Abstract
In humans, 1011 neutrophils are released from the bone marrow per day, and these cells have a half-life in the blood of only ∼6.5 h. Although it is generally believed that neutrophils are cleared from the circulation via the liver and spleen, in this study using 111In-labeled senescent neutrophils, we show that in mice, 32% of neutrophils are cleared from the circulation via the bone marrow. We have previously shown that senescent neutrophils home to the bone marrow in a CXCR4-dependent manner, and we show here that pretreatment of neutrophils with pertussis toxin significantly inhibits neutrophil clearance via the bone marrow (75%), consistent with a role for chemokines in this process. By labeling senescent neutrophils with inert fluorescent microspheres, we have tracked their fate and shown that in vivo, they are ultimately phagocytosed by bone marrow stromal macrophages. Finally, we show that under noninflammatory conditions, circulating levels of neutrophils are regulated by granulocyte-colony stimulating factor (G-CSF), but not interleukin-17. Interestingly, we report that the uptake of apoptotic neutrophils by bone marrow macrophages stimulates their production of G-CSF in vitro. Taken together, these data provide evidence that the bone marrow represents a major site of neutrophil clearance in mice.—Furze, R. C., Rankin, S. M. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse.
Keywords: macrophage, apoptosis, chemokine
Under homeostatic conditions 1011 neutrophils are released from the bone marrow each day. These cells have a half-life in the circulation of ∼6.5 h (1) and are thought to be cleared predominantly via the spleen and liver. As neutrophils age in the blood, they up-regulate cell-surface expression of CXCR4 within a matter of hours before becoming apoptotic (2, 3). These CXCR4high, preapoptotic neutrophils have the capacity to migrate to stromal cell-derived factor (SDF)-1α, the ligand for CXCR4 (2, 3). We term these CXCR4high neutrophils “senescent” neutrophils. These senescent neutrophils are therefore distinct from apoptotic neutrophils that do not have the capacity to migrate. The bone marrow expresses SDF-1α constitutively, and we have previously shown that senescent, CXCR4high neutrophils preferentially home back to the bone marrow in a CXCR4-dependent manner (3), which we suggested may represent a previously undefined pathway for neutrophil clearance. This hypothesis is consistent with the observation that, in a number of animal models and in humans, following their i.v. injection, large numbers of radiolabeled neutrophils are retained in the bone marrow (4,5,6,7). However, currently there is no direct evidence that neutrophils are destroyed in the bone marrow. Furthermore, the reason that the bone marrow should contribute to both neutrophil production and destruction is unclear.
Macrophages in the liver and spleen contribute to the clearance of senescent neutrophils from the circulation. These macrophages form part of the reticular endothelial system and are thus in direct contact with the blood, ideally positioned to recognize, bind, and phagocytose senescent/apoptotic neutrophils present in the circulation. In the bone marrow, macrophages are present in the hematopoietic cords, separated from the blood by the bone marrow sinusoidal endothelium. These stromal macrophages are an important source of cytokines and provide cell:cell contact necessary for hematopoiesis and lymphopoiesis (8,9,10,11). In addition, they are also important phagocytic cells, responsible for clearing cellular debris within the bone marrow and contributing to the removal of cells during the refinement of the lymphocyte repertoire. Thus, bone marrow macrophages phagocytose both nuclei expelled by developing erythroblasts (12) and pre-B cells, which have nonproductive B-cell receptor rearrangements and self-reactive immature B-cells (13, 14). The role of bone marrow macrophages in neutrophil clearance in vivo has not previously been investigated.
The cytokine G-CSF (granulocyte-colony stimulating factor) plays a critical role in regulating both granulopoiesis and the mobilization of neutrophils from the bone marrow under noninflammatory conditions (15). Thus, mice with a genetic deficiency of either G-CSF or granulocyte-colony stimulating factor receptor have markedly reduced numbers of neutrophils in their bone marrow reserve and in the circulation (16, 17). Whereas it is assumed that G-CSF is made locally in the bone marrow, the cellular source of this cytokine has not been identified and the factors regulating the production of G-CSF under noninflammatory conditions are unknown (18).
In this study, using a murine model system, we have specifically determined the relative contribution of the liver, spleen, and bone marrow in the clearance of senescent neutrophils from the circulation under homeostatic conditions and examined the fate of senescent neutrophils that home back to the bone marrow. Furthermore, we have demonstrated for the first time that G-CSF is produced by bone marrow macrophages in response to their uptake of apoptotic neutrophils.
MATERIALS AND METHODS
Reagents
Unless otherwise stated, all reagents were purchased from Sigma (Dorset, UK). Purified no azide/low endotoxin, anti-mouse interleukin (IL) -17, and isotype control 101.4 were kindly provided by Roger Palframan and Stephen Rapecki (UCB Celltech, Slough, UK). Recombinant mouse IL-17 (carrier free) and rat anti-mouse G-CSF [clone 67604; immunoglobulin G (IgG) 1] was obtained from R&D Systems (Abingdon, UK). Rat anti-mouse F4/80 was purchased from Serotec (Oxford, UK) and goat anti-rat IgG (H+L) Alexa Fluor® 594 from Invitrogen, Molecular Probes (Paisley, UK). Percoll was purchased from GE Healthcare UK Ltd. (Chalfont St. Giles, UK). Unless stated, cells were cultured in RPMI containing fetal calf serum (FCS; 10%), 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin (complete media) at 37°C in a 5% CO2 humidified incubator.
Mice
Female, 6- to 8-wk-old BALB/c mice were purchased from Harlan (Oxford, UK). Chow and water were available ad libitum. UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act 1986 were strictly observed.
