Abstract
The exercise pressor reflex arises from contracting skeletal muscle and is believed to play a role in evoking the cardiovascular responses to static exercise, effects that include increases in arterial pressure and heart rate. This reflex is believed to be evoked by the metabolic and mechanical stimulation of thin fiber muscle afferents. Lactic acid is known to be an important metabolic stimulus evoking the reflex. Until recently, the only antagonist for acid-sensitive ion channels (ASICs), the receptors to lactic acid, was amiloride, a substance that is also a potent antagonist for both epithelial sodium channels as well as voltage-gated sodium channels. Recently, a second compound, A-317567, has been shown to be an effective and selective antagonist to ASICs in vitro. Consequently, we measured the pressor responses to the static contraction of the triceps surae muscles in decerebrate cats before and after a popliteal arterial injection of A-317567 (10 mM solution; 0.5 ml). We found that this ASIC antagonist significantly attenuated by half (P < 0.05) the pressor responses to both contraction and to lactic acid injection into the popliteal artery. In contrast, A-317567 had no effect on the pressor responses to tendon stretch, a pure mechanical stimulus, and to a popliteal arterial injection of capsaicin, which stimulated transient receptor potential vanilloid type 1 channels. We conclude that ASICs on thin fiber muscle afferents play a substantial role in evoking the metabolic component of the exercise pressor reflex.
Keywords: groups III and IV muscle afferents, cats, neural control of the circulation, lactic acid, mechanogated channels, transient receptor potential vanilloid type 1 channels
the exercise pressor reflex arising from contracting skeletal muscles is one of the mechanisms responsible for the cardiovascular adjustments to static exercise (5, 20). These reflex adjustments include increases in arterial blood pressure, heart rate, and ventilation (22). The afferent arm of the exercise pressor reflex is composed of groups III and IV muscle afferents (20, 22) and is thought to be evoked by both mechanical and metabolic stimuli within the contracting muscles (14–16).
One of the metabolic by-products of muscle contraction that is thought to play a role in evoking the metabolic component of the exercise pressor reflex is lactic acid. Several lines of evidence suggest that lactic acid plays such a role. The most direct evidence is that lactic acid injected into the arterial supply of muscle stimulates groups III and IV afferents as well as evokes a reflex pressor response (25–27). Another line of evidence is that lactic acid concentrations in the muscle interstitium are increased by contraction (19). Finally, blunting lactic acid production with either dichloroacetate or glycogen depletion decreases the exercise pressor reflex (9, 28).
An important requirement of showing that a substance plays a role in evoking the metabolic component of the exercise pressor reflex is to demonstrate that this component is attenuated by the blockade of its receptor on groups III and IV muscle afferents. Recently, this demonstration was done using amiloride (4, 12). However, amiloride has many effects in addition to blocking the receptor for lactic acid. Two of the most troublesome are that amiloride blocks epithelial sodium channels (ENaCs) in lower concentrations than those needed to block acid-sensitive ion channels (ASICs) and that it blocks voltage-gated sodium channels in concentrations possibly higher than those needed to block ASICs. Blocking either ENaCs or voltage-gated sodium channels would interfere with the ability of the afferent to generate action potentials, thereby preventing it from evoking reflex effects regardless of the stimulus used. Because either of these effects could occur using amiloride, we are still uncertain whether ASICs play a role in evoking the reflex. To determine whether ASICs play a role in evoking the exercise pressor reflex, we used A-317567 to block this receptor to lactic acid on sensory nerve endings in skeletal muscle. A-317567 has been reported to have no effect on ENaCs (8), and in the doses that we used, it did not prevent reflex responses to stimuli other than lactic acid.
METHODS
All procedures were reviewed and approved by the Institutional Care and Use Committee of the Pennsylvania State University, Hershey Medical Center.
Surgical preparation.
