Abstract
Vascular remodeling after mechanoinjury largely depends on the migration of smooth muscle cells, an initial key step to wound healing. However, the role of the second messenger system, in particular, the cAMP signal, in regulating such remodeling remains controversial. Exchange protein activated by cAMP (Epac) has been identified as a new target molecule of the cAMP signal, which is independent from PKA. We thus examined whether Epac plays a distinct role from PKA in vascular remodeling. To examine the role of Epac and PKA in migration, we used primary culture smooth muscle cells from both the fetal and adult rat aorta. A cAMP analog selective to PKA, 8-(4-parachlorophenylthio)-cAMP (pCPT-cAMP), decreased cell migration, whereas an Epac-selective analog, 8-pCPT-2′-O-Me-cAMP, enhanced migration. Adenovirus-mediated gene transfer of PKA decreased cell migration, whereas that of Epac1 significantly enhanced cell migration. Striking morphological differences were observed between pCPT-cAMP- and 8-pCPT-2′-O-Me-cAMP-treated aortic smooth muscle cells. Furthermore, overexpression of Epac1 enhanced the development of neointimal formation in fetal rat aortic tissues in organ culture. When the mouse femoral artery was injured mechanically in vivo, we found that the expression of Epac1 was upregulated in vascular smooth muscle cells, whereas that of PKA was downregulated with the progress of neointimal thickening. Our findings suggest that Epac1, in opposition to PKA, increases vascular smooth muscle cell migration. Epac may thus play an important role in advancing vascular remodeling and restenosis upon vascular injury.
Keywords: cAMP, protein kinase A, vascular remodeling, exchange protein activated by cAMP
cellular proliferation and migration are fundamental processes that contribute to the injury response in major arteries (19, 21, 29). Such injury may commonly occur in patients with ischemic heart disease after balloon angioplasty and/or stenting, and the resultant pathology is the restenosis of arteries. Neointimal thickening precedes restenosis and can be induced in response to experimental arterial injury as well. Various vasoreactive Gs protein-coupled receptor stimuli, such as prostanoids or catecholamines, increase intracellular cAMP to evoke a variety of cellular responses (13). cAMP, a major second messenger that is produced by adenylyl cyclase, classically activates PKA to initiate multiple phosphorylation reactions and thus regulates various functions within cells (13). The activation of the cAMP-PKA signal has been reported to inhibit aortic smooth muscle cell (ASMC) migration/proliferation and thus reduce the formation of neointimal lesions after experimental arterial injury in vivo (11, 12, 15). However, a number of studies have also demonstrated controversial roles of cAMP in SMC migration and vascular remodeling (21, 27, 32). These data suggest that there are multiple or diverse pathways to regulate vascular SMC migration and remodeling by cAMP.
The recent cloning of an entirely new target of cAMP, i.e., exchange protein activated by cAMP (Epac), has elucidated the presence of a distinct cAMP signaling pathway that is independent of PKA activation (3). Epac is a guanine nucleotide exchange protein that regulates the activity of small G proteins Rap1 and Rap2. Various roles of Epac have been proposed, such as cell proliferation, migration, adhesion, secretion, and cellular Ca2+ handling (26, 33); however, as yet, the role of Epac in vascular SMC function is limited.
In the present study, we examined the role of Epac in cell migration, a fundamental process to induce neointimal thickening and, thus, vascular restenosis. In particular, we examined the differential role of Epac and PKA with the use of selective cAMP analogs specific to Epac or PKA and adenovirus-mediated gene transfer of Epac or PKA. We also examined changes in Epac and PKA expression in neointimal thickening after vascular injury in mice. We demonstrated that Epac promotes both fetal and adult rat ASMC migration and facilitates the development of neointimal thickening.
MATERIALS AND METHODS
Reagents.
8-(4-parachlorophenylthio)-cAMP (pCPT), 8-pCPT-2′-O-Me-cAMP (O8Me-cAMP), and anti-α-smooth muscle actin antibody were purchased from Sigma (St. Louis, MO). PDGF-BB was purchased from BD Biosciences (San Jose, CA). Antibodies of Epac1 were purchased from Santa Cruz Biotechnology (San Diego, CA) and Upstate Biotechnology (Charlottesville, VA). Antibodies of the PKA catalytic α-subunit, phalloidin, and von Willebrand factor were purchased from Cell Signaling (Danvers, MA), Molecular Probes (Carlsbad, CA), and Dako (Glostrup, Denmark), respectively.
Animals and tissue.
Wistar rat embryos obtained from timed-pregnant mothers, and adult Wister rats and 8-wk-old male wild-type ICR mice were purchased from Japan SLC (Shizuoka, Japan). This investigation conformed with the American Physiological Society “Guiding Principles in the Care and Use of Animals.” Experiments were approved by the Ethical Committee of Animal Experiments of Yokohama City University School of Medicine.
Cell culture.
ASMCs in primary culture were obtained from the aorta of Wistar rat embryos at embryonic day 21 and adult Wister rats as described previously (31). Briefly, the tissues were digested by a collagenase-dispase enzyme mixture [1.5 mg/ml collagenase-dispase (Roche, Basel, Switzerland), 0.5 mg/ml elastase type II-A (Sigma), 1 mg/ml trypsin inhibitor type I-S (Sigma), and 2 mg/ml BSA fraction V (Sigma) in HBSS (Sigma)] at 37°C for 15 min. Cell suspensions were centrifuged, and the medium was changed to a collagenase II enzyme mixture [1 mg/ml collagenase II (Worthington, Lakewood, NJ), 0.3 mg/ml trypsin inhibitor type I-S, and 2 mg/ml BSA fraction V in HBSS]. After 12 min of incubation at 37°C, cell suspensions were transferred to 10% FCS-DMEM in poly-l-lysine-coated dishes at 37°C in 5% CO2-95% ambient mixed air. Confluent cells between passages 4 and 6 were used in experiments.
