Abstract
Introduction
Reported increases in surgical wait times for cancer have intensified the focus on this quality of health care indicator and have created a very public, concerted effort by providers to decrease wait times for cancer surgery in Ontario. Delays in access to health care are multifactorial and their measurement from existing administrative databases can lack pertinent detail. The purpose of our study was to use a real-time surgery-booking software program to examine surgical wait times at a single centre.
Methods
The real-time wait list management system Axcess.Rx has been used exclusively by the department of urology at the Kingston General Hospital to book all nonemergency surgery for 4 years. We reviewed the length of time from the decision to perform surgery to the actual date of surgery for patients in our group urological practice. Variables thought to be potentially important in predicting wait time were also collected, including the surgeon’s assessment of urgency, the type of procedure (i.e., diagnostic, minor cancer, major cancer, minor benign, major benign), age and sex of the patient, inpatient versus outpatient status and year of surgery. Analysis was planned a priori to determine factors that affected wait time by using multivariate analysis to analyze variables that were significant in univariate analysis.
Results
There were 960 operations for cancer and 1654 for benign conditions performed during the evaluation period. The overall mean wait time was 36 days for cancer and 47 days for benign conditions, respectively. The mean wait time for cancer surgery reached a nadir in 2004 at 29.9 days and subsequently increased every year, reaching 56 days in 2007. In comparison, benign surgery reached a nadir wait time of 33.7 days in 2004 and in 2007 reached 74 days at our institution. Multivariate analysis revealed that the year of surgery was still a significant predictor of wait time. Urgency score, type of procedure and inpatient versus outpatient status were also predictive of wait time.
Conclusion
The application of a prospectively collected data set is an effective and important tool to measure and subsequently examine surgical wait times. This tool has been essential to the accurate assessment of the effect of resource allocation on wait times for priority and nonpriority surgical programs within a discipline. Such tools are necessary to more fully assess and follow wait times at an institution or across a region.
Surgical wait times have become a visible and contentious quality indicator in the universal health care systems of many nations. This important issue has had such influence that numerous surgical wait time initiatives have been formed1–4 and specialty groups, as well as the federal government, have set “benchmarks” for wait times in Canada.5
Cancer has been prioritized in Ontario as 1 of the 5 surgical areas in which to increase access.6 In the management of genitourinary diseases, wait times for both cancer and non-cancer surgery have demonstrated significant effects on outcomes. Unfortunately, there has been increasing evidence of significant delays in care to cancer surgery in Canada. A cohort of Ontario patients selected randomly from between 1990 and 1998 was analyzed and found to have increasing wait times for radical prostatectomy.7
Most wait time strategies tend to focus on the period of time from when the patient is pronounced “ready” for surgery to the actual surgery date.8 Administrative databases measuring this 1 summary aspect have confirmed that the median surgical wait time increased for all cancer surgeries studied from 1993 to 2000 in Ontario.9 Our objective was to examine the current wait times of the urological service at a single institution using prospectively collected data, and, in particular, any effects of priority programs on the wait times in general at this institution.
Methods
We based our study on data from real-time surgery-booking software at the Kingston General Hospital. This software has been used exclusively by our department to book all non-emergency surgery for several years. The data were entered by administrative staff, who received training on the software and used this program to schedule patients for the operating room. Demographic and surgical details of patients who underwent surgery were taken from the database housed by the software program Axcess.Rx (AdapCS.Canada). This research project was approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board.
We reviewed the length of time from the decision to perform surgery to the actual date of surgery for patients in our group urological practice. Variables thought to be potentially important in predicting wait time were also collected, including the surgeon’s assessment of urgency (coded 1–5, with 1 being the most urgent), the surgical category (diagnostic, e.g., a patient with suspicious cytology; minor cancer, e.g., bladder cancer treated by transurethral resection; major cancer, e.g., laparoscopic nephrectomy; minor benign, e.g., hydrocele repair; major benign, e.g., ureteropelvic junction open repair; detailed further in Appendix 1), age and sex of the patient, inpatient versus outpatient status and year of surgery. Minor procedures were classified as outpatient or endoscopic procedures, whereas major procedures required hospital admission and invasive open or laparoscopic procedures. Diagnostic procedures were those procedures in which the operation was completed to determine pathology (e.g., cystoscopy and retrograde pyelogram, or ureteroscopy for suspicious cytology).