Adult cats of either sex (n = 12; 2.9 ± 0.2 kg; range, 2.3–3.2 kg) were anesthetized with a mixture of 5% isoflurane and oxygen. The right jugular vein and common carotid artery were cannulated for the delivery of drugs and fluids and the measurement of arterial blood pressure, respectively. The carotid arterial catheter was connected to a pressure transducer (model P23 XL; Statham) to monitor blood pressure. Heart rate was calculated beat to beat from the arterial pressure pulse (Gould Biotech). The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus). Arterial blood gases and pH were measured by an automated blood gas analyzer (model ABL-700; Radiometer). PCO2 and arterial pH were maintained within normal range by adjusting either ventilation or the intravenous administration of sodium bicarbonate (8.5%). A temperature probe was passed through the mouth to the stomach. Temperature was continuously monitored and maintained at 37° to 38°C by a water-perfused heating pad.
The left common iliac artery and vein were isolated, and snares were placed around these vessels to trap the A-317567 in the circulation of the leg (see Experimental Protocol). The left triceps surae muscles, left popliteal artery, and tibial nerve were isolated. The cat was placed in a Kopf stereotaxic frame and spinal unit. The left calcaneal bone was cut, and its tendon was attached to a force transducer (model FT-10C; Grass) for the measurement of the tension developed during stretch and the static contraction of the left triceps surae muscles. The knee joint was secured to a post.
The cats were decerebrated at the midcollicular level under isoflurane anesthesia. Dexamethasone (4 mg) was injected intravenously just before the decerebration procedure to minimize brain edema. The left common carotid artery was tied off to reduce bleeding. All neural tissue rostral to the midcollicular section was removed, and the cranial vault was filled with agar.
Experimental protocol.
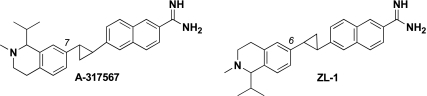
The effect of A-317567 (10 mM solution; 0.5 ml) and its structural analog ZL-1 (10 mM solution; 0.5 ml; Fig. 1) on the reflex pressor and cardioaccelerator responses to the following four stimuli was assessed: 1) static contraction of the left triceps surae muscles; 2) stretch of the left calcaneal tendon, which in turn stretched the triceps surae muscles; 3) injection of lactic acid (24 mM; 0.5–1.0 ml) into the left popliteal artery; and 4) injection of capsaicin (2 μg) into the left popliteal artery. In a 3-kg cat, the amount of A-317567 injected into the popliteal artery corresponded to a dose of about 1.7 μm/Kg. Contraction and tendon stretch lasted 60 s. The triceps surae muscles were contracted statically by electrical stimulating the left tibial nerve (40 Hz; 25 μs; 2× motor threshold); stretch was induced by turning a rack-and-pinion that was attached to the calcaneal tendon. Baseline tension was set at 0.3–0.5 kg. Arterial blood pressure and heart rate were recorded for 60 s before and during static contraction or tendon stretch. Injections of lactic acid and capsaicin were accomplished by gently inserting a 30-gauge needle into the popliteal artery and then injecting the compounds over ∼10 s into the vasculature of the triceps surae muscles. Before injecting lactic acid into the popliteal artery, we paralyzed the cat by intravenously injecting rocuronium bromide (0.5–0.7 mg/kg). These maneuvers were repeated at 10 and 45 min after the injection of A-317567 into the left popliteal artery. Immediately before injecting A-317567, we tightened the snare placed around the left common iliac artery and vein. A-317567 was then injected into the popliteal artery, and the snares were maintained for 10 min, after which they were released and the hind limb was freely perfused. At this point, the triceps surae muscles were contracted followed by stretch and an injection of lactic acid. A-317567 attenuated the exercise pressor response by 10 min and was no longer effective 45 min after its injection.
Fig. 1.
Two compounds, A-317567 and its structural analog ZL-1, were used in the present study. The 2 compounds are structurally identical except for their connectivity about their respective tetrahydroisoquinoline rings. As illustrated, the cyclopropane of A-317567 is attached to position 7 of the isoquinoline, whereas the cyclopropane in ZL-1 is connected at position 6. Both compounds were thoroughly characterized, and their structures were assigned using a combination of chemical intuition and spectroscopy. For the purposes of this work, A-317567 and ZL-1 behaved identically.
Data analysis.
