Abstract
Postconditioning (POC), a novel strategy of cardioprotection against ischemia-reperfusion injury, is clinically attractive because of its therapeutic application at the predictable onset of reperfusion. POC activates several intracellular kinase signaling pathways, including phosphatidylinositol 3-kinase (PI3K)-Akt (RISK). The regulation of POC-induced survival kinase signaling, however, has not been fully characterized. JAK-STAT activation is integral to cardiac ischemic tolerance and may provide upstream regulation of RISK. We hypothesized that POC requires the activation of both JAK-STAT and RISK signaling. Langendorff-perfused mouse hearts were subjected to 30 min of global ischemia and 40 min of reperfusion, with or without POC immediately after ischemia. A separate group of POC hearts was treated with AG 490, a JAK2 inhibitor, Stattic, a specific STAT3 inhibitor, or LY-294002, a PI3K inhibitor, at the onset of reperfusion. Cardiomyocyte-specific STAT3 knockout (KO) hearts were also subjected to non-POC or POC protocols. Myocardial performance (+dP/dtmax, mmHg/s) was assessed throughout each perfusion protocol. Phosphorylated (p-) STAT3 and Akt expression was analyzed by Western immunoblotting. POC enhanced myocardial functional recovery and increased expression of p-STAT3 and p-Akt. JAK-STAT inhibition abrogated POC-induced functional protection. STAT3 inhibition decreased expression of both p-STAT3 and p-Akt. PI3K inhibition also attenuated POC-induced cardioprotection and reduced p-Akt expression but had no effect on STAT3 phosphorylation. Interestingly, STAT3 KO hearts undergoing POC exhibited improved ischemic tolerance compared with KO non-POC hearts. POC induces myocardial functional protection by activating the RISK pathway. JAK-STAT signaling, however, is insufficient for effective POC without PI3K-Akt activation.
Keywords: phosphatidylinositol 3-kinase-Akt, STAT3, ischemia-reperfusion
reperfusion is essential for myocardial salvage following ischemic injury. The sudden restoration of blood flow and oxygen delivery to ischemic tissues, however, further injures cells altered but not irreversibly damaged by ischemia (26). Brief periods of ischemia and reperfusion applied at the onset of reperfusion following a prolonged period of ischemia is known as postconditioning (POC). This phenomenon modifies the onset and deleterious effects of reperfusion and attenuates myocardial injury by reducing endothelial dysfunction, cardiomyocyte apoptosis, and the inflammatory response to reperfusion (21, 22). POC has generated considerable interest because of its therapeutic potential. Unlike ischemic preconditioning (IPC), which is applied before an ischemic event, POC is applied at the predictable onset of reperfusion and does not require anticipation of ischemia (5, 25).
Preconditioning and POC strategies may use similar signal transduction pathways, however, the timing and/or mediators may differ (8, 9). Commonly initiated cellular signaling events include the activation of survival kinase pathways such as reperfusion injury salvage kinase (RISK) and JAK-STAT (9). RISK pathway initiation occurs via G protein-coupled receptor activation by ligands such as insulin, bradykinin, and cardiotrophin-1 (8). JAK-STAT signaling is induced by the binding of cytokines and growth factors (e.g., IL-6 and cardiotrophin-1) to gp130-associated transmembrane receptors (6). Previous studies have suggested that activation of either pathway may induce the protective effects of POC, however, the interaction between RISK and JAK-STAT signaling is unclear (2, 20).
The JAK-STAT pathway is comprised of a family of receptor-associated cytosolic tyrosine kinases (JAKs) that activate signal-dependent transcription factors (STATs). We and others have shown that this pathway is activated by stresses such as ischemia, mechanical stress, cytotoxic agents, or bacterial inflammation (4, 6, 10). Activation of STAT3 reduces infarct size in several POC protocols, however, its role in POC-induced functional protection and its interaction with other survival kinase pathways have not been fully characterized (2). Similarly, RISK activation via phosphatidylinositol 3-kinase (PI3K)-Akt has been implicated in the signaling mechanisms responsible for limiting infarct development in both IPC and POC, but few studies have investigated the interaction between RISK and JAK-STAT (8).
The purpose of our study was to characterize the interaction of two different survival kinase pathways activated by cardiac POC. We hypothesized that unlike preconditioning, POC requires the activation of both JAK-STAT and RISK signaling. Our results suggest that JAK-STAT signaling is insufficient for effective POC without PI3K-Akt activation.
MATERIALS AND METHODS
Animals.
All animal protocols conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23) and were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati. Wild-type (WT) male C57BL/6 mice at 6–8 wk of age (Harlan, Indianapolis, IN) and cardiomyocyte-specific STAT3-deleted [STAT3 knockout (KO)] animals were provided a standard mouse chow diet and water ad libitum and acclimatized for 2–3 days before experimentation. Hearts from STAT3 KO mice were subjected to isolated heart perfusion at 8–10 wk of age (11). WT (C57BL/6), age-matched animals served as controls.
Isolated heart perfusion.