After descriptive analysis, characteristics of those who underwent surgery were compared with those who did not, using independent sample t tests and χ2 tests. The analysis was planned a priori to identify factors associated with wait time by using multivariate analysis to analyze variables that were significant in univariate analysis. The wait time data were adjusted to take unavailable days into account. The associations between wait time and the independent variables listed above were tested using nonparametric statistics, as the wait time data were skewed. The associations between the number of cancellations and the independent variables were also assessed with nonparametric statistics. The wait time data were log-transformed for the multivariate linear regression, which was completed by using a manual approach to sequentially remove variables that did not contribute significantly to the model. No linear regression was done for the number of cancellations, as most of the patients had no cancellations, prohibiting log-transformation of these data. SPSS version 14.0 for Windows (SPSS, Inc.) was used for all analyses.
Results
The mean age of patients was 59 (range 0.5–97) years and 79.4% were male. From 2003 to June 2007, 2614 procedures were completed with 960 procedures done for malignancy (36.7%). Of the 2614 patients who underwent surgery, 9.8% (257 patients) had had their scheduled surgery cancelled at least once (range 1–6). Inpatient procedures had shorter wait times as compared with outpatient procedures (43 v. 56 d, p < 0.001).
Analysis of patients who underwent surgery versus those who never received surgery revealed no statistically significant differences in sex (p = 0.99). The difference in urgency score approached significance, with more urgent cases more likely to result in surgery (p = 0.07). Cancer status (p = 0.003), patient age (p = 0.016) and surgical category (p = 0.005) had significant differences between those who had surgery versus those who did not. Table 1 presents the mean and median wait times for the 5 surgical categories.
Table 1.
Wait times by surgical category
| Wait time, d
|
||
|---|---|---|
| Surgical category (n) | Mean (95% CI) | Median |
| Diagnostic procedures (381) | 45.9 (41.3–50.5) | 30 |
| Benign, minor (1198) | 46.7 (44.4–49.1) | 38 |
| Benign, major (184) | 64.3 (55.3–73.3) | 47 |
| Cancer, minor (470) | 33.2 (30.8–35.6) | 28 |
| Cancer, major (381) | 44.3 (40.8–47.7) | 38 |
| Total (2614) | 45.1 (43.5–46.6) | 35 |
CI = confidence interval.
Owing to the skewed distribution of waiting times, nonparametric statistical tests (Mann–Whitney U, Kruskal–Wallis and Spearman rank correlation) were employed to determine significant differences between groups. On univariate analysis, wait times were not significantly associated with sex (p = 0.79). Inpatient status (p < 0.001), cancer status (p < 0.001), urgency (p < 0.001), year of surgery (p < 0.001) and surgical category (p < 0.001) were all significantly associated with the outcome of wait time to surgery.
For the numerical variables of age and urgency, correlations with wait time were explored. Spearman rank correlation determined a positive correlation of 0.273 (p < 0.001) between urgency and wait time. Age was negatively correlated with wait time (−0.066, p = 0.001). Age and urgency were also associated, with a correlation of −0.323 (p < 0.001).