Mean arterial blood pressure and heart rate values are expressed as means ± SE. Baseline mean arterial blood pressure and heart rate were measured immediately before a maneuver, and peak mean arterial blood pressure and heart rate were measured during the injection of lactic acid and capsaicin as well as during the 60 s of tendon stretch or static muscle contraction. For statistical analyses, data from experiments using A-317567 and ZL-1 were pooled. Statistical comparisons were performed either with a one-way or two-way repeated-measures ANOVA. If significant main effects were found with an ANOVA, then post hoc tests were performed with the Student-Neuman-Keuls test between individual means. We used Bonferroni and the Dunnett's post hoc tests to determine significant differences between time course means. The criterion for statistical significance was P < 0.05.
RESULTS
We examined the effects of injecting A-317567 and ZL-1 (both 0.5 ml; 10 mM) into the popliteal artery on the pressor-cardioaccelerator responses to four stimuli, namely static contraction of the triceps surae muscles, stretch of the calcaneal tendon, which in turn stretched the triceps surae muscles, lactic acid injection (0.5 ml; 24 mM) into the popliteal artery, and capsaicin (0.5 ml; 2 μg) into the popliteal artery. The blockade of ASICs by A-317567 or ZL-1 was short lasting (about 15 min), and therefore not every stimulus was tested in every cat. Because A-317567 and ZL-1 produced similar effects on the cardiovascular responses to each maneuver, the data were pooled. The 10 mM concentration of A-317567 or ZL-1 was used because in three cats a 10-fold smaller concentration (i.e., 1 mM) in 0.5 ml had no effect on the pressor response to either lactic acid injection or static contraction of the triceps surae muscles. For example, the pressor responses to lactic acid injection before and 10 and 45 min after A-317567 averaged 32 ± 6 mmHg, 30 ± 6 mmHg, and 35 ± 5 mmHg, respectively (n = 3). These low concentration injections also acted as vehicle controls because the same volume of the vehicle (0.5 ml) was injected as with the larger (10 mM) doses.
Lactic acid injection.
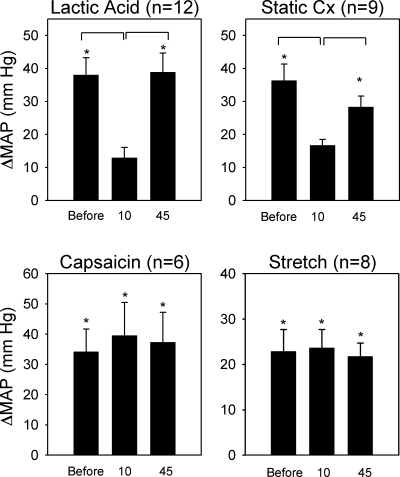
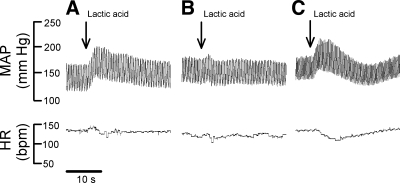
In each of the 12 cats tested, we found that A-317567 (n = 5) and ZL-1 (n = 7) attenuated the pressor response to lactic acid injection into the popliteal artery. On average, the pressor responses were decreased by almost 75% by the ASIC antagonist (P < 0.05). The interval between the injection of A-317567 and the second lactic acid injection was 10 min. On average, the pressor response to lactic acid was fully restored 45 min after the injection of the ASIC antagonist (Figs. 2 and 3 and Table 1). The cardioaccelerator responses to lactic acid injection were small and variable and were not significantly decreased by A-317567 (Table 1).
Fig. 2.
Effects of A-317567 on the pressor responses to arterial injection of lactic acid (0.2–0.5 ml; 24 mM), static contraction (Static Cx), arterial injection of capsaicin (1 to 2 μg; 0.25 ml), and stretch of the calcaneal tendon. Note each maneuver was performed before giving A-317567 and 10 and 45 min after giving A-317567. Bars represent changes in mean arterial pressure (MAP) above baseline. Horizontal brackets signify significant difference (P < 0.05) between pressor response before injection and that either 10 or 45 min after giving A-317567. Vertical brackets signify standard errors. *P < 0.05, significant difference between baseline and its corresponding peak effect.
