Abstract
Exercise in hypertensive individuals elicits exaggerated increases in mean arterial pressure (MAP) and heart rate (HR) that potentially enhance the risk for adverse cardiac events or stroke. Evidence suggests that exercise pressor reflex function (EPR; a reflex originating in skeletal muscle) is exaggerated in this disease and contributes significantly to the potentiated cardiovascular responsiveness. However, the mechanism of EPR overactivity in hypertension remains unclear. EPR function is mediated by the muscle mechanoreflex (activated by stimulation of mechanically sensitive afferent fibers) and metaboreflex (activated by stimulation of chemically sensitive afferent fibers). Therefore, we hypothesized the enhanced cardiovascular response mediated by the EPR in hypertension is due to functional alterations in the muscle mechanoreflex and metaboreflex. To test this hypothesis, mechanically and chemically sensitive afferent fibers were selectively activated in normotensive Wistar-Kyoto (WKY) and spontaneously hypertensive (SHR) decerebrate rats. Activation of mechanically sensitive fibers by passively stretching hindlimb muscle induced significantly greater increases in MAP and HR in SHR than WKY over a wide range of stimulus intensities. Activation of chemically sensitive fibers by administering capsaicin (0.01–1.00 μg/100 μl) into the hindlimb arterial supply induced increases in MAP that were significantly greater in SHR compared with WKY. However, HR responses to capsaicin were not different between the two groups at any dose. This data is consistent with the concept that the abnormal EPR control of MAP described previously in hypertension is mediated by both mechanoreflex and metaboreflex overactivity. In contrast, the previously reported alterations in the EPR control of HR in hypertension may be principally due to overactivity of the mechanically sensitive component of the reflex.
Keywords: blood pressure, heart rate, muscle afferents
the cardiovascular response to exercise is exaggerated in hypertension and is characterized by augmented increases in heart rate (HR), arterial blood pressure (ABP), and vascular resistance (3, 15, 19, 25, 38, 43). These abnormal hemodynamic responses to physical activity in hypertension are associated with an increased risk for adverse cardiovascular events during and after exercise such as myocardial ischemia or infarction, cardiac arrest, and stroke (13, 21, 35, 36). Therefore, dissection of the regulatory mechanisms underlying the altered hemodynamic responses to exercise in hypertension is important and clinically relevant.
The cardiovascular response to exercise is mediated by three neurophysiological mechanisms: the exercise pressor reflex (EPR), central command, and the arterial baroreflex. The EPR is a feedback peripheral neural drive originating in skeletal muscle that regulates changes in ABP and HR during physical activity predominantly via the sympathetic nervous system (1, 29, 34). Central command is a feed-forward mechanism that simultaneously activates motor areas within the cerebral cortex and cardiovascular centers within the brain stem (10, 22). During exercise, central command contributes to increases in ABP and HR via sympathetic activation and parasympathetic withdrawal. The cardiovascular responses mediated by the EPR and central command are modulated by the tonically active arterial baroreflex. Arterial baroreflex afferent fibers are stimulated by mechanically sensitive receptors located within the carotid arteries and aortic arch. These receptors respond to changes in blood pressure by altering HR, stroke volume, and vascular resistance on a beat-to-beat basis at rest and during exercise (28, 40). Although each of these mechanisms could potentially mediate the exaggerated circulatory response to exercise in hypertension, recent evidence from our laboratory suggests the EPR significantly contributes to this heightened cardiovascular responsiveness (49). However, the mechanisms driving EPR dysfunction in hypertension remain unclear.
EPR function is mediated by two reflex mechanisms: the muscle mechanoreflex and the muscle metaboreflex. The muscle mechanoreflex is activated by predominately mechanically sensitive group III afferent fibers (17, 18). Receptors associated with these fibers in skeletal muscle primarily respond to mechanical distortion of their receptive fields. The muscle metaboreflex is activated predominately by chemically sensitive group IV afferent fibers (17, 18). Receptors associated with these afferents are in close proximity to the vasculature of skeletal muscle and respond to the chemical by-products of muscle metabolism. Given that each of these inputs is essential to EPR activity (34), both the mechanoreflex and metaboreflex potentially mediate the EPR dysfunction that develops in hypertension (49). This is indeed the case in disease states closely related to hypertension (e.g., heart failure) where alterations in both mechanoreflex and metaboreflex activity have been shown to contribute to the development of EPR dysfunction (2, 26, 32, 47, 50).
The purpose of this investigation was, therefore, to determine the possible contributions of the muscle mechanoreflex and metaboreflex to EPR overactivity in hypertension. We hypothesized that the potentiated cardiovascular response to activation of the EPR in hypertension is driven by functional alterations in both the mechanically and chemically sensitive components of the reflex. To test this hypothesis, we preferentially stimulated mechanically and chemically sensitive skeletal muscle afferent fibers in decerebrated normotensive Wistar-Kyoto (WKY) and spontaneously hypertensive (SHR) rats. Determining which component of the EPR mediates its overactivity in hypertension is important since each arm of the reflex is activated by distinctly different stimuli. As such, the mechanoreflex and metaboreflex represent unique targets for the treatment of EPR dysfunction in hypertension.
MATERIALS AND METHODS
Subjects
Experiments were performed in 30 SHR and 35 WKY age-matched (14–20 wk) male rats (Harlan, Indianapolis, IN).Animals were housed in standard rodent cages on 12-h light-dark cycles and were given food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center at Dallas. In addition, all studies were conducted in accordance with the United States Department of Health and Human Services National Institutes of Health Guide for the Care and Use of Laboratory Animals.
