Abstract
Oxidized low-density lipoprotein (LDL) is proatherogenic and induces smooth muscle cell apoptosis, which contributes to atherosclerotic plaque destabilization. We showed previously that oxidized LDL downregulates insulin-like growth factor-1 receptor in human smooth muscle cells and that this is critical for induction of apoptosis. To identify mechanisms, we exposed smooth muscle cells to 60 μg/ml oxidized LDL or native LDL and assessed insulin-like growth factor-1 receptor mRNA levels, protein synthesis rate, and receptor protein stability. Oxidized LDL decreased insulin-like growth factor-1 receptor mRNA levels by 30% at 8 h compared with native LDL, and this decrease was maintained for up to 20 h. However, insulin-like growth factor-1 receptor protein synthesis rate was not altered by oxidized LDL. Pulse-chase labeling experiments revealed that oxidized LDL reduced insulin-like growth factor-1 receptor protein half-life to 12.2 ± 1.7 h from 24.4 ± 4.7 h with native LDL. This destabilization of insulin-like growth factor-1 receptor protein was accompanied by enhanced receptor ubiquitination. Overexpression of dominant-negative Nedd4 prevented oxidized LDL-induced downregulation of insulin-like growth factor-1 receptor, suggesting that Nedd4 was the ubiquitin ligase that mediated receptor downregulation. However, the proteasome inhibitors lactacystin, MG-132, and proteasome inhibitor-1 failed to block oxidized LDL-induced downregulation of insulin-like growth factor-1 receptor. Thus oxidized LDL downregulates insulin-like growth factor-1 receptor by destabilizing the protein via Nedd4-enhanced ubiquitination, leading to degradation via a proteasome-independent pathway. This finding provides novel insights into oxidized LDL-triggered oxidant signaling and mechanisms of smooth muscle cell depletion that contribute to plaque destabilization and coronary events.
Keywords: atherosclerosis, smooth muscle, oxidative stress
destabilization of advanced atherosclerotic lesions is characterized by erosion or rupture of the plaque, thrombosis, and subsequent partial or complete blockade of blood flow. Advanced atherosclerotic lesions that are prone to erosion and rupture typically present irregular thickening of the arterial intima, inflammatory cell accumulation, extracellular lipid, and fibrous tissue deposition (1, 9, 18, 26). Although accumulation of vascular smooth muscle cells (SMCs) (18) resulting from their migration and proliferation is an important feature of developing atherosclerotic lesions, as the lesion formation progresses, there is significant loss of SMC number and integrity in the newly formed intima. As a consequence, the advanced atherosclerotic plaque becomes rich in lipid-laden foam cells and generally has a thin fibrous cap (1, 9, 26), which increases the risk of destabilization. Oxidatively modified low-density lipoprotein (oxLDL), which is taken up by scavenger receptors on multiple cell types in the vascular wall, including SMCs, is a critical mediator of atherosclerotic plaque development (23). oxLDL stimulates proliferation of vascular cells (2, 4, 29), but we (12) and others (3, 13, 15) have reported that oxLDL can also contribute to vascular cell death. Thus oxLDL colocalizes with the pro-apoptotic Bcl-2 family protein BAX and TUNEL-positive SMC in the intima (20), suggesting that oxLDL has a pivotal role in vascular cell death and subsequent destabilization of the plaque. In previous studies, we have found that oxLDL markedly downregulates insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) expression in SMC (21). Since IGF-1 is a potent survival factor for vascular SMCs (5, 30), we have proposed that the oxLDL downregulation of IGF-1R expression and signaling (12) promotes SMC death in atherosclerotic plaque (14, 15, 17). Indeed, we have found that forced expression of IGF-1R in human SMC can counteract the cell-death-inducing effect of oxLDL (16). In this study, we determined mechanisms whereby oxLDL reduces IGF-1R protein expression. Our findings indicate that oxLDL shortens the half-life of the IGF-1R through an ubiquitin-dependent but proteasome-independent pathway. These results have important implications for understanding the process of SMC death leading to atherosclerotic plaque destabilization.
METHODS
Materials.
Rabbit anti-human IGF-1R β-chain antibody was from Santa Cruz Biotechnology (Santa Cruz, CA); anti-human β-actin monoclonal antibody (clone AC-15) was from Sigma-Aldrich (St. Louis, MO); MG-132, lactacystin, and rabbit anti-ubiquitin antibody were from Chemicon; [α-32P]uridine 5′-triphosphate and 35S-labeled l-methionine/cysteine mixture solution were from Perkin Elmer (Wellesley, MA); N-acetyl-Leu-Leu-Nle-CHO (ALLN), calpain inhibitor III, PD150606, calpeptin, and (2S,3S)-trans-epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester (EST) were from EMD Chemicals (Gibbstown, NJ).