Fig. 3.
Pressor (MAP) and heart rate (HR) responses to popliteal arterial injections of lactic acid (0.2–0.5 ml; 24 mM). A: before giving A-317567. B: 10 min after giving A-317567. C: 45 min after giving A-317567. Vertical arrow represents the beginning of the injection. Horizontal bar indicates 10 s. bpm, Beats/min.
Table 1.
Effects of A-317567 injected into the popliteal artery on the cardioaccelerator and pressor responses to lactic acid, static contraction, capsaicin, and tendon stretch
| n |
Heart Rate |
Mean Arterial Blood Pressure
|
|||
|---|---|---|---|---|---|
| Change | Baseline | Change | Baseline | ||
| Lactic acid | 12 | ||||
| Before | 11±4‡ | 145±14 | 38±5‡ | 121±7 | |
| 10 Min | 0±2† | 157±10 | 13±3† | 133±9 | |
| 45 Min | 7±2* | 163±11 | 39±6‡ | 131±9 | |
| Static contraction | 9 | ||||
| Before | 10±3‡ | 129±17 | 36±5‡ | 122±12 | |
| 10 Min | 4±2* | 148±14 | 17±2† | 134±11 | |
| 45 Min | 10±2‡ | 140±13 | 28±3‡ | 132±11 | |
| Capsaicin | 6 | ||||
| Before | 15±10 | 149±19 | 34±8 | 122±6 | |
| 10 Min | 8±3 | 164±13 | 40±11 | 129±8 | |
| 45 Min | 10±9 | 174±11 | 37±10 | 132±11 | |
| Tendon stretch | 8 | ||||
| Before | 5±2* | 142±21 | 23±5‡ | 117±13 | |
| 10 Min | 3±1 | 153±17 | 24±4‡ | 124±11 | |
| 45 Min | 4±2* | 156±17 | 22±3‡ | 126±10 | |
Values are means ± SE; n, no. cats/group.
P < 0.05 and
P < 0.01, significance from corresponding baseline value;
P < 0.05, significance from value before A-317567 injection.
Capsaicin injection.
In six cats, we found that A-317567 (n = 3) and ZL-1 (n = 3) had no effect on the pressor-cardioaccelerator responses to the capsaicin injection into the popliteal artery (Fig. 2).
Tendon stretch.
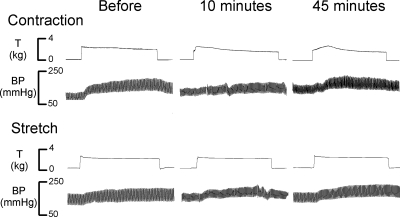
In eight cats, we found that A-317567 (n = 3) and ZL-1 (n = 5) had no effect on the pressor-cardioaccelerator responses to stretching the calcaneal tendon (Figs. 2 and 4 and Table 1). The ASIC blockers had no effect on either the magnitude of the pressor response or its onset latency. Specifically, the increase in blood pressure from the onset of tension development had a latency of 4.2 ± 1.5 s before A-317567 and 4.0 ± 1.5 s after A-317567 (P > 0.05; n = 8). The tension time indexes calculated before and 10 and 45 min after the injection of A-317567 were not significantly different from each other (P > 0.05; Table 2).
Fig. 4.
Pressor responses to static contraction and stretch of the calcaneal tendon before giving A-317567 and 10 and 45 min after giving A-317567. Note the top trace is muscle tension (T) and the lower trace is arterial blood pressure (BP).
Table 2.
The tension time indexes for static contraction and tendon stretch during reflex experiments
| Tendon Stretch | Static Contraction | |
|---|---|---|
| Before A-317567 | 146±16 | 143±17 |
| 10 Min | 165±18 | 135±13 |
| 45 Min | 163±14 | 119±9 |
Values are means ± SE. There were no significant differences (P > 0.05) between corresponding means before and 10 or 45 min after A-317567 injections (10 mM ia). All values are expressed in kilogram seconds.
Static contraction.