Mouse femoral artery injury model.
Surgery was carried out using a dissecting microscope (SMZ-800, Nikon). Transluminal mechanical injury of the femoral artery was induced by the insertion of a large wire (0.38 mm in diameter, no. C-SF-15-15, COOK) as previously described (25). Mice were euthanized by an intraperitoneal administration of an overdose of Nembutal at the time points indicated. At death, mice were perfused with 0.9% NaCl solution. Injured femoral arteries were harvested at the time points indicated. Femoral arteries were snap frozen in OCT compound (TissueTek, Tokyo, Japan) for immunohistochemistry. The neointima area was measured on digitized images using image-analysis software (Image-Pro Plus version 4.5, Media Cybernetics, San Diego, CA). Three to four sections every 100 μm were measured for each artery.
Quantitative RT-PCR.
Total RNA was isolated from each artery or ASMC using the RNeasy Minikit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Generation of cDNA was done as previously described (31). Quantitative RT-PCR was performed using the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). Amplifications were generated by 10 min at 95°C and then 40 cycles of denaturation at 95°C for 15 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s using a qPCR core kit for SYBR green (Eurogenetec, Seraing, Belgium). Primers for PCR amplification were designed between multiple exons based on rat and mouse nucleotide sequences of Epac1 and Epac2 (5′-GTGTTGGTGAAGGTCAATTCTG-3′ and 5′-CCACACCACGGGCATC-3′ for Epac1 and 5′-TGTTAAAGTGTCTGAGACCAGCA-3′ and 5′-AAAGGCTGTCCCAATTCCCAG-3′ for Epac2) and the regulatory IIa and catalytic subunit b of PKA (5′-AAAACTGACGAGCATGTCATTGA-3′ and 5′-CGGTTGTCATACTGACCAACAG-5′ for the regulatory IIa subunit and 5′-TGGTTATGGAATACGTCCCTGG-3′ and 5′-AATTAAGAGGTTTTCCGGCTTGA-3′ for the catalytic subunit b). Primers for human Epac1 (5′-GTGTTGGTGAAGGTCAATTCTG-3′ and 5′-CCACACCACGGGCATC-3′) were used to confirm adenovirus-mediated overexpression. Each template was tested at least three times to confirm the reproducibility of the assays. The abundance of each gene was determined relative to GAPDH using TaqMan Rodent GAPDH control reagents kits (Applied Biosystems). Amplifications for TaqMan assay were generated by 10 min at 95°C and then 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min.
Immunoblot analysis.
Immunoblot analysis was conducted as previously described (31). ASMCs were prepared in cell lysis buffer [50 mM Tris (pH 8.0), 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 200 mM sucrose, and protease inhibitor mixture (Roche)] and homogenized by sonication. Proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane by electroblotting. Membranes were blocked in 20 mM PBS-1% Tween containing 3% nonfat dry milk and incubated with primary antibody overnight at 4°C. Specific binding was visualized by the Super Signal West Dura extended duration substrate (Pierce, Rockford, IL).
Adenovirus construction.
For the construction of adenoviral vectors, a full-length cDNA-encoding human Epac1 (kindly provided by Dr. J. L. Bos) or the α1-catalytic subunit of PKA was cloned into a shuttle vector using an AdenoX adenovirus construction kit (Clontech, Tokyo, Japan). For controls, adenovirus vector harboring LacZ or green fluorescent protein (GFP) was used at the same multiplicity of infection (MOI). All experiments were performed 24 h after infection.
Immunocytochemistry.
ASMCs cultured on 12-mm glass coverslips were serum starved for 24 h and then stimulated for 1 h in media alone (control), O8Me-cAMP, or pCPT. Cells were then fixed in 10% buffered formalin for 10 min, washed twice with PBS, and permeabilized in 0.3% Triton X-100 and PBS for 10 min. ASMCs were washed twice with PBS-Tween 20 (0.1% Tween) and incubated with 1% BSA-PBS-Tween for 20 min and then with a FITC-conjugated phalloidin antibody for 16 h at 4°C. After five washes with PBS-Tween 20, coverslips were mounted for imaging.
Organ culture.
The descending aorta at embryonic day 19 was infected for 2 h in humidified 5% CO2-95% ambient mixed air at 37°C with 1.2 × 107 plaque-forming units (pfu)/ml of Epac1, PKA, or GFP adenovirus in 0.5% FCS containing DMEM (6). After infection, segments were cultured up to 2 days, fixed in 10% buffered formalin, and embedded in paraffin. Morphometric analyses were performed using Win Roof version 5.0 software (Mitani, Tokyo, Japan). Intimal cushion formation was defined as follows: [(neointimal area)/(medial area)] × 100%. The average of at least three sections was used as the value for each tissue.
RNA interference.
Double-stranded 21-bp short interfering (si)RNAs to the selected region of Epac1 cDNA were purchased from Ambion (Tokyo, Japan). The siRNA sequences targeting Epac1 were 5′-CCACAGAGCAUGUGCACAA(TT)-3′ and 5′-UUGUGCACAUGCUCUGUGG(TG)-3′. Negative control siRNA (Ambion) was used as a control. ASMCs were transfected with siRNA (100 pmol) using the Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions. Cells were kept serum free for an additional 24 h before experiments.
Migration assay.
