Abstract
This study was designed to delineate the medullary and spinal pathways mediating the cardiovascular responses to cold pressor test (CPT) and to identify neurotransmitters in these pathways. Experiments were done in barodenervated, urethane-anesthetized, male Wistar rats. The CPT was performed by immersing the limbs and ventral half of the body of the rat in ice-cold water (0.5°C) for 2 min. CPT elicited an immediate increase in mean arterial pressure (MAP), heart rate (HR), and greater splanchnic nerve activity (GSNA). Bilateral blockade of ionotropic glutamate receptors (iGLURs) in the rostral ventrolateral medullary pressor area (RVLM) significantly attenuated the CPT-induced responses. Bilateral blockade of γ-aminobutyric acid (GABA) receptors, but not iGLURs, in the nucleus ambiguus (nAmb) significantly reduced the CPT-induced increases in HR, but not MAP. Blockade of spinal iGLURs caused a significant reduction in CPT-induced increases in MAP and GSNA, whereas the increases in HR were reduced to a lesser extent. Combination of the blockade of spinal iGLURs and bilateral vagotomy or intravenous atropine almost completely blocked CPT-induced tachycardia. Midcollicular decerebration significantly reduced CPT-induced increases in MAP and HR. These results indicated that: 1) CPT-induced increases in MAP, HR, and GSNA were mediated by activation of iGLURs in the RVLM and spinal cord, 2) activation of GABA receptors in the nAmb also contributed to the CPT-induced tachycardic responses, and 3) brain areas rostral to the brain stem also participated in the CPT-induced pressor and tachycardic responses.
Keywords: arterial pressure, barodenervation, ionotropic glutamate receptors, nucleus ambiguus, sympathetic nerve activity
cold pressor test (CPT) has been used to evaluate the integrity of the autonomic nervous system in many clinical disorders (21, 25, 32, 38). In these studies, the test usually involved immersion of one of the limbs in ice cold water for 1–2 min and recording of variables such as arterial pressure (AP), heart rate (HR), and muscle or skin sympathetic nerve activity (SNA). Usual responses to the test included an increase in AP, HR, and SNA. These responses have been reported to be either absent or attenuated in pathological conditions such as Guillain-Barré syndrome, Parkinson's disease, idiopathic orthostatic hypotension, and diabetes mellitus with neuropathy while they are normal in other pathological situations such as Down's syndrome, arterial hypertension, and McArdle's disease (32). CPT has also been used to diagnose coronary artery disease (34). Hyperreactivity to CPT has been reported to be a predictor of the development (15) and severity (22) of hypertension in humans.
Despite the frequent use of CPT in clinical and experimental studies, little firm evidence is available regarding the neural pathways and neurotransmitters mediating the cardiovascular responses to this test. The rat is increasingly being used as an experimental animal in cardiovascular studies. The purpose of this investigation was to unravel the medullary and spinal pathways mediating the cardiovascular responses to this test in the rat and to identify the neurotransmitters in these pathways. The rostral ventrolateral medullary pressor area (RVLM) (11, 23, 30, 33, 42) and the nucleus ambiguus (nAmb) (24) are final structures in the medulla that are involved in the regulation of AP and HR, respectively. It is also well established that the intermediolateral cell column in the thoracolumbar cord (IML) is the final station in the central nervous system that is involved in the regulation of AP, HR, and SNA (1, 29, 35, 36). Therefore, this investigation was focused on the role of the RVLM, nAmb and spinal cord in mediating the cardiovascular responses to the CPT.
MATERIALS AND METHODS
General procedures.
Experiments were done in adult male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 300–360 g (n = 52). All animals were housed under controlled conditions with a 12:12-h light-dark cycle. Food and water were available to the animals ad libitum. The experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (7th Edition, 1996) and with the approval of the Institutional Animal Care and Use Committee of this university.
The general procedures have been described in detail elsewhere (17). Briefly, the rats were anesthetized with inhalation of isoflurane (2–3% in 100% oxygen), one of the femoral veins was cannulated, and urethane (1.2–1.4 g/kg) was injected intravenously in eight to nine aliquots at 2-min intervals (total volume of the anesthetic solution was 0.4–0.5 ml injected over a period of ∼16–18 min). The trachea was cannulated with polyethylene tubing (PE-240), and the rats were artificially ventilated using a rodent ventilator (model 683; Harvard Instruments, Holliston, MA). Isoflurane inhalation was terminated as soon as urethane administration was completed. Absence of withdrawal of the limb in response to pinching of a hind paw indicated that the rats were properly anesthetized. One of the femoral arteries was cannulated for monitoring AP. HR was monitored by a tachograph that was triggered by the AP waves. Mean arterial pressure (MAP) was derived electronically. The tidal volume and frequency were adjusted on the ventilator to maintain the end-tidal CO2 at 3.5–4.5%. The end-tidal CO2 was measured continuously in the expired gas collected via a side arm (PE-50 tubing) in the tracheal cannula. The connection between the side arm and the tracheal cannula was previously made leak proof by application of glue. An infrared CO2 analyzer modified for use in small animals (Micro-Capnometer; Columbus Instruments) was used for this purpose. Rectal temperature was maintained at 36.5 ± 0.5°C using a temperature controller (model TCAT-2AC; Physitemp Instruments, Clifton, NJ). All tracings were recorded on a polygraph (model 7D; Grass Instruments, West Warwick, RI).
Barodenervation.
Unless indicated otherwise, all rats were barodenervated. The carotid sinus nerves were sectioned bilaterally at their junction with glossopharyngeal nerves. The superior laryngeal nerves were sectioned bilaterally at their junction with the vagus nerves just below the nodose ganglia. Additionally, recurrent laryngeal nerves and aortic nerves were sectioned bilaterally low in the neck, and sympathetic nerves caudal to the superior cervical ganglia and pharyngeal branches of the vagus nerves were sectioned bilaterally. Following this procedure, bolus injections of phenylephrine (10 μg/kg iv) failed to elicit reflex inhibition of greater splanchnic nerve activity (GSNA) or bradycardia, indicating that the barodenervation was complete (18).
Adrenalectomy.
The rats were placed in a prone position in a stereotaxic instrument (model 1430; David Kopf Instruments, Tujunga, CA), and 1.5-cm-long incisions were made ∼1.5 cm lateral to the midline, just caudal to the last rib, parallel to the vertebral column. The adrenal glands were identified using an operating microscope, the blood vessels entering and emerging from the glands were ligated, and the glands were removed.
Vagotomy.