In each of nine cats tested, we found that A-317567 (n = 4) and ZL-1 (n = 5) attenuated the peak pressor response to static contraction, decreasing it on average by about 60% (P < 0.05). The interval between the injection of A-317567 and the second static contraction was 10 min. On average, the pressor response to static contraction was fully restored 45 min after the injection of the ASIC antagonist (Figs. 2 and 4 and Table 1). The cardioaccelerator responses to static contraction were small and variable and were not significantly decreased by A-317567 (Table 1).
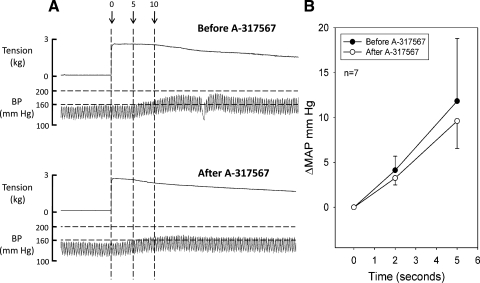
The ASIC blockers did not decrease the magnitude of the pressor response within the first 5 s of contraction. For example, at 2 s after the onset of tension development, the mean pressor response was 4.1 ± 1.6 mmHg before ASIC blockade and 3.3 ± 0.8 s afterward. Likewise, at 5 s after the onset of tension development, the mean pressor response was 11.8 ± 2.8 mmHg before ASIC blockade and 9.6 ± 3.1 mmHg afterward (Fig. 5, A and B). The ASIC blockers did not change the onset latency of the pressor response (Fig. 5A). Specifically, the increase in blood pressure from its onset of tension development had a latency of 1.9 ± 0.4 s before A-317567 and 2.1 ± 0.3 s after A-317567 (P > 0.05; n = 8). The tension time indexes calculated before and 10 and 45 min after the injection of A-317567 were not significantly different from each other (P > 0.05; Table 2).
Fig. 5.
Pressor responses to static contraction at the onset of tension development before and 10 min after acid-sensitive ion channel (ASIC) blockade. A: muscle tension and BP responses to static contraction before (top traces) and 10 min after (bottom traces) A-317567 injection. Dashed vertical lines represent 3 time points: onset of tension development (0), 5 s, and 10 s. Horizontal dashed lines represent BP at 200 and 160 mmHg. Note that in the first 5 s of contraction A-317567 had no significant effect on the pressor response. B: changes in MAP during contraction at 2 and 5 s after the onset of tension development. •, before A-317567; ○, after A-317567. Note there were no significant differences in the pressor responses at either 2 or 5 s.
Finally, in three cats we found that A-317567 injected intravenously into the jugular vein (0.5 ml; 10 mM) had no effect on the pressor responses to static contraction. Specifically, the change in mean arterial pressure during static contraction was 22.0 ± 1.5 mmHg before an intravenous injection of A-317567 and 22.5 ± 0.3 mmHg afterward.
DISCUSSION
A-317567 is an ASIC antagonist that is structurally dissimilar to either amiloride or its analogs (8). We have shown that the popliteal arterial injection of A-317567 markedly attenuated the pressor responses to both static contraction of the triceps surae muscles and lactic acid but had no effect on the pressor responses to either tendon stretch or capsaicin injection. Our findings are consistent with the hypothesis that A-317567 antagonized ASICs on groups III and IV afferents but had no effect on either transient receptor potential vanilloid type 1 channels or mechanogated channels. Previous evidence suggests that the dose of A-317567 used in our experiments did not antagonize ENaCs (8). Dube et al. (8) found in mice that an intraperitoneal injection of A-317567, in doses that blocked withdrawal responses to nociceptive stimuli, had no effect on urine output and sodium and potassium excretion (8). The strong possibility that A-317567 did not antagonize ENaCs distinguishes this compound from amiloride, which blocks both ENaCs and ASICs. In fact, the concentration of amiloride needed to block ENaCs is 10- to 100-fold lower than that needed to block ASICs (1, 17).