The migration assay was performed using 24-well Transwell culture inserts with polycarbontate membranes (8-μm pores, Corning, Acton, MA) as previously described (32). The membrane was coated with 75 μl fibronectin (50 μg/ml, BD Bioscience) for 16 h and washed by DMEM before cell suspension. ASMCs were harvested with trypsin-EDTA, resuspended in serum-free DMEM, counted, and distributed at a density of 1 × 105 cells/100 μl in inserts. Cells were allowed to settle in serum-free DMEM for 1 h before the addition of agents in the lower chamber. As the basal condition, the lower chambers were filled with 600 μl serum-free DMEM. ASMCs were then allowed to migrate to the underside of the insert's membrane for 4 h at 37°C at 5% CO2. At the end of the experiment, cells were fixed in 10% buffered formalin (Wako Pure Chemical Industries, Osaka, Japan). After cells on the upper surface of the membrane had been mechanically removed with a cotton swab, migrated cells on the lower surface of the membrane were stained with Cyto Quick (Muto Pure Chemicals, Tokyo, Japan) and were manually counted from three different fields (0.5 mm2/field) in a microscope using Image J software. We performed at least three independent experiments.
[3H]thymidine incorporation and cell counting.
Cell proliferation was measured using [3H]thymidine incorporation as previously described (31). Briefly, SMCs were reseeded into a 24-well culture plate at an initial density of 1 × 105 cells/well. The α1-catalytic subunit of PKA, Epac1, or LacZ was overexpressed with the use of adenovirus (5 MOI) for 24 h before the addition of 1 μCi [methyl-3H]thymidine for 4 h at 37°C. Cell numbers were counted 3 days after the incubation with drug or adenovirus containing Epac1 using trypan blue staining.
Immunohistochemistry.
Frozen sections of the femoral artery were fixed in acetone for 10 min and incubated for 5 min in peroxidase blocking reagent (Dako) to inactivate endogenous peroxidases. Tissue sections were then rinsed in PBS and incubated with anti-Epac1 antibody overnight at 4°C. Paraffin-embedded sections containing the aorta subjected to organ culture were stained as previously described (31). Slides were incubated with anti-α-smooth muscle actin or anti-von Willebrand factor antibody overnight at 4°C. Slides were washed with PBS for 5 min and sequentially incubated with streptavidin peroxidase at room temperature for 30 min and DAB chromogen substrate solution (Nichirei, Tokyo, Japan). Slides were counterstained with Mayer's hematoxylin, dehydrated, and mounted. The specificity of staining was examined by omission of the primary antibody.
Rap1 pull-down assay.
Rap1 activity assay was assayed with a Rap1 activation kit (Pierce) according to the manufacturer's instructions. Briefly, cells were serum starved for 48 h, incubated with drugs, lysed [1% Nonidet P-40, 25 mM Tris·HCl (pH 7.5), 150 mM NaCl, 5% glycerol, 5 mM MgCl2, 1 mM DTT, 2 μg/ml aportinin, and 1 mM PMSF], and centrifuged at 16,000 g for 1 min. Supernatants and 20 μg of GST-RalGDS-RBD were incubated on the glutathione disks for 1 h, and discs were washed three times with lysis buffer. Precipitates were separated by SDS-PAGE and analyzed by immunoblot analysis with anti-Rap1 monoclonal antibody.
Statistical analysis.
Data are means ± SE of n independent experiments. Statistical analysis was performed by the unpaired Student t-test or one-way ANOVA followed by the Tukey-Kramer multiple-comparison test. A value of P < 0.05 was considered significant.
RESULTS
Epac1 expression increases after vascular injury.
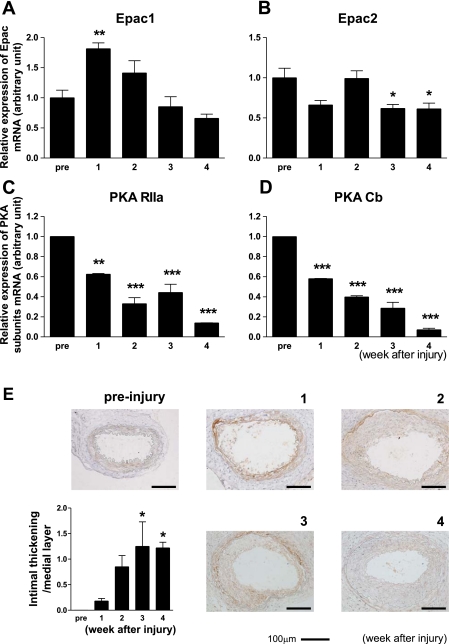
Transluminal mechanical injury was induced in mouse arteries to examine changes in the mRNA expression of PKA and Epac. We found that Epac1 mRNA, but not Epac2 mRNA (Fig. 1, A and B), was increased. The increased Epac1 mRNA expression was gradually returned to the basal level by 4 wk after the injury. In contrast, the expression of PKA subunits, i.e., the α-regulatory (RIIa) and the β-catalytic (Cb) subunits (Fig. 1, C and D), but not the β-regulatory (RIIb) and α-catalytic (Ca) subunits (data not shown), showed a decrease over the course of vascular injury and intimal thickening. These findings suggest that the expression of the two major target molecules of cAMP, Epac and PKA, are differently regulated after vascular injury and during the progression of neointimal thickening.
Fig. 1.
Changes in exchange protein activated by cAMP (Epac) and PKA expression upon vascular injury. A and B: Epac1 (A), but not Epac2 (B), mRNA increased after vascular injury. Transluminal mechanical injury of the femoral artery was induced by insertion of a large wire. mRNA expression of Epac1 was determined at 0 (pre), 1, 2, 3, and 4 wk by quantitative RT-PCR. The abundance of each mRNA was determined relative to GAPDH. Data are fold increases of control; n = 4. *P < 0.05; **P < 0.01. C and D: changes in PKA subunit mRNA expression after vascular injury. mRNA expression of α-regulatory (RIIa; C) and β-catalytic (Cb; D) subunits of PKA were determined in the same manner as that of Epac. Data are fold increases of control; n = 4. **P < 0.01; ***P < 0.001. E: changes in Epac1 protein expression and intimal thickening after vascular injury. Immunohistochemistry with anti-Epac1 antibody was performed at 0 (pre), 1, 2, 3, and 4 wk after injury to the mouse femoral artery. Representative images are shown. The ratio of intimal thickening to the medial layer was increased in a tine-dependent manner. n = 7–9. *P < 0.05.