General Surgical Procedures
Rats were anesthetized with isoflurane gas (2–3% in 100% oxygen), intubated, and mechanically ventilated (Harvard Apparatus). Levels of inhalant gas were increased as indicated by a withdrawal reflex to pinching of the hindpaw and/or spontaneous increases in HR. To minimize the development of edema, dexamethasone (0.2 mg) was given intramuscularly (53). Fluid-filled catheters (PE-50, polyethylene tubing) were placed in both common carotid arteries and the right jugular vein for the measurement of blood pressure and the administration of fluids, respectively. Arterial blood gases and pH were monitored throughout the experiment (50-μl blood samples; 4–5 times) using an automated blood gas analyzer (model ABL 5, Radiometer) to ensure these variables were maintained within normal ranges (arterial Po2 of >80 Torr; arterial Pco2 of 35–45 Torr; pH, 7.3–7.4). Body temperature was maintained between 36.5 and 38.0°C by an isothermal pad (Deltaphase). Animals were held in a stereotaxic head unit (Kopf Instruments), and a precollicular decerebration was performed. A bilateral craniotomy was conducted by drilling holes into the parietal skull. The bone superior to the central saggital sinus was removed. The dura mater covering the brain was cut, and the cerebrum was aspirated. The animal was rendered insentient by sectioning the brain rostral to the superior colliculus and aspirating the forebrain. To minimize cerebral hemorrhage, small pieces of oxidized regenerated cellulose (Ethicon, Johnson & Johnson) were placed on the internal skull surface, and the cranial cavity was packed with cotton. Immediately after the decerebration procedure was completed, gas anesthesia was discontinued. To maintain fluid balance and baseline ABP, a 1 M NaHCO3, 5% dextrose Ringer solution was continuously infused via the jugular vein at a rate of 3–5 ml·h−1·kg−1 (41). A minimum recovery period of 1 h was employed after decerebration before beginning any experimental protocol. This allowed sufficient time for the effects of isoflurane anaesthesia to completely dissipate and ABP to stabilize.
Mechanoreflex Testing
Preparation.
The additional surgical procedures subsequently described were conducted on 11 WKY and 11 SHR animals. To begin, a spinal laminectomy exposing the lower lumbar portions of the spinal cord (L2–L6) was performed. The dura of the cord was cut and reflected, allowing visual identification of the L4 and L5 spinal roots. The dorsal and ventral roots were carefully separated. The ventral roots were sectioned, and the cut peripheral ends were positioned on insulated bipolar platinum electrodes. The exposed neural tissue was covered in a pool of mineral oil. Animals were secured in a customized spinal frame by clamps placed on rostral lumbar vertebrae. The pelvis was stabilized with steel posts within the frame, and the right hindlimb was fixed in one position using clamps attached to the tibial bone. The gastrocnemius and soleus muscles were isolated, and the calcaneal bone was cut. Lastly, the Achilles' tendon was connected to a force transducer (Grass Instruments, FT10), allowing the measurement of muscle tension.
Experimental protocol.
During muscle contraction, both the mechanically and chemically sensitive components of the EPR are stimulated (34). Passively stretching hindlimb skeletal muscle does not increase muscle metabolism and, thus, is commonly used to selectively engage the mechanically sensitive component of the EPR (20, 26, 51). Therefore, to preferentially activate mechanically sensitive afferent fibers associated with the muscle mechanoreflex in WKY and SHR animals, the gastrocnemius and soleus muscles of the right hindlimb were passively stretched using a calibrated 9.5-mm rack and pinion system (Harvard Apparatus). To evoke a mechanical stimulus similar to that elicited during muscle contraction, care was taken to generate the same magnitude and pattern of muscle tension developed during contraction. This was achieved by first determining the maximal force developed during static contraction of the gastrocnemius and soleus muscles. These muscles were contracted by electrically stimulating the L4 and L5 ventral roots for 30 s. Constant-current electrical stimulation was used at three times motor threshold (defined as the minimum current required to produce a muscle twitch) with a pulse duration of 0.1 ms at 40 Hz. These stimulus parameters are known to generate maximal tension development during muscle contraction in rats (46). Once maximal tension development was established, the gastrocnemius and soleus muscles were stretched at this intensity a minimum of two times with a 15-min recovery period between each trial. In a subset of these animals (10 WKY and 9 SHR), additional stretches were performed at randomized submaximal intensities. Before all maneuvers, muscles were preloaded by stretching to 70–100 g of tension.
Metaboreflex Testing
Preparation.
The additional surgical procedures subsequently described were conducted on 19 WKY and 15 SHR animals. To administer drugs into the arterial supply of muscle within the right hindlimb, the circulation of the hindlimb was isolated. A catheter (PE-10, polyethylene tubing) was placed in the left common iliac artery, and its tip was advanced to the bifurcation of the abdominal aorta. This procedure allowed the injection of substances directly into the circulation of the right hindlimb via the right common iliac artery. To limit drug delivery to the hindlimb, a reversible vascular occluder was placed around the common iliac vein emptying the right hindlimb.
Experimental protocol.
Selective activation of chemically sensitive afferent fibers associated with the muscle metaboreflex was achieved by administering graded concentrations of capsaicin into the arterial supply of the right hindlimb of WKY and SHR animals. The capsaicin receptor, transient receptor potential vanilloid 1 (TRPv1), is a relatively selective marker of group IV afferent neurons, although the receptor is also present on a small number of group III afferent fibers (14, 31). Therefore, stimulation of this receptor predominately activates the neuronal population within skeletal muscle known to primarily mediate metaboreflex activity and is commonly used for this purpose (16, 26, 50). Capsaicin was injected directly into the arterial supply of the right hindlimb via the right common iliac artery as described. To limit drug delivery to the hindlimb being tested, the reversible ligature placed around the right common iliac vein was pulled for 2 min. In each animal, saline and/or the vehicle for capsaicin was injected first followed by the administration of capsaicin at five different dosages (0.01, 0.03, 0.10, 0.30, and 1.00 μg/100 μl). Animals underwent a recovery period of 15 min between each injection. Each injection was flushed by 0.2 ml of saline. Two minutes before each injection, 0.15 ml of the neuromuscular blocker vecuronium bromide was given intravenously to prevent muscle twitch and/or contraction during capsaicin injection.
Control experiments.
