Abstract
Fabry disease, an X-linked systemic vasculopathy, is caused by a deficiency of α-galactosidase A resulting in globotriaosylceramide (Gb3) storage in cells. The pathogenic role of Gb3 in the disease is not known. Based on previous work, we tested the hypothesis that accumulation of Gb3 in the vascular endothelium of Fabry disease is associated with increased production of reactive oxygen species (ROS) and increased expression of cell adhesion molecules. Gb3 loading resulted in increased intracellular ROS production in cultured vascular endothelial cells in a dose-dependent manner. Increased Gb3 also induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin. Reduction of endogenous Gb3 by treatment of the cells with an inhibitor of glycosphingolipid synthase or α-galactosidase A led to decreased expression of adhesion molecules. Plasma from Fabry patients significantly increased ROS generation in endothelial cells when compared with plasma from non-Fabry controls. This effect was not influenced by reduction of intracellular Gb3. This study provided direct evidence that excess intracellular Gb3 induces oxidative stress and up-regulates the expression of cellular adhesion molecules in vascular endothelial cells. In addition, other factors in patient’s plasma may also contribute to oxidative stress in Fabry vascular endothelial cells.
Keywords: Fabry disease, alpha-galactosidase A deficiency, globotriaosylceramide, endothelial cells, reactive oxygen species, cell adhesion molecule
Introduction
Fabry disease is an inborn error of glycosphingolipid catabolism caused by an insufficient activity of α-galactosidase A and progressive accumulation of globotriaosylceramide (Gb3) and related glycosphingolipids (GSL) in the vasculature, including the vascular endothelium [1; 2]. It is associated with a markedly increased risk of ischemic stroke of [3], manifold cardiac abnormalities and progressive renal failure.[4] The vascular complications are responsible for most symptoms and the premature mortality in Fabry disease. At present the pathophysiology of the disease is largely unknown.
Pathologically, vascular endothelial cells are the most predominantly affected cell type in this disease [2]. Clinical and animal studies demonstrated enhanced vascular thrombosis [5; 6; 7], and altered endothelial-dependent vascular reactivity in patients and in Fabry mouse model [8; 9; 10]. Increased markers of endothelial activation and endothelial microparticles were found in Fabry patients’ plasma [11; 12]. Others and we have found evidence for increased production of ROS [10; 13; 14].
We therefore hypothesized that Gb3 accumulation leads to increased production of ROS and other pathological changes in cultured Fabry vascular endothelial cells.
Materials and Methods
Cell cultures and treatments
Microvascular endothelial cells were isolated and purified from a forearm skin biopsy from Fabry patients and non-Fabry controls as described previously [15]. These primary cells, primary dermal microvascular endothelial cells (HMVEC, Clonetics, Cambrex, Walkersville, MD) and a Fabry disease endothelial cell line with an extended life span, IMFE1 cells were maintained in EGM-2 medium (Clonetics) as described [15]. HMVEC and other primary endothelial cells at passage 3–5 and IMFE1 cells at passage 17 to 26 were used in this study. For EtDO-P4 treatment, EtDO-P4, was dissolved in dimethylsulphoxide (DMSO) (Sigma, St. Louis, MO), aliquoted and stored at −20°C until use. IMFE1 cells were maintained in EGM-2 medium containing 1µM EtDO-P4 for 10–21 days. At a concentration of 1µmol/L, no significant changes in growth rate and morphology were observed. However, in a pilot study, a concentration of 2.5µmol/L led to significant IMFE1cell death. For enzyme treatment, cells were incubated with recombinant α-galactosidase A (Shire Human Genetic Therapies, Cambridge, MA) at 0.08 IU/ml (1:500 dilution of original solution) for 4 days. Medium and enzyme were replaced daily. For tumor necrosis factor-α (TNF-α) treatment, cells were incubated with TNF-α (R & D systems, Minneapolis, MN) at 100 units/ml for indicated lengths of time (4 or 6 hrs).
Gb3-loading
Gb3-loading was performed essentially as described with modifications [16]. An Aliquot of Gb3 (Matreya, Pleasant Gap, PA) dissolved in chloroform: methanol (2:1, v/v) was dried and DMSO was added. The mixture was heated at 90°C for 10 min with occasional vortexing. An appropriate amount of fatty acid-free bovine serum albumin (BSA, Sigma) in phosphate buffered saline (PBS) was added to obtain a 1:1 molar ratios of Gb3:albumin . The Gb3/albumin complex was sonicated for 5 min and was added to the medium containing 20% fetal bovine serum (FBS), 10 ng/ml recombinant human basic fibroblast growth factor (bFGF) (Invitrogen, Carlsbad, CA) and 100 µg/ml sodium heparin (Invitrogen) in human endothelial serum-free medium (Invitrogen). Cultures of endotheial cells at approximately 80% confluence were incubated with the medium containing Gb3/albumin complex for 2–3 days.