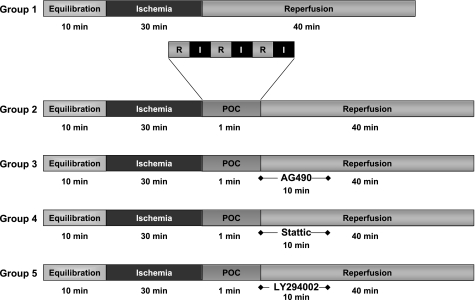
Randomly selected animals were anesthetized (90 mg/kg ketamine and 10 mg/kg xylazine) and heparinized (200 units) via intraperitoneal injection. The hearts were rapidly excised into warmed, oxygenated Krebs-Henseleit buffer, and the aorta was cannulated with a 23-gauge cannula. Langendorff perfusion commenced with normothermic (37°C), oxygenated (95% O2-5% CO2) Krebs-Henseleit buffer (in mM: 10 glucose, 118 NaCl, 2.5 CaCl2, 4.7 KCl, and 25 NaHCO3; pH 7.4) at a constant flow of 2–3 ml/min. Aortic pressure was maintained at 50–55 mmHg throughout the perfusion protocol. A cannula inserted into the left ventricle via an incision in the left atrium was connected to a pressure transducer (ADInstruments, Milford, MA) and coupled to a PowerLab/400 (ADInstruments) for continuous data recording. Hearts were equilibrated in a warmed (37°C), dry chamber for 10 min before any intervention. Hearts that could not achieve a minimal left ventricular pressure (LVP) of 85 mmHg and a coronary flow of 2–3 ml/min at the end of the equilibration period were discarded. A three-way stopcock above the aortic root was used to create global ischemia, during which hearts were immersed in a 37°C degassed organ bath. Following equilibration, WT hearts were randomly assigned (coin flip) to one of five treatment groups (Fig. 1). Group 1 hearts (n = 7), the control group (non-POC), underwent 30 min of global, normothermic ischemia and 40 min of reperfusion. Group 2 hearts (n = 7) were subjected to 30 min of global ischemia, POC (3 cycles of alternating 10-s intervals of reperfusion followed by 10 s of ischemia), and 40 min of reperfusion. Groups 3, 4, and 5 (n = 6 each) underwent protocols identical to that of group 2 but were treated with the JAK2 inhibitor AG 490 (20 μM), Stattic (100 μM), a small molecule direct STAT3 inhibitor, or the PI3K inhibitor LY-294002 (15 μM), respectively, during the first 10 min of reperfusion following the POC stimulus. Additional groups of hearts treated with the JAK-STAT or RISK inhibitors on reperfusion without POC served as drug delivery controls. Treatment with 0.4% DMSO in both POC and non-POC protocols was performed to detect any independent effects of the DMSO vehicle. STAT3 KO hearts were subjected to the previously described non-POC (group 6) and POC (group 7) protocols. Cardiac function was assessed at the end of equilibration (baseline) and at 10-min intervals during reperfusion by determining the maximum and minimum first derivative of developed pressure (DP) (+dP/dtmax and −dP/dtmin, respectively, in mmHg/s). Coronary flow (milliliters per minute) was measured by collecting pulmonary artery effluent at each time point.
Fig. 1.
Time course of experimental protocols. Hearts were randomized to 1 of 5 protocols. n = 7 for groups 1 and 2; n = 6 for groups 3, 4, and 5. POC, postconditioning; I, ischemia; R, reperfusion.
Pharmacological inhibitors.
AG 490 (Calbiochem, La Jolla, CA), a nonspecific tyrosine kinase inhibitor, limits JAK-STAT signaling via anti-JAK2 activity (IC50 = 10 μM) (23). AG 490 was reconstituted in DMSO, diluted in deionized water to 0.4% DMSO, and infused at 20 μM over 10 min (0.5 ml/min). Stattic (Calbiochem), a specific and direct inhibitor of the Src homology 2 (SH2) functional domain of STAT3 (17), was reconstituted in DMSO, diluted in Krebs-Henseleit buffer to 0.4% DMSO, and infused at 100 μM over 10 min (0.5 ml/min). The RISK pathway was interrogated by direct inhibition of PI3K with LY-294002 (Calbiochem; IC50 = 1.4 μM). After reconstitution in DMSO and dilution with Krebs-Henseleit buffer to 0.4% DMSO, the LY-294002 was infused at 15 μM over 10 min (0.5 ml/min).
Protein isolation and Western blot analysis.
At the completion of the perfusion protocols, the hearts were rapidly frozen in liquid nitrogen and stored at −80°C until analysis. Preparation of nuclear extracts to identify phosphorylated (activated) and translocated STAT protein was performed using NXTRACT (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's recommendations with the addition of phosphatase inhibitors (200 mM imidazole, 100 mM NaFl, 115 mM sodium molybdate, 100 mM sodium orthovanadate, 400 mM sodium tartrate dehydrate) comparable to Phosphatase Inhibitor Cocktail Set II (Calbiochem). Total protein concentrations were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL).
Aliquots of nuclear fractions, corresponding to 75 μg of protein, were separated by 10% SDS-PAGE (GeneMate Express gels; ISC BioExpress, Salt Lake City, UT), transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA), and blocked with 5% bovine serum albumin-TBS-Tween. STAT3 expression was analyzed using a primary monoclonal antibody against phosphorylated (p) STAT3705 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and total (t) STAT3 (1:200; Santa Cruz Biotechnology). Similarly, nuclear fractions were analyzed for p-STAT1 and t-STAT1 (1:200; Santa Cruz Biotechnology), p-STAT5 (1:1,000; Invitrogen, Carlsbad, CA), t-STAT5 (1:200; Santa Cruz Biotechnology), p-STAT6 (1:1,000; Millipore, Billerica, MA), and t-STAT6 (1:200; Santa Cruz Biotechnology). Cytosolic fractions using 100 μg of protein were analyzed for the expression of phosphorylated and total Akt (1:1,000; Cell Signaling Technology, Beverly, MA). To normalize protein loading, the membranes were analyzed with the primary antibody for calsequestrin (1:2,500; Affinity Bioreagents, Golden, CO). Intensity of protein expression is indicated by arbitrary units derived by dividing the densitometry values (AlphaEase) determined for each protein by their respective calsequestrin densitometry values. Immunoreactive signals were visualized with chemiluminescence luminal reagents (ECL; Amersham Pharmacia Biotech, Piscataway, NJ).
