Abstract
Flow-mediated dilation (FMD) has become a commonly applied approach for the assessment of vascular function and health, but methods used to calculate FMD differ between studies. For example, the baseline diameter used as a benchmark is sometimes assessed before cuff inflation, whereas others use the diameter during cuff inflation. Therefore, we compared the brachial artery diameter before and during cuff inflation and calculated the resulting FMD in healthy children (n = 45; 10 ± 1 yr), adults (n = 31; 28 ± 6 yr), and older subjects (n = 22; 58 ± 5 yr). Brachial artery FMD was examined after 5 min of distal ischemia. Diameter was determined from either 30 s before cuff inflation or from the last 30 s during cuff inflation. Edge detection and wall tracking of high resolution B-mode arterial ultrasound images was used to calculate conduit artery diameter. Brachial artery diameter during cuff inflation was significantly larger than before inflation in children (P = 0.02) and adults (P < 0.001) but not in older subjects (P = 0.59). Accordingly, FMD values significantly differed in children (11.2 ± 5.1% vs. 9.4 ± 5.2%; P = 0.02) and adults (7.3 ± 3.2% vs. 4.6 ± 3.3%; P < 0.001) but not in older subjects (6.3 ± 3.4% vs. 6.0 ± 4.2%; P = 0.77). When the diameter before cuff inflation was used, an age-dependent decline was evident in FMD, whereas FMD calculated using the diameter during inflation was associated with higher FMD values in older than younger adults. In summary, the inflation of the cuff significantly increases brachial artery diameter, which results in a lower FMD response. This effect was found to be age dependent, which emphasizes the importance of using appropriate methodology to calculate the FMD.
Keywords: methodology
flow-mediated dilation (FMD) describes the vasodilatation of a conduit artery following an increase in shear stress, typically induced by a 5-min period of ischemia induced by a limb cuff placed distal to the recording probe (3). With the assumption that appropriate methodology is utilized (12, 36), the FMD response is largely nitric oxide (NO) mediated (13, 19) and provides information about the integrity of the endothelium (38). Since impaired endothelial vasodilator function is considered an early and integral manifestation of vascular disease, which predicts cardiovascular events (10, 14, 18, 20, 25), the FMD approach has become a popular and frequently applied technique in clinical and physiological research.
FMD results depend critically upon the methodology used to assess and analyze vascular images. For example, several important issues relating to stimulus magnitude were recently outlined (30), and the time course of the response is also a critical consideration that impacts upon data interpretation (2). One important methodological issue that has not been critically examined relates to the baseline diameter selected for calculation of the FMD.
There are two commonly applied approaches used to assess baseline diameter, which differ according to whether measurements are collected before or during distal cuff inflation. In the classic studies, which introduced the technique (3), resting diameter preceding cuff inflation was used. This remains the most frequently applied method adopted in the literature (2, 21, 22, 34, 37). In contrast, some investigators assess baseline diameter from data collected while the adjacent occluding cuff is inflated (7, 23, 24, 28, 29). The latter approach is based on the assumption that the diameter immediately preceding cuff deflation more accurately reflects the starting point from which dilation occurs. However, it is also possible that tissue displacement, imaging artifact, or pressure changes (16) induced by cuff inflation may modify the resting diameter. The aim of this study was therefore to examine the impact of these two commonly utilized methods for the assessment of baseline diameter on the brachial artery diameter and, consequently, on calculating FMD responses in healthy young and older humans. We hypothesize that the inflation of the cuff would not impact upon baseline brachial artery or FMD responses.
METHODS
Subjects.
Healthy volunteers (n = 98) were recruited from the community and were stratified based on age into three different groups: children (n = 45; 9 to 10 yr), adults (n = 31; 20–41 yr), and older subjects (n = 22; 50–67 yr; Table 1). All subjects were nonsmokers and normotensive (<140/90 mmHg), and none had any history of diabetes, insulin resistance, or cardiovascular disease. None of the subjects used any medications. The Ethics Committee of the Liverpool John Moores University approved the study protocols, which adhered to the Declaration of Helsinki, and all subjects gave written informed consent.
Table 1.