Human plasma-loading study
Blood samples were obtained from Fabry patients who were receiving enzyme replacement therapy (ERT) by intravenous infusions of α-Gal A (Shire Human Genetic Therapies, Cambridge, MA) every 2 weeks in Institutional Review Board of the National Institute of Neurological Disorders and Stroke-approved studies. Blood samples were taken immediately before enzyme infusion, (i.e. 14 days after the previous infusion) to minimize the effects of ERT on plasma. Plasma was aliquoted and stored at −80°C until use. Plasma was also obtained from healthy male volunteers and was used as controls. Confluent or near confluent endothelial cells were incubated for 2 days with 20% human plasma in human endothelial serum-free medium (Invitrogen) supplemented with 10 ng/ml bFGF (Invitrogen) and 100 µg/ml sodium heparin (Invitrogen).
Immunostaining
Fluorescence immunostaining against Gb3 was performed as described previously with modification [15]. After fixation with 2% paraformaldehyde and permeablization with 0.3% Triton X-100, PBS was substituted for 0.01% Triton X-100 in all subsequent steps. We found that this omission of Triton X-100 moderately increases sensitivity of detection and that almost IMFE1 cells are positive for Gb3-immunoreactive signals using this procedure.
Thin-Layer Chromatography (TLC)
Glycosphingolipid extraction was performed as described previously [17]. Protein concentration was determined by BCA protein assay reagent (Pierce, Rockford, IL). Lipid extract corresponding to equal amounts of cell proteins were loaded on TLC plates (Si-HPF, Baker, Phillipsburg, NJ) and were separated for 45 min in a solvent system consisting of chloroform/methanol/water (65/25/4). GSL were detected by 0.1% 5-hydroxy-1-tetralone (Sigma) prepared in 80% sulfuric acid as described [18] and fluorescence was visualized and quantified using the Storm imaging system (Amersham Bioscience, Piscataway, NJ).
Determination of intracellular ROS: H2DCFDA dye assay
Intracellular ROS was monitored using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Molecular Probes, Eugene, OR) a cell-permeable indicator for ROS. H2DCFDA is nonfluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within the cell. Confluent cells grown on 96 well plates were washed and were incubated with 10 µM H2DCFDA in Hank’s balanced salt solution (HBSS) supplemented with 0.14 mg/ml CaCl2, 0.1 mg/ml MgCl2, 0.1 mg/ml MgSO4, 2% FBS, 10 ng/ml bFGF and 2mmol/L L-arginine for 40 min at 37°C in the dark. The dye was removed and 10 0 µl/well of the HBSS buffer was added to the cells and fluorescence was measured in a Victor plate reader (Perkinelmer, Waltham, MA) using excitation wavelength of 485 nm and emission wavelength of 535 nm. The fluorescence was measured every 2.5 min for up to 10 min and the rate of DCF signal increment was calculated and was used to express intracellular ROS level. ROS level was normalized for cell number determined by MTT assay or cell protein level. MTT assay was performed as described previously [15]. Protein concentration was determined using Micro BCA protein assay reagent (Pierce). Briefly, cells were rinsed with PBS, and were lysed by the addition of 25 µl/well 0.05 N NaOH. After addition of BCA reagents (150 µl/well), the plate was incubated at 37°C for 40 min and absorbance was measured at a wavelength of 562 nm.
Quantitative RT-PCR
Quantitative real time RT-PCR was performed as previously described [15]. Pre-designed TaqMan probes and primers for human ICAM-1, VCAM-1 and E-selectin were purchased from Applied Biosystems (ABI, Foster City, CA). 18S rRNA was used as internal control and detected by TaqMan probe and primers (ABI).
Western blot analysis
Western blot was performed as previously described [15] except for that denaturation and separation of the samples were done under non-reducing conditions, and that 10% Bis-Tris NuPAGE gels (invitrogen) were used to separate proteins. Primary antibodies used were: mouse monoclonal antibody to human ICAM-1 (R & D systems) and rabbit polyclonal antibody to human VCAM-1 (H-276; Santa Cruz Biotechnology, Santa Cruz, CA). Actin was detected with a monoclonal antibody to β-actin (Sigma) as loading control.