Preparation of senescent neutrophils from mouse bone marrow
Femurs, tibiae, and humeri were removed, and bone marrow was harvested by flushing with HBSS (Hanks’ balanced salt solution; without Ca2+/Mg2+), 30 mM Hepes, and 15 mM EDTA. A single-cell suspension was created by passing through a 21-gauge needle. Cells were centrifuged (Hettich Rotina 46R; Hettich, Tuttlingen, Germany) at 1200 rpm for 5 min, room temperature. Erythrocytes were lysed using hypotonic shock and leukocytes layered on a discontinuous (52%, 64%, and 72%) Percoll® gradient (100% Percoll=9:1 Percoll/10× PBS). Mature neutrophils (band and segmented) were isolated from the 64%/72% interface. Contaminating B cells were removed using anti-CD45R (B220) microbeads and MACS® MS separation column per manufacturer’s instructions (Miltenyi Biotec Ltd., Surrey, UK), and a purity of ≥97% was achieved. To obtain senescent cells, purified neutrophils were incubated overnight in complete media. Following overnight culture, apoptotic cells were removed using Annexin V+ microbeads per manufacturer’s instructions (Miltenyi Biotec Ltd.).
In vivo trafficking of Indium-111 (111In)-labeled neutrophils
Senescent neutrophils (maximum 2×107), in a volume of 1 ml, were incubated with 111InCl3 (∼100 μCi) chelated with 2-mercaptopyridine-N-oxide (0.4 mg in 1 ml of 50 mm PBS, pH 7.4) for 15 min at room temperature. Labeled neutrophils were then washed 2× and resuspended (HBSS with Ca2+/Mg2+, 30 mM Hepes, and 0.5% BSA). Percentage binding was >99%. Gαi signaling was inhibited where indicated by incubation for 1 h at 37°C with pertussis toxin (1 μg/ml). Cells were thoroughly washed before i.v. injection of 1 × 106 cells via the lateral tail vein. After 2 h, animals were sacrificed and tissue samples were removed. Blood was taken by cardiac puncture for determination of circulating neutrophil numbers, calculated based on a total blood volume of 80 ml/kg. Calculation of radioactivity in the bone marrow was determined assuming each femur represents 6% of total bone marrow (19). Accumulation was measured for 60 s over 100 to 500 KeV energy range in a 5-head gamma counter with automatic spillover and crosstalk correction (Packard Cobra, Meridien, CT, USA). The 111In content in tissues is expressed as a percentage of the total counts present in the entire mouse.
In vivo trafficking of latex (LX) -labeled neutrophils
Senescent neutrophils were obtained as described above with the following modification to ensure depletion of contaminating monocytes. After Percoll isolation, cells were incubated at room temperature for 20 min with rat α-mouse CD115 (eBioscience, San Diego, CA, USA), 0.2 μg/106 cells. Cells were washed 2× in depletion buffer (PBS, 0.5% BSA, and 2 mM EDTA, pH 7.2) and subsequently incubated with rat α-mouse CD45R (B220) microbeads per manufacturer’s instructions. After 2 washes, cells were incubated for 5 min with goat α-rat IgG microbeads (Miltenyi Biotec Ltd). Neutrophils were isolated on a MACS MS separation column and aged in vitro as described above. Senescent neutrophils were incubated for 1 h at 37°C in HBSS with Ca2+/Mg2+, 30 mM Hepes, and 0.1% BSA with 0.5 μm fluorescein isothiocyanate-conjugated (yellow gold) Fluoresbrite® YG microspheres [2.5% solids (w/v); Polysciences Inc., Warrington, PA, USA] diluted to a final concentration of 1 × 109 microspheres per 1 × 106 neutrophils. Cells were washed 3× to remove nonphagocytosed particles, and 5 × 106 LX+ neutrophils were delivered i.v. Cell-labeling efficiency was ≥95%. Bone marrow was collected from femurs after 2 or 24 h, and single-cell suspensions were centrifuged onto glass slides at 800 rpm for 5 min using a Cytospin 3 centrifuge (Shandon Scientific Ltd., Runcorn, UK) and allowed to dry for 20 min room temperature. Slides were stained using Kwik-Diff® staining kit (ThermoElectron, Pittsburgh, PA, USA) per manufacturer’s instructions. Slides were then mounted using Histomount mounting medium (VWR International Ltd., West Chester, PA, USA) and examined at ×100 using a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan). LX+ cells were identified by means of fluorescence and subsequently identified by morphological characteristics by light microscopy. A minimum of 200 LX+ cells per femur were counted. Images were obtained using a Nikon DMX1200 digital camera with LUCIA GF software and processed using Paint Shop Pro 7 (Jasc® Software Inc., Eden Prairie, MN, USA).
Immunohistochemistry
Cytospins of bone marrow from naive mice were prepared as described above and fixed immediately with 4% paraformadlehyde for 10 min at room temperature. To visualize, myeloperoxidase slides were washed 2× in PBS and incubated for 20 min at room temperature with 1.25 mg/ml o-dianisidine HCL and 0.05% H2O2 at 1:1. Slides were counterstained with hematoxylin for 3 min, mounted using Histomount mounting medium, and examined at ×100 using a Nikon Eclipse E400 microscope.