Multivariate linear regression was completed to determine the presence or absence of confounders and the predictive value of the model. For the categorical variables, the year 2003 and the diagnostic procedures were used as reference categories. Cancer status (p = 0.38) and age (p = 0.12) were sequentially removed from the model, as they were not significant. The multivariate model using the untransformed data was similar to the one using the transformed data, so the untransformed model is presented in Table 2 for ease of interpretation. Interaction between year of surgery and cancer status was also tested but was not significant (p = 0.26). On average, outpatients waited close to 6 days longer than inpatients did. As the urgency score decreased, the length of wait increased by an average of 14.1 days. Compared with the year 2003, 2004 had shorter wait times, and 2006 and 2007 saw longer wait times, as shown in Figure 1. As compared with the diagnostic category, the major benign and the major cancer categories had longer wait times.
Table 2.
Multivariate linear regression model for number of days waited*
| Variable | Unstandardized coefficients (95% CI) | p value |
|---|---|---|
| Constant | 2.5 (–5.6 to 10.5) | 0.55 |
| In- or outpatient† | 4.1 (0.2 to 8.0) | 0.041 |
| Year‡ | ||
| 2004 | −11.6 (−16.9 to −6.3) | < 0.001 |
| 2005 | 0.3 (−5.1 to 5.7) | 0.93 |
| 2006 | 5.8 (0.7 to 10.9) | 0.025 |
| 2007 | 21.5 (15.5 to 27.5) | < 0.001 |
| Surgical category§ | ||
| Benign, minor | −1.7 (−6.6 to 3.1) | 0.49 |
| Benign, major | 16.2 (8.9 to 23.5) | < 0.001 |
| Cancer, minor | −1.7 (−7.6 to 4.1) | 0.56 |
| Cancer, major | 11.6 (5.4 to 17.8) | < 0.001 |
| Urgency¶ | 14.1 (12.1 to 16.0) | < 0.001 |
CI = confidence interval
Model summary: F = 47.7, p < 0.001, adjusted r2 = 0.17.
Inpatients were coded as “0” in the model and outpatients as “1.”
Reference = 2003.
Reference = diagnostic.
Urgency was scored by the surgeon from 1 to 5, with 1 being the most urgent.
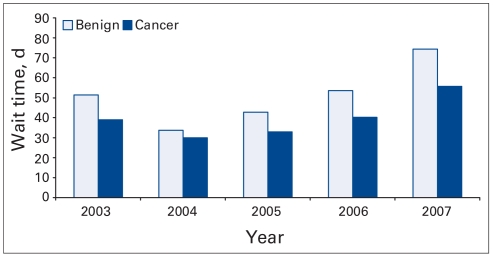
Fig. 1.
Wait time in days for benign and cancerous indications, by year.
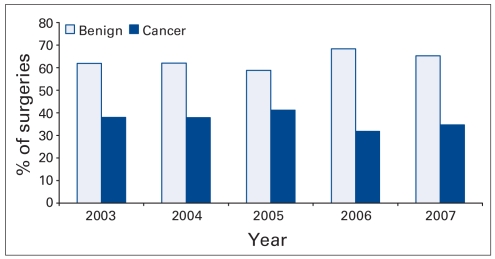
The proportion of surgery done for benign indications as opposed to cancer remained relatively stable during the years of study (range 35%–41%) as shown in Figure 1. The wait time did, however, increase significantly for both benign and cancerous surgeries. Figure 2 shows the increase in wait time for urological procedures stratified by cancerous versus noncancerous indications. The wait time was shortest in 2004 at 34 days for benign conditions and 29 days for cancerous conditions. The year 2007 (results up to June) has by far the longest wait times with 75 days (95% confidence interval [CI] 49–57) for benign procedures and 56 days (95% CI 67–82) for cancer surgery. The second longest wait times are from 2006 (54 and 40 days, respectively, for benign v. cancer surgery).
Fig. 2.
Percent of surgeries for benign and cancerous indications, by year.