Results
Gb3 increases ROS generation in cultured Fabry endothelial cells
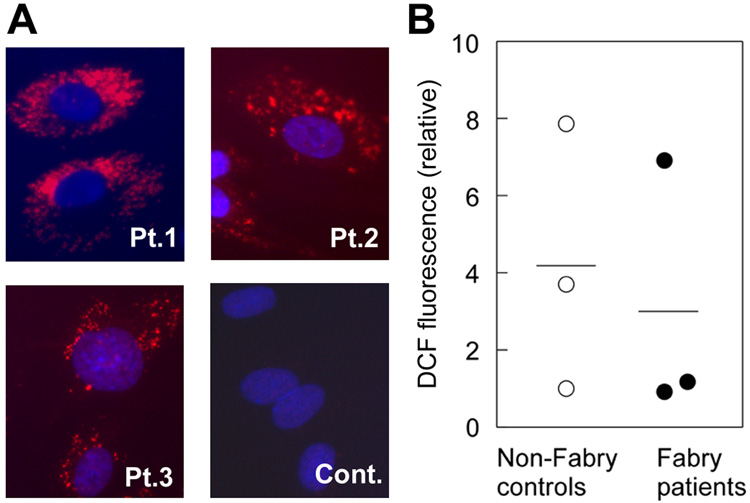
To study whether Gb3 accumulation can lead to the excessive generation of ROS, we initially assessed ROS production in primary cultured endothelial cells from Fabry patients and control subjects. Enzyme assay showed that Fabry endothelial cells (n = 3) had significantly reduced α-galactosidase A activities compared to non-Fabry controls (n = 3) (0, 0 and 6.6 nmol/mg/hr in Fabry cells vs. 56.7, 74.6 and 132.55 nmol/mg/hr in controls). In immunostaining for Gb3, Fabry endothelial cells showed characteristic punctate signals demonstrating lysosomal accumulation of Gb3 and no significant positive signal seen in control endothelial cells (Fig. 1A). No significant difference in ROS was shown between the two groups (Fig. 1B). We considered that this was due to the significant heterogeneity in genetic background of donors, the rapid senescence of primary vascular endothelial cells in culture, and the limited number of available primary lines.
Figure 1. Assessment of ROS production in primary ECs.
(A) Immunostaining for Gb3 in primary ECs. Significant lysosomal accumulation of Gb3 could be detected in all 3 Fabry patients’ ECs (Pt. 1–3) but not in control’s ECs (cont). (B) Intracellular ROS generation in primary ECs was assessed by DCF fluorescence dye. There was no significant difference between the cells from Fabry patients and controls (P > 0.5, Mann-Whitney U test). The results are presented as mean of the data from six wells for each cell line. The results shown are representative of three independent experiments.
We therefore decided to study the biological effects of different amounts of Gb3 in a well-characterized Fabry vascular endothelial line with extended lifespan, IMFE1 [15]. Three days after loading, cell-associated Gb3 in lipids extracts increased significantly when analyzed by TLC (Fig. 2A). Gb3 immunostaining showed higher intensities of positive intracellular signals in loaded cells as compared to untreated cells (Fig. 2B). This suggested that Gb3 added to the medium was taken up by the cells then transported and accumulated in endosomes and/or lysosomes. Increased intracellular Gb3 led to significantly increased ROS generation in the cells in a dose-dependent fashion (Fig. 2C). Treatment of IMFE1 with antioxidant, vitamin C (100 µmol/L) significantly decreased intracellular ROS level. No significant difference in ROS production could be detected between cells with and without Gb3-loading in the presence of vitamin C in the culture media (Fig. 2C).
Figure 2. Effects of Gb3 on the generation of ROS in IMFE1 cells.
IMFE1 cells were incubated with the medium containing Gb3/albumin complex for 3 days. Increase of intracellular Gb3 was verified by TLC (A) and immunostaining against Gb3 (B). Intracellular Gb3 was significantly increased after incubation with Gb3 (10 µmol/L) without significant changes in other GSLs. (C) Intracellular ROS generation was significantly increased in the cells loaded with exogenous Gb3 (5 and 10 µmol/L). The effect on ROS production was dependent on the concentrations of Gb3 in the media. Addition of 100 µmol/L vitamin C into the loading media significantly lowered ROS generation in the cells and abrogated the effect of Gb3 on ROS generation. The data are presented as mean ± SE (n = 6). Mann-Whitney U test was used to compare the statistical significance. Results shown are representative of three independent experiments.
Gb3 up-regulates the expression of adhesion molecules
IMFE1 cells were incubated with 10 µmol/L Gb3 for 2 days and mRNA levels of ICAM-1, VCAM-1 and E-selectin were measured by quantitative RT-PCR. Gb3-loading led to significant increment (up to 55% increase) of steady state mRNA levels of all three molecules (Fig. 3). Besides steady state, mRNA levels of these CAMs were also determined after TNF-α stimulation. TNF-α increased CAMs expression markedly. Gb3 significantly enhanced TNF-α-induced ICAM-1 expression when compared with unloaded cells (Fig. 3).
Figure 3. Effects of Gb3 on CAMs expression in IMFE1 cells.
IMFE1 cells were incubated in the medium containing Gb3 (10 µmol/L) for 2 days, then cells were treated with or without TNF-α (100 units/ml) for 4 hours. Total RNA was extracted and mRNA levels of CAMs were analyzed by TaqMan RT-PCR. Gb3-loading significantly increased steady state mRNA levels of all three CAMs analyzed, and increased ICAM-1 mRNA after TNF-α-stimulation. Results are obtained from three wells each and are representative of two independent experiments. The data are expressed as a ratio to untreated cells and presented as mean ± SE (n = 3), * P < 0.05, Mann-Whitney U test. Different y-axis scales are used for the absence (left) and presence (right) TNF-α treatment.