As a control, 1.00 μg/100 μl of capsaicin was injected directly into the systemic circulation via the jugular vein at the conclusion of the protocol. This maneuver was performed to evaluate the systemic response to capsaicin compared with that of its response when injected locally in the hindlimb circulation. As an additional control, a subset of WKY (n = 5) and SHR (n = 5) animals received injections of capsaicin in the hindlimb circulation together with capsazepine (100 μg/100 μl), a selective TRPv1 receptor antagonist. This experiment was performed to confirm the cardiovascular effects elicited by administration of capsaicin were the result of TRPv1 receptor activation. Finally, to eliminate the possibility that cutaneous afferent neurons may contribute to the cardiovascular response elicited by hindlimb injection of capsaicin, 0.3- and 1.0-μg/100 μl doses of capsaicin were injected into the arterial supply of the hindlimb before and after removing the skin from the leg in four WKY rats.
Corollary Experiments
To assess blood pressure responsiveness independent of EPR activation in normotensive and hypertensive animals, changes in ABP elicited by intravenous administration of the pressor agent phenylephrine (2 mg/kg) were determined in separate groups of WKY (n = 5) and SHR (n = 4) animals.
Morphological Measurements
At the conclusion of all experiments, decerebrated animals were euthanized by intravenous injection of saturated potassium chloride (4 M, 2 ml/kg). Use of this procedure adheres to the guidelines established by the Panel on Euthanasia of the American Veterinary Medical Association. In all animals, the heart and lungs were excised and weighed. Additionally, the tibia was harvested, weighed, and measured.
Data Acquisition
A pressure transducer (model DTX plus-DT-NN12, Ohmeda) connected to the left carotid arterial catheter was used to measure ABP. Mean arterial pressure (MAP) was determined by integrating the arterial pressure signal with a time constant of 1–4 s. HR was derived from the blood pressure pulse wave using a biotachometer (Gould Instruments). Hindlimb tension was measured by a force transducer (FT-10, Grass Instruments). Baseline values for all variables were determined by evaluating 30 s of recorded data before a given stretch or drug injection. The peak response of each variable was defined as the greatest change from baseline elicited by the experimental stimulus. All cardiovascular and contractile force data were acquired, recorded, and analyzed using data acquisition software (Spike 2, version 3, Cambridge Electronic Design) for the CED micro 1401 system (Cambridge Electronic Design).
Statistical Analyses
On all data sets, statistics were performed using correlation and regression analyses, Student t-tests, or ANOVA with Student-Newman-Keuls post hoc tests employed as appropriate. The significance level was set at P < 0.05. Results are presented as means ± SE. Statistical analyses were conducted using SigmaStat software (Jandel Scientific Software, SPSS).
RESULTS
Characterization of Hypertensive Model
Morphometric and baseline hemodynamic data for WKY and SHR animals are presented in Table 1. Body weights were not different between WKY and SHR. Ratios of heart weight to both body weight and tibial length were significantly greater in SHR than in WKY. However, the lung weight-to-body weight ratio was not different between the groups. Baseline MAP was significantly higher in SHR than in WKY animals, whereas baseline HR was not different.
Table 1.
Morphometric characteristics and baseline hemodynamics
| WKY | SHR | |
|---|---|---|
| n | 35 | 30 |
| Body weight, g | 353±8 | 351±6 |
| Heart weight/body weight, mg/g | 3.1±0.1 | 3.8±0.1* |
| Lung weight/body weight, mg/g | 6.3±0.3 | 6.9±0.3 |
| Heart weight/tibial length, mg/mm | 28.4±0.9 | 35.5±1.0* |
| MAP, mmHg | 104±6 | 160±6* |
| HR, beats/min | 438±10 | 446±11 |
Values are means ± SE. WKY, Wystar-Kyoto rats; SHR, spontaneously hypertensive rats; MAP, mean arterial blood pressure; HR, heart rate.
P < 0.05 compared with WKY rats.
The Cardiovascular Response to Activation of Mechanically Sensitive Afferent Fibers is Augmented in Hypertensive Rats
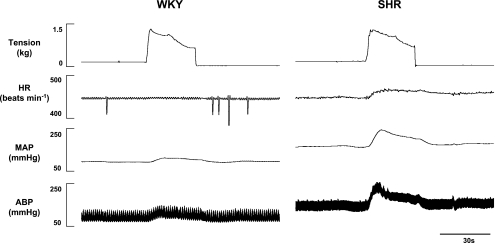
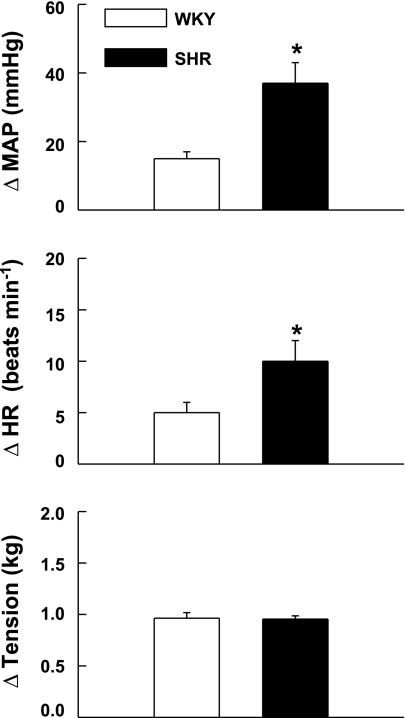
The cardiovascular response to passive muscle stretch was exaggerated in hypertensive compared with normotensive animals. An example of this finding is presented in representative tracings from WKY and SHR animals (Fig. 1). During passive stretch at maximal intensities (i.e., tension levels equivalent to that developed during maximal static contraction), activation of mechanically sensitive afferent fibers elicited significantly larger elevations in both MAP and HR in SHR (37 ± 6 mmHg and 10 ± 2 beats/min, respectively) compared with WKY (15 ± 2 mmHg and 5 ± 1 beats/min, respectively) animals (Fig. 2). Since resting blood pressures were markedly greater in SHR, the cardiovascular response to passive stretch was also quantified as a percentage of baseline MAP and HR. This analysis indicated that the percentage increase from baseline in MAP (SHR = 33.7 ± 6.4%; WKY = 21.1 ± 3.8%) and HR (SHR = 2.8 ± 0.5%; WKY = 1.3 ± 0.3%) in response to stretch was likewise significantly greater in SHR compared with WKY (P < 0.05).
Fig. 1.