Vagotomy was necessary in experiments designed to investigate the role of parasympathetic innervation to the heart in mediating the CPT-induced HR responses. In these experiments, silk sutures were placed loosely around the vagus nerves bilaterally for subsequent identification and sectioning of the nerves. In some experiments, instead of performing bilateral vagotomy, atropine methyl nitrate (50 μg/kg iv) was administered to block the vagal effects on the heart (12, 37).
Decerebration.
In one group of urethane-anesthetized rats, after performing the initial CPT, the rats were subjected to decerebration, and, after a stabilization period of 60 min, the CPT was performed again. The purpose of these experiments was to assess the role of brain structures located rostral to the brain stem. The procedure for decerebration involved the following steps: the common carotid arteries were ligated bilaterally, the rats were placed in a prone position in a stereotaxic instrument, the parietal bones were removed, the dura was incised, a transection was made at midcollicular level, the portion of the brain rostral to the transection was removed by suction, and the cranial cavity was loosely packed with cotton balls.
Intrathecal injections.
Intrathecal injections were performed in rats fixed in a stereotaxic instrument in a prone position. The dorsal neck muscles and parts of the occipital bone were removed, the atlanto-occipital membrane was incised, and a cannula (PE-10) connected to a Hamilton microsyringe and filled with drugs or artificial cerebrospinal fluid (aCSF) was inserted in the midline under the dura mater and advanced 6 cm caudally so that the tip of the tubing was located at the T9–T10 level (approximately middle of the thoracolumbar cord). The volume of intrathecal injection was 20 μl, and the duration of injection was 20–30 s. The location of the cannula tip at T9–T10 was confirmed by postmortem examination using an operating microscope. Intrathecal injections (20 μl) of aCSF were used as controls.
Microinjections.
The details of this technique are described elsewhere (17, 18). Briefly, the rats were placed in a prone position in a stereotaxic instrument with a bite bar 18 mm below the interaural line. The microinjections were made using a dorsal approach. Three-barreled glass micropipettes (tip size 20–40 μm) were mounted on a micromanipulator (model 1460 with an AP slide 1262; David Kopf Instruments), and each barrel was connected via PE tubing to one of the channels on a picospritzer (General Valve, Fairfield, NJ). One of the barrels contained l-glutamate (l-Glu). The contents of other barrels varied according to the requirements of the experiment. The coordinates for the RVLM were 1.6–2.0 mm rostral and 1.8–2.0 mm lateral to the calamus scriptorius (CS) and 3.1–3.2 mm deep from the dorsal medullary surface. The coordinates for the nAmb were 0.2–0.6 mm rostral and 1.8–1.9 mm lateral to the CS and 2.4–2.6 mm deep from the dorsal medullary surface. The sites eliciting pressor and tachycardic responses in the RVLM and bradycardia in the nAmb were identified by microinjections of l-Glu (5 mM). The volumes of microinjection in the RVLM and nAmb were 100 and 30 nl, respectively; the selection of these volumes was based on our previous studies in which these volumes of l-Glu elicited maximum responses from these brain areas (3, 17, 18). The volumes were pressure ejected (30–35 psi) and visually confirmed by the displacement of fluid meniscus in the barrel containing the solution using a modified binocular horizontal microscope with a graduated reticule in one eyepiece [model PZMH; World Precision Instruments (WPI), Sarasota, FL]. The duration of microinjection was 5–10 s. Microinjections of aCSF (pH 7.4) served as controls; the volumes of aCSF for microinjections in the RVLM and nAmb were 100 and 30 nl, respectively.
Greater splanchnic nerve recording.
The greater splanchnic nerve (GSN) was exposed using a retroperitoneal approach. A 1.5-cm-long incision was made just caudal to the last rib, parallel to the vertebral column and ∼1.5 cm lateral to the midline, on each side. The segment of the GSN immediately proximal to the celiac ganglion was identified using an operating microscope, sectioned at its junction with the celiac ganglion, and a few millimeters of the central end of the nerve were desheathed (31). The exposed portion of the nerve was placed on a bipolar electrode made of silver wire. The nerve, along with the tips of the electrode, was embedded in a silicone elastomer (Kwik-Sil; WPI, Florida) that was allowed to set for 5–10 min. The electrode was connected to a probe head stage (model Super-Z; CWE Systems, Philadelphia, PA), and the whole nerve discharge was amplified (X20,000–30,000, using model BMA-830 amplifier; CWE) and filtered (100–5,000 Hz). Amplified signals were digitized (22 kHz) using Neuro-Corder (Cygnus Technologies, Delaware Water Gap, PA), visualized on an oscilloscope (model R5103N; Tektronix, Beaverton, OR), and stored together with AP and HR on a video cassette recorder. The whole nerve activity was full-wave rectified, and an integrated signal was obtained (model MA-821 Integrator; time constant set at 100 ms; CWE Systems). At the end of the experiment, the GSN was sectioned centrally, and the remaining activity was considered to be the noise level.
Cold pressor test.
The ventral half of the body of the rat was shaved to expose the skin, the head was fixed in a stereotaxic instrument in a prone position, and the body was placed in a custom-made Plexiglas rectangular dish (21.5 cm long, 8.5 cm wide, and 2.5 cm deep). To perform the CPT, the dish was rapidly filled to the brim with ice-cold water (0.5°C) to immerse the rat's limbs and ventral half of the body. After 2 min immersion, the cold water was removed by rapid suction. As mentioned in General procedures, before starting the CPT, the rectal temperature was maintained at 36.5 ± 0.5°C (usual temperature in an anesthetized rat) using a temperature controller that was disconnected at the start of the CPT and reconnected 2 min after the removal of cold water from the dish. An interval of at least 40 min was allowed before another CPT was applied. In some rats, the temperature controller was not disconnected during the CPT.
Histology.
Typical sites of microinjections in the RVLM and nAmb were marked by microinjections of diluted India ink. The animals were perfused and fixed with 4% paraformaldehyde, and serial sections of the medulla were cut (40 μm) in a vibratome, mounted on slides, dehydrated, cleared, and stained with cresyl violet. The microinjection sites were identified using a microscope. The sections were photographed and compared with a standard atlas (28).
Drugs and chemicals.
The following drugs and chemicals were used: atropine methyl nitrate, d-(−)-2-amino-7-phosphono-heptanoic acid [d-AP7; N-methyl-d-alanine (NMDA) receptor antagonist], gabazine bromide [γ-aminobutyric acid (GABA) type A receptor antagonist], l-glutamate monosodium (l-Glu), 2-hydroxysaclofen (GABAB receptor antagonist), isoflurane, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-[f]quinoxaline-7-sulfonamide disodium (NBQX disodium salt; non-NMDA receptor antagonist), (±)-α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid hydrobromide (AMPA; a non-NMDA receptor agonist), l-phenylephrine hydrochloride, and urethane. All of the solutions for the microinjections were freshly prepared in aCSF. Where applicable, the concentration of drugs refers to their salts. Isoflurane was purchased from Baxter Pharmaceutical Products (Deerfield, IL). All other drugs and chemicals were obtained from Sigma Chemicals (St. Louis, MO).