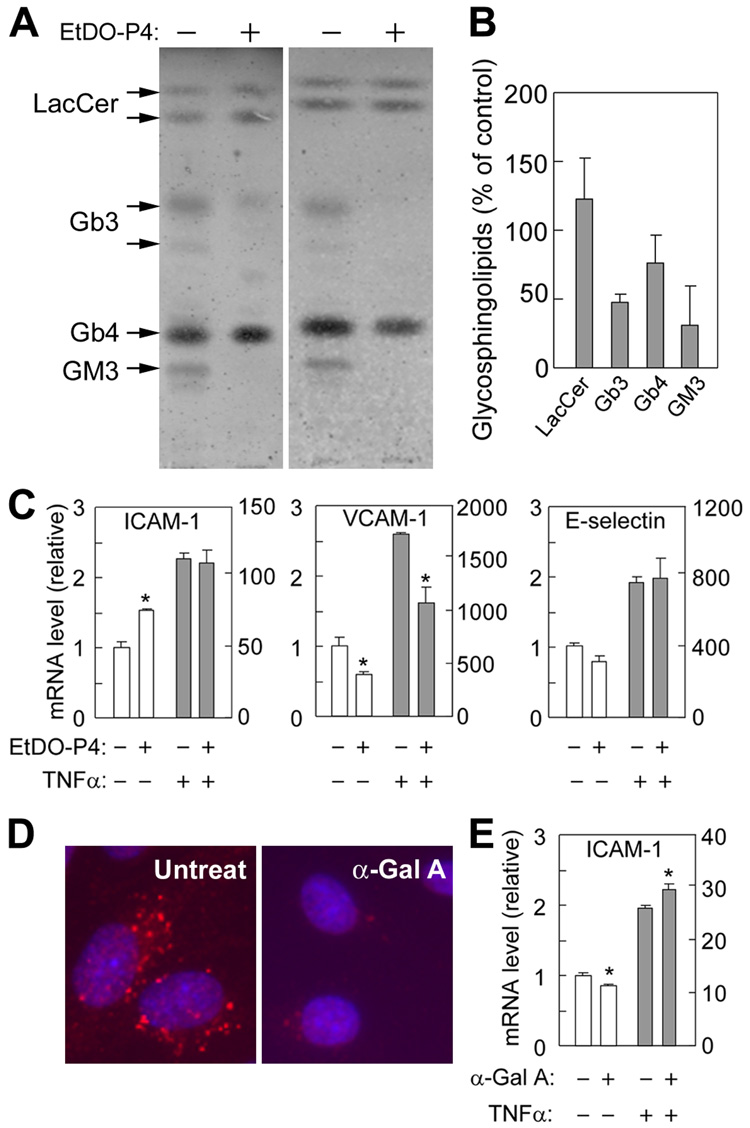
Treatment of IMFE1 cells with 1µmol/L of the GSL synthase inhibitor EtDO-P4 [19] resulted in significant decrement of Gb3 (Fig. 4A and B). EtDO-P4 also decreased Monosialoganglioside GM3 (GM3) but had minor effect on lactosylceramide (LacCer) and globosides (Gb4) (Fig. 4A and B). This heterogenic effect may be due to different turnover and uptake rates from the medium of individual GSL. Consistent to the observations on TLC, there was significant reduction of Gb3-immunoreactive signals in EtDO-P4-treated cells in immunostaining (data not shown). In EtDO-P4-treated cells, VCAM-1 mRNA decreased significantly compared with untreated cells in both steady state and after TNF-α-stimulation (Fig. 4C), while the steady state mRNA level of ICAM-1 was increased in EtDO-P4-treated cells.
Figure 4. Effects of EtDO-P4 and α-galactosidase A (α-Gal A) treatments on CAMs expression.
IMFE1 cells were treated with 1µmol/L EtDO-P4 for 10–17 days (A–C) or 0.08 IU/ml recombinant α-Gal A for 4 days (D and E). (A) Reduction of Gb3 in EtDO-P4 treated cells was verified with TLC. Graphs shown are the results of two independent experiments. (B) Quantitative data of GSLs levels obtained from the experiments shown in (A). The data are presented as mean ± SE (n = 2). (C) Effect of EtDO-P4 treatment on CAMs expression in presence and absence of TNF-α stimulation. (D) Lysosomal accumulation of Gb3 was reduced significantly by α-Gal A treatment when analyzed by immunostaining against Gb3. (E) Effect of α-Gal A treatment on ICAM-1 expression in the presence and absence of TNF-α stimulation. Different y-axis scales are used for the absence (left) and presence (right) of TNF-α treatment. Results are obtained from three wells and presented as mean ± SE (n = 3), * P < 0.05, Mann-Whitney U test.