Cell culture.
Cultured human aortic SMCs (HASMC; Lonza, Basel, Switzerland) were grown in SmBM medium (Lonza) supplemented with 5% fetal calf serum, antibiotics, human recombinant epidermal growth factor, insulin, and human recombinant fibroblast growth factor. The cells were used for experiment at passages 4–10.
Preparation of nLDL and oxLDL.
Native LDL (nLDL) was separated from human plasma of healthy donors (purchased from The Blood Center, New Orleans, LA) by sodium bromide stepwise density gradient centrifugation and then dialyzed against PBS containing 0.25 mM EDTA to remove sodium bromide. oxLDL was prepared as previously described. Briefly, an aliquot of the nLDL fraction was passed through a 10DG desalting column (Bio-Rad) to remove EDTA, then the nLDL fraction (0.2 mg/ml, diluted in PBS) was incubated with 5 μM CuSO4 at 37°C for 3 h. The reaction was stopped by adding EDTA (final concentration 0.25 mM). The oxLDL prepared under these conditions showed an increase in relative mobility on agarose gel electrophoresis, and the value for thiobarbituric acid-reactive substances (TBARS) in oxLDL was 37.2 ± 1.2 nmol malondialdehyde/mg protein. TBARS was not detectable in nLDL.
RNase-protection assay and real-time PCR.
To determine the effects of oxLDL on IGF-1R mRNA levels, cultured human aortic SMCs were exposed to either oxLDL or nLDL (60 μg/ml) for various times. Total RNA was extracted from the cells using the TriPure isolation reagent (Roche) and was subjected to solution hybridization/RNase protection assays, which were performed using RPAIII Ribonuclease Protection Assay Kit (Ambion). In brief, 30 μg of total RNA was hybridized to a [32P]UTP-labeled antisense riboprobe generated by T3 polymerase transcription of a linearized plasmid containing partial sequence of the human IGF-1R cDNA (nt no. 1-295). RNA was co-hybridized using a β-actin riboprobe (nt no. 327-464). After RNase digestion, RNase in the reaction mixture was inactivated, and RNA was precipitated by isopropanol and analyzed on a denaturing polyacrylamide urea gel. Protected IGF-1R and β-actin mRNA fragments are 295 and 138 bp, respectively. Autoradiograms were exposed for 1–3 days on X-ray films and protected bands were quantitated densitometrically. In addition, IGF-1R mRNA levels were measured using real-time PCR with primers for IGF-1R and for β-actin, GAPDH, 18s rRNA, or β2-microglobulin as controls. Assays were performed as previously described (24).
Western blot analysis.
Cells were washed with PBS and lysed in RIPA buffer, containing 150 mM NaCl, 20 mM Tris-Cl, pH 7.2, 1 mM EDTA, 1% NP40, 5 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 0.1 M okadaic acid, 0.1 μM aprotinin, 10 μg/ml leupeptin, and 10 mM NaF. Lysates were subjected to 10% SDS-PAGE and Western blotting analysis. Immunopositive bands were visualized by enhanced chemiluminescence (ECL; Amersham). Blots were stripped and reprobed with monoclonal anti-β-actin antibody as a control for equal loading. For experiments to determine effects of proteasome or proteinase inhibitors, cells were exposed to lipoproteins in the presence or absence of 10 μM inhibitor before analysis via Western blot. Inhibitors were added 1 h before exposure to lipoproteins.
Pulse labeling and chase.
Cells were metabolically labeled by 16 h of incubation in serum-free DMEM/F-12 (1/1; GIBCO) containing [35S]methionine and [35S]cysteine (final 100 μCi/ml). After labeling, cells were washed three times in medium containing an excess of unlabeled Met/Cys and chased in serum-free DMEM/F-12 for indicated time in figure legends. Cell lysates were obtained with RIPA buffer, and subjected to immunoprecipitation/SDS-PAGE and detection as described below.
Assessing radio-label incorporation into newly synthesized IGF-1R.
IGF-1R protein synthesis rate was assessed by measuring [35S]methionine and [35S]cysteine incorporation into newly synthesized protein. Cells were washed and incubated with serum-free DMEM/F-12 plus 60 μg/ml oxLDL or nLDL. After 12 h of incubation, a mixture of [35S]methionine and [35S]cysteine (final 100 μCi/ml) was added into the culture, and the incubation was continued for an additional 2, 4, and 8 h. Cell lysates were collected at each time point and then were subjected to the immunoprecipitation-SDS-PAGE analysis to measure the incorporation of radioactivity into specific proteins.
Immunoprecipitation.