Cancellations were significant at 9.8%, with the remaining 2357 (90.2%) having no cancellations. An additional 207 (7.9%) cancelled once, with an additional 36 (1.4%), 8 (0.3%), 4 (0.2%), 1 (0.0%) and 1 (0.0%) cancelling 2, 3, 4, 5 and 6 times, respectively. Table 3 contains the results of the univariate analysis for number of cancellations for all categorical variables. The correlation between age and number of cancellations was poor (Spearman rank correlation = 0.008, p = 0.68). The median value for all groups examined was equal to zero, so only the mean value and the 95% CI are presented. Regression analyses were not completed owing to the skewed nature of the data and the inability to log-transform the data because of the large number of zero values.
Table 3.
Univariate analysis of number of cancellations
| Variable (n) | Mean no. of cancellations (95% CI) | p value* |
|---|---|---|
| Sex | 0.61 | |
| Male (2076) | 0.13 (0.11–0.14) | |
| Female (538) | 0.13 (0.09–0.16) | |
| Patient status | 0.11 | |
| Inpatient (1701) | 0.11 (0.09–0.13) | |
| Outpatient (670) | 0.19 (0.15–0.22) | |
| Cancer status | 0.45 | |
| No cancer (1654) | 0.15 (0.13–0.17) | |
| Cancer† (960) | 0.09 (0.07–0.11) | |
| Year | ||
| 2003 (366) | 0.01 (0.00–0.01) | < 0.001 |
| 2004 (496) | 0.02 (0.01–0.04) | |
| 2005 (718) | 0.08 (0.06–0.10) | |
| 2006 (720) | 0.18 (0.15–0.22) | |
| 2007 (314) | 0.40 (0.31–0.50) | |
| Urgency code‡ | ||
| 1 (143) | 0.06 (0.02–0.09) | 0.020 |
| 2 (1014) | 0.12 (0.10–0.15) | |
| 3 (987) | 0.12 (0.09–0.14) | |
| 4 (335) | 0.16 (0.10–0.22) | |
| 5 (135) | 0.21 (0.12–0.31) | |
| Surgical category | < 0.001 | |
| Diagnostic procedures (381) | 0.23 (0.17–0.30) | |
| Benign, minor (1198) | 0.12 (0.09–0.14) | |
| Benign, major (184) | 0.15 (0.08–0.21) | |
| Cancer, minor (470) | 0.11 (0.08–0.15) | |
| Cancer, major (381) | 0.06 (0.03–0.08) | |
CI = confidence interval.
P values are based on nonparametric tests (Mann–Whitney and Kruskal–Wallis).
Those with a cancer flag (960) do not equal those identified as “Cancer, minor” and “Cancer, major” (851) since the flag is based on the presurgical impression.
1 = most urgent.
Discussion
Surgical wait times have become a frequently targeted barometer of the quality of our medical care, as prolonged wait times have been associated with poorer outcomes in several surgical procedures. Taking genitourinary surgery as an example, prolonged wait times have been shown to have significant effects on patients’ psychosocial well-being.10 Despite some controversy,11,12 studies have also demonstrated a deleterious effect of prolonged wait times on the outcomes of both prostate13 and bladder cancer.14,15
There is growing evidence that wait times for cancer treatment are increasing in Canada, particularly for urological cancers.7,16 As a result independent councils charged with monitoring the quality of health care and guiding change have emerged, but their work is unfortunately impeded by a lack of reliable and detailed data.17,18 The study of wait times in particular is plagued in many instances by bias or incomplete information. A prospective analysis of wait time data by surgeons was completed at cancer centres in Ontario and found that response bias and noncompletion of data forms was a problem, as only 72% of surgeries were tracked.19 Administrative data using detailed records still renders some patients with a paucity of information about care points despite rigorous retrospective study.20 Both these methods of study do not consider the effect that prioritizing a program (such as cancer care) can have on the wait times for other surgeries.