Treatment of IMFE1 cells with 0.08 IU/ml α-galactosidase A for 4 days significantly reduced Gb3 as shown by immunostaining (Fig. 4D). After enzyme treatment, steady state ICAM-1 mRNA level decreased moderately, but to a significant extent (P < 0.05). However, α-galactosidase A treatment enhanced TNF-α-induced ICAM-1 expression (Fig. 4E). There were no significant changes in mRNA levels of VCAM-1 and E-selectin after α-Gal A treatment (data not shown).
Steady state expression of ICAM-1 and VCAM-1 was almost undetectable with Western blot. The expression levels were markedly increased in TNF-α-treated cells. No significant difference was observed in VCAM-1 and ICAM-1 protein levels in cells with or without Gb3 loading at both steady state or after TNF-α-stimulation (Fig. 5A). Also, there was no significant difference in ICAM-1 expression level between the EtDO-P4-treated and untreated control cells (data not shown). However, VCAM-1 protein after TNF-α-stimulation was significantly decreased in EtDO-P4-treated cells comparing with untreated cells (Fig. 5B).
Figure 5. Western blot analysis for CAMs expression after Gb3-loading and EtDO-P4 treatment.
IMFE1 cells were incubated with 10 µmol/L Gb3 for 2 days (A) or 1 µmol/L EtDO-P4 for 17 days (B). The cells were treated with and without TNF-α (100 units/ml) for 6 hours before being harvested for Western blot analysis. β-actin was used as loading control. Results are representative of two independent experiments.
Plasma from Fabry patients increases ROS production in IMFE1 cells
We had previously found that incubation of normal and Fabry vascular endothelial cells with plasma of untreated patients with Fabry disease markedly increases intracellular Gb3 level (data not shown). The lysosomal accumulation of Gb3 in endothelium in Fabry patients is thought to originate in circulating Gb3 [2].
To explore the possible effects of plasma from Fabry patients on ECs under conditions that mimic in vivo environments, IMFE1 cells were incubated with 20% human plasma for 48 hrs and intracellular ROS production was analyzed. Fabry plasma led to significantly increased ROS production in IMFE1 cells when compared to that with non-Fabry plasma (Fig. 6A). To determine whether the increased ROS production was attributed to increased lysosomal Gb3 uptake from patients’ plasma, intracellular Gb3 levels were evaluated by immunostaining in these cells. Although there were moderate variations in the intensities of Gb3-positive signals among the cells incubated with individual plasma, no conclusion could be drawn regarding the effect of Fabry plasma on intracellular Gb3 storage. Since we used for these experiments plasma from patients on long-term ERT, the effect of Fabry plasma on ROS production in IMFE1 may not be directly related to an increased endosomal content of Gb3.
Figure 6. Effect of patients’ plasma on ROS generation in ECs.
Endothelial cells with or without indicated pre-treatments were incubated with 20% plasma obtained from either Fabry patients (n = 6) or non-Fabry controls (n = 6) for 2 days, and intracellular ROS generation was assessed. (A) Effect of plasma on IMFE1 cells. (B) Effect of plasma on primary normal ECs (HMVEC). (C) IMFE1 cells were treated with 1 µM EtDO-P4 for 3 weeks to reduce endogenous Gb3. The cells then were incubated with 20% plasma in the presence of EtDO-P4 for 2 days. (D) IMFE1 cells were incubated with 0.08 IU/ml recombinant α-Gal A for 4 days to deplete endogenous Gb3. Then, the cells were incubated with 20% human plasma or FBS in the absence of α-Gal A for 2 days before DCF dye analysis. * P < 0.02, cells incubated with Fabry plasma were compared with the cells incubated with non-Fabry plasma; † P < 0.05, cells treated with α-Gal A were compared with un-treated cells (both cells were incubated with non-Fabry plasma after enzyme treatment); ‡ P < 0.03, cells treated with α-Gal A were compared with un-treated cells (both cells were incubated with FBS after treatment). Mann-Whitney U test was used to compare for statistical significance.
Plasma-loading study was also performed on non-Fabry primary vascular endothelial cells (HMVEC). There was no statistical significance (P > 0.05) in ROS generation in HMVEC incubated with Fabry and non-Fabry plasma, although there was a trend for higher ROS levels in cells incubated with patients’ plasma (Fig. 6B).
Fabry plasma incubated with IMFE1 cells pretreated with EtDO-P4 caused significantly higher ROS production than non-Fabry plasma on these cells (Fig. 6C). Fabry plasma also caused higher ROS production in IMFE1 cells pretreated with recombinant α-galactosidase A to reduce Gb3 (Fig. 6D). These data suggested that intracellular Gb3, or other affected GSL, had no or little synergistic effect on plasma-induced ROS generation. Unexpectedly, α-galactosidase A treatment significantly increased ROS generation in the IMFE1 cells incubated with non-Fabry plasma or FBS compared with untreated cells (Fig. 6D).