Supernatants of cell lysates after centrifugation at 12,000 g for 10 min at 4°C were incubated with anti-IGF-1R antibody or anti-β-actin antibody for 1 h with gentle rotation at 4°C. After addition of protein A/G-agarose (Santa Cruz Biotechnology), the incubation continued overnight at 4°C. The precipitates were washed with RIPA buffer for three times and then were suspended in Sample buffer (10% SDS, 2 M 2-mercaptoethanol, 20% sucrose, 0.015% bromophenol blue, 50 mM Tris, pH 6.8), followed by heating in boiling water for 5 min to extract precipitated proteins. Extracted proteins were resolved on 10% SDS-PAGE gels and detected by using FLA-3000 Bio-Imaging Analysis System (Fujifilm).
Plasmid and transfection.
CMV5-DnNedd4 plasmid DNA, which encodes dominant-negative Nedd4 (C854S), is a kind gift from Dr. Shin-Ichiro Takahashi (The University of Tokyo, Tokyo, Japan). The plasmid was introduced into cells by electroporation, using a Nucleofector Kit for primary SMCs in the Nucleofector device according to the manufacturer's instructions (Amaxa, Gaithersburg, MD). The cells were subjected to experiments 24 h after transfection.
RESULTS
oxLDL decreased IGF-1R mRNA levels but did not alter IGF-1R synthesis rate.
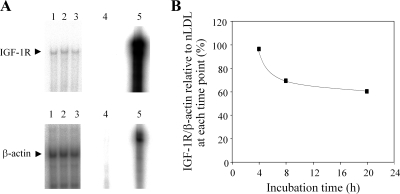
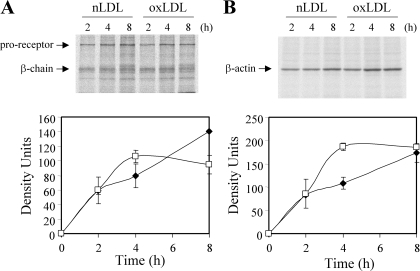
We confirmed that oxidatively modified LDL (oxLDL) downregulates IGF-1R protein levels in human aortic SMCs by 70–80% after 24 h of co-incubation (data not shown), as we have reported previously (12). To determine whether oxLDL modulates IGF-1R mRNA levels, we exposed SMCs to oxLDL or nLDL for 4–20 h and analyzed total RNA by solution hybridization-RNase protection assays. A small (∼30%) but significant decrease in IGF-1R mRNA levels was observed as early as 8 h after exposure to oxLDL (Fig. 1B), and this was sustained for up to 20 h. To confirm this result, we performed real-time PCR and found that oxLDL reduced IGF-1R by 53, 48, 38, and 43% with β-actin, 18s rRNA, β2-microglobulin, and GAPDH as internal controls at 20 h. To evaluate the significance of this decrease in mRNA levels in downregulation of IGF-1R protein, we assessed IGF-1R de novo synthesis. We determined the incorporation of radiolabeled methionine and cysteine into IGF-1R protein starting at 12 h up to 20 h of co-incubation with oxLDL, which is the time window corresponding to downregulation of IGF-1R by oxLDL (12). As shown in Fig. 2, radiolabel incorporation increased linearly for the initial 4 h, which corresponds to a time duration from 12 to 16 h of co-incubation with oxLDL. The incorporation rate was comparable between nLDL- and oxLDL-treated cells. It is noteworthy that radiolabel incorporation reached a plateau after 4 h of radiolabel coincubation in oxLDL-treated cells, although it increased linearly until 8 h or longer in nLDL-treated cells. We assume that the lower incorporation rate of radiolabel in oxLDL-treated cells after 4 h resulted from increased degradation of synthesized IGF-1R in oxLDL-treated cells. The results shown here clearly indicate that, although oxLDL decreases IGF-1R mRNA levels, there is no change in receptor synthesis rate. β-Actin synthesis rate as measured by radiolabel incorporation between 0 and 2 hs was also not different between nLDL- and oxLDL-treated cells (Fig. 2B).
Fig. 1.
Oxidatively modified low-density lipoprotein (oxLDL) decreased levels of insulin like growth factor-1 receptor (IGF-1R) mRNA in human aortic smooth muscle cells. Human aortic smooth muscle cells (SMCs) were cultured in serum-free medium and incubated with lipoproteins and IGF-1R, and β-actin mRNA levels were determined by solution hybridization and RNase-protection assay. A: representative RNase protection assay following 20 h of co-incubation with native LDL (nLDL) or oxLDL. Lane 1, serum-free; lane 2, 60 μg/ml nLDL; lane 3, 60 μg/ml oxLDL; lane 4, digested probe; lane 5, undigested probe. B: time course change in IGF-1R mRNA levels in oxLDL-treated cells expressed relative to the levels in nLDL-treated cells. This data represents similar results obtained from three independent experiments.