For identification of F4/80+ cells, slides were blocked for 30 min at room temperature using goat IgG before incubation for 1 h at room temperature with purified rat anti-mouse F4/80 (diluted 1/50). Cells were washed in PBS and incubated in the dark with a secondary goat anti-rat IgG Alexa594 (diluted 1/1000) for 30 min. Uridine triphosphate nick end-labeling (TUNEL) staining was performed using an in situ cell death detection kit, TMR red (Roche, Burgess Hill, UK) according to manufacturer’s instructions. Slides were washed in PBS and mounted using Vectashield mounting media with 4′,6′-diamidino-2-phenylidole (DAPI; Vector Laboratories Inc., Burlingame, CA, USA). Images were obtained as above.
Transmission electron microscopy (TEM) of bone marrow
Femoral bone marrow was fixed by infusion of 2.5% glutaraldehyde in a modified Krebs-Ringer solution for 15 min using an in situ perfusion technique previously described (3). The femur was removed and placed in fresh phosphate-buffered 2.5% glutaraldehyde fixative for 12 h. The femur was then cut in half to expose the marrow, rinsed with PBS, postfixed in 1% OsO4 buffered with 0.05 M sodium cacodylate, and dehydrated through an ethanol series (50–100%). During dehydration, the marrow was stained en bloc with a saturated solution of uranyl acetate in 50% ethanol. Marrow samples were treated with propylene oxide and embedded in Araldite® (Ciba Geigy Plc, Stein, Switzerland), and ultrathin sections were prepared using an ultramicrotome (Ultracut E; Leica Microsystems, Wetzlar, Germany). Sections were placed on 200-mesh copper grids, further stained with uranyl acetate and lead citrate, and examined and photographed in a Hitachi 7000 transmission electron microscope (Hitachi, Tokyo, Japan).
Neutralization of G-CSF and IL-17 in vivo
Anti-G-CSF (7.5 mg/kg), anti-IL-17 (10 mg/kg), or matched isotype control antibodies were injected i.p. in a final volume of 200 μl PBS. For longer term IL-17 neutralization, mice were administered a second dose i.p. 2 days following the initial injection. At indicated times, peripheral blood samples were obtained from the mouse tail vein and smeared onto microscope slides. Samples were stained using the Kwik-Diff method, and the neutrophil content was determined by differential leukocyte counts.
Recruitment of cells to IL-17 in vivo
Mice were injected i.p. with 0.5 μg rIL-17 in combination with either control IgG or anti-IL-17 in a final volume of 200 μl PBS. PBS alone was used as a control. After 4 h, peritoneal lavages were performed using 8 ml of cold PBS and cytospin prepared as described previously. Slides were stained using the Kwik-Diff method, and the neutrophil content was determined by differential leukocyte counts.
Peritoneal macrophage isolation and culture
The peritoneal cavity of BALB/c mice was lavaged with 8 ml of cold PBS. Cell viability was >97% as determined by trypan blue dye exclusion. Cells were washed, and macrophages were adhered to 96-well, flat-bottomed tissue culture plates for 3 h in complete media. Nonadherent cells were removed by gentle washing.
Bone marrow-derived macrophage (BMDM) cell culture
Bone marrow was harvested as described previously, and 4 × 106 cells were plated per petri dish (9 cm diameter; Sterilin, South Wales, UK) in 8 ml of media [DMEM containing 10% FCS, 20% L-cell-conditioned medium (from L929 cell line), 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin]. After 4 days, 10 ml of media was added per dish. After 7 days, nonadherent cells were removed by washing once with cold PBS. Adherent cells were harvested by addition of 15 ml/dish ice-cold PBS, 15 min at 4°C. Cells were washed, and cell viability was >95% as determined by trypan blue dye exclusion.
Macrophage and apoptotic neutrophil interaction
Macrophages were adhered to 96-well, flat-bottomed tissue culture plates at 2 × 105/well for 3 h in complete media. Nonadherent cells were removed by gentle washing. Purified neutrophils were incubated overnight in complete media at 37°C in a 5% CO2 humidified incubator. Senescent neutrophils were added at a 1:1 or 5:1 ratio to 2 × 105 peritoneal or BMDM (day 7) macrophages, in the presence or absence of 100 ng/ml lipopolysaccharide (LPS). A final volume of 200 μl/well was used in all cases. Proportional numbers of neutrophils were cultured under the same conditions in the absence of macrophages. Samples were assayed using 3–6 replicates per condition. Cells were allowed to interact for 48 h, and cell-free supernatants were harvested and stored at −20°C.
ELISAs
Concentrations of G-CSF in culture supernatants were determined by sandwich ELISA according to manufacturer’s instructions using a murine G-CSF ELISA development kit (PeproTech EC, London, UK). Plates were developed using enhanced K-blue tetramethylbenzidine substrate solution (Neogen Corporation, Ayr, UK), and the reaction was stopped by addition of 1.8 M H2SO4. Plates were read on a spectrophotometer (Tecan Sunrise using Magellan software; Tecan Group Ltd., Männedorf, Switzerland) at 450 nm with a wavelength correction of 540 nm. Data were corrected for background production of G-CSF from neutrophils cultured alone.
Statistics
Data are shown as mean ± se. Data were analyzed using 2-way analysis of ANOVA and Bonferroni’s posttest (see Figs. 2and 5A), Student’s unpaired t test assuming unequal variance (see Fig. 3G) and 1-way ANOVA and Dunnet’s posttest (see Figs. 5B and 6). Values of P ≤ 0.05 were considered statistically significant.
Figure 2.