Discussion
Our study showed direct evidence that accumulated Gb3, in a dose-dependent manner, induces oxidative stress and up-regulates adhesion molecules expression in Fabry vascular endothelial cells indicating a potential mechanism for the vascular involvement in Fabry disease. Factors in patients’ plasma may also contribute to the overproduction of ROS. These in vitro data are further supported by previous clinical findings showing excessive formation of ROS in the patients with Fabry disease [10; 13; 20].
Growing evidence indicate that excessive production of ROS has a causal role in the development of atherosclerosis and other cardiovascular disorders through a number of mechanisms such as oxidation of low-density lipoprotein and modification of endothelial functions and adhesion molecules [21; 22]. At the cellular level, overproduced ROS damages DNA, lipids and proteins through oxidation and leads to cellular dysfunction.[23] The present study suggests that oxidative stress could participate in the pathogenesis of vascular complications in Fabry disease.
The mechanism by which elevated intracellular Gb3 induces ROS production in vascular endothelial cells is unclear. It is possible that Gb3 activates oxidative enzymes such as nicotinamide adenine dinucleotide (phosphate) (NADPH) oxidases the major source of ROS generation in vascular endothelial cells [23]. In addition, it has been shown that endothelial nitric oxide synthase (eNOS) is another important source of ROS generation under certain pathological conditions [24; 25; 26]. Superoxide generated by this mechanism (referred as eNOS uncoupling) plays an important role in endothelial dysfunction [27]. Interestingly, a recent study showed increased Gb3 and related GSL in the caveolar fractions of endothelial cells from a mouse model of Fabry disease [28]. It is possible that altered lipid rafts in caveolae by Gb3 accumulation causes eNOS uncoupling increased ROS generation such as superoxide.
Another important finding of the present study is that increased Gb3 content up-regulates the expression of CAMs in vascular endothelial cells at the transcriptional level with a down-regulation of CAMs expression in response to reduced endogenous Gb3 both by EtDO-P4 and enzyme treatment. This is consistent with findings in patients with Fabry disease [11]. It has been shown that the amount of soluble CAMs correlates directly with intracellular expression of the CAMs in vitro [29]. E-selectin is involved in the rolling of leukocytes, and ICAM-1 and VCAM-1 induce subsequent firm adhesion of leukocytes to endothelium [30], and its over-expression may play a role in the vasculopathy of Fabry disease.
The induction of ICAM-1, VCAM-1 and E-selectin in endothelial cells is predominantly regulated at a transcriptional level. ROS are involved in the up-regulation of endothelial CAMs through the activation of the transcription factors, mainly nuclear factor-kappa B (NF-κB) [31]. It is possible that the up-regulation of CAMs induced by Gb3 in this study is mediated by excess ROS. Despite the increased transcription of CAMs induced by Gb3-loading, no changes of steady state expression of these molecules could be detected by Western blot analysis. The possible explanation is the low expression level of these proteins on inactivated vascular endothelial cells is below detection limit of Western blot.
Our results suggest that intracellular Gb3 does not have synergistic effect to patients’ plasma on ROS generation because reduction of Gb3 by EtDO-P4 and α-galactosidase A couldn’t eliminate the effect of plasma. Fabry patients’ plasma also increased ROS generation in normal ECs (HMVEC), although not significantly so. We speculate that certain altered circulating factors (possibly inflammatory cytokines or other GSL) in Fabry plasma resulting lead to excess production of ROS. We did not exclude that circulating globotriaosylsphingosine is responsible for some of the effects we observed [32]. The methods we used were not designed to detect the very low concentration of, in the nM range, of the more water soluble globotriaosylsphingosine (Gb3 level is in the µM range). Interestingly, patients with some residual α-galactosidase A activity had normal globotriaosylsphingosine levels in plasma [32].
Unexpectedly, treatment of IMFE1cells with recombinant α-galactosidase A led to increased generation of ROS. The enzyme treatment also enhanced the TNF-α-induced increment of ICAM-1 mRNA although it was associated with decreased steady state ICAM-1 mRNA after enzyme treatment. α-Galactosidase A treatment under these in vitro conditions appears to enhance oxidative stress and the reactivity of the vascular endothelial cells to inflammatory cytokines suggesting that the biological effects of ERT may be more complicated than depletion of accumulated Gb3. However, it should be noted that the observations in this study consist of “short-term” experiments. Although α-galactosidase A is specific to GSL with terminal α-D-galactosyl residues, acute catabolism of its substrates may cause excessive generation of their metabolic products and temporary imbalance in the biosynthesis and degrative pathways of other GSL. These changes in GSL may influence ROS generation and adhesion molecules expression. However, these findings may be relevant to the fact that strokes continue to occur in patients on long-term ERT [33; 34; 35; 36].