Fig. 2.
oxLDL did not alter IGF-1R synthesis rate. Human aortic SMCs were co-incubated with 60 μg/ml nLDL (▪) or oxLDL (□) in serum-free medium for a total of 20 h. At 12 h (corresponds to time 0 on the graphs), 35S-Met/Cys was added into the medium, and cell lysates were collected after 2, 4, and 8 h. IGF-1R (A) and β-actin (B) were immunoprecipitated and resolved by SDS-PAGE and autoradiography. This data represents similar results obtained from two independent experiments.
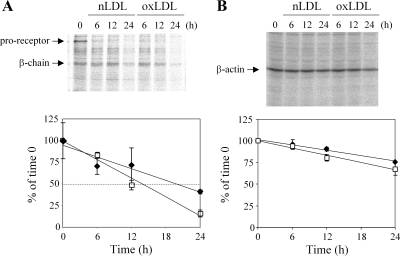
oxLDL shortens IGF-1R protein half-life.
To examine potential destabilization of IGF-1R protein by oxLDL, we performed metabolic label and chase analysis. The half-life of IGF-1R protein was 12.2 ± 1.7 and 24.4 ± 4.7 h in oxLDL- and nLDL-treated cells, respectively (Fig. 3; P = 0.025 by Student's t-test). β-Actin protein half-life was not altered significantly by oxLDL co-incubation (Fig. 3). This result indicates that oxLDL destabilizes IGF-1R protein in human SMC.
Fig. 3.
oxLDL shortened IGF-1R half-life in human aortic SMCs. SMCs were labeled with 35S-Met/Cys for 16 h, washed, and then chased for 24 h in serum-free medium with lipoproteins. Cell lysates were collected at 0, 6, 12, and 24 h of chase and subjected to immunoprecipitation for IGF-1R (A) and β-actin (B), followed by SDS-PAGE and autoradiography. The time-dependent change in radioactivity is expressed relative to the time 0 in nLDL (60 μg/ml; ▪) and oxLDL (60 μg/ml; □) treated cells. Graphs were averaged from four independent experiments.
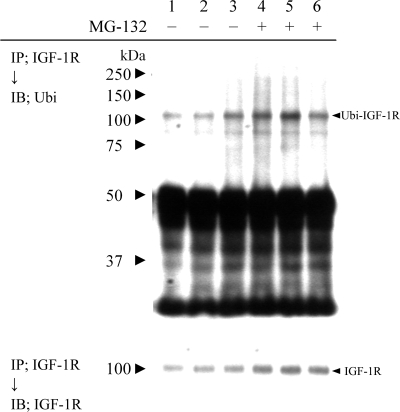
oxLDL enhances ubiquitination of the IGF-1R.
Because our data suggested that oxLDL may enhance degradation of the IGF-1R, we examined potential receptor ubiquitination, which reportedly contributes to the downregulation of IGF-1R induced by the lower availability of a tumor suppressor, p53 (8, 27), or by IGF-1-ligand binding (27). SMCs were exposed to lipoproteins for 6 h with or without the proteasome inhibitor MG-132, and IGF-1R was immunoprecipitated and associated ubiquitination was determined by Western blot analysis (Fig. 4). oxLDL caused an increase in ubiquitin-associated signals (Fig. 4, lane 3); intriguingly, MG-132 produced an increase in IGF-1R β-chain ubiquitination in nLDL- but not in oxLDL-treated cell lysates (Fig. 4, lanes 4–6). The lack of elevation in ubiquitinated IGF-1R level in oxLDL-treated cell lysates with MG-132 could be due to limited availability of monomer ubiquitin molecules for proteins with a long half-life, such as IGF-1R, or alternatively it may be caused by enhanced oxLDL-induced degradation of IGF-1R by a proteolytic activity other than the proteasome. In serum-free medium and nLDL-treated cells, the ubiquitinated IGF-1R appeared to be degraded by the proteasome, as suggested by the elevation in levels of ubiquitinated IGF-1R following exposure to the proteasome inhibitor MG-132 (Fig. 4, lanes 4 and 5). We reprobed the same membrane with anti-IGF-1R antibody (Fig. 4, bottom) and found no significant difference in IGF-1R levels between nLDL- and oxLDL-treated cell lysates, likely because the co-incubation time with oxLDL was too short to produce significant receptor downregulation. Interestingly, MG-132 increased IGF-1R levels (Fig. 4, bottom, compare lanes 1–3 and lanes 4–6), consistent with basal turnover of the IGF-1R being mediated by the ubiquitin-proteasome pathway. Taken together, our results indicate that the IGF-1R undergoes ubiquitination and that oxLDL increases IGF-1R ubiquitination. However, our results suggest that, contrary to the case with nLDL or serum-free treated cells, oxLDL induces ubiquitination of the IGF-1R, which is then degraded by a pathway that is not MG132 inhibitable and therefore likely proteasome-independent.