Statistical analyses.
For comparison of MAP and HR responses, the mean and SE were calculated for maximum changes in these values in response to CPT. In tachyphylaxis tests, microinjections of d-AP7 and NBQX in the RVLM, microinjections of gabazine and 2-hydroxysaclofen in the nAmb, comparisons of the maximum increases in MAP and HR during CPT were made by using repeated-measures ANOVA followed by Tukey-Kramer's multiple-comparison test. Comparisons of the maximum increases in MAP and HR during CPT before and after the bilateral barodenervation, bilateral adrenalectomy, bilateral vagotomy, intravenous injections of atropine, microinjections of d-AP7 and NBQX in the nAmb, and intrathecal injections of d-AP7 and NBQX were made by using the Student's paired t-test. Comparisons of the CPT-induced decreases in rectal temperature in which the temperature controller remained connected and disconnected during the CPT were made by using the unpaired t-test. For the analyses of nerve activities, the integrated signals obtained just before and during maximal responses to CPT were averaged over a period of 35 s. The means and SE of the integrated signals before and during CPT were compared using Student's paired t-test. In all cases, the differences were considered significant at P < 0.05.
RESULTS
CPT in intact and barodenervated rats.
Baseline values for MAP and HR in intact rats (n = 5) were 105.0 ± 4.1 mmHg and 374.0 ± 18.6 beats/min, respectively. In the same group of rats, barodenervation initially elicited increases in MAP (38.0 ± 4.0 mmHg) and HR (30.0 ± 5.4 beats/min) that lasted for ∼60 min. After this period of time following the barodenervation, the baseline MAP (96.0 ± 4.3 mmHg) and HR (348.0 ± 19.8 beats/min) were not statistically different (P > 0.05) from the baseline values before the barodenervation. The CPT was performed before and after barodenervation in the same group of rats. The increases in MAP in response to CPT were significantly greater (P < 0.05) in barodenervated rats (51.0 ± 7.6 mmHg) compared with these responses in intact rats (30.0 ± 2.2 mmHg). However, increases in HR in response to CPT in intact (74.0 ± 9.2 beats/min) and barodenervated (76.0 ± 10.7 beats/min) rats were not significantly different (P > 0.05). To avoid baroreflex effects on pressor and tachycardic responses to CPT, all other experiments were done in barodenervated rats.
The changes in rectal temperature in response to CPT were noted in all rats (this and other series, n = 44). The rectal temperature before the CPT was 36.56 ± 0.01°C. After CPT (2 min), the rectal temperature decreased to 36.43 ± 0.01°C (P < 0.05). After the removal of cold water, the temperature controller was reconnected, and the rectal temperature returned to the pre-CPT level within 30 min. In some rats (n = 8), the temperature controller was not disconnected during the CPT. In these rats, the rectal temperature before the CPT was 36.56 ± 0.02°C. After CPT (2 min), the rectal temperature decreased to 36.41 ± 0.02°C (P < 0.05). After the removal of cold water, the rectal temperature returned to the pre-CPT level within 30 min. The CPT-induced decreases in rectal temperature were not statistically different (P > 0.05) in rats in which the temperature controller remained connected or disconnected during the CPT.
Reproducibility of CPT responses.
Baseline values for MAP and HR in another group of barodenervated rats (n = 4) were 107.5 ± 11.6 mmHg and 415.0 ± 28.7 beats/min, respectively. In this group of rats, the CPT was performed four times at 40-min intervals. The MAP responses to these tests were 45.0 ± 9.3, 47.5 ± 10.3, 38.7 ± 9.4, and 36.2 ± 8.9 mmHg, respectively. The HR responses for these tests were 38.7 ± 15, 35.0 ± 13.2, 32.5 ± 10.3, and 30.0 ± 4.0 beats/min, respectively. The MAP and HR values for these repeated tests were not significantly different (P > 0.05), indicating that the CPT responses were reproducible if the interval between the tests was at least 40 min.
Effect of adrenalectomy.
The baseline values for MAP and HR in another group of barodenervated rats (n = 4) were 88.7 ± 5.5 mmHg and 397.5 ± 8.5 beats/min, respectively. The baseline values for MAP (82.5 ± 1.4 mmHg) and HR (377.5 ± 16.5 beats/min) in this group of rats after bilateral adrenalectomy were not statistically different (P > 0.05) compared with the corresponding values before adrenalectomy. The CPT was performed before and after bilateral adrenalectomy in this group of rats. Increases in MAP and HR in response to CPT were 47.5 ± 3.2 mmHg and 45.0 ± 9.5 beats/min, respectively, in intact rats. After bilateral adrenalectomy, increases in MAP and HR in response to CPT were 46.2 ± 6.5 mmHg and 57.5 ± 7.5 beats/min, respectively. Thus the cardiovascular responses to CPT before and after adrenalectomy were not significantly different (P > 0.05).
Effect of ionotropic glutamate receptor blockade in the RVLM.
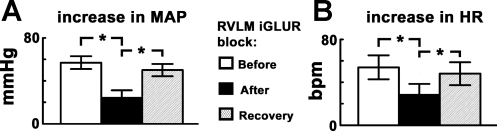
The effect of ionotropic glutamate receptor (iGLUR) blockade in the RVLM on CPT-induced responses was studied in another group of barodenervated rats (n = 5). After performing the initial CPT, the RVLM was identified on one side by microinjections (100 nl) of l-Glu (5 mM). Within 2 min, NBQX (2 mM, 50 nl) and d-AP7 (5 mM, 50 nl) were microinjected sequentially within 1 min via adjacent barrels at the same site. Within 2 min, the procedure was repeated on the contralateral RVLM. Bilateral blockade of iGLURs in the RVLM was completed within 5–6 min. Both MAP and HR responses to CPT after the blockade of iGLURs in the RVLM were significantly (P < 0.05) smaller compared with the responses before the blockade (Fig. 1, A and B). The increases in MAP and HR in response to CPT recovered within 60 min (Fig. 1, A and B). Microinjections of aCSF in the RVLM did not alter CPT-induced responses. The baseline MAP and HR before (88.0 ± 7.1 mmHg and 392.0 ± 17.7 beats/min, respectively) and after (92.0 ± 7.6 mmHg and 398.0 ± 18.2 beats/min, respectively) the iGLUR blockade in the RVLM were not statistically different (P > 0.05). Tracings showing attenuation of the pressor and tachycardic responses to CPT after the blockade of iGLURs in the RVLM are shown in Fig. 2, A–C.