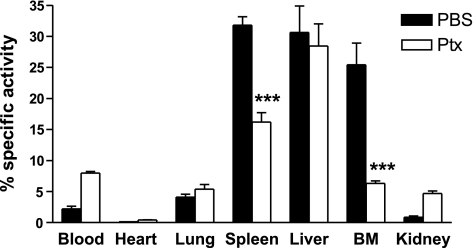
Pertussis toxin inhibits neutrophil clearance by the spleen and bone marrow. Purified, senescent neutrophils labeled with 111In were incubated for 1 h with 1 μg/ml pertussis toxin or PBS and subsequently administered i.v. into recipient mice. Organs were harvested after 2 h, and the radioactivity in each tissue was calculated as a percentage of the total present in the whole animal. Data are presented as mean ± se; n = 3 experiments, 3 mice/group. ***P ≤ 0.001; 2-way ANOVA and Bonferroni’s posttest.
Figure 5.
Neutralization of IL-17 does not significantly alter circulating neutrophil numbers in naive mice. A) Anti-G-CSF (7.5 mg/kg), anti-IL-17 or control IgG (10 mg/kg) was administered to naive mice at days 0 and 2 (anti-IL-17 only). The percentage of circulating neutrophils was determined by differential cell counts on the days indicated. Data are presented as mean ± se; n = 4–10. **P ≤ 0.01; 2-way ANOVA and Bonferroni’s posttest. B) To demonstrate the effectiveness of the anti-IL-17 in vivo, neutrophil recruitment to the peritoneum was assessed 4 h after the i.p. injection of 0.5 μg/mouse rIL-17. Neutrophil numbers were determined by differential cell counts. Data are presented as mean ± se, n = 4. **P ≤ 0.01; 1-way ANOVA and Dunnet’s posttest.
Figure 3.
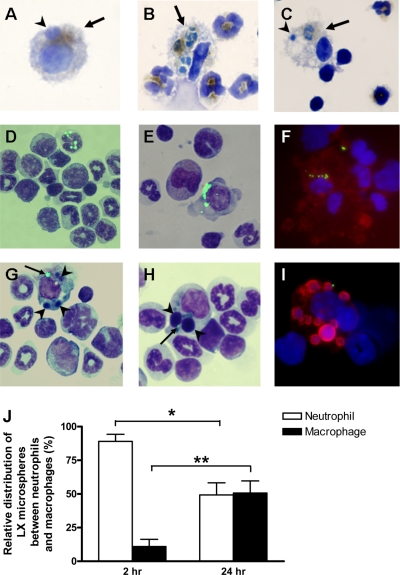
Senescent neutrophils become apoptotic and are phagocytosed by resident F4/80+ macrophages within the bone marrow. Cytospins were prepared from freshly isolated bone marrow. Neutrophils phagocytosed by macrophages (arrows) were detected by staining for MPO. Slides were counterstained with hematoxylin. A) MPO+ macrophages in conjunction with apoptotic bodies (arrowheads) are observed. B, C) MPO+ macrophages containing partially degraded neutrophils are also identified. Senescent neutrophils were labeled with fluorescent LX microspheres and delivered i.v. into recipient mice. Bone marrow was harvested after 2 or 24 h as indicated and either prepared for TEM or used for cytospins. D) LX+ neutrophils are observed in bone marrow cytospins at 2 h. E) LX+ mononuclear cells are visible in bone marrow cytospins after 24 h. F) F4/80 staining (red) with a DAPI counterstain (blue) confirms mononuclear cells as macrophages. G, H) Macrophages containing LX microspheres (arrows) and apoptotic bodies (arrowheads) are observed at 24 h. I) TUNEL staining (red) confirms the presence of apoptotic bodies in macrophages. J) Cytospins were prepared and counterstained with Kwik-Diff for morphological identification of LX+ cells. Data are presented as mean ± se; n = 3 mice; ≥200 LX+ cells counted per animal. *P ≤ 0.05, **P ≤ 0.001; Student’s unpaired t test assuming unequal variance.
Figure 6.
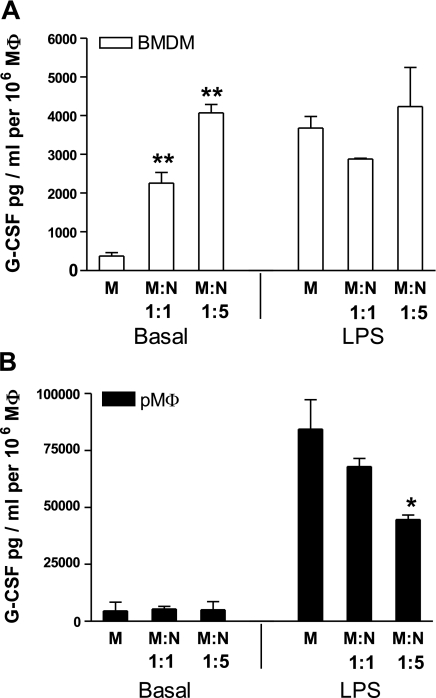
G-CSF production by BMDMs (A) and peritoneal macrophages (B) following their phagocytosis of senescent neutrophils. Senescent neutrophils (N) were added to BMDMs or peritoneal macrophages (pMφ) in 1:1 and 5:1 ratios, in the presence or absence of 100 ng/ml LPS. Supernatants were harvested after 48 h and G-CSF production was determined by ELISA. Data are presented as mean ± se, n = 3 experiments; samples assayed in triplicate and neutrophil background levels subtracted. *P ≤ 0.05, **P ≤ 0.01; 1-way ANOVA and Dunnet’s posttest.