We also examined whether intimal thickening developed after injury and also examined changes in Epac1 expression (Fig. 1E). When Epac1 protein expression was examined in injured vessels by immunostaining, we found that Epac1 expression was very low in medial smooth muscle layers, even if detectable before injury. By the end of 1 wk following injury, the vessels were dilated and Epac1 expression became readily detectable in the smooth muscle layer. At 2 wk, diffuse neointimal thickening occurred. At 3 wk, neointimal thickening progressed with a thickened smooth muscle layer. Epac1 expression was detectable in both thickened neointimal lesions and smooth muscle layers. At 4 wk, neointimal thickening peaked, whereas staining for Epac1 was decreased. Immunofluorescent data of Epac1 protein also indicated that Epac1 protein was increased after vascular injury (data not shown). Although immunostaining findings are not necessarily quantitative, in conjunction with our findings from RT-PCR, these results suggest that Epac1 protein expression is upregulated after injury in smooth muscle layers and then neointimal lesions during the progression of neointimal thickening.
Epac plays an opposite role from PKA in regulating fetal ASMC migration.
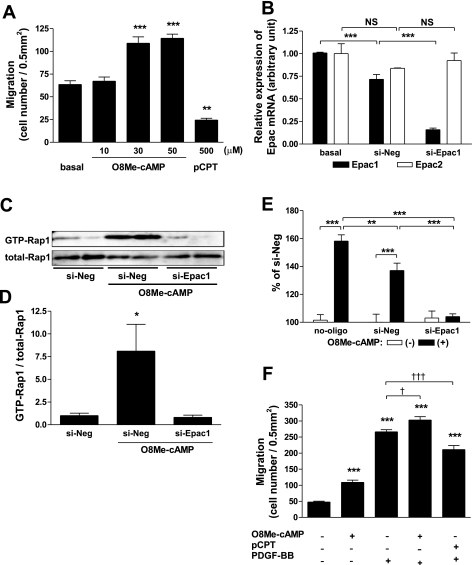
Since migration is a fundamental process contributing to neointimal formation (19, 21, 29), we compared the role of PKA and Epac with the use of cAMP analogs that can selectively stimulate either of the two molecules in the regulation of cell migration in fetal ASMCs, a common cell system for such assays. Activation of PKA with pCPT (16) was inhibitory to ASMC migration, as previously shown (11, 12), even at a relatively high concentration (7, 16). In contrast, we found that stimulation of Epac with O8Me-cAMP (7) was stimulatory to migration. Indeed, the enhancement of ASMC migration paralleled Epac activation with an increasing concentration of O8Me-cAMP (Fig. 2A). The use of siRNA decreased Epac1 mRNA expression to ∼30% of basal and did not affect Epac2 mRNA (Fig. 2B). Epac1-targeted siRNA abolished O8Me-cAMP-induced Rap1 activation (Fig. 2, C and D) and negated the enhancement of migration induced by O8Me-cAMP (Fig. 2E). These findings suggest that Epac1 was mostly responsible for the O8Me-cAMP-induced ASMC migration. Furthermore, the enhancement of migration in the presence of O8Me-cAMP was additive to that in the presence of PDGF-BB, a potent enhancer of cell migration (19) (Fig. 2F). In contrast, PKA activation was inhibitory and decreased the enhanced cell migration induced by PDGF-BB (Fig. 2F).
Fig. 2.
Effect of Epac and PKA activation on fetal aortic smooth muscle cell (ASMC) migration. A: Epac and PKA were activated by selective cAMP analogs. ASMC migration was examined using a modified Boyden chamber method in the absence (basal) or presence of either 8-(4-parachlorophenylthio)-cAMP [pCPT-cAMP (pCPT)], a selective PKA activator, or 8-pCPT-2′-O-Me-cAMP (O8Me-cAMP), an Epac activator. n = 4–9. **P < 0.01; ***P < 0.001. B: selective silencing of Epac1 mRNA. Changes in the mRNA expression of Epac1 were determined by quantitative RT-PCR. Epac1 mRNA was significantly decreased in cells transfected with Epac1-targeted short interfering (si)RNA. Epac1-targeted siRNA did not affect Epac2 mRNA expression. Data were normalized to GAPDH; n = 4. ***P < 0.001. NS, not significant. C and D: Epac1-targeted siRNA (si-Epac1) abolished O8Me-cAMP-induced Rap1 activation. Rap1 activation was determined in the presence of oligonucleotides [negative control (si-Neg) or si-Epac1]. Cells were incubated with or without Epac activator (30 μmol/l O8Me-cAMP) for 15 min and assayed for Rap1 activation. Precipitates (C, top) and total cell lysates (C, bottom) were analyzed by immunoblot analysis with an anti-Rap1 antibody. Changes in GTP-bound Rap1 were normalized to total Rap1 (D). n = 4. *P < 0.05. E: siRNA-mediated negation of Epac-induced migration. Migration was determined in the presence or absence of oligonucleotides (si-Neg or si-Epac1). Changes in cell migration in the absence or presence of Epac activator (30 μmol/l O8Me-cAMP) are shown as percent increases from the negative control in the absence of Epac activator. n = 9–12. **P < 0.01; ***P < 0.001. F: effect of Epac or PKA activation on growth factor-stimulated migration. ASMCs were stimulated with either 30 μmol/l O8Me-cAMP, an Epac activator, or 500 μmol/l pCPT, a selective PKA activator, to determine cell migration in the presence of 10 ng/ml PDGF-BB for 4 h. n = 4. ***P < 0.001 compared with basal; †P < 0.05; †††P < 0.001.