The availability of an authentic in vitro model system is important for the study of disease mechanisms. IMFE1 cells were established by introduction of human telomerase and have a significantly extended lifespan. This cell line has a stable phenotype and expresses the major constitutive and inducible endothelial markers (ICAM-1, VCAM-1 and E-selectin) as shown in this study and retains functional characteristics of vascular endothelial cells [15]. The ease of manipulating Gb3 level makes IMFE1 an ideal model to study biological effects of Gb3 in endothelial cells. In contrast, comparing a limited number of different cell lines from Fabry patients and non-Fabry controls may be inadequate for mechanistic studies. The intrinsic differences in genetic background and endothelial characteristics of different isolates could make analysis difficult to perform.
Taken together, the present study demonstrates that Gb3 induces ROS production and regulates transcription of CAMs in vascular endothelial cells of Fabry patients. Besides pathological conditions, our results may have implications in providing new insights to biological role of Gb3 and/or α-galactosidase A in maintaining vascular functions in physiological conditions. Further studies of endothelial cells in vitro, together with animal and clinical studies would lead to better understanding of pathogenesis of Fabry disease and developing new therapeutic strategies.
Acknowledgments
We thank Drs. R.O. Brady and G. J. Murray for discussions.
Sources of Funding
This work was funded by the Intramural Program of the National Institute of Neurological Disorders and Stroke, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry's disease. eramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- 2.Desnick RJ, Ioannou YA, Eng CM. a-Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3733–3774. [Google Scholar]
- 3.Rolfs A, Bottcher T, Zschiesche M, Morris P, Winchester B, Bauer P, Walter U, Mix E, Lohr M, Harzer K, Strauss U, Pahnke J, Grossmann A, Benecke R. Prevalence of Fabry disease in patients with cryptogenic stroke: a prospective study. Lancet. 2005;366:1794–1796. doi: 10.1016/S0140-6736(05)67635-0. [DOI] [PubMed] [Google Scholar]
- 4.Brady RO, Schiffmann R. Clinical features of and recent advances in therapy for Fabry disease. Jama. 2000;284:2771–2775. doi: 10.1001/jama.284.21.2771. [DOI] [PubMed] [Google Scholar]
- 5.Mitsias P, Levine SR. Cerebrovascular complications of Fabry's disease. Ann Neurol. 1996;40:8–17. doi: 10.1002/ana.410400105. [DOI] [PubMed] [Google Scholar]
- 6.Eitzman DT, Bodary PF, Shen Y, Khairallah CG, Wild SR, Abe A, Shaffer-Hartman J, Shayman JA. Fabry disease in mice is associated with age-dependent susceptibility to vascular thrombosis. J Am Soc Nephrol. 2003;14:298–302. doi: 10.1097/01.asn.0000043901.45141.d4. [DOI] [PubMed] [Google Scholar]
- 7.Moore DF, Kaneski CR, Askari H, Schiffmann R. The cerebral vasculopathy of Fabry disease. J Neurol Sci. 2007;257:258–263. doi: 10.1016/j.jns.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 8.Altarescu G, Moore DF, Pursley R, Campia U, Goldstein S, Bryant M, Panza JA, Schiffmann R. Enhanced endothelium-dependent vasodilation in Fabry disease. Stroke. 2001;32:1559–1562. doi: 10.1161/01.str.32.7.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heare T, Alp NJ, Priestman DA, Kulkarni AB, Qasba P, Butters TD, Dwek RA, Clarke K, Channon KM, Platt FM. Severe endothelial dysfunction in the aorta of a mouse model of Fabry disease; partial prevention by N-butyldeoxynojirimycin treatment. J Inherit Metab Dis. 2007;30:79–87. doi: 10.1007/s10545-006-0473-y. [DOI] [PubMed] [Google Scholar]
- 10.Moore DF, Ye F, Brennan ML, Gupta S, Barshop BA, Steiner RD, Rhead WJ, Brady RO, Hazen SL, Schiffmann R. Ascorbate decreases Fabry cerebral hyperperfusion suggesting a reactive oxygen species abnormality: an arterial spin tagging study. J Magn Reson Imaging. 2004;20:674–683. doi: 10.1002/jmri.20162. [DOI] [PubMed] [Google Scholar]
- 11.DeGraba T, Azhar S, Dignat-George F, Brown E, Boutiere B, Altarescu G, McCarron R, Schiffmann R. Profile of endothelial and leukocyte activation in Fabry patients. Ann Neurol. 2000;47:229–233. [PubMed] [Google Scholar]
- 12.Gelderman MP, Schiffmann R, Simak J. Elevated endothelial microparticles in Fabry children decreased after enzyme replacement therapy. Arterioscler Thromb Vasc Biol. 2007;27:e138–e139. doi: 10.1161/ATVBAHA.107.143511. [DOI] [PubMed] [Google Scholar]