Fig. 4.
oxLDL increases IGF-1R ubiquitination. Human aortic SMCs were incubated in serum-free medium (lanes 1 and 4) or in serum-free medium with 60 ug/ml nLDL (lanes 2 and 5) or 60 μg/ml oxLDL (lanes 3 and 6), in the absence or presence of 10 μM MG-132 for 6 h. IGF-1R was immunoprecipitated and subjected to Western blot using anti-ubiquitin polyclonal antibody (top). Ubiquitin-associated IGF-1R signals are indicated by an arrowhead (Ubi-IGF-1R). The same blot was reprobed with anti-IGF-1R antibody (bottom).
The ubiquitin ligase Nedd4 mediates oxLDL-induced IGF-1R ubiquitination.
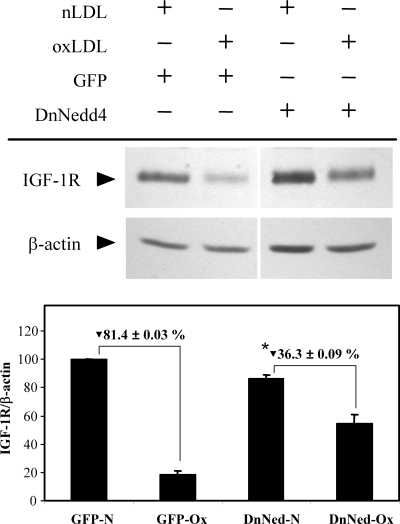
To determine the role of oxLDL-induced ubiquitination in the downregulation of IGF-1R, we transfected SMCs with an expression plasmid for a dominant negative form of Nedd4 (DnNedd4), which has a single amino acid substitution at Cys-854 to a serine residue to eliminate its enzymatic activity (7) and exposed the cells to oxLDL. Nedd4 is a ubiquitin ligase that has been reported to mediate ligand-induced downregulation of IGF-1R in mouse embryo fibroblasts (27). The transfection efficiency was ∼50–60% based on green fluorescent protein expression, which was transfected simultaneously (data not shown). Western blot analysis indicated that exposure to oxLDL for 24 h resulted in an 80% decrease in IGF-1R, and this decrease was markedly inhibited by DnNedd4 (Fig. 5; 55% inhibition), indicating involvement of Nedd4 in IGF-1R downregulation.
Fig. 5.
Dominant-negative Nedd4 overexpression attenuated oxLDL downregulation of IGF-1R. Human aortic SMCs were transfected with GFP- or dominant-negative Nedd4-expression plasmid and exposed to nLDL or oxLDL (60 μg/ml) for 24 h, followed by Western blot analysis. The graph indicates relative IGF-1R levels normalized to β-actin levels. GFP-N, GFP-expressing and nLDL treated; GFP-Ox, GFP-expressing and oxLDL treated; DnNed-N, dominant-negative Nedd4-expressing and nLDL treated; DnNed-Ox, dominant-negative Nedd4-expressing and oxLDL treated. *Dominant-negative Nedd4 inhibition of oxLDL induced IGF-1R downregulation is statistically significant (P < 0.01, n = 4).
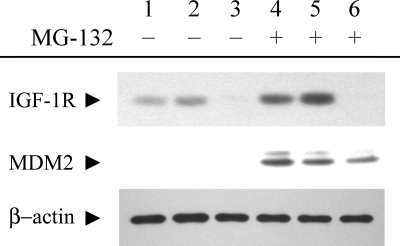
oxLDL-induced IGF-1R degradation is mediated by a proteasome-independent pathway.
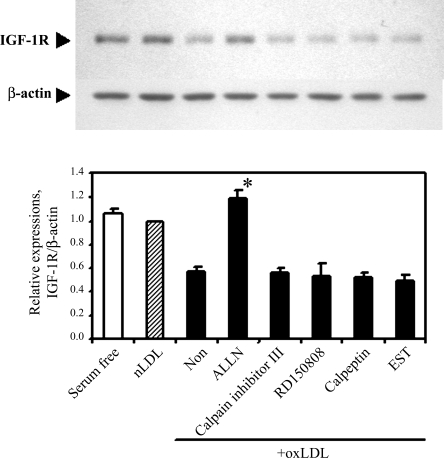
To determine whether the ubiquitination-dependent proteasomal degradation is involved in oxLDL-induced IGF-1R downregulation, cells were co-incubated with lipoproteins and MG-132 for 24 h, and IGF-1R expression was determined by Western blotting. MG-132 increased levels of MDM2 protein, a known substrate for the ubiquitin-proteasomal degradation pathway, indicating that MG-132 effectively blocked proteasome activity (Fig. 6). However, oxLDL-induced downregulation of IGF-1R was not prevented by MG-132 (Fig. 6). Other proteasome inhibitors, namely lactacystin and proteasome inhibitor-1, also failed to prevent oxLDL-induced downregulation of IGF-1R (data not shown). We tested other protease inhibitors to characterize the oxLDL-induced degradation pathway for IGF-1R (Fig. 7). Three of the tested inhibitors (calpain inhibitor III, PD150606, and calpeptin) inhibit the calpain-dependent pathway, and the other two (ALLN and EST) possess a range of inhibitory specificities toward proteolytic enzymes, including calpain, lysosomal cathepsins, and the proteasome (ALLN). Among those tested, only ALLN blocked oxLDL downregulation of IGF-1R (Fig. 7).