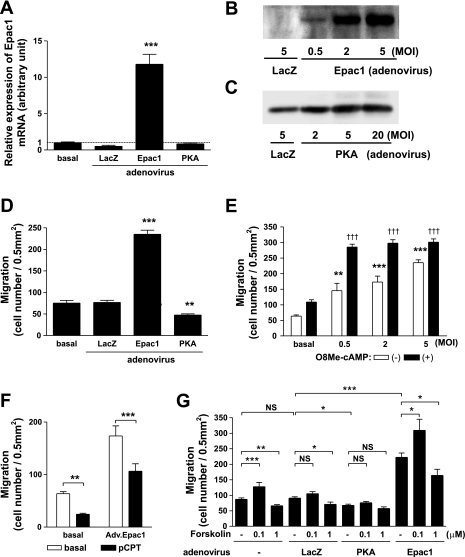
The above findings suggested that PKA activation inhibited fetal ASMC migration; this was in agreement with findings from a previous study (11). However, we found that Epac activation instead increased migration. To further confirm this opposing effect of Epac, we examined the impact of adenovirus-mediated gene transfer of Epac1. Levels of protein of Epac1 and PKA were both increased in a dose-dependent manner after adenovirus-mediated gene transfer of Epac1 and PKA, respectively (Fig. 3, A–C). We confirmed that a longer exposure gave a immunoreactive band for Epac1 in cells infected with adenovirus LacZ. Gene transfer of PKA decreased cell migration, whereas that of Epac1 increased migration (Fig. 3D). Indeed, cell migration was dramatically increased with an increasing viral dose of Epac1 and was further enhanced by the addition of the Epac activator O8Me-cAMP (Fig. 3E). This led to the maximal activation of migration, which was comparable with that of PDGF-BB, one of the most potent migratory stimulators (Fig. 2F). The response to O8Me-cAMP appeared weaker in cells infected with 5 MOI of adenovirus Epac1 than that in cells infected with a low titer of adenovirus Epac1. We think that this is because the response of migration measured in our system is limited (350 migrated cells/0.5 mm2). In contrast, PKA activation with pCPT decreased cell migration at basal and also in cells overexpressing Epac1, suggesting that PKA can counteract Epac in regulating migration (Fig. 3F). To determine the outcome of cAMP signaling, we examined migration using forskolin (1 or 0.1 μM), which activates PKA and Epac nonselectively through activating adenylyl cyclase (Fig. 3G). The lower concentration of forskolin (0.1 μM) significantly promoted migration in cells in the absence and presence of Epac1 overexpression. The lower concentration of forskolin-induced migration did not reach a statistical difference in cells infected with LacZ. In contrast, the higher concentration of forskolin (1 μM) inhibited migration in uninfected cells. Forskolin at 1 μM also inhibited migration in cells infected with LacZ or Epac1 adenovirus. Although further inhibition of migration using the higher concentration of forskolin was not observed in cells infected with the PKA catalytic subunit, these data suggest that the higher concentration of forskolin inhibits ASMC migration most likely via PKA. We also obtained similar findings using 10 μM forskolin and confirmed the viability of cells (data not shown). The effect of nonselective cAMP-raising drug on migration was not uniform and, at least in part, appeared to depend on the concentration of cAMP.
Fig. 3.
Effect of adenovirus-mediated gene transfer of Epac1 and PKA in fetal ASMCs. A: overexpression of Epac1. Changes in the mRNA expression of Epac1 were determined by quantitative RT-PCR. Adenovirus-mediated gene transfer of Epac1 for 24 h significantly increased Epac1 mRNA. Data were normalized to GAPDH; n = 4. ***P < 0.001. B and C: changes in Epac1 (B) and PKA (C) protein after adenovirus-mediated gene transfer. Changes in the protein expression of Epac1 and PKA were determined by immunoblot analysis. Epac1 and PKA α-catalytic subunit protein were increased in overexpressing cells in a dose-dependent manner 24 h after infection. An equal amount of protein from each sample was loaded. MOI, multiplicity of infection. D: effect of adenovirus-mediated gene transfer of PKA or Epac1 on migration. The α1-catalytic subunit of PKA, Epac1, or Lac Z was overexpressed with the use of adenovirus (MOI 5). Twenty-four hours after infection, ASMC migration was compared with that in the absence of infection (basal). n = 8. **P < 0.01; ***P < 0.001 compared with basal. E: Epac1 dose-dependent increases in migration. Epac1 was overexpressed with the use of adenovirus with an increasing MOI (0.5, 2, and 5). Twenty-four hours after infection, ASMC migration was determined in the presence or absence of 30 μmol/l O8Me-cAMP. n = 6–8. **P < 0.01, ***P < 0.001, and †††P < 0.001 compared with basal. F: PKA-mediated inhibition of Epac-induced migration. ASMCs in the presence or absence of a selective PKA activator (500 μmol/l pCPT) were subjected to a cell migration assay in the presence or absence of Epac1 overexpression. n = 4–9. **P < 0.01; ***P < 0.001. G: effect of forskolin on migration. ASMCs were infected with or without 5 MOI of LacZ, PKA, or Epac1 adenovirus for 24 h. ASMC migration was determined in the presence or absence of 1 μM or 100 nM forskolin for 4 h. n = 4–15. *P < 0.05; **P < 0.01; ***P < 0.001.
Epac promotes and PKA inhibits migration in adult ASMCs.
Because fetal cells, which are commonly used for migration assays, sometimes have different properties from those of adult cells, we also examined the effect of Epac and PKA activation in adult ASMCs. Activation of Epac by O8Me-cAMP and overexpression of Epac1 promoted migration, whereas activation of PKA by pCPT and overexpression of PKA inhibited adult ASMC migration, indicating the presence of similar signaling between fetal and adult cells (Fig. 4, A and B). We further examined the differing effects of Epac and PKA. O8Me-cAMP promoted PKA-inhibited migration (Fig. 4A). pCPT decreased overexpression of Epac1-induced migration, and O8Me-cAMP increased overexpression of PKA-inhibited migration (Fig. 4C). These results suggest that Epac and PKA have opposing effects on migration, even though both are targets of cAMP.