Subject characteristics of participants
| Children | Adults | Older Subjects | ANOVA | |
|---|---|---|---|---|
| n | 45 | 31 | 22 | |
| Age, yr | 10±1* | 28±6* | 58±5* | <0.001 |
| Sex, male:female | 15:28 | 27:4 | 13:9 | |
| Blood pressure, mmHg | ||||
| Systolic | 106±8* | 113±9 | 118±15 | 0.002 |
| Diastolic | 63±3* | 67±7 | 69±10 | 0.01 |
| Mean | 77±4* | 82±7 | 85±11 | 0.002 |
| Height, cm | 141±7* | 179±8* | 170±10* | <0.001 |
| Weight, kg | 38.2±7.7* | 79.5±11.7* | 77.2±1.7* | <0.001 |
| Body mass index, kg·m2 | 19.1±2.7* | 24.7±3.0 | 26.4±5.0 | <0.001 |
Data are means ± SD; n, no. of participants/group. Participants were divided into three groups: children, adults, and older subjects.
Post hoc significant from other groups at P < 0.05.
Experimental design.
After patients reported to the laboratory, the brachial artery FMD response was measured after a resting period of at least 20 min. All measures were performed under standardized conditions in a quiet, temperature-controlled room and after at least 6 h of fast and at least 8 h of abstinence from caffeine or alcohol. No subject performed strenuous physical activity for at least 24 h before testing.
Brachial artery FMD.
Patients rested supine with the right arm extended and immobilized with foam, supported at an angle of ∼80° from the torso. Heart rate and mean arterial pressure were determined from an automated sphygmomanometer (Dinamap; GE Pro 300V2; Tampa, FL) on the contralateral arm. For the assessment of the FMD response, a rapid inflation/deflation pneumatic cuff was positioned on the imaged arm distal to the olecranon process to provide a stimulus to forearm ischemia. A 7.5 (Aspen; Acuson, Mountain View, CA) or 10 MHz (T3000; Terason, Aloka, UK) multifrequency linear array probe attached to a high-resolution ultrasound machine was used to image the brachial artery in the distal third of the upper arm. Ultrasound parameters were set to optimize longitudinal, B-mode images of the lumen/arterial wall interface. Continuous Doppler velocity assessment was obtained simultaneously and was collected using the lowest possible insonation angle (always <60°), which did not vary during each study (26). After a resting period of at least 20 min, 1 min of baseline recording of the brachial artery diameter and velocity was performed. Subsequently, the occlusion cuff was inflated to >200 mmHg for 5 min. Brachial artery diameter and velocity recording were restarted at least 30 s before cuff deflation and continued at least 3 min after deflation. Peak artery diameter and flow, and the time to reach this peak after cuff deflation, were recorded.
Brachial artery diameter and blood flow.
Posttest analysis of the brachial artery diameter was performed using custom-designed edge-detection and wall-tracking software, which is independent of investigator bias (39). Briefly, the echo Doppler signal was real-time encoded and stored as a digital file when using the Terason ultrasound machine. When the Aspen machine was used, the video signal was taken directly from the ultrasound machine and, using an IMAQ-PCI-1407 card, was encoded and stored as a digital DICOM file on a personal computer. In both situations, a subsequent software analysis of the data was performed at 30 Hz using an icon-based graphical programming language and toolkit (LabVIEW 6.02; National Instruments, Austin, TX). The initial phase of image analysis involved the identification of regions of interest (ROI) on the first frame of every individual study.
These ROI allowed automated calibration for diameters on the B-mode image and velocities on the Doppler strip. An ROI was drawn around the optimal area of the B-mode image, and within this ROI a pixel-density algorithm automatically identified the angle-corrected near- and far-wall e-lines for every pixel column within the ROI. The algorithm begins by dividing the ROI into an upper half, containing the near-wall lumen-intima interface, and a lower half containing the far-wall interfaces. The near-wall intimal edge is identified by a Rake routine that scans from the bottom to the top of the upper half of the ROI. The position of the edge is established by determining the point where the pixel intensity changes most rapidly. Typical B-mode ROI, therefore, contained ∼200–300 diameter measures/frame, the average of which was calculated and stored. This process occurred at 30 frames/s. A final ROI was drawn around the Doppler waveform and automatically detected the peak of the waveform. The mean diameter measure derived from within the B-mode ROI (above) was synchronized with the velocity measure derived from the Doppler ROI at 30 Hz. Ultimately, from this synchronized diameter and velocity data, blood flow [the product of cross-sectional area and Doppler velocity and shear rate (4 times velocity divided by diameter)] were calculated at 30 Hz. All data were written to file and retrieved for analysis in a custom-designed analysis package. We have shown that the reproducibility of diameter measurements using this semiautomated software is significantly better than manual methods, reduces observer error significantly, and possesses an intraobserver coefficient of variation of 6.7% (39).