RESULTS
The bone marrow accounts for >30% of neutrophil clearance under homeostatic conditions
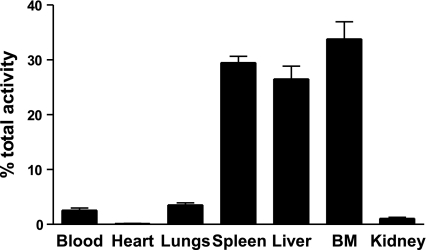
Under homeostatic conditions, senescent CXCR4high neutrophils preferentially home to the bone marrow (3). However, the relative contribution of the bone marrow vs. the liver and spleen to the clearance of aged neutrophils has not been determined. To address this, senescent CXCR4high neutrophils were radiolabeled with 111In and injected i.v. into recipient mice. Mice were culled after 2 h, a time when 97% of the injected cells had cleared from the blood and the percentage of radioactivity in each organ determined. Data presented in Fig. 1demonstrate retention of these senescent neutrophils in the bone marrow, 33.8 ± 3.1%; spleen, 29.4 ± 1.2%; and liver, 26.5 ± 2.3%. These data suggest that neutrophils may be cleared in the bone marrow in addition to the liver and spleen.
Figure 1.
Under homeostatic conditions, senescent neutrophils are cleared in equal proportions by the spleen, liver, and bone marrow. Purified, senescent neutrophils labeled with 111In were administered i.v. into recipient mice. Organs were harvested after 2 h, and the radioactivity in each tissue was calculated as a percentage of the total present in the whole animal. Data are presented as mean ± se; n = 7 experiments, 3 mice/group.
We have previously shown that the return of senescent CXCR4high neutrophils to the bone marrow under homeostatic conditions is dependent on the SDF-1α/CXCR4 chemokine axis. To determine the contribution of chemokine-driven migration of neutrophils to the bone marrow, liver, and spleen, 111In-labeled neutrophils were treated with pertussis toxin before injection into recipient mice. Pretreatment of neutrophils with pertussis toxin significantly inhibited neutrophil accumulation in the bone marrow (75%) and spleen (49%), indicating that neutrophil clearance via these tissues is an active process that is mediated via Gαi-coupled receptors (Fig. 2). In contrast, clearance by the liver was unaffected by pertussis toxin pretreatment. Interestingly, when the chemokine-driven migration of neutrophils was blocked, ∼28% of neutrophils accumulated in other tissues, further suggesting that these chemokine-dependent pathways are critical for normal efficient clearance of neutrophils. Further uptake of neutrophils into other tissues occurred when clearance via the spleen and bone marrow was compromised. These data are consistent with our previous data indicating that trafficking back to the bone marrow is dependent on CXCR4/SDF-1α. The results also suggest that trafficking back to the spleen may be partially driven by a chemoattractant acting via Gαi-coupled receptors.
Senescent neutrophils return to the bone marrow and are cleared by resident macrophages
The fate of neutrophils that home to the bone marrow has not been previously defined. We hypothesized that, on return to the bone marrow, senescent CXCR4high neutrophils are phagocytosed by resident stromal macrophages. To look for evidence that neutrophils were destroyed in the bone marrow under homeostatic conditions, we performed myeloperoxidase (MPO) staining of bone marrow cytospins using bone marrow harvested from naive mice. As shown in Fig. 3, MPO together with either apoptotic bodies (Fig. 3A) or engulfed, partially degraded neutrophils (Fig. 3B, C) can be detected in stromal macrophages. Previous studies have shown that it takes only 20–60 min for apoptotic neutrophils to be phagocytosed and degraded by macrophages (20). The detection of MPO in stromal macrophages is consistent with the hypothesis that neutrophils are being destroyed within the bone marrow under homeostatic conditions.
To quantify the uptake of neutrophils by macrophages, senescent neutrophils were labeled with fluorescent 0.5 μm inert LX microspheres before i.v. injection. These microspheres are nondegradable and can therefore be used to track the fate of neutrophils in vivo. Labeling does not result in activation of the neutrophils, as assessed by CD62L shedding and does not affect neutrophil chemotaxis toward SDF-1α or KC in vitro, or accumulation of 111In-labeled neutrophils to the bone marrow in vivo (data not shown).
From the data presented in Fig. 1, we calculate that labeled neutrophils would represent <0.13% of the total bone marrow population, and therefore we could not accurately quantify their cellular distribution by FACs analysis. However, these LX+ cells were easily identifiable on cytospins of the bone marrow and were thereby quantified (Fig. 3J). Two hours after injection, the majority of LX+ cells were neutrophils (Fig. 2D); however, after 24 h, >50% of LX+ cells were macrophages (identified by their distinct morphology and positive staining for F4/80 (Fig. 2E, F). These data suggest that after their recruitment back to the bone marrow, senescent neutrophils undergo apoptosis and are subsequently phagocytosed by stromal macrophages. Indeed, apoptotic bodies were seen in some of the LX+ macrophages, indicative of their phagocytosis of apoptotic neutrophils (Fig. 2G, H). This finding was confirmed with the use of terminal deoxynucleotide transferase-mediated TUNEL staining (Fig. 2I). TEM of the bone marrow, 24 h after injection of LX+ neutrophils, revealed the presence of LX microspheres and apoptotic bodies in bone marrow stromal macrophages (Fig. 4A–C). Taken together, these data provide the first direct evidence that senescent neutrophils homing back to the bone marrow are ultimately phagocytosed and destroyed by bone marrow stromal macrophages.
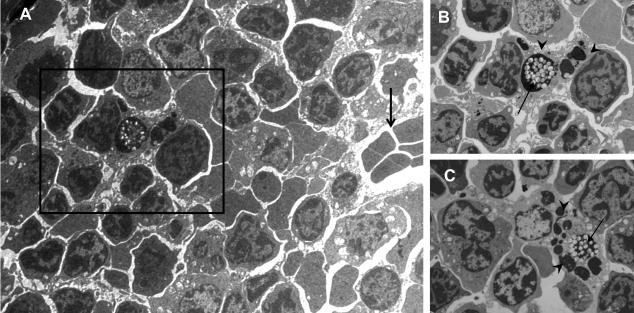
Figure 4.