Statistical analysis.
Data are expressed as means ± SE. Statistical analysis was performed by two-tailed t-test or one-way ANOVA with Tukey's post hoc analysis for multiple comparisons; P < 0.05 was considered significant.
RESULTS
Baseline cardiodynamics.
Baseline cardiodynamics were obtained following 10 min of equilibration. There were no significant differences among the experimental groups in any of the baseline cardiodynamic variables assessed (Table 1).
Table 1.
Baseline cardiodynamics
| DP, mmHg | LVP, mmHg | EDP, mmHg | CF, ml/min | HR, beats/min | +dP/dtmax, mmHg/s | −dP/dtmin, mmHg/s | |
|---|---|---|---|---|---|---|---|
| Non-POC (n = 7) | 107±5 | 100±5 | −8±2 | 2.2±0.1 | 386±28 | 3,164±185 | −3,386±279 |
| POC (n = 7) | 115±5 | 103±5 | −13±1 | 2.5±0.2 | 387±14 | 3,226±252 | −3,569±326 |
| POC + AG 490 (n = 6) | 137±10 | 122±8 | −15±2 | 2.6±0.1 | 345±26 | 3,759±312 | −4,146±354 |
| POC + Stattic (n = 6) | 121±5 | 112±4 | −9±1 | 2.8±0.1 | 354±13 | 3,058±138 | −3,198±231 |
| POC + LY-294002 (n = 6) | 120±5 | 111±5 | −9±1 | 2.9±0.1 | 350±27 | 3,110±140 | −3,387±151 |
Values are expressed as means ± SE. POC, postconditioning; DP, developed pressure; LVP, left ventricular pressure; EDP, end-diastolic pressure; CF, coronary flow; HR, heart rate; +dP/dtmax and −dP/dtmin, the maximum and minimum 1st derivative of developed pressure, respectively.
POC and ischemic tolerance.
Ischemia-reperfusion challenge without postconditioning (non-POC) resulted in considerable impairment in both myocardial systolic and diastolic function. At end-reperfusion, non-POC hearts recovered only 25% of their baseline DP (end-reperfusion vs. baseline DP, 28 ± 5 vs. 107 ± 5 mmHg; P < 0.01). POC significantly enhanced end-reperfusion myocardial performance. Compared with non-POC hearts, POC significantly improved end-reperfusion DP, LVP, +dP/dtmax, and −dP/dtmin (Table 2). No differences in end-reperfusion coronary flow or heart rate were identified.
Table 2.
End-reperfusion cardiodynamics
| DP, mmHg | LVP, mmHg | EDP, mmHg | CF, ml/min | HR, beats/min | +dP/dtmax, mmHg/s | −dP/dtmin, mmHg/s | |
|---|---|---|---|---|---|---|---|
| Non-POC (n = 7) | 28±5 | 47±16 | 20±14 | 1.9±0.1 | 362±19 | 929±180 | −1,013±183 |
| Non-POC + AG 490 (n = 3) | 35±18 | 47±25 | 12±9 | 1.6±0.2 | 357±17 | 1,013±473 | −1,019±505 |
| Non-POC + Stattic (n = 3) | 20±10 | 52±17 | 32±17 | 1.8±0.6 | 309±30 | 667±306 | −575±263 |
| Non-POC + LY-294002 (n = 3) | 25±6 | 58±22 | 34±23 | 1.0±0.1* | 382±58 | 789±132 | −809±119 |
| POC (n = 7) | 64±8* | 91±5* | 27±11 | 2.2±0.2 | 381±14 | 1,950±156* | −1,967±210* |
| POC + AG 490 (n = 6) | 16±4† | 92±16 | 77±17† | 2.1±0.1 | 431±28 | 584±125† | −589±135† |
| POC + Stattic (n = 6) | 11±3† | 95±3 | 84±4† | 2.3±0.2 | 438±27 | 382±121† | −364±95† |
| POC + LY-294002 (n = 6) | 11±4† | 82±16 | 69±14† | 1.4±0.2† | 399±37 | 477±170† | −458±138† |
Values are expressed as means ± SE.
P < 0.05 vs. non-POC;
P < 0.05 vs. POC.