Data analysis.
Peak diameter following cuff deflation was automatically detected according to an algorithm that identified the maximum bracket of data subsequent to the performance of a moving window smoothing function. This smoothing routine calculates the median value from 100 consecutive samples before the window shifts to the next bracket of data, which shares 20% overlap with the preceding bracket. The maximum value of all the calculated median values is then automatically detected and chosen to represent the peak of the diameter curve. FMD was calculated as the percent rise of this peak diameter from the preceding baseline diameter.
Baseline diameter and blood flow before, as well as during, cuff inflation were determined from the same B-mode ROI. A time frame of at least 20 s was used to calculate baseline diameter before inflation and during cuff inflation. Baseline diameter before inflation was assessed in the final 30 s before cuff inflation, whereas baseline diameter during inflation data were collected during the final 30 s before cuff deflation. To examine the impact of these different baseline diameters on FMD, we calculated the FMD from an identical peak diameter, but using the baseline diameters before and during cuff inflation.
Statistics.
Statistical analyses were performed using SPSS 14.0 (SPSS, Chicago, IL) software. All data are reported as means (SD), and statistical significance was assumed at P ≤ 0.05. Paired t-tests were used to examine differences between the two baseline diameters and between the FMD percentages in the different age groups. A two-way ANOVA was performed (group × FMD measurement) to examine whether the impact of the FMD calculation using baseline diameter during or before inflation differed between groups. When a significant interaction was present, post hoc tests were performed with a Bonferroni correction for multiple comparison.
RESULTS
Subject characteristics.
Systolic, diastolic, and mean arterial blood pressure and body mass index (BMI) were significantly lower in children compared with adults and older subjects, whereas adults and older men did not differ in blood pressure or BMI (Table 1). Age, height, and weight were significantly different between the groups (Table 1).
Preinflation versus inflation brachial diameter by age group.
When data were clustered for all groups, brachial artery baseline diameter during inflation of the cuff was significantly higher than the baseline collected before cuff inflation (Table 2). Within individual groups, children and adults demonstrated a significant increase in diameter during cuff inflation compared with the preinflation baseline diameter (Table 2). In contrast, older subjects exhibited no difference between the brachial artery baseline diameters (Table 2).
Table 2.
Average diameter and the consequent FMD response before and during cuff inflation
| Shear Rate AUC, 104 | Dpreinfl | Dinfl | P Value | FMD Dpreinfl | FMD Dinfl | P Value | |
|---|---|---|---|---|---|---|---|
| Total | 2.7±1.7 | 3.46±1.0 | 3.51±1.0 | <0.001 | 8.9±4.7 | 7.1±4.9 | <0.001 |
| Children | 3.6±1.8* | 2.63±0.5 | 2.67±0.5 | 0.02 | 11.2±5.1 | 9.4±5.2 | 0.02 |
| Adults | 1.7±1.0 | 4.17±0.5 | 4.27±0.5 | <0.001 | 7.3±3.2 | 4.6±3.3 | <0.001 |
| Older subjects | 2.1±1.1 | 4.13±0.8 | 4.15±0.9 | 0.59 | 6.3±3.4 | 6.0±4.2 | 0.77 |
Data are means ± SD; n, no. of participants/group. Average diameter before inflation (Dpreinfl) and during cuff inflation (Dinfl) and the consequent flow-mediated dilation (FMD) response using the baseline diameter before FMD Dpreinfl or during FMD Dinfl presented for all subjects (n = 98) and in three groups: children (n = 45), adults (n = 31), and older subjects (n = 22). Shear rate area under the curve (AUC) peak diameter is presented as being the most appropriate eliciting stimulus for the FMD response (Ref. 29) for all subjects (n = 91), children (n = 44), adults (n = 25), and older subjects (n = 22). P values represent a paired t-test.