Fig. 4.
Effect of Epac and PKA activation on adult ASMC migration. A: activation of Epac and PKA by selective cAMP analogs. Rat adult ASMC migration was examined using same methods as in Fig. 2. The Epac activator O8Me-cAMP (30 μmol/l) promoted migration, and the selective PKA activator pCPT (500 μmol/l) inhibited migration. Epac-induced migration was decreased by the selective PKA activator. Serum was depleted from the culture medium. CTRL, control. n = 4–9. ***P < 0.001; †P < 0.05. B: effect of adenovirus-mediated gene transfer of PKA or Epac 1 on migration. The α1-catalytic subunit of PKA (PKA), Epac1, or LacZ was overexpressed with the use of adenovirus (5 MOI). Overexpression of Epac1 promoted and that of PKA inhibited adult ASMC migration. Twenty-four hours after infection, migration assays were conducted. n = 4–10. ***P < 0.001. C: Epac and PKA counteract in ASMC migration. pCPT (500 μmol/l) decreased the overexpression of Epac-induced migration. O8Me-cAMP (30 μmol/l) increased the overexpression of PKA-induced migration. n = 4–10. *P < 0.05; ***P < 0.001. D: effect of Epac and PKA on DNA synthesis. Adult ASMCs were overexpressed with 5 MOI of LacZ, Epac1, or the α-catalytic subunit of PKA. Twenty-four hours after infection, thymidine uptake was conducted for 4 h. All treatments did not show a significant difference compared with LacZ. n = 16–20. E and F: effect of Epac on proliferation. Cell numbers were counted 3 days after an incubation with drug (E) or adenovirus containing Epac1 (F). We used PDGF-BB as a positive control. Epac activation by O8Me-cAMP (30 μmol/l) and overexpression of Epac1 did not affect cell numbers. n = 5–8. ***P < 0.001.
Since SMC growth also contributes to vascular remodeling, we also examined the effect of Epac and PKA on DNA synthesis and proliferation in adult ASMCs. Adenovirus-mediated gene transfer of both Epac1 and PKA did not affect [3H]thymidine uptake in adult ASMCs (Fig. 4D). Epac activation by O8Me-cAMP and overexpression of Epac1 did not affect cell numbers 3 days after treatment (Fig. 4, E and F).
Effect of Epac activation on cell morphology in fetal and adult ASMCs.
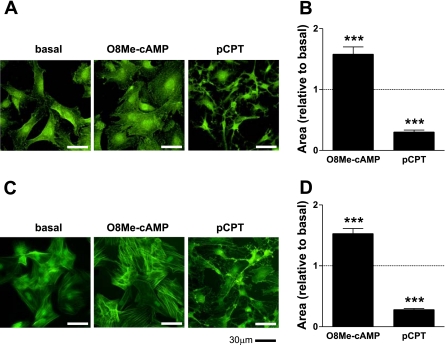
We also found that changes in SMC morphology after Epac activation were quite different from those after PKA activation. Fetal and adult ASMCs treated with either O8Me-cAMP or pCPT were compared after being stained with phalloidin (green) (Fig. 5, A and C, respectively). In accordance with a previous report (23), PKA activation showed a rapid and striking morphological change characterized by the presence of long branching processes. This classic change in stellate cell shape was readily detectable within 1 h of treatment. However, we found that Epac-activated cells showed a diffusely expanded cell shape without dendritic extensions. This suggests that the same cAMP signal can induce distinct morphological phenotypes in addition to functional differences, i.e., migration, depending on which of its downstream effectors, PKA or Epac, is activated. Cell surface areas of fetal and adult ASMCs were significantly increased with Epac activation, whereas they were significantly decreased with PKA activation (Fig. 5, B and D, respectively).
Fig. 5.
Effect of Epac on cell morphology in fetal and adult ASMCs. A: fetal ASMCs were incubated in the presence of either a selective PKA activator (500 μmol/l pCPT) or an Epac activator (30 μmol/l O8Me-cAMP) for 1 h followed by immunostaining using anti-phalloidin antibody (green). B: changes in the cell surface area of fetal ASMCs in the presence of pCPT or O8Me-cAMP. n = 10–15. ***P < 0.001 compared with basal. C: adult ASMCs were incubated in the presence of either pCPT (500 μmol/l) or O8Me-cAMP (30 μmol/l) for 1 h followed by immunostaining using anti-phalloidin antibody (green). D: changes in the cell surface area of adult ASMCs in the presence of pCPT or O8Me-cAMP. n = 20. ***P < 0.001 compared with basal.
Development of neointimal thickening aorta by Epac overexpression.
We examined the effect of 4 days of incubation with the Epac activator O8Me-cAMP on intimal thickening in the rat aorta at embryonic day 19 in organ culture (Fig. 6A). Intimal thickening, as determined by the ratio between neointimal area and media area, was increased with Epac activator (Fig. 6B). Finally, to examine whether overexpression of Epac1 indeed promotes the development of neointimal thickening and thus luminal occlusion, we overexpressed Epac1 in the same system and examined the changes in intimal thickening at 48 h after gene transfer (Fig. 6C). Overexpression of Epac1 in the cultured tissues was confirmed by real-time RT-PCR (Fig. 6E). Intimal thickening was dramatically increased with Epac1 gene transfer (Fig. 6D). Cells in the area of intimal thickening were SMCs and not endothelial cells (Fig. 6F). These data suggest that Epac1 indeed promoted neointimal thickening, at least in rat fetal tissues.
Fig. 6.
