We found that there were significant increases in the levels of RyR, Cav1.2, and SERCA2a, following the temporally controlled overexpression of cardiac-specific PI3Kα (Fig. 4). In cardiomyocytes, Cav1.2 is critical for Ca2+ influx and, along with RyR, is responsible for Ca2+-induced Ca2+ release during the systolic period. Ca2+ ions are actively transported back into the sarcoplasmic reticulum by SERCA2a or pumped out by the Na+-Ca2+ exchanger (NCX) during the diastole (6, 15). The upregulation of these proteins should favor improved contraction and relaxation in cardiomyocytes and may represent a mechanism underlying the increase in contractility following the temporal activation of cardiac PI3Kα. These data set the tone for the future study of other Ca2+-regulating proteins, e.g., NCX, phospholamban (PLB), calsequestrin (CSQ), and calreticulin. It is also crucial to examine the phosphorylation status of some of these proteins including CSQ, RyR, and PLB. To our knowledge, nevertheless, our data represent the first demonstration of the PI3Kα activation-induced induction of Ca2+-regulating proteins in cardiomyocytes. It will be interesting to investigate the downstream factors involved in the transduction of the PI3Kα cascade.
Fig. 4.
Temporal overexpression of cardiac-specific PI3Kα induces upregulation of Ca2+-regulating proteins. Three-month-old male double transgenic littermates were maintained with Dox water (control; n = 4) or regular drinking water (PI3Kα; n = 3) for 2 wk. Western blotting was performed using antibodies against L-type voltage-gated Ca2+ channel (Cav1.2), ryanodine receptor (RyR) type 2, and sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a; top). Actin levels were used as the loading control. Bottom: results of densitometric measurement of each antibody from 3 separate experiments. *P < 0.05 vs. control.
In summary, we have engineered a new conditional transgenic mouse line that allows the temporally-controlled overexpression of cardiac-specific PI3Kα. The temporal activation of cardiac PI3Kα in these animals does not cause cardiomyopathy or hypertrophy but induces an increase in contractility and the upregulation of Ca2+-regulating proteins. These changes may represent the early phenotypic characteristics following the activation of cardiac PI3Kα.
GRANTS
This work was supported by National Institutes of Health Grant 1-P20-RR018728.
Acknowledgments
We thank Drs. T. Franke and S. Izumo for the iSH2-p110α cDNA construct, L. C. Cantley and J. Luo for helpful insights in PI3K signaling, and Ulrike Mende for critical reading of the manuscript. We also thank Sandy Falcone for the excellent work in pronuclear injection.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J Jr. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA 99: 12333–12338, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 110: 737–749, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275: 665–668, 1997. [DOI] [PubMed] [Google Scholar]
- 4.García Z, Kumar A, Marqués M, Cortés I, Carrera AC. Phosphoinositide 3-kinase controls early and late events in mammalian cell division. EMBO J 25: 655–661, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science 287: 1049–1053, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda Y, Hoshijima M, Chien KR. Toward biologically targeted therapy of calcium cycling defects in heart failure. Physiology (Bethesda) 23: 6–16, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA 102: 802–807, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YK, Kim SJ, Yatani A, Huang Y, Castelli G, Vatner DE, Liu J, Zhang Q, Diaz G, Zieba R, Thaisz J, Drusco A, Croce C, Sadoshima J, Condorelli G, Vatner SF. Mechanism of enhanced cardiac function in mice with hypertrophy induced by overexpressed Akt. J Biol Chem 278: 47622–47628, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Maier U, Babich A, Nurnberg B. Roles of non-catalytic subunits in Gβγ-induced activation of class I phosphoinositide 3-kinase isoforms β and γ. J Biol Chem 274: 29311–29317, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 277: 22896–22901, 2002. [DOI] [PubMed] [Google Scholar]
- 11.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase (p110α) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA 100: 12355–12360, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murga C, Fukuhara S, Gutkind JS. A novel role for phosphatidylinositol 3-kinase β in signaling from G protein-coupled receptors to Akt. J Biol Chem 275: 12069–12073, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res 92: 609–616, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science 287: 1040–1046, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Seidler T, Hasenfuss G, Maier LS. Targeting altered calcium physiology in the heart: translational approaches to excitation, contraction, and transcription. Physiology (Bethesda) 22: 328–334, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J 19: 2537–2548, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol 22: 2799–2809, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115: 2108–2118, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res 94: 884–891, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Tseng YT, Yano N, Rojan A, Stabila JP, McGonnigal BG, Ianus V, Wadhawan R, Padbury JF. Ontogeny of phosphoinositide 3-kinase signaling in developing heart: effect of acute β-adrenergic stimulation. Am J Physiol Heart Circ Physiol 289: H1834–H1842, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70: 535–602, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Yano N, Ianus V, Zhao TC, Tseng A, Padbury JF, Tseng YT. A novel signaling pathway for β-adrenergic receptor-mediated activation of phosphoinositide 3-kinase in H9c2 cardiomyocytes. Am J Physiol Heart Circ Physiol 293: H385–H393, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Zhao TC, Cheng G, Zhang LX, Tseng YT, Padbury JF. Inhibition of histone deacetylases triggers pharmacologic preconditioning effects against myocardial ischemic injury. Cardiovasc Res 76: 473–481, 2007. [DOI] [PubMed] [Google Scholar]