Fig. 1.
Cold pressor test (CPT) responses: effect of blockade of ionotropic glutamate receptors (iGLURs) in the rostral ventrolateral medullary (RVLM) pressor area. A and B: increases in mean arterial pressure (MAP) and heart rate (HR) in response to initial CPT were 57.0 ± 6.0 mmHg and 54.0 ± 11.2 beats/min, respectively (n = 5). After the blockade of iGLURs in the RVLM, by microinjections of 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo-[f]quinoxaline-7-sulfonamide disodium (NBQX; 2 mM, 50 nl) and d-(−)-2-amino-7-phosphono-heptanoic acid (d-AP7; 5 mM, 50 nl), the increases in MAP and HR in response to CPT were 25.0 ± 6.3 mmHg and 29.0 ± 9.5 beats/min, respectively; both MAP and HR responses to CPT after the blockade of iGLURs in the RVLM were significantly (*P < 0.05) smaller compared with the responses before the blockade. After ∼60 min, increases in MAP and HR in response to CPT were 50.0 ± 5.7 mmHg and 48.0 ± 10.6 beats/min, respectively, indicating recovery of responses.
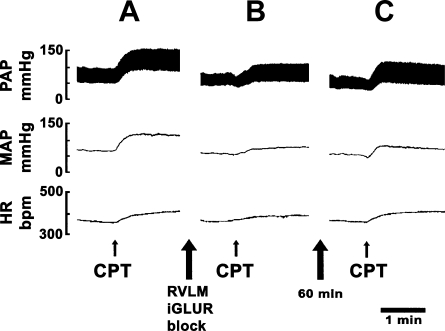
Fig. 2.
Attenuation of CPT responses by iGLUR blockade in the RVLM. A: pulsatile arterial pressure (PAP; trace on top), MAP (trace in middle), and HR (trace on bottom). The PAP, MAP, and HR were increased rapidly after CPT. B: after an interval of 40 min, NBQX (2 mM) and d-AP7 (5 mM) were microinjected bilaterally in the RVLM to block iGLURs. Within 5 min, CPT was performed again; the PAP, MAP, and HR responses were reduced. C: after an interval of 60 min, the CPT was performed again; the PAP, MAP, and HR responses recovered to the initial revels.
Effect of GABA receptor blockade in the nAmb.
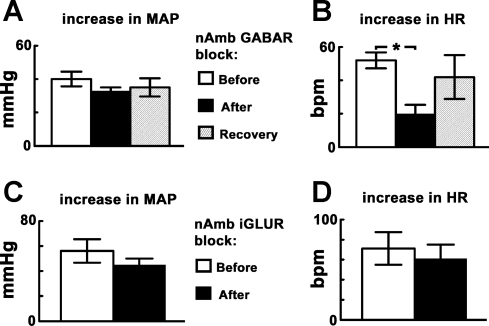
The CPT was performed before and after the blockade of GABA receptors in the nAmb in another group of barodenervated rats (n = 5). After performing the initial CPT, the nAmb was identified bilaterally by microinjections (30 nl) of l-Glu (5 mM). Within 2 min, gabazine (2 mM, 15 nl) and 2-hydroxysaclofen (100 mM, 15 nl) were microinjected in the same nAmb sites via adjacent barrels. The HR responses were significantly (P < 0.05) reduced after the blockade of GABA receptors in the nAmb, whereas the differences in MAP responses were not statistically significant (P > 0.05). The HR responses to CPT recovered within ∼60 min after the blockade of GABA receptors in the nAmb (Fig. 3, A and B). Microinjections of aCSF into nAmb had no effect on CPT-induced responses. The baseline MAP before the GABA receptor blockade in the nAmb (94.0 ± 5.0 mmHg) was not statistically different (P > 0.05) from the corresponding value after the GABA receptor blockade (78.0 ± 4.6 mmHg). However, the baseline HR after the blockade of GABA receptors in the nAmb (196.0 ± 25.4 beats/min) was significantly decreased (P < 0.05) compared with the value before the blockade (376.0 ± 9.7 beats/min).
Fig. 3.
CPT responses: effect of blockade of γ-aminobutyric acid (GABA) receptors or iGLURs in the nucleus ambiguus (nAmb). A and B: increases in MAP and HR to initial CPT were 40.0 ± 4.4 mmHg and 52.0 ± 4.8 beats/min, respectively (n = 5). The increases in MAP and HR to CPT after the blockade of GABA receptors in the nAmb, by microinjections of gabazine (2 mM, 15 nl) and 2-hydroxysaclofen (100 mM, 15 nl), were 33.0 ± 2.0 mmHg and 20.0 ± 5.4 beats/min, respectively; the HR responses were reduced significantly (*P < 0.05), whereas the differences in MAP responses were not statistically significant (P > 0.05). The HR responses to CPT recovered (42.0 ± 13.1 beats/min) within ∼60 min after the blockade of GABA receptors in the nAmb. C and D: increases in MAP and HR in response to initial CPT were 56.2 ± 9.4 mmHg and 71.2 ± 16.3 beats/min, respectively (n = 4). After the blockade of iGLURs in the nAmb, by microinjections of NBQX (2 mM, 15 nl) and d-AP7 (5 mM, 15 nl), the increases in MAP and HR in response to CPT were 45.0 ± 5.0 mmHg and 61.2 ± 13.9 beats/min, respectively; thus, both MAP and HR responses to CPT were not significantly altered (P > 0.05).
Effect of iGLUR blockade in the nAmb.
The CPT was performed before and after the blockade of iGLURs in the nAmb in another group of barodenervated rats (n = 4). After performing the initial CPT, the nAmb was identified bilaterally by microinjections (30 nl) of l-Glu (5 mM). Within 2 min, NBQX (2 mM, 15 nl) and d-AP7 (5 mM, 15 nl) were microinjected in the same nAmb sites via adjacent barrels. After the blockade of iGLURs in the nAmb, neither the MAP nor HR responses to CPT were significantly altered (P > 0.05) (Fig. 3, C and D). The baseline MAP and HR before (88.7 ± 6.5 mmHg and 375.0 ± 11.9 beats/min, respectively) and after (101.2 ± 3.1 mmHg and 370.0 ± 14.7 beats/min, respectively) the iGLUR blockade in the nAmb were not statistically different (P > 0.05).