Effect of Epac on neointimal thickening in the fetal aorta. A: the rat fetal aorta was incubated with or without O8Me-cAMP in organ culture for 4 days. Tissues were stained with elastica-van Gieson stain to visualize intimal thickening. B: thickness of the intimal cushion presented as the ratio of the area of the intimal cushion to the area of whole smooth muscle layers. n = 4–5. *P < 0.05. C: the rat fetal aorta was infected with green fluorescent protein (GFP) or Epac1 adenovirus followed by an incubation in organ culture for 48 h. Tissues were stained with elastica-van Gieson stain to visualize intimal thickening. D: thickness of the intimal cushion presented as the ratio of the area of the intimal cushion to the area of whole smooth muscle layers. n = 3–5. *P < 0.05. E: the rat fetal aorta was infected with GFP or Epac1 adenovirus. mRNA were extracted 48 h after incubation and subjected to real-time RT-PCR. n = 4. ***P < 0.001 compared with GFP adenovirus. F: rat fetal aortas infected with Epac1 adenovirus in the organ culture system were stained with anti-α-smooth muscle actin or von Willebrand factor antibody to visualize SMCs or endothelial cells, respectively.
DISCUSSION
We demonstrated that the two major effecter molecules of cAMP, i.e., PKA and Epac, play differential roles in regulating ASMC migration and induce different morphological changes. PKA inhibited, whereas Epac promoted, cell migration in both fetal and adult rat ASMCs, as demonstrated with the use of selective cAMP analogs and/or adenovirus-mediated gene transfer.
Although it has been demonstrated that a distinct signaling pathway is involved for Epac from that of PKA in other cell types (18, 33), the biological significance of Epac signaling has been poorly characterized in SMCs. Epac is one of major targets of cAMP and is abundantly expressed in cardiac myocytes, fibroblasts, and vascular smooth muscle tissues (17, 33). It has been reported that Epac activation promotes monocytes or cardiac fibroblast migration (1, 33). in contrast to these reports, Epac activation inhibits the migration of endothelial cells (10), implying that the role of Epac, compared with that of PKA, may be different in these tissues. Our findings show that a distinct role of Epac in SMC migration from PKA.
We do not know the exact molecular mechanisms that lead to Epac activation and increased migration in ASMCs, which is, at least, additive to that of growth factor-mediated migration, as shown above. The activation of Rap1, Akt, and/or ERK may be well involved, as demonstrated in the regulation of cell adhesion and migration (8, 14, 18, 20, 24, 30, 33), which does not depend on the classical activation of PKA and may play a role in regulating migration in vascular SMCs as well. Our results showed that stimulation of Epac or PKA induced different morphological changes. It has been reported that adhesion is increased by Epac and decreased by PKA (2, 28) and plays an important role in cell morphology and migration (4). Especially, turnover of focal adhesion has been reported to be important for cell migration (9). Morphological changes might be related to migration changes induced by Epac versus PKA. We also showed that overexpression of Epac1 promoted intimal thickening formation in the fetal rat aorta using an organ culture system, although a similar demonstration was difficult to make with adult organs (data not shown).
The higher concentration of forskolin (1 μM) inhibited migration, whereas the lower concentration of forskolin (0.1 μM) promoted migration in cells without infection or even in cells with overexpression of Epac1. It is thus tentative to speculate that the outcome of cAMP signaling as induced by forskolin, a direct adenylyl cyclase activator, was not uniform and may depend on the actual concentration and kinetics of the cAMP signal, as previously reported (33).
Epac1 expression was upregulated, whereas PKA RIIa and Cb subunits were downregulated, upon vascular injury in mice, when cell migration is known to be enhanced. Overexpression of Epac1 and PKA led to opposite changes in such cellular behaviors in vitro. ASMC migration was promoted in cells with overexpression of Epac1 and inhibited by overexpression of the PKA catalytic subunit. Although we did not show the direct involvement of Epac in vascular injury, e.g., vascular injury in Epac-null mice, our findings suggest that, at least in part, enhanced expression of Epac1 and decreased PKA expression were coordinated during the process of increased cell migration.
Because siRNA-mediated disruption of Epac1 expression attenuated cAMP-induced enhancement of cell migration, it is tentative to speculate that the inhibition of the Epac1 signal, at least when Epac1 expression is transiently upregulated upon vascular injury, may be considered as a therapeutic strategy to prevent the progress of vascular restenosis. This may be considered in addition to, or potentially over and above, the previous proposals, which used cAMP analogs to activate PKA to prevent neointimal thickening (11, 12), simply because PKA was downregulated or not changed and Epac1 was upregulated upon vascular injury, as demonstrated in this study. A limitation in our study is that we examined a vascular injury model in mice and migration in rat ASMCs. Although mice and rats are similar in some aspects, they are not identical in histological and genetic features (5, 22). Further study will be needed to investigate a regulation of intimal thickening by Epac in other species, including humans.
Our findings indicated that the role of Epac is opposite to that of PKA and that Epac can promote cell migration. The stoichiometry of Epac may be increased to enhance such cellular migration upon vascular injury. The robust nature of our findings is to suggest, however, that the two major effectors of cAMP, i.e., Epac and PKA, have differential roles in regulating cell migration, directing distinct pathophysiological processes upon vascular injury.
GRANTS
This work was in part supported by funds from the Mother and Child Health Foundation (to S. Minamisawa), Yokohama Foundation for Advanced Medical Science (to U. Yokoyama, S. Minamisawa, T. Akaike, and Y. Ishikawa), the Ministry of Education, Science, Sports and Culture of Japan (MEXT) (to S. Minimisawa and Y. Ishikawa), Japan Cardiovascular Research Foundation (to S. Minimisawa), Waseda University Grant for Special Research Projects 2007A-854 (to S. Minimisawa), Miyata Cardiology Research Promotion Founds (to S. Minimisawa), Takeda Science Foundation (to S. Minimisawa and Y. Ishikawa), Foundation for Growth Science (to S. Minimisawa), a grant from the Mitsubishi Pharma Research Foundation (to S. Minimisawa), the Special Coordination Funds for Promoting Science and Technology, MEXT (to S. Minimisawa), Uehara Memorial Foundation (to U. Yokoyama), Yokohama Academic Foundation (to U. Yokoyama and T. Akaike), Inoue Foundation for Science (to T. Akaike), Naito Foundation (to T. Akaike), Kitsuen Research Foundation (to Y. Ishikawa), Japan Space Forum (to Y. Ishikawa), and National Institute of General Medical Sciences Grant RO1-GM-067773 (to Y. Ishikawa).