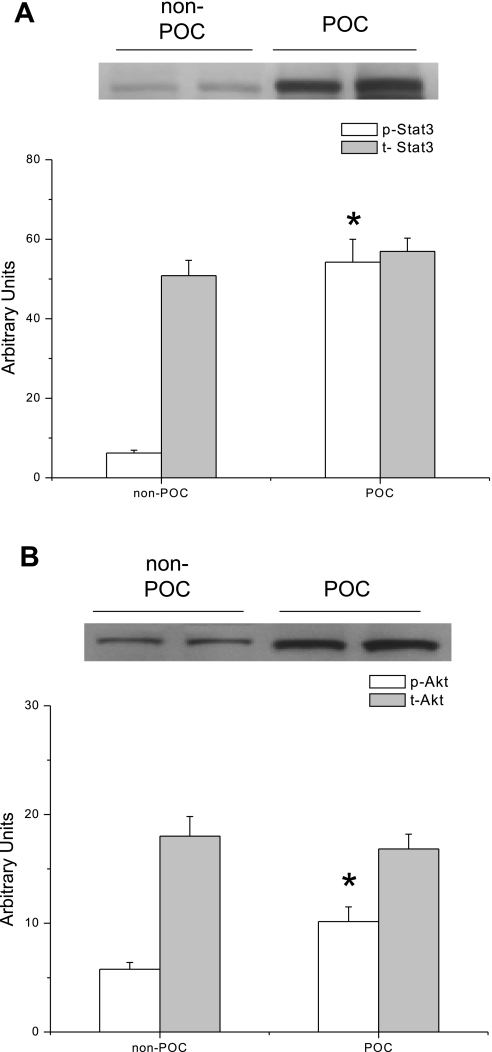
Western immunoblot analysis of myocardial nuclear fractions revealed significantly increased expression of phosphorylated STAT3 (Fig. 2A) and Akt (Fig. 2B) following POC. There were no significant differences in total or phosphorylated STAT1, -5, or -6 expression following POC (data not shown).
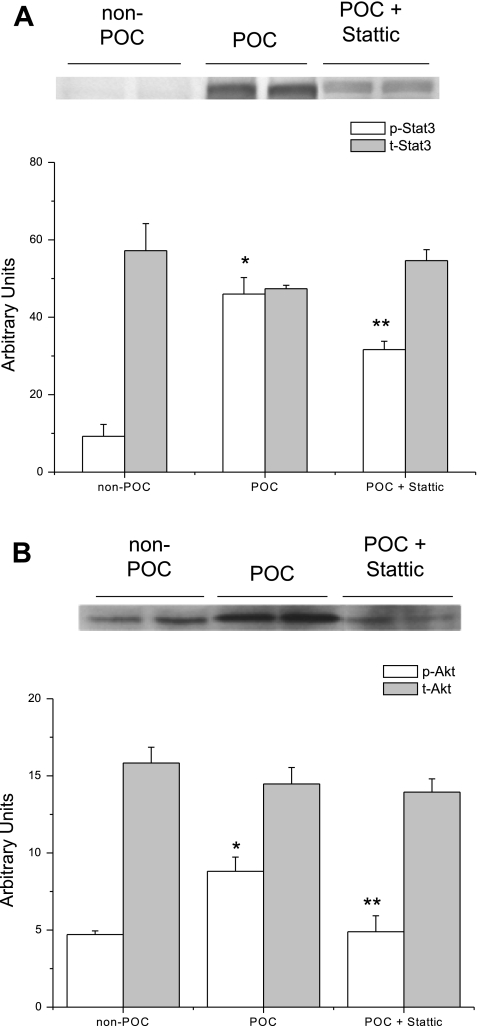
Fig. 2.
Representative immunoblots of protein expression in hearts that underwent no experimental protocol (non-POC) or POC. A: phosphorylated (p-) STAT3 expression is significantly increased in POC hearts compared with non-POC hearts. *P = 0.001 vs. non-POC. B: POC significantly increased the expression of p-Akt in POC hearts compared with non-POC hearts. *P = 0.04 vs. non-POC. t-, Total.
JAK-STAT signaling and POC.
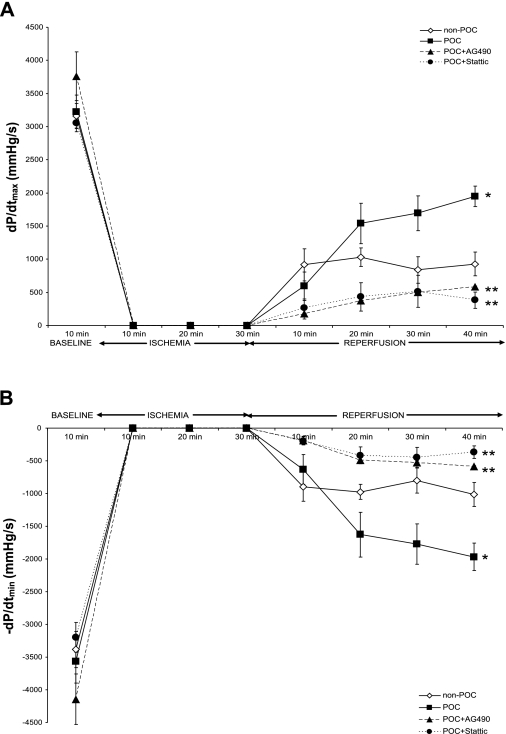
Treatment with inhibitors of two different components of the JAK-STAT pathway (JAK2 and STAT3) significantly attenuated recovery of myocardial function at end-reperfusion. AG 490 and Stattic significantly abrogated POC-induced improvements in +dP/dtmax (Fig. 3A) and −dP/dtmin (Fig. 3B) following ischemia-reperfusion challenge. End-reperfusion DP was significantly lower, and end-diastolic pressure (EDP) significantly higher in the presence of JAK2 and STAT3 inhibition (Table 2). Control hearts treated with Stattic or AG 490 during ischemia-reperfusion challenge without POC were not different from untreated hearts subjected to ischemia-reperfusion (Table 2). No differences in coronary flow were identified.
Fig. 3.