Senescent neutrophils within bone marrow macrophages can be visualized by electron microscopy. Senescent neutrophils were labeled with fluorescent LX microspheres and delivered i.v. into recipient mice. Bone marrow was harvested after 24 h and either prepared for TEM or used for cytospins. A) TEM of bone marrow sections at 24 h further confirms the presence of LX microspheres within cells situated in close proximity to a blood vessel (open arrow). B, C). LX microspheres (closed arrow) and apoptotic bodies (arrowhead) within stromal macrophages. Panel B shows enlarged image from boxed area in panel A.
Under homeostatic conditions, G-CSF, but not IL-17, regulates circulating neutrophil numbers
To determine the relative roles of G-CSF and IL-17 in regulating circulating numbers of neutrophils under noninflammatory conditions, mice were treated with neutralizing monoclonal antibodies (mAbs) to either G-CSF or IL-17. As expected, 2 days after the administration of anti-G-CSF-blocking mAbs, there was a significant reduction in circulating neutrophils (57% decrease; Fig. 5A). In contrast, neutralization of IL-17 did not affect neutrophil numbers in the blood at day 2 or day 5, whereas Fig. 5B shows that the anti-IL-17 mAb caused a significant inhibition in IL-17-stimulated neutrophil recruitment into the peritoneum. Together, these data suggest that under homeostatic conditions, G-CSF, but not IL-17, regulates circulating numbers of neutrophils.
Uptake of senescent neutrophils induces G-CSF production by bone marrow macrophages
The factors regulating G-CSF production under homeostatic conditions are unknown. Furthermore, it is not known whether cells within the bone marrow generate G-CSF. In contrast, G-CSF is known to be produced by tissue macrophages in response to inflammatory stimuli, such as LPS. The uptake of apoptotic neutrophils by macrophages at sites of inflammation has been shown to regulate their production of inflammatory cytokines and chemokines (21,22,23). Because macrophages from different tissues are phenotypically distinct, in this study we sought to investigate whether the uptake of apoptotic neutrophils by bone marrow and peritoneal macrophages differentially affected their production of G-CSF under inflammatory and noninflammatory conditions.
Unexpectedly, we found that the uptake of apoptotic neutrophils by bone marrow macrophages stimulates their production of G-CSF in a dose-dependent manner. Specifically, when the ratio of bone marrow macrophages to apoptotic neutrophils was 1:5, there was an 11-fold increase in G-CSF production (Fig. 6A). Addition of LPS alone stimulated a similar level of G-CSF production by these macrophages, but this was not further modulated by the addition of apoptotic neutrophils.
In contrast, when the same experiment was performed with peritoneal macrophages, the uptake of apoptotic neutrophils by these cells was not found to stimulate G-CSF production. Moreover, although LPS stimulated peritoneal macrophages to generate G-CSF, this response was significantly inhibited by their uptake of apoptotic neutrophils (Fig. 6B). Taken together, these data demonstrate that G-CSF production is differentially regulated in these 2 distinct populations of tissue macrophages.
DISCUSSION
In this study, we have shown that in addition to the liver and spleen, the bone marrow represents an important site of neutrophil clearance. We provide the first direct evidence that neutrophils homing back to the bone marrow are phagocytosed by bone marrow stromal macrophages. It is well known that G-CSF regulates both granulopoiesis and neutrophil mobilization under homeostatic conditions (15), and it has recently been suggested (24) that IL-17 may indirectly regulate the production of G-CSF. We show here, however, that G-CSF, but not IL-17, regulates circulating numbers of neutrophils under noninflammatory conditions. Moreover, we show that the uptake of apoptotic neutrophils by bone marrow macrophages stimulates these cells to produce G-CSF.
SDF-1α, the ligand for CXCR4, is expressed constitutively in the bone marrow, and our previous studies (3) showed that homing of senescent CXCR4high neutrophils back to the bone marrow was CXCR4-dependent. In this study, pertussis toxin pretreatment of neutrophils inhibited their homing to the bone marrow by 75%, consistent with the hypothesis that this process is driven by the CXCR4/SDF-1α chemokine axis. We have previously shown that up-regulation of CXCR4 precedes neutrophil apoptosis (3); indeed, whereas SDF-1α is chemotactic for senescent, preapoptotic neutrophils, it is not chemotactic for apoptotic neutrophils. Our data support a model whereby CXCR4high senescent neutrophils actively traffic back to the bone marrow, their final apoptosis occurring locally in the bone marrow. Unexpectedly, we also observed a 49% inhibition of neutrophil retention in the spleen after pertussis toxin treatment. This finding suggests that there may be 2 distinct pathways for neutrophil clearance via the spleen, one dependent on a Gαi-linked receptor in addition to a Gαi-independent mechanism, an area that warrants further investigation.