Effect of spinal iGLUR blockade.
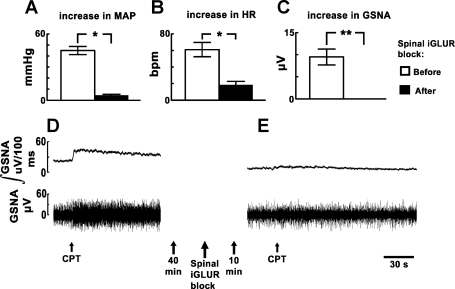
The CPT was performed before and after intrathecal injections of NBQX and d-AP7 in another group of barodenervated rats (n = 10). After performing initial CPT, NBQX (2 mM, 10 μl) and d-AP7 (5 mM, 10 μl) were injected intrathecally. Both MAP and HR responses to CPT were significantly reduced (P < 0.001) after the blockade of spinal iGLURs, but the reduction in the CPT-induced increases in MAP (90%) were much greater than the reduction in HR (69.67%) increases (Fig. 4, A and B). In this and other experiments, intrathecal administration of aCSF (20 μl) had no effect on the CPT-induced responses. The baseline MAP and HR were significantly decreased (P < 0.05) after the blockade of iGLURs in the spinal cord (58.5 ± 5.9 mmHg and 366.6 ± 7.2 beats/min, respectively) when compared with the values before (76.5 ± 2.7 mmHg and 383.3 ± 11.4 beats/min, respectively) the blockade. In this group of rats, five rats were selected randomly for recording changes in the efferent GSNA after the blockade of spinal iGLURs. The baseline GSNA was 18.0 ± 1.2 μV. Initial CPT increased the GSNA. After intrathecal injections of NBQX and d-AP7, the baseline GSNA decreased slowly and reached the nadir (12.5 ± 1.1 μV) within 10 min. At this time, application of CPT failed to elicit an increase in GSNA (Fig. 4C). Tracings of these responses are shown in Fig. 4, D and E.
Fig. 4.
Effect of spinal iGLUR blockade on CPT responses. A and B: initial increases in MAP and HR in response to CPT were 45.0 ± 3.7 mmHg and 61.0 ± 8.6 beats/min, respectively (n = 10). After the blockade of spinal iGLURs, by intrathecal injections of NBQX (2 mM, 10 μl) and d-AP7 (5 mM, 10 μl), the increases in MAP and HR in response to CPT were 4.5 ± 0.8 mmHg and 18.5 ± 4.2 beats/min, respectively; both MAP and HR responses to CPT were significantly (*P < 0.001) reduced, but the reduction in the CPT-induced increases in MAP (90%) was much greater than the reduction in HR increases (69.67%). C: increase in greater splanchnic nerve activity (GSNA) in response to CPT before the blockade of spinal iGLURs was 9.5 ± 1.8 μV. After the blockade of spinal iGLURs, application of CPT failed to elicit an increase in GSNA (**). D: integrated GSNA (trace on top) and raw GSNA (trace on bottom). The integrated and raw GSNA were increased rapidly after CPT. The onset of the increase in GSNA was immediate, and the peak response was observed within 10–15 s. E: later (40 min), intrathecal injections of NBQX (2 mM) and d-AP7 (5 mM) caused a decrease in GSNA. Later (10 min), the response to CPT was blocked.
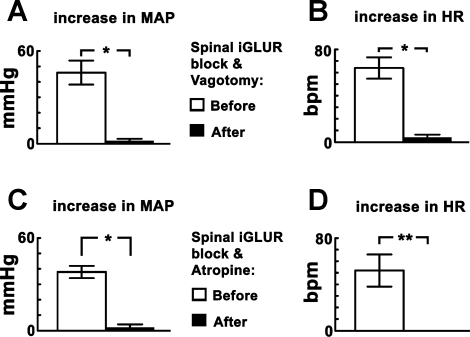
Effect of bilateral vagotomy or intravenous atropine combined with spinal iGLUR blockade.
In one group of rats (n = 5), after performing initial CPT, the rats were vagotomized bilaterally. After a stabilization period of ∼40 min, NBQX (2 mM, 10 μl) and d-AP7 (5 mM, 10 μl) were injected intrathecally, and CPT was performed again within 10 min. After the combination of blockade of spinal iGLURs and bilateral vagotomy, the reductions of CPT-induced increases in MAP (95.6%) and HR (93.7%) were highly significant (P < 0.005) (Fig. 5, A and B). The baseline HR values before (380.0 ± 10.4 beats/min) and after (386.0 ± 16.6 beats/min) the combination of blockade of spinal iGLURs and bilateral vagotomy were not statistically different (P > 0.05). However, the baseline MAP (68.0 ± 5.1 mmHg) after the combination of blockade of spinal iGLURs and bilateral vagotomy was significantly decreased (P < 0.05) when compared with the value before (100.0 ± 6.8 mmHg) these procedures. These experiments were repeated in another group of rats (n = 5) in which the blockade of spinal iGLURs was combined with administration of atropine methyl nitrate instead of performing vagotomy. After performing initial CPT, NBQX (2 mM, 10 μl) and d-AP7 (5 mM, 10 μl) were injected intrathecally. Within 10 min, atropine methyl nitrate (50 μg/kg iv) was administered. After the combination of blockade of spinal iGLURs and cardiac cholinergic muscarinic receptors, the reduction of CPT-induced increase in MAP (94.7%) was highly significant (P < 0.005), and the CPT-induced increase in HR was completely blocked (Fig. 5, C and D). The baseline HR values before (400.0 ± 14.8 beats/min) and after (382.0 ± 13.5 beats/min) the combination of blockade of spinal iGLURs and cardiac muscarinic receptor blockade were not statistically different (P > 0.05). However, the baseline MAP (72.0 ± 6.2 mmHg) after the combination of blockade of spinal iGLURs and cardiac muscarinic receptor blockade was significantly decreased (P < 0.05) when compared with the value before (98.0 ± 4.6 mmHg) these procedures.
Fig. 5.
CPT responses: effect of combination of blockade of spinal iGLURs and interruption of parasympathetic innervation to the heart. A and B: in one group of rats (n = 5), increases in MAP and HR in response to initial CPT were 46.0 ± 7.8 mmHg and 64.0 ± 9.2 beats/min, respectively. After the combination of blockade of spinal iGLURs and bilateral vagotomy, the increases in MAP and HR in response to CPT were 2.0 ± 1.2 mmHg and 4.0 ± 2.4 beats/min, respectively; thus, CPT-induced increases in MAP and HR were reduced significantly (*P < 0.005). C and D: in another group of rats (n = 5), increases in MAP and HR in response to initial CPT were 38.0 ± 4.0 mmHg and 52.0 ± 13.9 beats/min, respectively. After the combination of blockade of spinal iGLURs and cardiac cholinergic muscarinic receptors, by injections of atropine methyl nitrate (50 μg/kg iv), the CPT-induced increases in MAP (2.0 ± 2.0 mmHg) were significantly reduced (*P < 0.005), and the increase in HR was completely blocked (**).