Significantly different from young and older adults (unpaired t-test).
FMD calculation using preinflation versus inflation brachial diameter by age group.
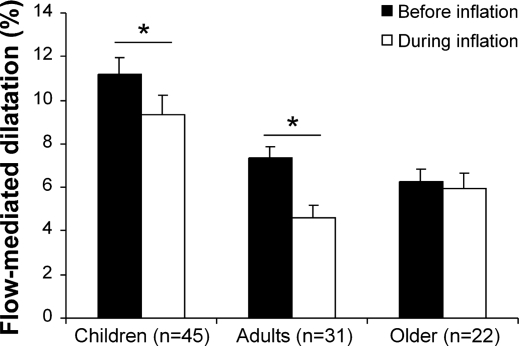
When the FMD response using diameters during inflation was calculated, a significantly lower FMD response was evident in children and adults when the cuff inflation baseline diameter was used (Fig. 1). In contrast, baseline diameters did not alter FMD in older adults (Fig. 1). The two-way ANOVA calculated between young and older adults revealed a significant interaction (P = 0.05). This indicates that the method used to calculate FMD has a different impact in young versus older adults; young adults had a lower FMD response when diamteter during inflation was used, whereas there was no difference in FMD in older adults using both methods (Fig. 1).
Fig. 1.
Brachial artery flow-mediated dilation (FMD; presented as percent change from baseline diameter) in children (n = 45), adults (n = 31), and older subjects (n = 22). The FMD is calculated using the diameter before (black bars) and during (white bars) cuff inflation. *Significantly different between FMD calculation using the diameter before or during cuff inflation at P < 0.05 (paired t-test). Error bars represent SE.
DISCUSSION
This is the first study, to our knowledge, that has directly examined the impact of the two commonly used methods of baseline brachial artery diameter assessment to calculate FMD responses. We observed that the brachial artery during cuff inflation is significantly larger than before inflation. Consequently, a significantly lower FMD was evident when this larger cuff inflation diameter was used to calculate the FMD. More importantly, the impact of cuff inflation on the baseline diameter was found to be group specific. Although we found a significant increase in diameter during cuff inflation in children and healthy younger adults, no differences were reported for the older subjects. The impact of cuff occlusion on brachial artery diameter is, therefore, age dependent. This resulted in a significant impact of baseline selection on FMD in children and younger adults but had no effect in older individuals. Taken together, these observations indicate the method chosen to calculate baseline diameter importantly impacts upon comparisons undertaken between groups. This finding has implications for future studies using the FMD technique where groups are compared or individuals undergo interventions that may affect baseline diameters.
Thousands of articles have reported the use of FMD as an outcome measure, but optimal methodological approaches continue to evolve. Although attempts have been made to standardize some aspects of the methodology (5), these have ignored important issues that may impact upon the validity and interpretation of FMD measurements. For example, recent attention has focused on characterizing and normalizing FMD responses for their eliciting shear stress or shear rate stimulus (27, 29, 30). Other studies have reported important impacts on the interpretation of FMD findings according to the method used to identify the peak diameter following cuff deflation (2). Although baseline diameter importantly contributes to the calculation of the FMD, this study is the first, to our knowledge, to consider the impact of using different approaches to baseline assessment. In marked contrast to our hypothesis, cuff inflation resulted in a small, but significant, increase in brachial artery diameter, which impacts significantly on the calculation of FMD. The increase in brachial artery diameter during cuff inflation is not due to the accumulation of ischemic metabolites, since the artery is scanned above the cuff location. Given that shear stress on the endothelium is a potent vasodilator stimulus (15), it is possible that cuff inflation increases diameter via a rise in shear stress during cuff inflation. However, previous studies have reported lower brachial artery shear rates during cuff inflation (1, 7), so this is unlikely to explain the increase in diameter that we observed. Other possibilities likely include local arterial pressure changes immediately proximal to the occluding cuff, influencing brachial diameter, and a recent review has suggested that transmural pressure gradients induce changes in vasodilator function (16). Finally, cuff inflation may also alter diameter measurements as a consequence of local tissue displacement, which influence the image quality.