Acknowledgments
The authors are grateful to Mayumi Watanabe, Yukari Kaneda, and Fumi Nakamura for technical assistance and animal care.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Basoni C, Nobles M, Grimshaw A, Desgranges C, Davies D, Perretti M, Kramer IM, Genot E. Inhibitory control of TGF-β1 on the activation of Rap1, CD11b, and transendothelial migration of leukocytes. FASEB J 19: 822–824, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bos JL Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bos JL Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4: 733–738, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol 20: 85–90, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur J Cancer 41: 1911–1922, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Engelse MA, Lardenoye JH, Neele JM, Grimbergen JM, De Vries MR, Lamfers ML, Pannekoek H, Quax PH, De Vries CJ. Adenoviral activin a expression prevents intimal hyperplasia in human and murine blood vessels by maintaining the contractile smooth muscle cell phenotype. Circ Res 90: 1128–1134, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Enyeart JJ, Mlinar B, Enyeart JA. Adrenocorticotropic hormone and cAMP inhibit noninactivating K+ current in adrenocortical cells by an A-kinase-independent mechanism requiring ATP hydrolysis. J Gen Physiol 108: 251–264, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grande-Garcia A, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J Cell Biol 177: 683–694, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong J, Doebele RC, Lingen MW, Quilliam LA, Tang WJ, Rosner MR. Anthrax edema toxin inhibits endothelial cell chemotaxis via Epac and Rap1. J Biol Chem 282: 19781–19787, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Indolfi C, Avvedimento EV, Di Lorenzo E, Esposito G, Rapacciuolo A, Giuliano P, Grieco D, Cavuto L, Stingone AM, Ciullo I, Condorelli G, Chiariello M. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med 3: 775–779, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Indolfi C, Di Lorenzo E, Rapacciuolo A, Stingone AM, Stabile E, Leccia A, Torella D, Caputo R, Ciardiello F, Tortora G, Chiariello M. 8-chloro-cAMP inhibits smooth muscle cell proliferation in vitro and neointima formation induced by balloon injury in vivo. J Am Coll Cardiol 36: 288–293, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa Y, Homcy CJ. The adenylyl cyclases as integrators of transmembrane signal transduction. Circ Res 80: 297–304, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Jeon TJ, Lee DJ, Merlot S, Weeks G, Firtel RA. Rap1 controls cell adhesion and cell motility through the regulation of myosin II. J Cell Biol 176: 1021–1033, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson R, Webb JG, Newman WH, Wang Z. Regulation of human vascular smooth muscle cell migration by beta-adrenergic receptors. Am Surg 72: 51–54, 2006. [PubMed] [Google Scholar]
- 16.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem 278: 8279–8285, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 282: 2275–2279, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277: 11497–11504, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Millette E, Rauch BH, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB transactivates the fibroblast growth factor receptor to induce proliferation in human smooth muscle cells. Trends Cardiovasc Med 16: 25–28, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Murphy MM, Zayed MA, Evans A, Parker CE, Ataga KI, Telen MJ, Parise LV. Role of Rap1 in promoting sickle red blood cell adhesion to laminin via BCAM/LU. Blood 105: 3322–3329, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol 190: 300–309, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Obrosova IG, Drel VR, Kumagai AK, Szabo C, Pacher P, Stevens MJ. Early diabetes-induced biochemical changes in the retina: comparison of rat and mouse models. Diabetologia 49: 2525–2533, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelletier S, Julien C, Popoff MR, Lamarche-Vane N, Meloche S. Cyclic AMP induces morphological changes of vascular smooth muscle cells by inhibiting a Rac-dependent signaling pathway. J Cell Physiol 204: 412–422, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol 160: 487–493, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sata M, Tanaka K, Ishizaka N, Hirata Y, Nagai R. Absence of p53 leads to accelerated neointimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol 23: 1548–1552, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt M, Sand C, Jakobs KH, Michel MC, Weernink PA. Epac and the cardiovascular system. Curr Opin Pharmacol 7: 193–200, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Slomp J, van Munsteren JC, Poelmann RE, de Reeder EG, Bogers AJ, Gittenberger-de Groot AC. Formation of intimal cushions in the ductus arteriosus as a model for vascular intimal thickening. An immunohistochemical study of changes in extracellular matrix components. Atherosclerosis 93: 25–39, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Swaney JS, Patel HH, Yokoyama U, Head BP, Roth DM, Insel PA. Focal adhesions in (myo)fibroblasts scaffold adenylyl cyclase with phosphorylated caveolin. J Biol Chem 281: 17173–17179, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 52: 639–672, 2000. [PubMed] [Google Scholar]
- 30.Ulucan C, Wang X, Baljinnyam E, Bai YZ, Okumura S, Sato M, Minamisawa S, Hirotani S, Ishikawa Y. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol 293: H1662–H1672, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama U, Minamisawa S, Adachi-Akahane S, Akaike T, Naguro I, Funakoshi K, Iwamoto M, Nakagome M, Uemura N, Hori H, Yokota S, Ishikawa Y. Multiple transcripts of Ca2+ channel α1-subunits and a novel spliced variant of α1C-subunit in the rat ductus arteriosus. Am J Physiol Heart Circ Physiol 290: H1660–H1670, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, Iwasaki S, Iwamoto M, Misra S, Tamura K, Hori H, Yokota S, Toole BP, Sugimoto Y, Ishikawa Y. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest 116: 3026–3034, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci USA 105: 6386–6391, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]