Use of amiloride as an antagonist to ASICs has a second complication in addition to its blockade of ENaCs. Specifically, amiloride in relatively high concentrations appears to block voltage-gated sodium channels (3, 12, 18), an effect that impairs the ability of sensory nerves to generate action potentials. This complication was not a factor in past studies from our laboratory because the dose of amiloride used was an order of magnitude lower than that needed to block voltage-gated sodium channels (12, 21). Moreover, if this complication occurred in the present experiments, one would expect to see an A-317567-induced attenuation of the pressor responses to the capsaicin injection and to tendon stretch, effects which did not occur.
ASICs found in dorsal root ganglion cells are encoded by three genes and two splice variants (2). A-317567 probably does not distinguish between the various ASICs. Nevertheless, the ASIC3, which is also known as DRASIC, stands out because it is, for the most part, only found on dorsal root ganglion cells, whereas the other ASICs are distributed throughout the spinal cord and brain (33). In vitro, the ASIC3 opens when exposed to pHs of 7.4–7.0, proton concentrations that are found in the interstitium of skeletal muscle when it contracts (13). These findings led us to speculate that ASIC3 on the endings of groups III and IV muscle afferents was blocked in our experiments by A-317567.
In humans, the role played by lactic acid in evoking the metabolic component of the exercise pressor reflex is controversial. In particular, this controversy has arisen from the study of the responses to exercise by patients with McArdle's disease, a pathological condition in which individuals produce little lactic acid because of a myophosphorylase deficiency. Two studies using McArdle's patients have shown that the pressor and muscle sympathetic nerve responses to static exercise are almost nonexistent (10, 24), whereas two others have shown that these responses to handgrip are preserved (31, 32). In humans, substantial evidence has accumulated that both the pressor and muscle sympathetic nerve responses to static exercise are caused by the exercise pressor reflex (23, 30), and as a consequence these responses are important indications of its presence or absence. Past (12, 21) and present findings from our laboratory demonstrate that lactic acid does play a role in the generation of the metabolic component of the exercise pressor reflex, although we can offer no insights into the controversial findings in humans about this issue.
Our findings suggest that ASIC blockade inhibited the discharge of metaboreceptors, most of which respond to contraction with a latency of at least 5 s, but had little, if any, effect on the discharge of mechanoreceptors, most of which respond to contraction with a latency of 1 s or less (15). In a previous study, our laboratory found that amiloride, an ASIC antagonist, did not significantly attenuate the contraction-induced increase in renal nerve sympathetic activity for the first 10 s of the contraction period (21). Similarly, in the present study, we found that A-317567 attenuated the peak pressor responses to contraction but had no effect on the pressor responses to tendon stretch. Moreover, the pressor response to the first 5 s of the contraction period was not on average attenuated by ASIC blockade (Figs. 4 and 5); likewise, the onset latency of this response was not changed either. Based on these findings, we offer the speculation that A-317567 attenuated input from metaboreceptors, most of which are innervated group IV afferents (15, 16). Usually group IV afferents do not respond much during the first 5 s of contraction, a period of time that usually does not allow metabolic by-products of contraction to accumulate to threshold levels.
Findings from isolated dorsal root ganglion cells taken from transgenic mice suggest that ASICs play a little role in nonnoxious mechanosensation but may play an important role in transducing pain. For example, the currents generated by wild-type dorsal root ganglion cells responding to mechanical stimuli were no different from those generated by ASIC2 and -3 knockout dorsal root ganglion cells (6). In addition, ASIC3, but not ASIC1, knockout mice failed to develop chronic pain caused by the repeated intramuscular injection of acid (29). Finally, A-317567 has been shown in rats to be a more effective blocker than amiloride of postoperative pain, which in turn was thought to be caused by proton release from the wound site (8).
ASICs in dorsal root ganglion cells are believed to be heteromultimeric (2). Not only can different ASICs combine to form channels on sensory nerves, but ASICs can combine with ENaCs to form channels as well (7). This latter type of combination might be especially relevant to acid sensing by thin fiber afferents in skeletal muscle. Specifically, the δ-ENaC protein is thought to confer proton sensitivity to multimeric channels (7) and in combination or in isolation with the ASIC3 protein might determine proton sensitivities to lactic acid generation by exercising muscles.