Fig. 6.
The proteasome inhibitor MG-132 did not block oxLDL downregulation of IGF-1R. Human aortic SMCs were incubated in serum-free medium (lanes 1 and 4) or in serum-free medium with 60 μg/ml nLDL (lanes 2 and 5) or oxLDL (lanes 3 and 6), in the presence or absence of 10 μM MG-132, and lysates were subjected to Western blot analysis.
Fig. 7.
The effect of proteinase inhibitors on oxLDL downregulation of IGF-1R. Human aortic SMCs were exposed to native LDL (60 μg/ml, shaded column) and oxLDL (60 μg/ml, filled column) for 24 h with the indicated proteinase inhibitors (10 μM). IGF-1R levels were determined by Western blot analysis, and relative expression levels are summarized from three independent experiments to illustrate means ± SE for each group. *P < 0.01 vs. oxLDL co-incubated with no proteinase inhibitor (Non; n = 3) by Student's t-test.
DISCUSSION
Studies from our laboratory have shown that there is decreased expression of IGF-1R in SMCs or smooth muscle-derived cells in neointima of atherosclerotic plaque (20) and that downregulation of IGF-1R co-localizes with presence of oxLDL (19). Futhermore, we have shown that oxLDL decreases IGF-1R in cultured human SMC (12). In this study, we investigated potential mechanisms for oxLDL downregulation of IGF-1R. We found that oxLDL caused a decrease in IGF-1R mRNA levels by ∼30% after 8 h of exposure (Fig. 1). Despite the apparent decrease in IGF-1R mRNA, direct assessment of IGF-1R protein synthesis rate by measuring 35S-Met/Cys incorporation indicated that there was no obvious decrease in IGF-1R protein synthesis (Fig. 2). The oxLDL-induced decrease in mRNA could have been compensated for by other mechanisms; as we reported previously (12), oxLDL induces generation of reactive oxygen species, which can stimulate multiple signaling pathways, including those upstream of the translational machinery, leading to phosphorylation on eukaryotic initiation factor 4E to enhance its activity(6, 28). Thus it is possible that the small decrease in IGF-1R mRNA levels induced by oxLDL was compensated for by increased translational activity.
Since changes in IGF-1R mRNA levels were unlikely to account for the receptor protein downregulation, we examined the half-life of IGF-1R protein and found that it was reduced from 24.4 ± 4.7 to 12.2 ± 1.7 h by oxLDL, suggesting that destabilization of IGF-1R protein is the primary mechanism for the downregulation by oxLDL. The turnover of proteins expressed on the plasma membrane results from highly regulated processes, such as internalization, targeting to endosomes/lysosomes, and sorting for either recycling or degradation. It has been shown that, for some membrane proteins, such as ErbB, ubiquitination is a key regulatory step for their turnover (reviewed in Ref. 25); in fact, it has also been suggested that ubiquitination is an important event for IGF-1R downregulation in response to reduced p53 expression (8), ligand-binding (27), and Herbimycin A (8, 22, 27). Thus we addressed potential involvement of the ubiquitin-proteasome system in oxLDL downregulation of IGF-1R in human SMC and found that the IGF-1R is a substrate for and, moreover, oxLDL enhances conjugation with ubiquitin (Fig. 4). A well documented consequence of ubiquitination on proteins is the degradation of the particular protein by the proteasome, and this is the case with IGF-1R expressed in human aortic SMCs, at least in serum-free and nLDL co-incubated conditions; thus MG-132 increased IGF-1R-ubiquitin conjugates (Fig. 4, top), demonstrating that IGF-1R is a substrate for ubiquitination, and also increased levels of IGF-1R protein after 24 h (Fig. 4, bottom), consistent with proteasome-dependent degradation. The importance of ubiquitination for oxLDL downregulation of IGF-1R is supported by the results obtained using dominant-negative Nedd4 overexpression (Fig. 5). Nedd4 is an ubiquitin ligase that mediates IGF-1R ubiquitination on ligand binding, leading to receptor downregulation (27). Our results indicate that dominant negative Nedd4 overexpression markedly attenuates oxLDL downregulation of IGF-1R (Fig. 5). It is likely that the incomplete inhibition resulted from the transfection efficiency of dominant-negative Nedd4 expression plasmid being 50–60%. However, MG-132 and other proteasome inhibitors (lactacystin, proteasome inhibitor 1) failed to prevent oxLDL downregulation of IGF-1R (Fig. 6), indicating that oxLDL-induced degradation of IGF-1R is not mediated by proteasome activity.