Myocardial function during ischemia-reperfusion, POC, or POC with AG 490 or Stattic, as reflected by maximum positive (+dP/dtmax; A) and negative (−dP/dtmin; B) 1st derivative of developed pressure. *P < 0.01 vs. non-POC; **P < 0.001 vs. POC.
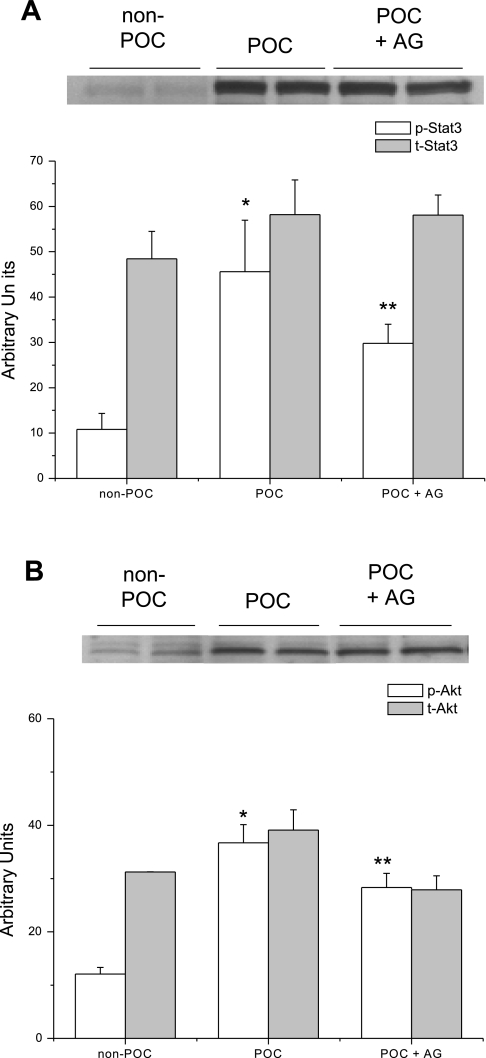
JAK2 inhibition with AG 490 had no effect on the expression of p-STAT3 (Fig. 4A) or p-Akt (Fig. 4B) compared with POC hearts (Fig. 4). Administration of Stattic, however, significantly decreased expression of p-STAT3 (Fig. 5A) and p-Akt (Fig. 5B). There were no differences in the expression of phosphorylated or total STAT1, -5, or -6 compared with POC hearts perfused in the absence of JAK-STAT inhibition (data not shown).
Fig. 4.
Representative immunoblots of phosphorylated protein expression in hearts that underwent no experimental protocol (non-POC), POC, or POC + 20 μM AG 490 (POC + AG). A: AG 490 treatment did not significantly reduce p-STAT3 expression compared with POC hearts without AG 490. *P = 0.04 vs. non-POC; **P = 0.02 vs. non-POC. B: similarly, expression of p-Akt was not significantly reduced following AG 490 treatment compared with POC hearts without the inhibitor. *P = 0.003 vs. non-POC; **P = 0.006 vs. non-POC.
Fig. 5.
Representative immunoblots of phosphorylated protein expression in hearts that underwent no experimental protocol (non-POC), POC, or POC + 100 μM Stattic. A: p-STAT3 expression is significantly decreased in POC + Stattic hearts compared with POC hearts without Stattic. *P = 0.003 vs. non-POC; **P = 0.01 vs. POC. B: similarly, Stattic significantly reduced expression of p-Akt compared with POC hearts without the inhibitor. *P = 0.01 vs. non-POC; **P < 0.05 vs. POC.
PI3K-Akt signaling and POC.
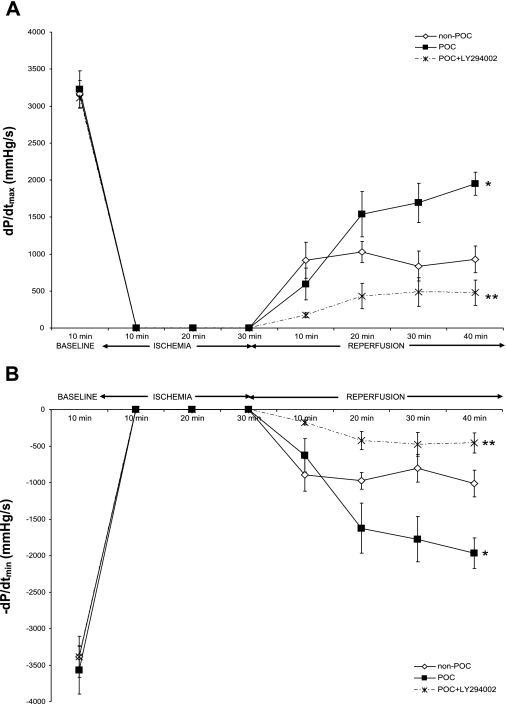
Administration of LY-294002 significantly diminished POC-induced cardioprotection. Similar to the effects of JAK-STAT inhibitors, LY-294002 caused a significant reduction in end-reperfusion DP, +dP/dtmax (Fig. 6A), and −dP/dtmin (Fig. 6B) and increased end-reperfusion EDP compared with untreated POC hearts (Table 2). In contrast to the Stattic- and AG 490-treated hearts, however, PI3K inhibition significantly attenuated end-reperfusion coronary flow following POC (POC + LY-294002 vs. POC, 1.4 ± 0.2 vs. 2.2 ± 0.2 ml/min; P = 0.03). Treatment with LY-294002 in the absence of POC had no significant effect on myocardial functional recovery compared with non-POC hearts (Table 2); however, end-reperfusion coronary flow was decreased (non-POC + LY-294002 vs. non-POC, 1.0 ± 0.1 vs. 1.9 ± 0.1 ml/min; P = 0.03).