Characteristic cardiovascular responses to passive stretch in representative Wistar-Kyoto (WKY) and spontaneously hypertensive (SHR) rats. In response to passive hindlimb muscle stretch of the same tension, mean arterial blood pressure (MAP) and heart rate (HR) were consistently larger in hypertensive compared with normotensive animals.
Fig. 2.
Cardiovascular responses to activation of mechanically sensitive afferent fibers in WKY and SHR animals. Passive stretch of hindlimb skeletal muscle induced increases in MAP and HR that were significantly greater in SHR compared with WKY rats at maximal levels of tension development. *P < 0.05 compared with WKY rats.
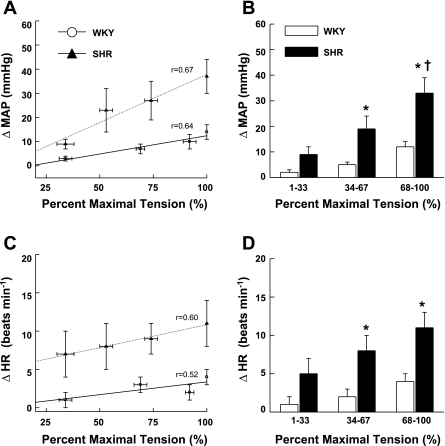
The cardiovascular responses to graded intensities of passive stretch are shown in Fig. 3. As presented in Fig. 3A, graded stretch at submaximal intensities elicited pressor responses that were positively correlated to tension development in both WKY (r = 0.64; P < 0.01) and SHR (r = 0.67; P < 0.01). Linear regression analyses determined that the slope of this relationship was significantly greater (P < 0.05) in SHR (0.41 ± 0.07) than in WKY (0.21 ± 0.06). When data were binned into low (1–33%), medium (34–67%), and high (68–100%) levels of maximal tension (Fig. 3B), the MAP response to stretch was found to be elevated in SHR at all levels of intensity and became significantly different from WKY in the medium range. Similarly, graded stretch-induced HR responses were positively correlated to tension development in WKY (r = 0.52; P < 0.01) and SHR (r = 0.60; P < 0.01) animals (Fig. 3C). Linear regression analyses determined that the slope of this relationship was larger in SHR (0.09 ± 0.02) than in WKY (0.05 ± 0.02), although a significant difference was not established. As with MAP, binning HR data into low, medium, and high levels of maximal tension (Fig. 3D) determined that the HR response to stretch was larger in SHR at every level of tension developed, again reaching statistical significance from WKY at the medium range of intensity.
Fig. 3.
Cardiovascular responses to graded activation of mechanically sensitive afferent fibers. A: passive stretch of hindlimb skeletal muscle at graded intensities elicited pressor responses that were positively correlated to tension development in both WKY and SHR rats (P < 0.01). B: the stretch-induced increases in MAP were significantly larger in SHR than in WKY rats beginning at intensity levels corresponding to 34–67% of maximal tension development. C: HR was also found to be positively correlated to tension development in each group of animals (P < 0.01). D: likewise, the stretch-induced increases in HR were significantly larger in hypertensive than in normotensive rats beginning at intensity levels corresponding to 34–67% of maximal tension development. In A and C, when regression analyses were applied, the slopes of the relationships were greater in SHR compared with WKY. All data points within a group were used to determine the correlation coefficient (r) and regression slope and averaged for presentation only. *P < 0.05 compared with WKY rats. †P < 0.05 compared with all preceding lower levels of tension development.
The Pressor Response to Activation of Chemically Sensitive Afferent Fibers is Potentiated in Hypertensive Rats
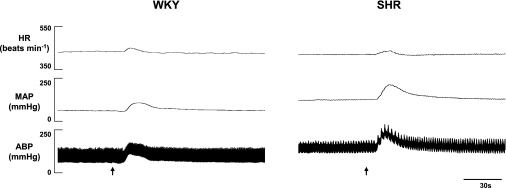
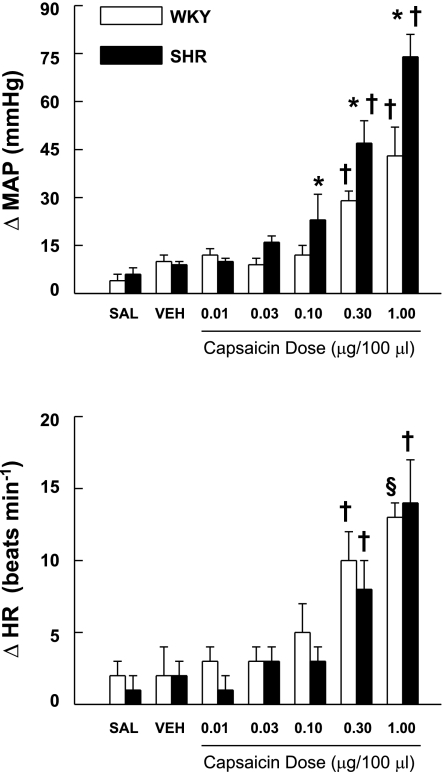
The pressor, but not tachycardic, response to intra-arterial administration of capsaicin within the hindlimb was consistently augmented in hypertensive compared with normotensive animals. A representative example of this finding is presented in Fig. 4. Figure 5 demonstrates the changes in MAP and HR elicited by activation of chemically sensitive afferent fibers via the administration of increasing doses of capsaicin within the hindlimb circulation. Both groups of animals displayed dose-related increases in MAP and HR. However, beginning at capsaicin doses of 0.10 μg/100 μl or higher, the elevations in MAP were significantly greater in SHR than in WKY. For example, at the 0.30 μg/100 μl dose of capsaicin, the increase in MAP was 47 ± 7 mmHg in SHR compared with 29 ± 3 mmHg in WKY. When these responses were quantified as a percent change from resting blood pressure, the percent increase from baseline in MAP was consistently larger in SHR than in WKY, but statistical significance was not reached. Interestingly, the HR response to capsaicin was not significantly different between the two groups of animals at any dose of capsaicin administered.
Fig. 4.
Characteristic cardiovascular responses to capsaicin in representative WKY and SHR rats. In response to the intra-arterial administration of capsaicin (1.00 μg/100 μl) within the hindlimb, MAP was consistently larger in hypertensive compared with normotensive animals. The HR response to capsaicin was similar in WKY and SHR. Arrows demarcate administration of capsaicin.