Our evaluation of a real-time booking software to report on wait times is, to our knowledge, the first to consider other important and broad-ranging variables for analysis, such as type of surgery, benign versus cancerous indication, patient age and sex, admission postsurgery to hospital, urgency and cancellation data. Assessing wait times with this tool, we have confirmed a significant recent trend of prolonged wait times for urological diseases. Steady increases in surgical wait times have resulted in an average wait time of 56 days for cancer and 74 days for benign conditions. These progressively increasing wait times are concerning, especially for the cancer cases, and are longer than national and international recommendations1–4 of 2–4 weeks for oncologic surgery.
Improving access to cancer surgery is one of the Wait Times Information Strategy’s 5 priority programs,21 yet the effect was not observed at our centre. Possible reasons for this phenomenon include an increase in the technical complexity of oncologic cases (e.g., laparoscopic partial nephrectomy v. open partial nephrectomy) leading to longer operative times for the same oncologic outcome, a relatively static distribution of surgery time for urological surgery at our centre, and a multifaceted issue of resource allocation for the operating room and ancillary services required, such as postoperative anesthesia unit access. Overall, the demographic shift upwards in age leads to the principle of increased demand for services without a concomitant increase in supply of surgical time.
Evaluation with this prospective, real-time booking software has afforded significant insight into wait times. Our final model determined factors that appeared to decrease wait time, including surgeon’s assessment of higher urgency, inpatient status and earlier year of surgery. Confirming results from other studies in universal health care systems,22,23 it is apparent that significant triaging of patients occurs at our institution. Examination of the results stratified by the priority program (cancer surgery) displays a predilection for cancer cases to be performed ahead of benign indications. In fact, cancer status was undoubtedly factored into the urgency scoring of cases, and once urgency was accounted for, cancer status was no longer significant in multivariate analysis. Similar results were found for patient age, with younger patients triaged as more urgent cases.
The surgical time available to urological service did not change over time, but the absolute number of surgical cases did vary over the years of study. Although we detected a recent, and concerning, 9.8% cancellation rate, other variables such as the increase in technically advanced surgeries and changes in surgical personnel likely explain some of the variance in the case completion rate. However, the aging demographic in Ontario, as well as increasing detection and surgical management of many urological diseases has likely resulted in an increased demand and is a major factor in prolongation of wait times.
Our study does have limitations. It is retrospective (although the software was designed and implemented specifically to track wait times and data was entered prospectively), the pathological outcomes of patients stratified by wait time are not presented, and there are likely biases in data entry with regard to surgical decision to treat. However, the bias is likely to actually underestimate the wait time, as operative room paperwork is received by staff after the decision to treat is made. This implies that the date entered in the software program, if not entered as the correct date, would be 1 to a few days later. Furthermore, the summary measurement of surgical wait time from decision-making to surgery is limiting in the overall depiction of access to care issues.
The strength of our study is the detailed description of variables associated with prolonged surgical wait times for urological surgery. Despite government initiatives to decrease wait times for cancer surgery, our wait times for urological oncology have steadily increased. It is apparent from our local experience that simplistically applied financial incentives are not sufficient to decrease wait times without more detailed examination of the origins of prolonged surgical wait times on a departmental, institutional or regional basis. Furthermore, it is apparent from the triaging described in our study that there is a need to closely evaluate resource allocation to priority programs, ensuring that any effects on nonpriority surgery are minimized.
Conclusion
Our institution’s real-time surgical booking software is an excellent tool for tracking surgical wait times and subsequently examining resource allocation. We recommend that health care units at the departmental, institutional and regional level require more of the detailed information collection afforded by real-time, prospectively collected data in order to improve the complex problem of prolonged surgical wait times in a universal health care system.
Acknowledgements
Kingston General Hospital Research Initiation Grant, CUA Scholarship.