Fig. 6.
Myocardial function following ischemia-reperfusion, POC, or POC with LY-294002 (LY) as reflected by maximum positive (+dP/dtmax; A) and negative (−dP/dtmin; B) 1st derivative of developed pressure. *P < 0.01 vs. non-POC; **P < 0.001 vs. POC.
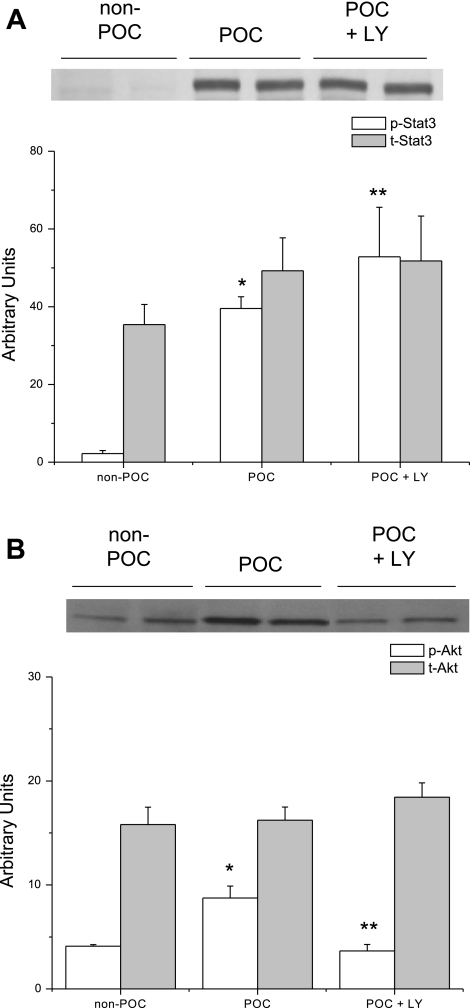
As expected, LY-294002 treatment reduced p-Akt expression but interestingly had no effect on p-STAT3 expression compared with POC hearts not treated with the inhibitor (Fig. 7). These results suggest that RISK signaling does not have a downstream effect on STAT3 activation following POC.
Fig. 7.
Representative immunoblots of phosphorylated protein expression in hearts that underwent no experimental protocol (non-POC), POC, or POC + 15 μM LY. A: p-STAT3 expression is significantly increased in POC + LY hearts compared with POC hearts without LY. *P < 0.001 vs. non-POC; **P = 0.03 vs. POC. B: LY significantly reduced the expression of p-Akt compared with POC hearts without the inhibitor. *P = 0.02 vs. non-POC; **P = 0.003 vs. POC.
Myocardial recovery and cardiac-specific STAT3 deletion.
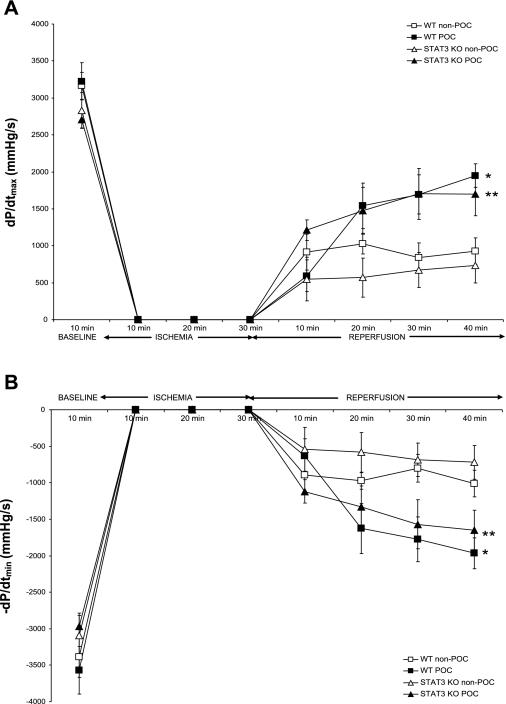
To determine if p-STAT3 is necessary for POC, we examined myocardial performance following POC in STAT3 KO hearts. The recovery of myocardial function in STAT3 KO hearts was not different from that of WT hearts following ischemia-reperfusion challenge. Interestingly, POC effectively protected the cardiac function in STAT3 KO hearts as demonstrated by enhanced recovery of end-reperfusion +dP/dtmax (Fig. 8A) and −dP/dtmin (Fig. 8B).
Fig. 8.
Myocardial function during ischemia-reperfusion or POC in wild-type (WT) and cardiomyocyte-restricted STAT3 knockout (KO) hearts, as reflected by maximum positive (+dP/dtmax; A) and negative (−dP/dtmin; B) 1st derivative of developed pressure. *P < 0.01 vs. WT non-POC; **P = 0.02 vs. STAT3 KO non-POC.