Little is known about cellular mechanisms of IGF-1R turnover and in particular degradation. Girnita et al. (8) showed that decreased expression of tumor suppressor p53 protein causes ubiquitination and subsequent degradation of the IGF-1R via the proteasome. Sepp-Lorenzino et al. (22) reported that IGF-1R downregulation induced by Herbimycin A is mediated by the ubiquitin-proteasome system. Vecchinoe et al. (27) demonstrated that IGF-1 binding causes IGF-1R-ubiquitination and subsequent proteolysis via both the proteasomal and lysosomal pathways. Our results suggest that, in human aortic SMCs, ubiquitination and subsequent degradation by the proteasome contribute to basal turnover of IGF-1R protein. However, whereas ubiquitination contributes to the oxLDL downregulation of IGF-1R, proteasome involvement is unlikely. Rather, oxLDL-induced IGF-1R protein degradation is likely mediated via a proteasome-independent pathway. Candidates include the lysosomal pathway and calpain-dependent pathway. We examined bafilomycin A1 and chloroquine as lysosomal pathway inhibitors; however, these agents were toxic after prolonged (18–24 h) cell culture periods, which is the time frame required to observe IGF-1R downregulation; thus we were unable to determine a potential involvement of the lysosomal pathway. We also screened calpain-dependent proteolysis inhibitors (calpain inhibitor III, PD150606, and calpeptin) and cysteine protease inhibitors (ALLN and EST). Only ALLN effectively blocked oxLDL-induced IGF-1R downregulation. ALLN is known to inhibit calpains I and II, cathepsins B and L, and even the proteasome. Considering the ineffectiveness of other inhibitors for calpain and the proteasome, it is likely that ALLN is effective by inhibiting lysosomal protease activity, such as cathepsins B and L. The mechanism we have suggested for IGF-1R downregulation by oxLDL involves ubiquitination followed by proteasome-independent degradation. This implicates a functional role of protein ubiquitination other than targeting the particular protein to the proteasome-system (11). An analogous mechanism has been reported for ligand-induced degradation of EGF receptors, namely that the protein ubiquitination leads to lysosomal degradation (10).
In this study, we have shown that oxLDL destabilizes IGF-1R protein via a proteasome-independent pathway that requires the ubiquitin ligase Nedd4. IGF-1R is critical for prevention of SMC death caused by oxLDL in cell culture (12, 16) and in vivo (19, 20, 24), and oxLDL downregulation of IGF-1R is one of the foremost causes of cell death induced by oxLDL. Prevention of Nedd4-mediated IGF-1R ubiquitination and downregulation offers a potential therapeutic target to limit apoptotic cell death and subsequent destabilization of atherosclerotic plaques.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-070241 and HL-080682 and a Research Enhancement Fund from Tulane University (P. Delafontaine).
Acknowledgments
The authors acknowledge Dr. Shin-Ichiro Takahashi for providing expression-plasmid encoding dominant-negative Nedd4.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arroyo LH, Lee RT. Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res 41: 369–375, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Auge N, Garcia V, Maupas-Schwalm F, Levade T, Salvayre R, Negre-Salvayre A. Oxidized LDL-induced smooth muscle cell proliferation involves the EGF receptor/PI-3 kinase/Akt and the sphingolipid signaling pathways. Arterioscler Thromb Vasc Biol 22: 1990–1995, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter KL, Challis IR, Arends MJ. Mildly oxidised LDL induces more macrophage death than moderately oxidised LDL: roles of peroxidation, lipoprotein-associated phospholipase A2 and PPARgamma. FEBS Lett 553: 145–150, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Chai YC, Binion DG, Macklis R, Chisolm GM, 3rd. Smooth muscle cell proliferation induced by oxidized LDL-borne lysophosphatidylcholine. Evidence for FGF-2 release from cells not extracellular matrix. Vascul Pharmacol 38: 229–237, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci USA 94: 4772–4777, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan RF, Peterson H, Hagedorn CH, Sevanian A. Oxidative stress increases eukaryotic initiation factor 4E phosphorylation in vascular cells. Biochem J 369: 213–225, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukushima T, Hakuno F, Nagata S, Takahashi SI. Nedd4 associates with insulin receptor substrates (IRSs) leading to enhancement of IGF signals with its ubiquitin ligase activity. In: The Third International Congress of the GRS and the IGF Society, Kobe, Japan. Growth Hormone & IGF Research, 2006, p. S5.