In previous studies (4, 5, 7), when freshly isolated 111In-labeled neutrophils were injected into mice and humans, radioactivity was detected in the bone marrow up to 24 h after injection. However, it was not known whether these neutrophils were cleared in this tissue or whether they subsequently recirculate. In this study, by labeling senescent neutrophils with nondegradable fluorescent LX microspheres, we tracked the fate of neutrophils in vivo. We show that 2 h after their i.v. injection, the majority of LX+ cells in the bone marrow are indeed neutrophils. In contrast, 24 h after the i.v. injection of senescent LX+ neutrophils, the microspheres are equally distributed between macrophages and neutrophils. The presence of apoptotic bodies and TUNEL-positive staining alongside the LX microspheres, in many of these macrophages, is indicative of their phagocytosis of apoptotic neutrophils. Further, by transmission electron microscopy, LX microspheres and apoptotic bodies are clearly visible in stromal macrophages in situ. Taken together, these data constitute the first direct evidence that neutrophils, which migrate to the bone marrow, are subsequently phagocytosed and destroyed by resident stromal macrophages. Further examination of cytospins from naive mice reveals stromal macrophages staining positively for MPO containing apoptotic bodies or partially degraded neutrophils, suggesting that these cells are destroying neutrophils in the bone marrow under homeostatic conditions. In the bone marrow, macrophages constitute ∼30% of the leukocyte population, and previous studies show that these cells play a central role both in supporting hematopoiesis and clearing cellular debris, notably the nuclei extruded from erthyroblasts (12) and apoptotic B lymphocytes (13, 14). The data presented here suggest a further role of these stromal macrophages in the clearance of apoptotic neutrophils in murine bone marrow. Electron micrographs showing apoptotic neutrophils contained within bone marrow macrophages in humans suggest that this pathway may also exist in humans (25).
The cytokine IL-17 stimulates the production of G-CSF and ELR+CXC chemokines by stromal cells and has been shown to play a role in neutrophil recruitment in various models of inflammation (26,27,28,29,30,31). More recently, it has been postulated that IL-17 is also critical in regulating granulopoiesis under homeostatic conditions (32). However, these studies involved the use of mice with genetic deficiencies in adhesion molecules that have spontaneously high numbers of IL-17 producing splenocytes (up to 100-fold higher than wild type) and consequently exhibit high plasma levels of IL-17. These adhesion molecule-deficient mice exhibit high circulating numbers of neutrophils that can be reduced significantly by neutralization of IL-17 (24). In contrast, we show here that in naive mice, under noninflammatory conditions, circulating numbers of neutrophils are not affected by blockade of IL-17, whereas neutralization of G-CSF causes a significant reduction. Our data are consistent with the observation that under basal conditions, IL-17R knockout mice have absolute blood neutrophil counts comparable to wild-type mice (28). Therefore, we conclude that G-CSF, but not IL-17, regulates circulating numbers of neutrophils under homeostatic conditions.
The cellular source of G-CSF in the bone marrow is unknown (18). In this study, we show, for the first time, that the uptake of apoptotic neutrophils by bone marrow macrophages directly stimulates their production of G-CSF. In contrast, the uptake of apoptotic neutrophils did not affect the basal production of G-CSF by peritoneal macrophages. The fact that peritoneal and bone marrow macrophages behaved differently with respect to G-CSF production is consistent with a previous study (33) that showed that bone marrow and peritoneal macrophages utilize distinct receptors for the recognition/phagocytosis of apoptotic neutrophils. These differences probably reflect the fundamentally different functional responses of unstimulated bone marrow and activated tissue macrophages encountering apoptotic neutrophils. Thus, at sites of inflammation where tissue macrophages are activated, these cells are important sources of inflammatory mediators; their uptake of apoptotic neutrophils has previously been shown to dampen the inflammatory response, thereby promoting the resolution of inflammation (21,22,23). In contrast, in the bone marrow under homeostatic conditions, our data indicate that the phagocytosis of apoptotic neutrophils by resident macrophages stimulates their production of G-CSF. This suggests a positive feedback mechanism whereby the elimination of neutrophils by bone marrow macrophages potentially stimulates local granulopoiesis, a hypothesis that warrants further investigation.
CONCLUSIONS
The present study provides evidence that under homeostatic conditions, circulating neutrophils are cleared equally by the liver, spleen, and bone marrow. Our data support a model in which up-regulation of CXCR4 on aging neutrophils allows these cells to actively migrate back to the bone marrow, where they apoptose and are subsequently phagocytosed by the large population of stromal macrophages. The finding that the uptake of apoptotic neutrophils by stromal macrophages stimulates their production of G-CSF suggests a potential mechanism for regulating the replenishment of neutrophils in the blood, which warrants further investigation.
Acknowledgments
The authors thank C. A. Dewar for help with the TEM. The authors also thank R. Palframan and S. Rapecki (UCB Celltech, Slough, Berkshire, UK) for kindly providing the anti-murine IL-17 and isotype control 101.4 used in this study. This work was funded by the Wellcome Trust. The authors declare no competing financial interests.