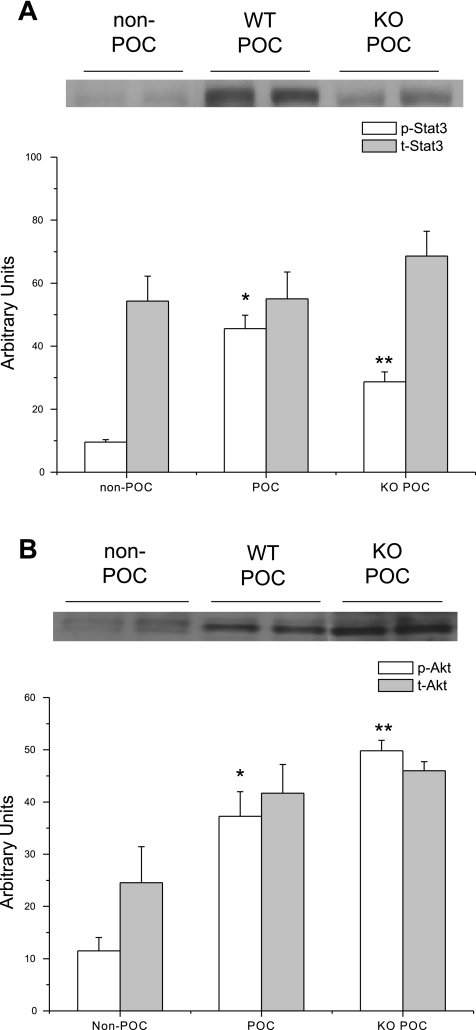
Left ventricular nuclear fractions from STAT3 KO POC hearts demonstrated increased expression of p-Akt but reduced expression of p-STAT3 compared with WT POC hearts (Fig. 9). The results of the physiological and molecular analysis in STAT3 KO POC hearts implicate a critical role for RISK signaling in effective POC.
Fig. 9.
Representative immunoblots of protein expression in STAT3 and WT hearts subjected to no experimental protocol (non-POC) or POC. A: p-STAT3 expression is significantly reduced following POC in STAT3 KO hearts compared with WT hearts. *P = 0.001 vs. non-POC; **P = 0.03 vs. WT POC. B: POC in STAT3 KO hearts significantly increased the expression of p-Akt compared with POC in WT hearts. *P = 0.02 vs. non-POC; **P = 0.04 vs. WT POC.
DISCUSSION
The results of this study demonstrate that POC protects myocardial function from ischemia-reperfusion injury by activating the RISK and JAK-STAT pathways in the isolated mouse heart. Moreover, POC-induced functional protection is dependent on an intact PI3K-Akt signaling pathway and is independent of JAK-STAT signaling. Importantly, our results suggest that POC induces significant cross talk between the RISK and JAK-STAT pathways.
Reperfusion injury and POC cardioprotection.
Few studies have examined the impact of POC on cardiac functional recovery (12, 13). Our results demonstrate that POC protects cardiac contractility and relaxation and maintains coronary flow in an ex vivo murine model. Kaljusto et al. (12) similarly demonstrated significant POC-induced improvements in DP, +dP/dtmax, and −dP/dtmin, without an improvement in LV EDP. Models of in vivo regional ischemia, however, have failed to demonstrate enhanced hemodynamic variables by POC despite significant decreases in infarct size (13). Comparisons of infarct size alone may underestimate the effects of POC-mediated attenuation of reperfusion injury because infarct size does not reflect the impact of myocardial stunning. Moreover, measurements of cardiac mechanical performance incorporate more of the immediate effects of reperfusion-induced cardiac dysfunction, such as dysrhythmias and stunning (3).
JAK-STAT signaling induced by POC.
Activation of STAT3 provides cardioprotection in response to ischemic stress and is an integral component of the JAK-STAT pathway. The impact of other STAT subtype activation in POC, however, has not been fully characterized. Moreover, previous studies have demonstrated opposing effects of STAT subtypes on myocyte viability (1). Activation of STAT1 may induce cellular apoptosis and extend reperfusion injury via its proapoptotic effect (18). The effects of STAT5 and -6 activation are less well known but have been associated with reduced infarct size and decreased myocyte apoptosis (24). In this study, we demonstrate that POC specifically activates STAT3 with little effect on the expression of STAT1, -5, and -6.
Previous studies with AG 490 inhibition demonstrate attenuation of POC-induced reduction of infarct size (2). AG 490 primarily targets JAK2 activity, but it is also known to inhibit JAK1 and -3 (23). This inhibitor has also been shown to have variable effects on infarct development when administered to hearts undergoing ischemia-reperfusion alone. Whereas Mascareno et al. (14) demonstrated an infarct size reduction following AG 490 pretreatment, Xuan et al. (23) found no effect on infarct development in hearts treated with the inhibitor before ischemia-reperfusion. The permissiveness of the inhibitor may account for these variable effects through downstream modification of other signaling pathways such as PI3K-Akt and MAPK (2). Furthermore, our results show that JAK2 inhibition by AG 490 attenuated POC-enhanced functional recovery without significantly reducing the expression of p-STAT3. This finding may be attributed to the concentration of AG 490 used, a lack of inhibitor specificity, or the pharmacokinetics of AG 490 allowing for rephosphorylation of STAT3 after an initial crucial period of inhibition. By contrast, Stattic irreversibly and specifically inhibits STAT3 with negligible effects on JAK1, JAK2, Akt, JNK, and ERK1/2 (17). Moreover, we demonstrate for the first time, to our knowledge, that Stattic specifically inhibits STAT3 phosphorylation in the isolated ex vivo murine heart and is more effective than AG 490 at limiting STAT3 activation by POC.