Our findings indicate that the change in diameter during cuff inflation is age dependent since the brachial artery diameter increased during cuff inflation in children and adults but not in older subjects. At first glance, the finding contrasts with that of Parker and coworkers (23, 24), who did not report a significant difference between preinflation and inflation baseline data. However, although not significant, young women reported a larger diameter during inflation compared with preinflation (+0.08 mm) (23), a value that corresponds with that observed in the current study in young individuals (+0.10 mm). Both the present study and that of Parker et al. (23) demonstrated similarly diminished effects of cuff inflation on brachial diameter in older (+0.02 mm) compared with younger (+0.08–0.10 mm) individuals. The absence of significant differences in this previous study might therefore relate to the relatively small numbers studied (n = 14–16). The results of Parker et al. (23) therefore essentially reinforce our findings of an age-dependent effect of cuff inflation on baseline brachial artery diameter. We suggest that such differences can importantly impact upon the interpretation of studies that compare disparate groups.
The age-dependent effect on the response of the brachial artery diameter to cuff inflation might be explained by changes in arterial wall characteristics, which accompany age. For example, the age-related increase in arterial stiffness, commonly reported when cross-sectional comparison is made between age groups (17, 32, 33), could explain the smaller response to cuff inflation in these individuals. Increased stiffness of the vessel would likely result in less reactivity to any given stimulus, including small changes in pressure or tissue translocation. Another possible explanation for the lack of response in diameter with age relates to the attenuation in endothelial function (6, 31). Taken in the context of these studies, our findings suggest that differences in arterial wall characteristics with age likely impact the diameter change we observed in response to cuff inflation.
In a recent paper, Gori et al. (11) reported a decrease in the radial artery diameter when examining this conduit artery within the ischemic zone during cuff inflation and hypothesized that this response is related to vascular health, since constriction is attenuated using the endothelium-derived hyperpolarizing factor inhibition. Although this study (11) and our results both examined diameter changes in a low-flow state, we observed an increase, whereas Gori et al. (11) reported a decrease in diameter. Differences in methodology (diameter assessment within and above the ischemic zone) likely explain the differences between these studies, since relative placements of the cuff and probe will impact upon the contribution of metabolite build-up and transmural pressures. Gori et al. (11) observed an impaired constricting response in smokers, hypertensives, and subjects with coronary disease, suggesting the presence of impaired vasoactive function in subjects at increased cardiovascular risk. In keeping with these results, we observed no dilating response in older subjects, a group also at increased cardiovascular risk. Taken together, these studies suggest that the arteries of subjects with cardiovascular disease risk factors are less sensitive to physiological stimuli. The clinical relevance and utility of the assessment of proximal and distal cuff inflation effects on artery diameter remains largely unexplored, and basic physiological mechanistic studies before their widespread adoption to this end are needed.
The age-specific changes in baseline diameters we observed have widespread consequences for the calculation of the FMD response. When the diameter before cuff inflation was used, an age-dependent gradual decrease in brachial artery FMD response was evident in our data set. This finding is in agreement with several previous studies using the noninvasive FMD technique (2) or intrabrachial infusion of vasoactive substances in animals (4) and humans (6, 9, 31, 35). In marked contrast, when the diameter during cuff inflation was used, FMD responses in older adults were greater than those we observed in younger adults; that is, the generally accepted age-dependent decline in endothelial function was not apparent when the cuff inflation baseline diameter was used. Despite identical peak FMD diameters being used in both calculations, the differences in baseline diameter impacted on the interpretation of age-related changes in endothelial function. These unexpected findings highlight the importance of choosing an appropriate and consistent baseline diameter in FMD studies to reduce the variability of FMD measurements between studies. The recent study of Gori et al. (11), which also reported altered arterial diameters in response to cuff inflation, reinforces this suggestion.
Limitations.