References
- Mauer A M, Athens J W, Ashenbrucker H, Cartwright G E, Wintrobe M M. Leukokinetic studies II. A method for labelling granulocytes in vitro with radioactive diidopropylfluorophosphate (DFP) J Clin Invest. 1960;39:1481–1486. doi: 10.1172/JCI104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Miyamasu M, Yamaguchi M, Imanishi M, Tsuno N H, Matsushima K, Yamamoto K, Morita Y, Hirai K. Cytokine-mediated regulation of CXCR4 expression in human neutrophils. J Leukoc Biol. 2002;71:711–717. [PubMed] [Google Scholar]
- Martin C, Burdon P C E, Bridger G, Gutierrez-Ramos J C, Williams T J, Rankin S M. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Thakur M L, Lavender J P, Arnot R N, Silvester D J, Segal A W. Indium-111-labeled autologous leukocytes in man. J Nucl Med. 1977;18:1014–1021. [PubMed] [Google Scholar]
- Suratt B T, Young S K, Lieber J, Nick J A, Henson P M, Worthen G S. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol. 2001;281:L913–L921. doi: 10.1152/ajplung.2001.281.4.L913. [DOI] [PubMed] [Google Scholar]
- Lovas K, Knudsen E, Iversen P O, Benestad H B. Sequestration patterns of transfused rat neutrophilic granulocytes under normal and inflammatory conditions. Eur J Haematol. 1996;56:221–229. doi: 10.1111/j.1600-0609.1996.tb01933.x. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S H, Peters A M, Keshavarzian A, Reavy H J, Lavender J P. The kinetics of 111indium distribution following injection of 111indium labelled autologous granulocytes in man. Br J Haematol. 1985;61:675–685. doi: 10.1111/j.1365-2141.1985.tb02882.x. [DOI] [PubMed] [Google Scholar]
- Temeles D S, McGrath H E, Kittler E L, Shadduck R K, Kister V K, Crittenden R B, Turner B L, Quesenberry P J. Cytokine expression from bone marrow derived macrophages. Exp Hematol. 1993;21:388–393. [PubMed] [Google Scholar]
- Dexter T M, Wright E G, Krizsa F, Lajtha L G. Regulation of haemopoietic stem cell proliferation in long term bone marrow cultures. Biomedicine. 1977;27:344–349. [PubMed] [Google Scholar]
- Kittler E L, McGrath H, Temeles D, Crittenden R B, Kister V K, Quesenberry P J. Biologic significance of constitutive and subliminal growth factor production by bone marrow stroma. Blood. 1992;79:3168–3178. [PubMed] [Google Scholar]
- King A G, Wierda D, Landreth K S. Bone marrow stromal cell regulation of B-lymphopoiesis. I. The role of macrophages, IL-1, and IL-4 in pre-B cell maturation. J Immunol. 1988;141:2016–2026. [PubMed] [Google Scholar]
- Sadahira Y, Mori M. Role of the macrophage in erythropoiesis. Pathol Int. 1999;49:841–848. doi: 10.1046/j.1440-1827.1999.00954.x. [DOI] [PubMed] [Google Scholar]
- Dogusan Z, Montecino-Rodriguez E, Dorshkind K. Macrophages and stromal cells phagocytose apoptotic bone marrow-derived B lineage cells. J Immunol. 2004;172:4717–4723. doi: 10.4049/jimmunol.172.8.4717. [DOI] [PubMed] [Google Scholar]
- Osmond D G, Rico-Vargas S, Valenzona H, Fauteux L, Liu L, Janani R, Lu L, Jacobsen K. Apoptosis and macrophage-mediated cell deletion in the regulation of B lymphopoiesis in mouse bone marrow. Immunol Rev. 1994;142:209–230. doi: 10.1111/j.1600-065x.1994.tb00891.x. [DOI] [PubMed] [Google Scholar]
- Semerad C L, Liu F, Gregory A D, Stumpf K, Link D C. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- Lieschke G J, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler K J, Basu S, Zhan Y F, Dunn A R. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- Metcalf D, Robb L, Dunn A R, Mifsud S, Di R L. Role of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in the development of an acute neutrophil inflammatory response in mice. Blood. 1996;88:3755–3764. [PubMed] [Google Scholar]
- Hareng L, Hartung T. Induction and regulation of endogenous granulocyte colony-stimulating factor formation. Biol Chem. 2002;383:1501–1517. doi: 10.1515/BC.2002.172. [DOI] [PubMed] [Google Scholar]
- Chervenick P A, Boggs D R, Marsh J C, Cartwright G E, Wintrobe M M. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968;215:353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- Savill J S, Wyllie A H, Henson J E, Walport M J, Henson P M, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Stuart L M, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–2615. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- Fadok V A, Bratton D L, Konowal A, Freed P W, Westcott J Y, Henson P M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V A, McDonald P P, Bratton D L, Henson P M. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. 1998;26:653–656. doi: 10.1042/bst0260653. [DOI] [PubMed] [Google Scholar]
- Forlow S B, Schurr J R, Kolls J K, Bagby G J, Schwarzenberger P O, Ley K. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood. 2001;98:3309–3314. doi: 10.1182/blood.v98.12.3309. [DOI] [PubMed] [Google Scholar]
- Dresch C, Flandrin G, Breton-Gorius J. Phagocytosis of neutrophil polymorphonuclears by macrophages in human bone marrow: importance in granulopoiesis. J Clin Pathol. 1980;33:1110–1113. doi: 10.1136/jcp.33.11.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl G M, Frei U, Jorres A. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GROα chemokine from mesothelial cells. J Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- Ye P, Garvey P B, Zhang P, Nelson S, Bagby G, Summer W R, Schwarzenberger P, Shellito J E, Kolls J K. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez F H, Kanaly S, Stocking K L, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito J E, Bagby G J, Nelson S, Charrier K, Peschon J J, Kolls J K. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan M, Cui Z H, Hoshino H, Lotvall J, Sjostrand M, Gruenert D C, Skoogh B E, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170:4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- Witowski J, Ksiazek K, Warnecke C, Kuzlan M, Korybalska K, Tayama H, Wisniewska-Elnur J, Pawlaczyk K, Trominska J, Breborowicz A, Jorres A. Role of mesothelial cell-derived granulocyte colony-stimulating factor in interleukin-17-induced neutrophil accumulation in the peritoneum. Kidney Int. 2007;71:514–525. doi: 10.1038/sj.ki.5002082. [DOI] [PubMed] [Google Scholar]
- Stark M A, Huo Y, Burcin T L, Morris M A, Olson T S, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Fadok V A, Savill J S, Haslett C, Bratton D L, Doherty D E, Campbell P A, Henson P M. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]