Boengler et al. (2) demonstrated that JAK-STAT inhibition with AG 490 attenuated POC-induced reduction in infarct size. In female cardiomyocyte-specific STAT3 KO hearts, POC was ineffective in limiting infarct size following ischemia-reperfusion challenge (2). Our results demonstrate that POC improved myocardial systolic and diastolic function following ischemia-reperfusion challenge and interestingly was effective in male cardiomyocyte-specific STAT3 KO hearts. The different effects of POC may be attributed to the POC protocol used or perhaps sex-dependent differences in ischemic tolerance. However, our results with PI3K inhibition suggest that compensatory RISK activation accounts for the preserved myocardial performance seen in STAT3 KO POC hearts.
Interaction of JAK-STAT and RISK signaling.
RISK pathway activation through PI3K and its downstream effector, Akt, may be an integral point of convergence for signal transduction pathways controlling cardiomyocyte function, survival, and recovery from ischemic insult (8, 15). Cross talk between the PI3K-Akt and JAK-STAT pathways is implicated in alternative models of cardioprotection, including IPC (7, 19). In contrast to the mechanisms proposed by studies of opioid- and IPC-induced cardioprotection, our results of PI3K inhibition following POC suggest that STAT3 signaling may be parallel to, if not upstream of, PI3K-Akt activation. Our data are consistent with the findings that STAT3 phosphorylation does not require PI3K activation in cardiac myocytes and furthermore that Akt regulation may occur through PI3K-independent signals (16). We acknowledge, however, that additional survival kinases previously demonstrated to be activated by both IPC and POC, including members of the protein kinase C and MAPK pathways, may additionally regulate JAK-STAT and RISK pathway activation following the POC stimulus. The impact of survival protein kinase cross talk following POC and its potential pharmacological modulation remain important areas for future investigation.
Summary.
Our results suggest a compelling role for the interaction of JAK-STAT and RISK signaling in the cardioprotective response induced by POC following an ischemic insult. The present data suggest that JAK-STAT signaling may provide upstream initiation of RISK pathway signaling via PI3K-Akt activation. JAK-STAT signaling, however, is insufficient to provide cardioprotection following POC without subsequent RISK activation. RISK pathway activation may therefore represent a critical target to ameliorate the cardiomyocyte injury and dysfunction induced by ischemia-reperfusion injury.
GRANTS
This study was supported by NIH Grants T32-HL-07382 (A. Schwartz) and HL-68867 (K. L. Butler).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barry SP, Townsend PA, Latchman DS, Stephanou A. Role of the JAK-STAT pathway in myocardial injury. Trends Mol Med 13: 82–89, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R. Cardioprotection by ischemic postconditioning is lost in aged and STAT3 deficient mice. Circ Res 102: 131–135, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R Mechanisms of myocardial “stunning.” Circulation 82: 723–738, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Butler KL, Huffman LC, Koch SE, Hahn HS, Gwathmey JK. STAT-3 activation is necessary for ischemic preconditioning in hypertrophied myocardium. Am J Physiol Heart Circ Physiol 291: H797–H803, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Crisostomo PR, Wairiuko GM, Wang M, Tsai BM, Morrell ED, Meldrum DR. Preconditioning versus postconditioning: mechanisms and therapeutic potentials. J Am Coll Surg 202: 797–812, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol 102: 279–297, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Gross ER, Hsu AK, Gross GJ. The JAK-STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3β. Am J Physiol Heart Circ Physiol 291: H827–H834, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Hausenloy AJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med 15: 69–75, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res 70: 240–253, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Huffman LC, Koch SE, Butler KL. Coronary effluent from a preconditioned heart activates the JAK-STAT pathway and induces cardioprotection in a donor heart. Am J Physiol Heart Circ Physiol 294: H257–H262, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, Ji L, Iwamoto Y, Li E, Schneider M, Russell KS, Fu XY. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA 100: 12929–12934, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaljusto ML, Mori T, Rizvi SM, Galagudza M, Frantzen ML, Valen G, Vaage J. Postconditioning in rats and mice. Scand Cardiovasc J 40: 332–341, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res 62: 74–85, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Mascareno E, El-Shafei M, Maulik N, Sato M, Guo Y, Das DK, Siddiqui MA. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation 104: 325–329, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI3-kinase and Akt. J Mol Cell Cardiol 38: 63–71, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, Yamauchi-Takihara K. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem 273: 9703–9710, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol 13: 1235–1242, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Stephanou A Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J Cell Mol Med 9: 519–525, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res 79: 127–133, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res 95: 230–232, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Tsang A, Hausenloy DJ, Yellon DM. Myocardial postconditioning: reperfusion injury revisited. Am J Physiol Heart Circ Physiol 289: H2–H7, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Vinten-Johansen J Postconditioning: a mechanical maneuver that triggers biological and molecular cardioprotective responses to reperfusion. Heart Fail Rev 12: 235–244, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci USA 98: 9050–9055, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaura G, Turoczi T, Yamamoto F, Siddqui MA, Maulik N, Das DK. STAT signaling in ischemic heart: a role of STAT5A in ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H476–H482, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Yang XC, Liu Y, Wang LF, Cui L, Wang T, Ge YG, Wang HS, Li WM, Xu L, Ni ZH, Liu SH, Zhang L, Jia HM, Vinten-Johansen J, Zhao ZQ. Reduction in myocardial infarct size by postconditioning in patients after percutaneous coronary intervention. J Invasive Cardiol 19: 424–430, 2007. [PubMed] [Google Scholar]
- 26.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007. [DOI] [PubMed] [Google Scholar]