Appendix 1. Classification of operative procedures
Diagnostic procedures
Diagnostic procedures can have cancer flag "yes"
Diagnosis should not be "urolithiasis"
Minor
Biopsy, bladder
Biopsy, penis
Biopsy, prostate needle
Biopsy, renal
Biopsy, testis
Cystoscopy
Cystoscopy laser lithotripsy
Ureteroscopy
Cystoscopy ureteroscopy, flexible/rigid
Cystoscopy with retrogrades
Benign conditions
Cancer flag must be “no,” except for diagnostic procedures and resection, prostate transurethral
Major
Bladder suspension, 4 corner
Burch procedure
Burch suspension, open
Suspension, bladder neck
Sling, pubovaginal
Minor
Repair tension-free vaginal tape
Vaginal wall sling
Bladder stone
Minor
Cystolithopaxy
Major
Cystolithotomy, open
Benign prostatic hyperplasia
Minor
Resection, prostate transurethral
Cystoscopy insertion, UroLume (American Medical Systems, Inc.)
Major
Prostatectomy, simple
Stricture disease
Minor
Visual internal urethrotomy
Incision, bladder neck contracture
Resection, bladder neck transurethral
Cystoscopy urethral dilatation
Phimosis
Minor
Circumcision
Dorsal slit
Kidney stone
Major
Percutaneous laser lithotripsy
Nephrolithotomy, percutaneous
Percutaneous nephroscopy
Stone disease
For stone disease diagnosis should be “urolithiasis,” otherwise procedure category should be “diagnostic” for the 4 codes below
Minor
Cystoscopy laser lithotripsy
Ureteroscopy
Cystoscopy ureteroscopy, flexible/rigid
Cystoscopy with retrogrades
Elective procedures
Minor
Vasectomy
Varicocelectomy, single
Major
Vasovasostomy
Scrotal surgery
Minor
Hydrocelectomy
Spermatocelectomy
Epididymectomy
Other
If cancer flag is “no” and not diagnostic
Cancer operations
Cancer flag must be “yes” for these to be cancer operations, i.e., nephrectomy may be for benign reason and therefore should be excluded
Bladder cancer
Minor
Resection, bladder tumour transurethral
Resection, bladder tumour transurethral, recheck
Major
Cystectomy, radical
Cystectomy with ileal conduit
Cystectomy, partial
Testis cancer
Minor
Orchidectomy, unilateral
Orchidectomy, radical
Major
Lymphadenectomy, retroperitoneal
Kidney cancer
Major
Nephrectomy
Nephrectomy, laparoscopic
Nephrectomy, partial
Nephrectomy, radical
Upper tract transitional cell carcinoma
Major
Nephroureterectomy
Nephroureterectomy, laparoscopic
Prostate cancer
Major
Prostatectomy, radical
Prostatectomy, retropubic
Penile cancer
Major
Penectomy
“Other”
If cancer flag is “yes,” not categorized as diagnostic, and not in above list
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
References
- 1.Bell D, Morash C, Dranitsaris G, et al. Does prolonging the time to testicular cancer surgery impact long-term cancer control: a systematic review of the literature. Can J Urol. 2006;13(Suppl 3):30–6. [PubMed] [Google Scholar]
- 2.Fradet Y, Aprikian A, Dranitsaris G, et al. Does prolonging the time to bladder cancer surgery affect long-term cancer control: a systematic review of the literature. Can J Urol. 2006;13(Suppl 3):37–47. [PubMed] [Google Scholar]
- 3.Jewett M, Rendon R, Dranitsaris G, et al. Does prolonging the time to renal cancer surgery affect long-term cancer control: a systematic review of the literature. Can J Urol. 2006;13(Suppl 3):54–61. [PubMed] [Google Scholar]
- 4.Canadian Surgical Wait Times (SWAT) Initiative. Consensus document: recommendations for optimal surgical wait times for patients with urological malignancies. Can J Urol. 2006;13(Suppl 3):62–4. [PubMed] [Google Scholar]
- 5.Eggertson L. Wait Time Alliance first to set benchmarks. CMAJ. 2005;172:1277. doi: 10.1503/cmaj.050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trypuc J, Hudson A, MacLeod H. Ontario’s wait time strategy: part 1. Healthc Q. 2006;9:44–51. 2. doi: 10.12927/hcq.2006.18101. [DOI] [PubMed] [Google Scholar]