Fig. 5.
Cardiovascular responses to activation of chemically sensitive afferent fibers in WKY and SHR animals. Administration of graded doses of capsaicin into the arterial supply of hindlimb skeletal muscle induced increases in MAP that were significantly greater in SHR compared with WKY rats. Although capsaicin evoked increases in HR, the chronotropic response was not different between normotensive and hypertensive animals at all doses administered. *P < 0.05 compared with WKY rats. †P < 0.05 compared with all preceding lower doses of capsaicin. §P < 0.05 compared with all doses of capsaicin of <0.30 μg/100 μl.
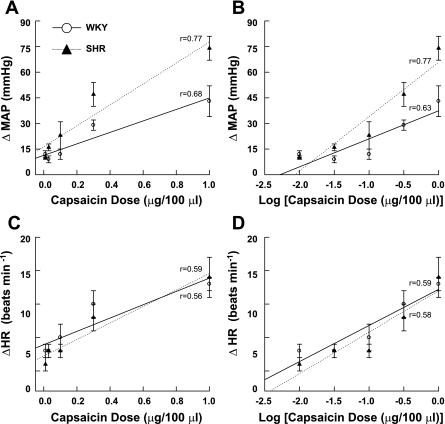
As presented in Fig. 6A, graded administration of capsaicin elicited pressor responses that were positively correlated to the dose given in both WKY (r = 0.68; P < 0.01) and SHR (r = 0.77; P < 0.01). Linear regression analyses determined that the slope of this relationship was significantly greater (P < 0.05) in SHR (64.5 ± 7.8) than in WKY (34.6 ± 9.2). Since capsaicin was administered on a log scale, the pressor response was also plotted against the log of the capsaicin dose (Fig. 6B) to correct for skewness within the distribution of data. This analysis produced similar results determining the MAP response was positively correlated to the log of the capsaicin dose administered in WKY (r = 0.63; P < 0.01) and SHR (r = 0.77; P < 0.01). Again, linear regression analyses determined that the slope of this relationship was significantly greater (P < 0.05) in SHR (31.0 ± 3.5) than in WKY (17.1 ± 3.6). As presented in Fig. 6C, graded administration of capsaicin elicited HR responses that were positively correlated to the dose given in both WKY (r = 0.56; P < 0.01) and SHR (r = 0.59; P < 0.01). Similar results were produced for WKY (r = 0.59; P < 0.01) and SHR (r = 0.58; P < 0.01) when the HR response was plotted against the log of the capsaicin dose administered (Fig. 6D). In both sets of analyses, no difference existed in the slopes of the relationships between WKY and SHR animals.
Fig. 6.
Cardiovascular responses to graded activation of chemically sensitive afferent fibers. A: injection of capsaicin into the arterial supply of hindlimb skeletal muscle elicited pressor responses that were positively correlated to the dose of capsaicin administered in both WKY and SHR rats (P < 0.01). B: when MAP was plotted against the log of the capsaicin dose, this relationship remained. In A and B, when regression analyses were applied, the slopes of the relationships were significantly greater in SHR compared with WKY. C: HR was likewise found to be positively correlated to the dose of capsaicin administered in each group of animals (P < 0.01). D: when HR was plotted against the log of the capsaicin dose, this relationship was again the same. When regression analyses were applied in C and D, the slopes of the relationships were not different between SHR and WKY. In A–D, all data points within a group were used to determine the correlation coefficient (r) and regression slope. Data were averaged for presentation only.
To ensure that the cardiovascular responses to capsaicin were due to local activation of chemically sensitive afferent fibers within the hindlimb and not to systemic activation of TRPv1 receptors, capsaicin (1.00 μg/100 μl) was also administered via the jugular vein at the conclusion of each experiment. The intravenous administration of capsaicin into the general circulation elicited smaller increases in MAP than those seen during intra-arterial hindlimb injection in WKY (27 ± 14 vs. 43 ± 9 mmHg, respectively) and SHR (35 ± 9 vs. 74 ± 7 mmHg, respectively). Importantly, the intravenous administration of capsaicin induced decreases in HR in both WKY (−103 ± 39 beats/min) and SHR (−79 ± 28 beats/min). These data are in contrast to the tachycardic response elicited by hindlimb intra-arterial injections. The small increases in MAP and decreases in HR in response to the systemic administration of capsaicin are consistent with the effects of capsaicin on receptors in the general circulation (6). This control experiment validates the contention that the administration of capsaicin was limited to the hindlimb circulation in this study.
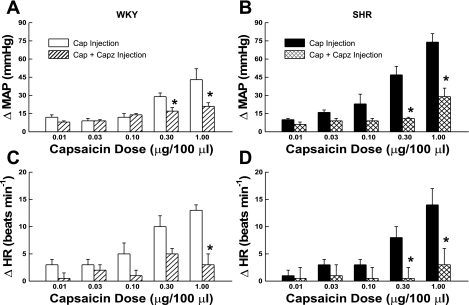
The effect of the TRPv1 antagonist capsazepine on the cardiovascular response to the intra-arterial hindlimb injection of capsaicin is presented in Fig. 7. Capsazepine significantly attenuated the pressor response to capsaicin beginning at the 0.30 μg/100 μl dose in WKY (Fig. 7A). In SHR, the increase in MAP was reduced at all doses of capsaicin when co-administered with capsazepine becoming significant at the 0.30 μg/100 μl dose (Fig. 7B). Capsazepine had similar effects on the HR response to capsaicin in both WKY (Fig. 7C) and SHR (Fig. 7D) animals. These findings support the contention that the cardiovascular responses evoked by administration of capsaicin were mediated by TRPv1 receptor activation.
Fig. 7.
Antagonizing the TRPv1 receptor within the hindlimb attenuates the cardiovascular response to capsaicin (Cap). The MAP response to injection of capsaicin into the arterial supply of the hindlimb was significantly reduced when co-administered with the selective TRPv1 antagonist capsazepine (Capz; 100 μg/100 μl) in both WKY (A) and SHR (B) animals. Capsazepine had a similar effect on the HR response to capsaicin in WKY (C) and SHR (D). *P < 0.05 compared with trials in which only capsaicin was injected.