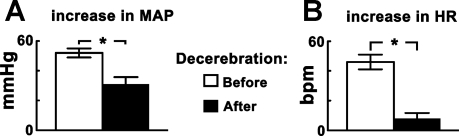
Effect of midcollicular decerebration.
The effect of midcollicular decerebration on CPT-induced responses was studied in another group of barodenervated, urethane-anesthetized rats (n = 5). After monitoring the initial CPT response, midcollicular decerebration was performed. After a stabilization period of ∼60 min, the CPT was repeated. Both increases in MAP and HR in response to CPT were significantly smaller (P < 0.05) after the decerebration compared with the corresponding values before the decerebration (Fig. 6, A and B). The baseline MAP before (98.0 ± 4.0 mmHg) and after (75.0 ± 6.5 mmHg) the decerebration and the baseline HR before (400.0 ± 13.0 beats/min) and after (432.0 ± 36.2 beats/min) the decerebration were not statistically different (P > 0.05).
Fig. 6.
Effect of midcollicular decerebration on CPT responses. A: CPT-induced increases in MAP were 52.0 ± 3.0 and 31.0 ± 4.8 mmHg before and after the decerebration, respectively (*P < 0.05) (n = 5). B: in the same group of rats, the CPT-induced increases in HR were 46.0 ± 5.0 and 8.0 ± 3.7 beats/min before and after the decerebration, respectively (*P < 0.05).
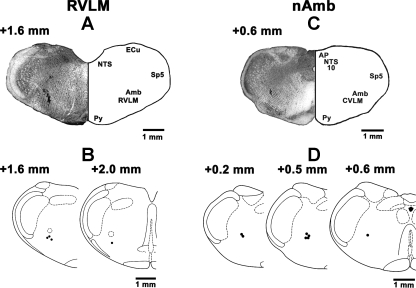
Histology.
The RVLM sites, where microinjections of l-Glu elicited pressor and tachycardic responses, were marked in four rats; a typical RVLM site marked with India ink (100 nl) is shown in Fig. 7A. Figure 7B represents composite diagrams of RVLM sites. The nAmb sites, where microinjections of l-Glu elicited bradycardic responses, were marked in seven rats; a typical nAmb site marked with India ink (30 nl) is shown in Fig. 7C. Figure 7D represents composite diagrams of nAmb sites.
Fig. 7.
Histological identification of microinjection sites. A: coronal section of the medulla at a level 1.6 mm rostral to the calamus scriptorius (CS) showing a typical RVLM site marked with India ink (100 nl) where NBQX and d-AP7 microinjections were made. The center of the spot was 1.8 mm lateral to the midline and 2.7 mm deep from the dorsal medullary surface. B: drawings of coronal sections at a level 1.6 and 2.0 mm rostral to the CS. In this and other panels, microinjection sites are shown as dark spots; each spot represents a site in one animal. C: coronal section of the medulla at a level 0.6 mm rostral to the CS showing a typical nAmb site marked with India ink where NBQX and d-AP7 or hydroxysaclofen and gabazine microinjections were made. The center of the spot was 1.9 mm lateral to the midline and 2.3 mm deep from the dorsal medullary surface. D: drawings of coronal sections at a level 0.2, 0.5, and 0.6 mm rostral to the CS. Amb: nucleus ambiguus; AP: area postrema; CVLM: caudal ventrolateral medullary depressor area; ECu: external cuneate nucleus; NTS: nucleus tractus solitarius; Py: pyramids; Sp5: spinal trigeminal tract; 10: dorsal motor nucleus of vagus.
DISCUSSION
The major findings of the present study were as follows: 1) bilateral blockade of iGLURs in the RVLM significantly attenuated the CPT-induced increases in MAP and HR, 2) bilateral blockade of GABA receptors, but not iGLURs, in the nAmb significantly reduced the CPT-induced increases in HR, 3) blockade of spinal iGLURs resulted in significant attenuation of CPT-induced increases in MAP while the reduction in HR responses was less, and 4) combination of blockade of spinal iGLURs with bilateral vagotomy or cardiac cholinergic muscarinic receptors abolished the CPT-induced increases in MAP and HR.
It is well established that the neurons involved in pain and temperature sensations are located in the dorsal root ganglia, and the peripheral free nerve endings of their axons are located throughout the skin. The afferent fibers (type Aδ and C) mediating pain and temperature sensations terminate on second-order neurons in the lamina I of the dorsal horn in the ipsilateral spinal cord where anatomically discrete sets of neurons mediating pain and temperature sensations are located (13). The axons of these neurons cross in the midline and ascend contralaterally as an anterolateral tract system, including the spinothalamic, spinoreticular, and spinomesencephalic tracts (14). There are differences in the ascending projections of the nociceptive-specific, polymodal nociceptive, and thermoreceptive-specific neurons in lamina I. For example, projections of nociceptive-specific neurons ascend in the lateral mesencephalon, whereas those from the thermoreceptive-specific neurons ascend in the medial mesencephalon. Furthermore, nociceptive-specific neurons do not project to the dorsomedial part of the ventroposterior medial nucleus of the thalamus, whereas nearly all thermoreceptive-specific neurons project to this nucleus (2). CPT has been used as one of the methods of administering noxious stimuli under experimental conditions and has been reported to have excellent reliability and validity (9).
Based on the above-mentioned information, the mechanism of CPT-induced increases in MAP and SNA in our study can be explained as follows. CPT stimulated the pain- and temperature-sensitive free nerve endings in the skin and activated the spinal anterolateral system of ascending tracts. Ascending fibers of these tracts, especially the spinoreticular tract projecting to the medullary reticular formation, activated presympathetic RVLM neurons. Activation of the projection from the RVLM to the IML in the thoracolumbar cord resulted in excitation of the sympathetic preganglionic neurons causing increases in SNA and MAP (11, 23, 30, 33, 42). The stimulation of RVLM neurons was mediated via iGLURs because bilateral microinjections of iGLUR antagonists attenuated the increases in MAP and SNA. We have previously reported that the projection from the RVLM to the IML is glutamatergic (36). Our observation in the rat regarding the CPT-induced increase in GSNA is consistent with earlier reports in humans in which CPT increased muscle or skin SNA (5, 10, 40).