In conclusion, we have shown that A-317567 injected into the arterial supply of hind limb muscle attenuated the reflex pressor responses to lactic acid and static contraction. Injection of this purported ASIC antagonist had no effect, however, on the reflex pressor responses to either the capsaicin injection or tendon stretch. The former stimulus is an agonist for transient receptor potential vanilloid type 1 channels and the latter for mechanogated channels, which can be blocked by a gadolinium injection into the arterial supply of the hind limb muscles (11). A-317567 in doses that appear to block ASICs appears to have little effect on ENaCs. Consequently, with the combination of its apparent blocking effect on lactic acid-evoked pressor reflex and its lack of effect on ENaCs (8), A-317567 is at the present time the compound of choice with which to determine the role played by ASICs on thin fiber muscle afferents in reflexly activating the cardiovascular system during exercise.
GRANTS
This work was supported by National Institutes of Health Grant HL-30710.
Acknowledgments
We thank Jennifer Probst for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Benos DJ Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol Cell Physiol 242: C131–C145, 1982. [DOI] [PubMed] [Google Scholar]
- 2.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr MJ, Gover TD, Weinreich D, Undem BJ. Inhibition of mechanical activation of guinea-pig airway afferent neurons by amiloride analogues. Br J Pharmacol 133: 1255–1262, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol 556: 691–710, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension 51: 1265–1271, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain 117: 88–96, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger S, Gray K, Whisler S, Sinoway L. Dichloroacetate reduces sympathetic nerve responses to static exercise. Am J Physiol Heart Circ Physiol 261: H1653–H1658, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Fadel PJ, Wang Z, Tuncel M, Watanabe H, Abbas A, Arbique D, Vongpatanasin W, Haley RW, Victor RG, Thomas GD. Reflex sympathetic activation during static exercise is severely impaired in patients with myophosphorylase deficiency. J Physiol 548: 983–993, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol 280: H2153–H2161, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581: 1271–1282, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron 37: 75–84, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am Physiol Soc, 1996, sect. 12, chapt. 10, p. 381–447.
- 15.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984. [DOI] [PubMed] [Google Scholar]
- 17.Kleyman TR, Cragoe EJ Jr. Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105: 1–21, 1988. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Maile MD, Sinoway A, Sinoway LI. The muscle pressor reflex: the potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol 97: 1709–1714, 2004. [DOI] [PubMed] [Google Scholar]
- 19.MacLean DA, La Noue KF, Gray KS, Sinoway LI. Effects of hindlimb contraction on pressor and muscle interstitital metabolite responses in the cat. J Appl Physiol 85: 1583–1592, 1998. [DOI] [PubMed] [Google Scholar]
- 20.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCord JL, Hayes SG, Kaufman MP. Acid sensing ion and epithelial sodium channels do not contribute to the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol (July 3, 2008). doi: 10.1152/ajpheart.00450.2008. [DOI] [PMC free article] [PubMed]
- 22.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell JH, Reeves DR, Rogers HB, Secher NH. Epidural anesthesia and cardiovascular responses to static exercise in man. J Physiol 417: 13–24, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pryor SL, Lewis SF, Haller RG, Bertocci LA, Victor RG. Impairment of sympathetic activation during static exercise in patients with muscle phosphorylase deficiency (McArdle's disease). J Clin Invest 85: 1444–1449, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol 67: 256–263, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol 69: 1053–1059, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, Miller G, Moore RL. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. Am J Physiol Heart Circ Physiol 263: H1499–H1505, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106: 229–239, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res 65: 468–476, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Vissing J, MacLean D, Vissing SF, Sander M, Saltin B, Haller RG. The exercise metaboreflex is maintained in the absence of muscle acidosis: insights from muscle microdialysis in humans with McArdle's disease. J Physiol 537: 641–649, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vissing J, Vissing SF, MacLean DA, Saltin B, Quistorff B, Haller RG. Sympathetic activation in exercise is not dependent on muscle acidosis. J Clin Invest 101: 1654–1660, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem 272: 20975–20978, 1997. [DOI] [PubMed] [Google Scholar]