To determine whether activation of cutaneous afferents contributed to the cardiovascular response elicited by the injection of capsaicin into the arterial supply of the hindlimb, capsaicin was administered before and after removing the skin from the leg. This procedure had no effect on the HR and MAP responses to capsaicin. For example, capsaicin (0.30 μg/100 μl) induced a 26 ± 8 mmHg increase in MAP before the skin was removed from the hindlimb and a 25 ± 10 mmHg increase after the skin was removed.
Blood Pressure Responsiveness to Phenylephrine is Attenuated in Hypertensive Rats
To determine whether the blood pressure response to a nonreflex-mediated stimulus is likewise exaggerated in hypertensive compared with normotensive rats, the pressor agent phenylephrine was administered intravenously. Phenylephrine evoked a 55 ± 9 mmHg increase in MAP in SHR animals. Despite this robust pressor response in hypertensive rats, it was significantly less than that elicited in normotensive WKY animals (104 ± 12 mmHg).
DISCUSSION
It is well established that the cardiovascular response to exercise is potentiated in hypertensive patients (3, 15, 19, 43). We have recently determined that this accentuated cardiovascular response to physical activity is mediated, in part, by EPR overactivity (49). In the current study, we report, for the first time, that selective activation of mechanically sensitive skeletal muscle afferent fibers (associated with the muscle mechanoreflex) elicits exaggerated increases in MAP and HR in hypertensive compared with normotensive rats. We have further demonstrated that the pressor response to selective activation of chemically sensitive afferent fibers within skeletal muscle (associated with the muscle metaboreflex) is enhanced in hypertension.
Identifying alterations in the cardiovascular response to activation of afferent fibers associated with the mechanically and chemically sensitive components of the EPR was expected in the current study. This is a logical hypothesis given the essential contributions of both the mechanoreflex and metaboreflex to normal EPR function (17, 34). However, the nature in which the mechanically and chemically sensitive components would be altered in hypertension could not be anticipated. Changes in the circulatory response to activation of mechanically and chemically sensitive fibers could manifest in the same or opposite direction. For example, in heart failure (a disease state closely related to hypertension), accumulating evidence suggests that EPR overactivity is driven primarily by an exaggerated muscle mechanoreflex, whereas muscle metaboreflex activity appears to be reduced (26, 32, 47, 50, 52). Therefore, the finding that the pressor response to activation of both mechanically and chemically sensitive muscle afferent fibers is exaggerated in hypertensive rats is unique. In itself, this is not surprising given the differences in the etiology of various cardiovascular diseases. For example, although hypertension can often lead to congestive heart failure, heart failure can be produced by a variety of cardiac abnormalities (e.g., myocardial infarction) that do not involve chronic increases in systemic pressure. Thus the pathophysiology of EPR dysfunction in hypertension may be distinct from other forms of cardiovascular disease. In the future, this could prove critical in developing strategies targeted at improving EPR dysfunction in hypertension.
As stated previously, the cardiovascular response to exercise is not exclusively mediated by the EPR. On the contrary, the circulatory response to physical activity is determined by integrating input from the EPR, central command, and the arterial baroreflex (10, 28, 40). For example, recent reports suggest that EPR activity can be modulated by central command input (7–9). Therefore, it was important to control for central command activity in the current study. This was achieved by utilizing a previously developed decerebrate model. Although the decerebration procedure does not eliminate all the putative components of the central command pathway (e.g., the mesencephalic locomotor region remains intact), it does remove the areas of the cerebral cortex from which central command originates. Thus central command activity is unlikely to contribute to the cardiovascular responses reported. Likewise, the EPR and baroreflex are known to modify one another functionally (30, 37, 39, 48). Indeed, evidence suggests that the baroreflex buffers EPR activity under normal conditions (49, 55). Furthermore, it has been clearly established that baroreflex sensitivity is reduced in hypertension (23, 33). Therefore, it is possible that the exaggerated cardiovascular response to activation of mechanically and chemically sensitive afferent fibers is due to a decrease in the buffering capacity of the baroreflex. This is unlikely, however, since we have previously established that the baroreflex maintains its ability to buffer the EPR in hypertensive rats (49).
Baseline Evidence for Muscle Mechanoreflex Overactivity in Hypertension
Clearly, the MAP and HR responses to passive stretch at maximal intensity were significantly greater in SHR compared with WKY. Perhaps more interesting, however, was the finding that pressor and tachycardic responses to activation of mechanically sensitive afferent fibers were significantly larger in hypertensive animals over a wide range of submaximal stimulus intensities. For example, even at low stretch intensities (i.e., 1–33% of maximal tension), the MAP response in SHR was more than four times that of WKY, whereas the HR response was five times greater in hypertensive animals. Combined with the finding that the slope of the relationship characterizing MAP and tension was significantly larger in SHR than in WKY, these data suggest that the sensitivity of the reflex response to activation of mechanically sensitive afferent fibers is enhanced in hypertension. These results further suggest that, at low to moderate work intensities, activation of mechanically sensitive afferent neurons may be capable of driving abnormally large cardiovascular responses to exercise. Theoretically, this could include relatively nonstrenuous exercise that could potentially increase the risk for the occurrence of deleterious cardiovascular events during the performance of common daily activities.
It should be noted that, although passively stretching muscle is a useful experimental tool, this technique may not completely mimic the pattern or mode by which the muscle mechanoreflex is activated during exercise. For example, it has been recently reported that muscle stretch stimulates a different population of mechanically sensitive muscle fibers than does muscle contraction, although there is some overlap between populations (12). With this limitation in mind, passive stretch is commonly used because it can selectively activate mechanically sensitive afferent fibers, associated with the muscle mechanoreflex, in a controlled and graded fashion (26, 45, 51). In the current study, use of this strategy has provided initial evidence consistent with the tenant that the muscle mechanoreflex is overactive in hypertension. Although this line of evidence represents a valuable starting point, additional experimentation designed to assess the activity of the mechanoreflex during exercise in hypertension is warranted.