The pressor responses to CPT were not completely blocked by the iGLUR blockade in the RVLM, suggesting that other pathways bypassing the RVLM and projecting directly to the IML may also be activated. Consistent with this notion was the observation that blockade of spinal iGLURs almost completely reduced the pressor and SNA responses to the CPT. The selection of concentrations of the iGLUR antagonists (NBQX and d-AP7) was based on our earlier reports in which these concentrations were found to block the effects of their respective agonists (AMPA and NMDA, respectively) in a specific manner (7, 39).
The CPT-induced increases in HR, even though attenuated, persisted after the blockade of spinal iGLURs, suggesting other pathways were involved in the tachycardic responses. When the blockade of spinal iGLURs was combined with bilateral vagotomy or blockade of cardiac cholinergic muscarinic receptors by intravenous atropine, the tachycardic responses to CPT were abolished. Thus CPT-induced increases in HR were mediated via both activation of sympathetic and inhibition of parasympathetic input to the heart. Our results suggested that CPT may have caused inhibition of parasympathetic preganglionic neurons innervating in the nAmb (24). Inhibition of these nAmb neurons may have caused withdrawal of the vagal input to the heart, resulting in tachycardia. The inhibition of nAmb was mediated via GABAergic mechanisms because blockade of GABAA and GABAB receptors in the nAmb (by gabazine and 2-hydroxysaclofen, respectively) attenuated the CPT-induced tachycardic responses. The selection of concentrations of gabazine and 2-hydroxysaclofen was based on our earlier reports in which these antagonists were found to block the effects of their respective agonists (muscimol and baclofen, respectively) in a specific manner (2, 16). The blockade of iGLURs in the nAmb did not attenuate CPT-induced increases in HR. Complete blockade of iGLURs in the nAmb was confirmed by microinjecting l-Glu at the same site; l-Glu-induced bradycardia was abolished. The blockade of iGLURs in the nAmb did not attenuate CPT-induced increases in HR; complete blockade of iGLURs was confirmed by microinjections of l-Glu in these experiments. On the other hand, GABA receptor blockade in the nAmb did attenuate CPT-induced increases in HR. Based on these results, it was concluded that GABAergic neurons may lie between the ascending pain and temperature afferent projections and the nAmb. The location of the GABAergic neuron is not clear at this time. The presence of GABAergic inputs to the nAmb is well documented (41). Although the nucleus tractus solitarius (NTS) has been identified as one of the sources of GABAergic inputs to the nAmb (41), other brain regions, which remain to be identified, may also provide such inputs to the nAmb. In our experiments, GABA receptor blockade in the nAmb significantly decreased the baseline HR. This observation is consistent with the notion that parasympathetic preganglionic neurons innervating the heart receive tonically active GABAergic inputs.
The parasympathetic nervous system is predominant in the control of HR (24). Because it has been proposed that cardiac vagal preganglionic neurons are activated by a glutamatergic projection from the NTS to the nAmb and this pathway may mediate baroreceptor-induced bradycardia (12, 24), microinjections of iGLUR antagonists in the nAmb should increase baseline HR, which was not observed in our study. This apparent discrepancy could be explained by lack of vagal tone on the heart in anesthetized animals (24). Moreover, other excitatory neurotransmitters (e.g., serotonin) have been implicated in the nAmb in mediating reflexly induced bradycardia (19); in this case, microinjections of iGLUR antagonists in the nAmb are not expected to increase baseline HR, consistent with our observations.
Blockade of spinal iGLURs decreased baseline HR and AP. Subsequent bilateral vagotomy or intravenous atropine increased HR. These effects indicated the presence of tonic sympathetic and parasympathetic control of HR despite anesthesia. The result of the two opposing effects was that HR remained near the levels before the two procedures were performed.
CPT-induced increases in MAP and HR were significantly reduced after midcollicular transection of the brain, suggesting that projections located rostral to the brain stem may also participate in CPT-induced cardiovascular responses. Identification of individual rostral brain structures involved in CPT-induced cardiovascular responses was beyond the scope of this investigation and may be attempted in the future.
The blockade or attenuation of CPT-induced responses after different pharmacological manipulations was not due to repetition of CPTs because these responses were reproducible when the tests were applied repeatedly at 40-min intervals. It has been reported that the baroreceptors remain capable of modulating SNA during CPT (5). To avoid the reflex modulation of CPT responses, our experiments were carried out in barodenervated rats. Acute barodenervation resulted in increases in AP and HR that returned to basal levels within 60 min. The recovery of AP and HR after barodenervation has been attributed to the failure to maintain sustained elevation of SNA (27). The CPT-induced increases in MAP, but not HR, were significantly greater in barodenervated rats. This difference in the effect of barodenervation on CPT-induced MAP and HR responses can be attributed to the CPT-induced changes in baroreflex sensitivity. It has been reported that CPT increases the sensitivity of baroreflex control of SNA (5). Increased baroreflex sensitivity is likely to exert an increased inhibitory effect on the CPT-induced increases in SNA and pressor responses. Abolition of this inhibitory effect by barodenervation, therefore, exaggerated CPT-induced increases in SNA and MAP. On the other hand, CPT has been reported to have no effect on the sensitivity of baroreflex control of HR (5). In the absence of increased baroreflex sensitivity on HR control, the inhibitory effect on the CPT-induced increases in HR may be minimal. Thus barodenervation did not exaggerate CPT-induced increases in HR.
Adrenalectomy has been reported to have no effect on CPT-induced responses (6, 20). Because these observations were made in human subjects, it became necessary to confirm these findings in our rat model. Our results regarding the persistence of cardiovascular response after adrenalectomy in the rat are in agreement with the observations made in human subjects. Other investigators have reported that the CPT did not stimulate the release of renin, aldosterone, or vasopressin in human subjects (8, 26).
In conclusion, in the rat, CPT-induced increases in MAP were mediated by activation of the RVLM. Activation of brain areas, located rostral to the brain stem and projecting directly to the IML, also played a role in the CPT-induced pressor responses. Activation of the RVLM as well as the spinal cord was mediated via iGLURs. CPT-induced increases in HR were also mediated via the activation of iGLURs in the RVLM and spinal cord. In addition, inhibition of the nAmb via activation of GABA receptors also contributed to the CPT-induced tachycardic responses.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-024347 and HL-076248 awarded to H. N. Sapru.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barman SM Spinal cord control of the cardiovascular system. In: Nervous Control of Cardiovascular Function, edited by Randall WC. New York: Oxford Univ Press, 1984, p. 321–345.