- 8.Girnita L, Girnita A, Larsson O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA 100: 8247–8252, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutstein DE, Fuster V. Pathophysiology and clinical significance of atherosclerotic plaque rupture. Cardiovasc Res 41: 323–333, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Haglund K, Shimokawa N, Szymkiewicz I, Dikic I. Cbl-directed monoubiquitination of CIN85 is involved in regulation of ligand-induced degradation of EGF receptors. Proc Natl Acad Sci USA 99: 12191–12196, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann J, Lerman LO, Lerman A. Ubiquitin and ubiquitin-like proteins in protein regulation. Circ Res 100: 1276–1291, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Higashi Y, Peng T, Du J, Sukhanov S, Li Y, Itabe H, Parthasarathy S, Delafontaine P. A redox-sensitive pathway mediates oxidized LDL-induced downregulation of insulin-like growth factor-1 receptor. J Lipid Res 46: 1266–1277, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh CC, Yen MH, Yen CH, Lau YT. Oxidized low density lipoprotein induces apoptosis via generation of reactive oxygen species in vascular smooth muscle cells. Cardiovasc Res 49: 135–145, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Jing Q, Xin SM, Cheng ZJ, Zhang WB, Zhang R, Qin YW, Pei G. Activation of p38 mitogen-activated protein kinase by oxidized LDL in vascular smooth muscle cells: mediation via pertussis toxin-sensitive G proteins and association with oxidized LDL-induced cytotoxicity. Circ Res 84: 831–839, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Lee T, Chau L. Fas/Fas ligand-mediated death pathway is involved in oxLDL-induced apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol 280: C709–C718, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Higashi Y, Itabe H, Song YH, Du J, Delafontaine P. Insulin-like growth factor-1 receptor activation inhibits oxidized LDL-induced cytochrome C release and apoptosis via the phosphatidylinositol 3 kinase/Akt signaling pathway. Arterioscler Thromb Vasc Biol 23: 2178–2184, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Napoli C, Quehenberger O, De Nigris F, Abete P, Glass CK, Palinski W. Mildly oxidized low density lipoprotein activates multiple apoptotic signaling pathways in human coronary cells. FASEB J 14: 1996–2007, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Newby AC, Zaltsman AB. Fibrous cap formation or destruction: the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res 41: 345–360, 1999. [PubMed] [Google Scholar]
- 19.Okura Y, Brink M, Itabe H, Scheidegger KJ, Kalangos A, Delafontaine P. Oxidized low-density lipoprotein is associated with apoptosis of vascular smooth muscle cells in human atherosclerotic plaques. Circulation 102: 2680–2686, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Okura Y, Brink M, Zahid AA, Anwar A, Delafontaine P. Decreased expression of insulin-like growth factor-1 and apoptosis of vascular smooth muscle cells in human atherosclerotic plaque. J Mol Cell Cardiol 33: 1777–1789, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Scheidegger KJ, James RW, Delafontaine P. Differential effects of low density lipoproteins on insulin-like growth factor-1 (IGF-1) and IGF-1 receptor expression in vascular smooth muscle cells. J Biol Chem 275: 26864–26869, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Sepp-Lorenzino L, Ma Z, Lebwohl DE, Vinitsky A, Rosen N. Herbimycin A induces the 20S proteasome- and ubiquitin-dependent degradation of receptor tyrosine kinases. J Biol Chem 270: 16580–16587, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev 84: 1381–1478, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 27: 2684–2690, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney C, Carraway KL 3rd. Negative regulation of ErbB family receptor tyrosine kinases. Br J Cancer 90: 289–293, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Wal AC, Becker AE. Atherosclerotic plaque rupture–pathologic basis of plaque stability and instability. Cardiovasc Res 41: 334–344, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Vecchione A, Marchese A, Henry P, Rotin D, Morrione A. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol Cell Biol 23: 3363–3372, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Flynn A, Waskiewicz AJ, Webb BL, Vries RG, Baines IA, Cooper JA, Proud CG. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J Biol Chem 273: 9373–9377, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Zettler ME, Prociuk MA, Austria JA, Massaeli H, Zhong G, Pierce GN. oxLDL stimulates cell proliferation through a general induction of cell cycle proteins. Am J Physiol Heart Circ Physiol 284: H644–H653, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Zheng WH, Kar S, Quirion R. Insulin-like growth factor-1-induced phosphorylation of the forkhead family transcription factor FKHRL1 is mediated by Akt kinase in PC12 cells. J Biol Chem 275: 39152–39158, 2000. [DOI] [PubMed] [Google Scholar]