- 13.Moore DF, Scott LT, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, Pease-Fye M, Ferri R, Brady RO, Herscovitch P, Schiffmann R. Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation. 2001;104:1506–1512. doi: 10.1161/hc3801.096352. [DOI] [PubMed] [Google Scholar]
- 14.Bodary PF, Shen Y, Vargas FB, Bi X, Ostenso KA, Gu S, Shayman JA, Eitzman DT. Alpha-galactosidase A deficiency accelerates atherosclerosis in mice with apolipoprotein E deficiency. Circulation. 2005;111:629–632. doi: 10.1161/01.CIR.0000154550.15963.80. [DOI] [PubMed] [Google Scholar]
- 15.Shen JS, Meng XL, Schiffmann R, Brady RO, Kaneski CR. Establishment and characterization of Fabry disease endothelial cells with an extended lifespan. Mol Genet Metab. 2007;92:137–144. doi: 10.1016/j.ymgme.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monti E, Preti A, Novati A, Aleo MF, Clemente ML, Marchesini S. Uptake and metabolism of a fluorescent sulfatide analogue in cultured skin fibroblasts. Biochim Biophys Acta. 1992;1124:80–87. doi: 10.1016/0005-2760(92)90129-j. [DOI] [PubMed] [Google Scholar]
- 17.Ullman MD, McCluer RH. Quantitative analysis of plasma neutral glycosphingolipids by high performance liquid chromatography of their perbenzoyl derivatives. J Lipid Res. 1977;18:371–378. [PubMed] [Google Scholar]
- 18.Watanabe K, Mizuta M. Fluorometric detection of glycosphingolipids on thin-layer chromatographic plates. J Lipid Res. 1995;36:1848–1855. [PubMed] [Google Scholar]
- 19.Lee L, Abe A, Shayman JA. Improved inhibitors of glucosylceramide synthase. J Biol Chem. 1999;274:14662–14669. doi: 10.1074/jbc.274.21.14662. [DOI] [PubMed] [Google Scholar]
- 20.Kaneski CR, Moore DF, Ries M, Zirzow GC, Schiffmann R. Myeloperoxidase predicts risk of vasculopathic events in hemizgygous males with Fabry disease. Neurology. 2006;67:2045–2047. doi: 10.1212/01.wnl.0000247278.88077.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 22.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 23.Napoli C, de Nigris F, Palinski W. Multiple role of reactive oxygen species in the arterial wall. J Cell Biochem. 2001;82:674–682. doi: 10.1002/jcb.1198. [DOI] [PubMed] [Google Scholar]
- 24.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Wang S, Yan L, Madara P, Del Pilar Cintron A, Wesley RA, Danner RL. Superoxide production and reactive oxygen species signaling by endothelial nitric-oxide synthase. J Biol Chem. 2000;275:16899–16903. doi: 10.1074/jbc.M000301200. [DOI] [PubMed] [Google Scholar]
- 27.Munzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 28.Shu L, Shayman JA. Caveolin-associated accumulation of globotriaosylceramide in the vascular endothelium of alpha-galactosidase A null mice. J Biol Chem. 2007;282:20960–20967. doi: 10.1074/jbc.M702436200. [DOI] [PubMed] [Google Scholar]
- 29.Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543–549. [PMC free article] [PubMed] [Google Scholar]
- 30.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 31.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. Faseb J. 1995;9:899–909. [PubMed] [Google Scholar]
- 32.Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, van Roomen C, Mirzaian M, Wijburg FA, Linthorst GE, Vedder AC, Rombach SM, Cox-Brinkman J, Somerharju P, Boot RG, Hollak CE, Brady RO, Poorthuis BJ. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008;105:2812–2817. doi: 10.1073/pnas.0712309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ries M, Clarke JT, Whybra C, Timmons M, Robinson C, Schlaggar BL, Pastores G, Lien YH, Kampmann C, Brady RO, Beck M, Schiffmann R. Enzyme-replacement therapy with agalsidase alfa in children with Fabry disease. Pediatrics. 2006;118:924–932. doi: 10.1542/peds.2005-2895. [DOI] [PubMed] [Google Scholar]
- 34.Beck M, Ricci R, Widmer U, Dehout F, de Lorenzo AG, Kampmann C, Linhart A, Sunder-Plassmann G, Houge G, Ramaswami U, Gal A, Mehta A. Fabry disease: overall effects of agalsidase alfa treatment. Eur J Clin Invest. 2004;34:838–844. doi: 10.1111/j.1365-2362.2004.01424.x. [DOI] [PubMed] [Google Scholar]
- 35.Wilcox WR, Banikazemi M, Guffon N, Waldek S, Lee P, Linthorst GE, Desnick RJ, Germain DP. Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet. 2004;75:65–74. doi: 10.1086/422366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jardim L, Vedolin L, Schwartz IV, Burin MG, Cecchin C, Kalakun L, Matte U, Aesse F, Pitta-Pinheiro C, Marconato J, Giugliani R. CNS involvement in Fabry disease: clinical and imaging studies before and after 12 months of enzyme replacement therapy. J Inherit Metab Dis. 2004;27:229–240. doi: 10.1023/B:BOLI.0000028794.04349.91. [DOI] [PubMed] [Google Scholar]