- 2.Chitravanshi VC, Sapru HN. GABA receptors in the phrenic nucleus of the rat. Am J Physiol Regul Integr Comp Physiol 276: R420–R428, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Chitravanshi VC, Sapru HN. Microinjections of nociceptin into the nucleus ambiguus elicit tachycardia in the rat. Brain Res 1051: 199–204, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Craig AD, Dostrovsky JO. Differential projections of thermoreceptive and nociceptive lamina I trigeminothalamic and spinothalamic neurons in the cat. J Neurophysiol 86: 856–870, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Cui J, Wilson TE, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during cold pressor test in humans. Am J Physiol Heart Circ Physiol 282: H1717–H1723, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Cummings MF, Steele PM, Mahar LJ, Frewin DB, Russell WJ. The role of adrenal medullary catecholamine release in the response to a cold pressor test. Cardiovasc Res 17: 189–191, 1983. [DOI] [PubMed] [Google Scholar]
- 7.Dhruva A, Bhatnagar T, Sapru HN. Cardiovascular responses to microinjections of glutamate into the nTS of unanesthetized supracollicular decerebrate rats. Brain Res 801: 88–100, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Edelson JT, Robertson GL. The effect of the cold pressor test on vasopressin secretion in man. Psychoneuroendocrinology 11: 307–316, 1986. [DOI] [PubMed] [Google Scholar]
- 9.Edens JL, Gil KM. Experimental induction of pain: utility in the study of clinical pain. Behav Ther 26: 197–216, 1995. [Google Scholar]
- 10.Fagius J, Blumberg H. Sympathetic outflow to the hand in patients with Raynaud's phenomenon. Cardiovasc Res 19: 249–253, 1985. [DOI] [PubMed] [Google Scholar]
- 11.Guyenet PG The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Guyenet PG, Filtz TM, Donaldson SR. Role of excitatory amino acids in rat vagal and sympathetic baroreflexes. Brain Res 407: 272–284, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Han ZS, Zhang ET, Craig AD. Nociceptive and thermoreceptive lamina I neurons are anatomically distinct. Nat Neurosci 1: 218–225, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science (4th ed.). New York: McGraw-Hill, 2000.
- 15.Kasagi F, Akahoshi M, Shimaoka K. Relation between cold pressor test and development of hypertension based on 28-year follow-up. Hypertension 25: 71–76, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Kasamatsu K, Chitravanshi VC, Sapru HN. Depressor and bradycardic responses to microinjections of endomorphin-2 into the NTS are mediated via ionotropic glutamate receptors. Am J Physiol Regul Integr Comp Physiol 287: R715–R728, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular effects of adrenocorticotropin microinjections into the rostral ventrolateral medullary pressor area of the rat. Brain Res 1102: 117–126, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular function of a glutamatergic projection from the hypothalamic paraventricular nucleus to the nucleus tractus solitarius. Neuroscience 153: 605–617, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellett DO, Ramage AG, Jordan D. Central 5-HT7 receptors are critical for reflex activation of cardiac vagal drive in anaesthetized rats. J Physiol Lond 563: 319–331, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenders JW, Peters JH, Pieters GF, Willemsen JJ, Thien T. Hemodynamic reactivity to sympathoadrenal stimulation in adrenalectomized women. J Clin Endocrinol Metab 67: 139–143, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Lovallo W The cold pressor test and autonomic function: a review and integration. Psychophysiology 12: 268–282, 1975. [DOI] [PubMed] [Google Scholar]
- 22.Loyke HF Cold pressor test as a predictor of the severity of hypertension. South Med J 88: 300–304, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Madden CJ, Sved AF. Rostral ventrolateral medulla C1 neurons and cardiovascular regulation. Cell Mol Neurobiol 23: 739–749, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendelowitz D Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci 14: 155–161, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Mizushima T, Tajima F, Nakamura T, Yamamoto M, Lee KH, Ogata H. Muscle sympathetic nerve activity during cold pressor test in patients with cerebrovascular accidents. Stroke 29: 607–612, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Mizushima T, Tajima F, Okawa H, Umezu Y, Furusawa K, Ogata H. Cardiovascular and endocrine responses during the cold pressor test in subjects with cervical spinal cord injuries. Arch Phys Med Rehab 84: 112–118, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Osborn JW, England SK. Normalization of arterial pressure after barodenervation: role of pressure natriuresis. Am J Physiol Regul Integr Comp Physiol 259: R1172–R1180, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed.). New York: Academic, 1986.
- 29.Sapru HN Spinal mechanisms in the sympathetic control of cardiac function. In: Central Neural Mechanisms in Blood Pressure Regulation, edited by Kunos G and Ciriello J. Boston, MA: Birkhauser, 1991, p. 183–208.
- 30.Sapru HN Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exp Pharmacol Physiol 29: 491–496, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Sapru HN, Gonzalez ER, Krieger AJ. Greater splanchnic nerve activity in the rat. Brain Res Bull 8: 267–272, 1982. [DOI] [PubMed] [Google Scholar]
- 32.Scharf M, Korczyn AD. Cold pressor test. In: Handbook of Autonomic Nervous System Dysfunction, edited by Korczyn AD. New York: Decker, 1995, p. 557–562.
- 33.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 514–521, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Seneviratne BI, Linton I, Wilkinson R, Rowe W, Spice M. Cold pressor test in diagnosis of coronary artery disease: echophonocardiographic method. Br Med J (Clin Res Ed) 286: 1924–1926, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundaram K, Murugaian J, Sapru HN. Cardiac responses to the microinjections of excitatory amino acids into the intermediolateral cell column of the rat spinal cord. Brain Res 482: 12–22, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Sundaram K, Sapru HN. NMDA receptors in the intermediolateral column of the spinal cord mediate sympathoexcitatory responses elicited from the ventrolateral medullary pressor area. Brain Res 544: 33–41, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol 504: 479–488, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velasco M, Gomez J, Blanco M, Rodriguez I. The cold pressor test: pharmacological and therapeutic aspects. Am J Ther 4: 34–38, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Viard E, Sapru HN. Cardiovascular responses to activation of metabotropic glutamate receptors in the NTS of the rat. Brain Res 952: 308–321, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Victor RG, Leimbach WN Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Wang JJ, Irnaten M, Mendelowitz D. Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain Res 889: 78–83, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Willette RN, Barcas PP, Krieger AJ, Sapru HN. Vasopressor and depressor areas in the rat medulla: identification by l-glutamate microinjections. Neuropharmacology 22: 1071–1079, 1983. [DOI] [PubMed] [Google Scholar]