Assessment of arterial dilation in response to ischemic stimuli is a valuable and frequently applied technique to examine conduit artery function and health. Our findings, however, specifically relate to a 5-min ischemic stimulus where the occluding cuff was placed distal to the ultrasound probe. Previous evidence suggests that this methodology results in flow-mediated dilator responses, which are largely NO dependent. Our findings cannot be generalized to protocols that examine the artery within the ischemic zone or using ischemic stimuli exceeding 5 min.
In conclusion, we found that the two commonly utilized approaches for assessment of baseline arterial diameter result in significant differences in calculated FMD and the interpretation of group differences in artery function. We suggest that using the baseline diameter prior to cuff inflation for FMD studies will make comparisons to prior work more valid, since most of these studies have used this baseline. The FMD percentage reported using the predeflation diameter may also be more valid because it may reduce confounding of this measurement by age and others risk factors and because it has the potential to reduce any direct effects of cuff inflation on FMD measures.
GRANTS
D. H. J. Thijssen is financially supported by The Netherlands Organization for Scientific Research Grant 82507010. M. A. Black is supported the British Heart Foundation Grant FS/05/117/19971.
Acknowledgments
We thank Chris Reed for assistance with development of the edge detection and wall-tracking software.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Betik AC, Luckham VB, Hughson RL. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol Heart Circ Physiol 286: H442–H448, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res 83: 279–286, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. [DOI] [PubMed] [Google Scholar]
- 6.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol 290: H1446–H1453, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27: 849–853, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Gocke N, Keaney JF, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 105: 1567–1572, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD. Conduit artery constriction mediated by low flow. A novel non-invasive method for the assessment of vascular function. J Am Coll Cardiol 51: 1953–1958, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Green D Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1233–1234, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Kuvin JT, Patel RR, Sliney KA, Pandian NG, Rand WM, Udelson JE, Karas RH. Peripheral vascular endothelial function testing as a non-invasive indicator of coronary artery disease. J Am Coll Cardiol 38: 1843–1849, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231: 405–407, 1986. [DOI] [PubMed] [Google Scholar]
- 16.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 104: 588–600, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyachi M, Donato AJ, Yamamoto K, Takahashi K, Gates PE, Moreau KL, Tanaka H. Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension 41: 130–135, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 40: 505–510, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner G, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol 86: 207–210, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol 103: 843–851, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Ou P, Celermajer DS, Mousseaux E, Giron A, Aggoun Y, Szezepanski I, Sidi D, Bonnet D. Vascular remodeling after “successful” repair of coarctation: impact of aortic arch geometry. J Am Coll Cardiol 49: 883–890, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol 291: H3043–H3049, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Parker BA, Smithmyer SL, Jarvis SS, Ridout SJ, Pawelczyk JA, Proctor DN. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol 292: H1148–H1156, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Perticone F, Ceravolo R, Puji A, Ventura G, Iacopino S, Scozzafva A, Ferraro A, Chello M, Mastroroberto P, Verdechhia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Potter K, Reed CJ, Green DJ, Hankey GJ, Arnolda LF. Ultrasound settings significantly alter arterial lumen and wall thickness measurements. Cardiovasc Ultrasound 6: 6, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol 97: 499–508, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Pyke KE, Poitras V, Tschakovsky ME. Brachial artery flow-mediated dilation during handgrip exercise: evidence for endothelial transduction of the mean shear stimulus. Am J Physiol Heart Circ Physiol 294: H2669–H2679, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka H, Seals DR, Monahan KD, Clevenger CM, DeSouza CA, Dinenno FA. Regular aerobic exercise and the age-related increase in carotid artery intima-media thickness in healthy men. J Appl Physiol 92: 1458–1464, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Thijssen DH, de Groot PC, Smits P, Hopman MT. Vascular adaptations to 8-week cycling training in older men. Acta Physiol (Oxf) 190: 221–228, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Thijssen DH, Rongen GA, van Dijk A, Smits P, Hopman MT. Enhanced endothelin-1-mediated leg vascular tone in healthy older subjects. J Appl Physiol 103: 852–857, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1235–1237, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Vita JA, Holbrook M, Palmisano J, Shenouda SM, Chung WB, Hamburg NM, Eskenazi BR, Joseph L, Shapira OM. Flow-induced arterial remodeling relates to endothelial function in the human forearm. Circulation 117: 3126–3133, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]