Baseline Evidence for Muscle Metaboreflex Overactivity in Hypertension
Activation of chemically sensitive skeletal muscle afferent fibers by the intra-arterial administration of capsaicin within the hindlimb elicited increases in MAP that were greater in SHR than in WKY over a relatively large range of stimulus intensities. Furthermore, the slope of the relationship characterizing MAP and capsaicin dose was significantly larger in SHR than in WKY. With regard to the control of blood pressure, these results indicate the sensitivity of the reflex response to activation of chemically sensitive afferent fibers is augmented in hypertension. However, the HR response to capsaicin was not found to be different between hypertensive and normotensive animals. Adding more intrigue, a recent report in humans intimates that the metaboreflex control of muscle sympathetic nerve activity may be reduced in hypertension (42). Collectively, these findings in animals and humans suggest that activation of chemically sensitive afferent fibers in skeletal muscle may differentially regulate blood pressure, HR, and sympathetic nerve activity in hypertension.
Administering chemical agents into the arterial supply of muscle is commonly used to activate chemically sensitive afferent fibers (16). Since the endogenous metabolite that stimulates this population of afferent fibers during muscle contraction is still unknown, capsaicin is often used for this purpose (16, 26, 50). The capsaicin receptor, TRPv1, is predominately located on chemically sensitive group IV afferent neurons as well as a small number of group III afferent fibers (14, 31). Thus stimulation of this receptor excites neurons associated with the muscle metaboreflex. However, capsaicin is not an endogenously produced by-product of muscle metabolism. Therefore, it is possible that capsaicin activates these neurons in a manner that is not completely consistent with their stimulation during exercise. Despite this limitation, the administration of capsaicin is a useful tool since it allows baseline assessment of predominately group IV afferent fiber behavior. It has been used for this purpose in the current study and provides initial insight that the muscle metaboreflex may be altered in hypertension. However, additional experimentation is needed to definitively characterize changes in metaboreflex function during exercise in this disease.
Study Limitations
Several limitations to the interpretation of the study are acknowledged. It is possible that the exaggerated blood pressure responses to stretch and capsaicin in hypertensive rats were not the product of alterations in the sensitivity of mechanically and chemically sensitive reflexes but rather reflective of vascular hyperreactivity. Conceptually, if this is the case, an exaggerated pressor response would be evoked in SHR by any type of stimulus that raises blood pressure. To assess this possibility, phenylephrine was administered intravenously in WKY and SHR. In agreement with previously published reports (24, 54), the blood pressure response to phenylephrine was significantly larger in normotensive WKY rats rather than in hypertensive SHR animals. It has been reported that this absence of hyperresponsiveness in SHR in response to a circulating pressor agent is not due to an increase in the activity of attenuating reflexes (54). It should be noted that in situ and isolated vessel preparations have demonstrated that vasoconstrictor responses are enhanced in SHR compared with WKY (5, 27). However, this often does not translate into an exaggerated increase in blood pressure responsiveness in vivo (54). This may be due to the finding that increases in shear stress can induce greater vasodilation in SHR compared with WKY, thus balancing enhancements in vasoconstrictor drive (5). Therefore, it is unlikely that the exaggerated blood pressure responses to stretch and capsaicin in this study were primarily due to vascular hyperreactivity. More likely, the enhanced blood pressure responses in SHR were mediated by reflex-induced exaggerations in sympathetic nerve activity.
Primary afferent neurons within the hindlimb innervate the skin, joints, and skeletal muscle. In addition, afferent fibers innervating skeletal muscle respond to both noxious (nociceptors) and nonnoxious (ergoreceptors of the EPR) stimuli. It is possible that the techniques used to preferentially activate mechanically and chemically sensitive afferent fibers (associated with ergoreceptors) also stimulated nociceptors as well as joint afferent fibers, thus contributing to the cardiovascular responses observed. In contrast, since there was no difference in the cardiovascular response to capsaicin before or after removing the skin from the hindlimb, it is unlikely that cutaneous afferents contributed appreciably to the changes in MAP and HR reported.
Conclusions and Clinical Significance
In summary, the findings of this investigation provide evidence consistent with the concept that EPR dysfunction in hypertension is mediated, in part, by both mechanoreflex and metaboreflex overactivity. Since regular exercise has been shown to increase resting arterial compliance and lower basal sympathetic nerve activity, as well as normalize pressure and baroreceptor function (4, 11, 44), physical activity can be used as an effective nonpharmacological treatment in hypertensive patients. Unfortunately, exercise training is often accompanied by risks for adverse cardiac events (e.g., acute myocardial infarction) or stroke limiting its prescription in these individuals (13, 21, 35, 36). Increasing our understanding of mechanoreflex and metaboreflex dysfunction in hypertension could potentially lead to the development of therapeutic interventions designed to make exercise a safer modality of treatment in hypertension.
GRANTS
This research was supported by grants from the American Heart Association (SDG 0735355N to S. A. Smith), the National Institutes of Health (HL-088422 to S. A. Smith), and the Lawson & Rogers Lacy Research Fund in Cardiovascular Diseases (to J. H. Mitchell).