- 7.Siemens DR, Schulze K, Groome PA, et al. Increasing wait times for radical prostatectomy: a registry-based observational study on the surgical management of prostate cancer in Ontario. Can J Urol. 2004;9:1515. [Google Scholar]
- 8.Hudson AR. Ontario wait time strategy. Can J Urol. 2006;13(Suppl 3):14–5. [PubMed] [Google Scholar]
- 9.Simunovic M, Theriault ME, Paszat L, et al. Using administrative databases to measure waiting times for patients undergoing major cancer surgery in Ontario, 1993–2000. Can J Surg. 2005;48:137–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Sampalis J, Boukas S, Liberman M, et al. Impact of waiting time on the quality of life of patients awaiting coronary artery bypass grafting. CMAJ. 2001;165:429–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Liedberg F, Anderson H, Mansson W. Treatment delay and prognosis in invasive bladder cancer. J Urol. 2005;174:1777–81. doi: 10.1097/01.ju.0000177521.72678.61. [DOI] [PubMed] [Google Scholar]
- 12.Andrews SF, Horwitz EM, Feigenberg SJ, et al. Does a delay in external beam radiation therapy after tissue diagnosis affect outcome for men with prostate carcinoma? Cancer. 2005;104:299–304. doi: 10.1002/cncr.21184. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen PL, Whittington R, Koo S, et al. The impact of a delay in initiating radiation therapy on prostate-specific antigen outcome for patients with clinically localized prostate carcinoma. Cancer. 2005;103:2053–9. doi: 10.1002/cncr.21050. [DOI] [PubMed] [Google Scholar]
- 14.Fahmy NM, Mahmud S, Aprikian AG. Delay in the surgical treatment of bladder cancer and survival: systematic review of the literature. Eur Urol. 2006;50:1176–82. doi: 10.1016/j.eururo.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 15.Mahmud SM, Fong B, Fahmy N, et al. Effect of preoperative delay on survival in patients with bladder cancer undergoing cystectomy in Quebec: a population based study. J Urol. 2006;175:78–83. doi: 10.1016/S0022-5347(05)00070-4. [DOI] [PubMed] [Google Scholar]
- 16.Johnston GM, MacGarvie VL, Elliott D, et al. Radiotherapy wait times for patients with a diagnosis of invasive cancer, 1992–2000. Clin Invest Med. 2004;27:142–56. [PubMed] [Google Scholar]
- 17.Miller DC, Montie JE, Wei JT. Measuring the quality of care for localized prostate cancer. J Urol. 2005;174:425–31. doi: 10.1097/01.ju.0000165387.20989.91. [DOI] [PubMed] [Google Scholar]
- 18.Spencer BA, Steinberg M, Malin J, et al. Quality-of-care indicators for early-stage prostate cancer. J Clin Oncol. 2003;21:1928–36. doi: 10.1200/JCO.2003.05.157. [DOI] [PubMed] [Google Scholar]
- 19.Simunovic M, Gagliardi A, McCready D, et al. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001;165:421–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Saint-Jacques N, Younis T, Dewar R, et al. Wait times for breast cancer care. Br J Cancer. 2007;96:162–8. doi: 10.1038/sj.bjc.6603523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ontario Ministry of Health and Long-Term Care. [accessed 2008 Nov 6];Wait times in Ontario. Available: www.health.gov.on.ca/transflrmation/wait_times/providers/wt_pro_mn.html.
- 22.Comber H, Cronin DP, Deady S, et al. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98:238–9. [PubMed] [Google Scholar]
- 23.Gaudet MC, Ehrmann FD, Rossignol M, et al. The wait for total hip replacement in patients with osteoarthritis. Can J Surg. 2007;50:101–9. [PMC free article] [PubMed] [Google Scholar]