Acknowledgments
The authors thank Margaret Robledo, Martha Romero, and Julius Lamar, Jr. for expert technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alam M, Smirk FH. Observation in man upon a blood pressure raising reflex arising from voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansorge EJ, Augustyniak RA, Perinot ML, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O'Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Aoki K, Sato K, Kondo S, Pyon CB, Yamamoto M. Increased responses of blood pressure to rest and handgrip in subjects with essential hypertension. Jpn Circ J 47: 802–809, 1983. [DOI] [PubMed] [Google Scholar]
- 4.Cameron J, Dart A. Exercise training increases total systemic arterial compliance in humans. Am J Physiol Heart Circ Physiol 266: H693–H701, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Chang HR, Lee RP, Wu CY, Chen HI. Nitric oxide in mesenteric vascular reactivity: a comparison between rats with normotension and hypertension. Clin Exp Pharmacol Physiol 29: 275–280, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Coleridge HM, Coleridge JCG. Role of the pulmonary arterial baroreceptors in the effects produced by capsaicin in the dog. J Physiol 170: 272–285, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degtyarenko AM, Kaufman MP. Fictive locomotion and scratching inhibit dorsal horn neurons receiving thin fiber afferent input. Am J Physiol Regul Integr Comp Physiol 279: R394–R403, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Degtyarenko AM, Kaufman MP. Stimulation of the mesencephalic locomotor region inhibits the discharge of neurons in the superficial laminae of the dorsal horn of cats. Neurosci Lett 296: 109–112, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Degtyarenko AM, Kaufman MP. Stimulation of the MLR inhibits the discharge of dorsal horn neurons responsive to muscular contraction. Brain Res 880: 178–182, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grassi G, Seravalle G, Calhoun DA, Mancia G. Physical training and baroreceptor control of sympathetic nerve activity in humans. Hypertension 23: 294–301, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hoberg E, Schuler G, Kunze B, Obermoser AL, Hauer K, Mautner HP, Schlierf G, Kubler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol 65: 583–589, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Jansco G, Kiraly E, Jansco-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 270: 741–743, 1977. [DOI] [PubMed] [Google Scholar]
- 15.Kahn JF The static exercise-induced arterial hypertension test. Presse Medicine 20: 1067–1071, 1991. [PubMed] [Google Scholar]
- 16.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res 50: 133–139, 1982. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscle contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984. [DOI] [PubMed] [Google Scholar]
- 19.Kazatani Y, Hamada M, Shigematsu Y, Hiwada K, Kokubu T. Beneficial effect of a long-term antihypertensive therapy on blood pressure response to isometric handgrip exercise in patients with essential hypertension. Am J Ther 2: 165–169, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Kim JK, Hayes SG, Kindig AE, Kaufman MP. Thin-fiber mechanoreceptors reflexly increase renal sympathetic nerve activity during static contraction. Am J Physiol Heart Circ Physiol 292: H866–H873, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C, Fernhall B. Determinants of exercise blood pressure response in normotensive and hypertensive women: role of cardiorespiratory fitness. J Cardiopulm Rehab 22: 178–183, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscle work. J Physiol 47: 112–136, 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol 283: R815–R826, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Leenen FH, Yuan B, Tsoporis J, Lee RM. Arterial hypertrophy and pressor responsiveness during development of hypertension in spontaneously hypertensive rats. J Hypertens 12: 23–32, 1994. [PubMed] [Google Scholar]
- 25.Lever AF, Boushel R. Hypertension. In: Exercise and Circulation in Health and Disease. Champaign, IL: Human Kinetics, 2000.
- 26.Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation 110: 3049–3054, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Lockette W, Otsuka Y, Carretero O. The loss of endothelium-dependent relaxation in hypertension. Hypertension 8: 61–66, 1986. [DOI] [PubMed] [Google Scholar]
- 28.Mancia G, Mark AL. Arterial baroreflexes in humans. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Blood Flow. Bethesda, MD: Am. Physiol. Soc., 1983, sect. 2, vol. III, pt. 2, chapt. 20, p. 755–793.
- 29.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory response originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIlveen SA, Hayes SG, Kaufman MP. Both central command and the exercise pressor reflex reset the carotid sinus baroreflex. Am J Physiol Heart Circ Physiol 280: H1454–H1463, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Michael GJ, Preistly JV. Differential expression of mRNA for the vanilloid receptor subtype 1 in cells of adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci 5: 1844–1854, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Gonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation 101: 784–789, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Minami N, Yoshikawa T, Kataoka H, Mori N, Nagasaka M, Kurosawa H, Kanazawa M, Kohzuki M. Effects of exercise and beta-blocker on blood pressure and baroreflexes in spontaneously hypertensive rats. Am J Hypertens 16: 966–972, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 35.Mittleman M, Siscovick D. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin 14: 263–270, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. N Engl J Med 329: 1677–1683, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Papelier Y, Escourrou P, Helloco F, Rowell LB. Muscle chemoreflex alters carotid sinus baroreflex response in humans. J Appl Physiol 82: 577–583, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Pickering TG Pathophysiology of exercise hypertension. Herz 12: 119–124, 1987. [PubMed] [Google Scholar]
- 39.Potts JT, Hand GA, Li J, Mitchell JH. Central interaction between carotid baroreceptors and skeletal muscle receptors inhibits sympathoexcitation. J Appl Physiol 84: 1158–1165, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Potts JT, Shi X, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 265: H1928–H1938, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Quintin L, Gillon JY, Saunier CF, Ghignone M. Continuous volume infusion improves circulatory stability in anesthetized rats. J Neurosci Meth 30: 77–83, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Rondon MU, Laterza MC, Matos LDd Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrao CE. Abnormal muscle metaboreflex control of sympathetic activity in never-treated hypertensive subjects. Am J Hypertens 19: 951–957, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Seguro C, Sau F, Zedda N, Scano G, Cherchi A. Arterial blood pressure behavior during progressive muscular exercise in subjects with stable arterial hypertension. Cardiologia 36: 867–877, 1991. [PubMed] [Google Scholar]
- 44.Silva GJ, Brum PC, Negrao CE, Krieger EM. Acute and chronic effects of exercise on baroreflexes in spontaneously hypertensive rats. Hypertension 30: 714–719, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Smith SA, Mammen PPA, Mitchell JH, Garry MG. The role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation 108: 1126–1132, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol 551: 1013–1021, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SA, Williams MA, Mitchell JH, Mammen PPA, Garry MG. The capsaicin sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111: 2056–2065, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effects of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988. [DOI] [PubMed] [Google Scholar]
- 52.Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation 84: 2034–2039, 1991. [DOI] [PubMed] [Google Scholar]
- 53.Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 111: 178–186, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Toal CB, Leenen FH. Blood pressure responsiveness during the development of hypertension in conscious spontaneously hypertensive rat. Can J Physiol Pharmacol 63: 1258–1262, 1985. [DOI] [PubMed] [Google Scholar]
- 55.Waldrop TG, Mitchell JH. Effects of barodenervation on cardiovascular responses to muscle contraction. Am J Physiol Heart Circ Physiol 249: H710–H714, 1985. [DOI] [PubMed] [Google Scholar]