Summary
Signals from fibronectin-binding integrins promote neural crest cell motility during development in part through protein-tyrosine kinase (PTK) activation. Neuroblastoma (NB) is a neural crest malignancy with high metastatic potential. We find that α4 and α5 integrins are present in late-stage NB tumors and cell lines derived thereof. To determine the signaling connections promoting either α4β1- or α5β1-initiated NB cell motility, pharmacological, dominant-negative, and short-hairpin RNA (shRNA) inhibitory approaches were undertaken. shRNA knockdown revealed that α5β1-stimulated NB motility is dependent upon focal adhesion kinase (FAK) PTK, Src PTK, and p130Cas adaptor protein expression. Cell reconstitution showed that FAK catalytic activity is required for α5β1-stimulated Src activation in part through direct FAK phosphorylation of Src at Tyr-418. Alternatively, α4β1-stimulated NB cell motility is dependent upon Src and p130Cas but FAK is not essential. Catalytically-inactive receptor protein-tyrosine phosphatase α over-expression inhibited α4β1-stimulated NB motility and Src activation consistent with α4-regulated Src activity occurring through Src Tyr-529 dephosphorylation. In α4 shRNA-expressing NB cells, α4β1-stimulated Src activation and NB cell motility were rescued by wild type but not cytoplasmic domain truncated α4 re-expression. These studies, supported by results using reconstituted fibroblasts, reveal that α4β1-mediated Src activation is mechanistically distinct from FAK-mediated Src activation during α5β1-mediated NB migration and support the evaluation of inhibitors to α4, Src, and FAK in the control of NB tumor progression.
Keywords: Src, FAK, α4 integrin, α5 integrin, signaling, motility, neuroblastoma
Introduction
Neuroblastoma (NB) represents 8-10% of childhood cancers (Brodeur, 2003) and originates from precursor cells of the peripheral sympathetic nervous system. NB metastatic spread is a major obstacle to clinical treatment. Integrins mediate cell-extracellular matrix interactions that modulate cell adhesion, migration, survival, and growth (Guo and Giancotti, 2004). Unligated integrins negatively effect NB survival and metastasis (Stupack et al., 2006) whereas integrin-stimulated signaling cascades controlling NB cell motility remain largely undefined.
Fibronectin (FN) signals can facilitate tumor development (Ruoslahti, 1999). α5β1 is a classical FN receptor with binding through FN repeats III-9 and III-10 (Pankov and Yamada, 2002). Upon cell binding and spreading on FN, focal adhesion kinase (FAK) is recruited to sites of α5β1 clustering through FAK C-terminal domain interactions with β1-integrin binding proteins such as talin and paxillin (Parsons, 2003). FN-stimulated FAK activation increases FAK Tyr-397 phosphorylation (pY397) and promotes the binding of Src-family PTKs to FAK, potentially leading to conformational Src activation (Mitra and Schlaepfer, 2006). Maximal Src activation requires Tyr-418 phosphorylation within the kinase domain (Roskoski, 2005). FAK-Src activation leading to p130Cas or paxillin phosphorylation is associated with cell motility (Mitra et al., 2005), but the molecular controls regulating these events remain loosely defined.
Cellular FN (cFN) contains an alternately spliced region termed connecting segment 1 (CS-1) that binds α4β1 and α4β7 (Pankov and Yamada, 2002). α4β1 also binds to vascular cell adhesion molecular 1 (VCAM-1) expressed on activated endothelium during inflammation (Rose et al., 2002). Studies with chimeric α4 integrin subunits showed that the α4 cytoplasmic domain can confer enhanced migratory properties to cells (Chan et al., 1992) and that α4β1 may promote motility through different molecular mechanisms than α5β1 (Mostafavi-Pour et al., 2003). In mouse fibroblasts, we previously showed that human α4 expression can create a functional α4β1 pair and promote cell motility through Src activation (Hsia et al., 2005). However, it remains unclear whether endogenous α4 motility-promoting signals occur through similar or distinct mechanisms (Huttenlocher, 2005).
Here, we show that α4 integrin is expressed in late-stage NB tumors and on a variety of human NB cells. We evaluate NB motility and PTK activation to cFN and to specific recombinant FN ligands for α4β1 or α5β1. We find that α4β1-stimulated NB cell motility requires Src but not FAK whereas FAK expression and activity are required to promote α5β1-stimulated NB motility. As recombinant FAK can phosphorylate Tyr-418 within the Src kinase domain, our studies also provide a novel mechanism of direct FAK-mediated Src activation.
Results
α4 and α5 integrins are expressed in late-stage tumors and promote NB cell motility
NB is derived from neural crest cell progenitors and the α4-subunit enhances neural crest motility and survival (Kil et al., 1998; Testaz and Duband, 2001). Staining of NB tumors with anti-α4 or anti-α5 antibodies revealed their presence in late but not early stage NB tumors whereas an NB marker (anti-disialoganglioside, GD2) was detected in all tumor samples (Fig. 1a, 1b). Flow cytometry analyses were performed to determine whether α4 expression is maintained in cell lines derived from patient's tissues (Table I). α4 surface expression was variable with high expression found in NB cells isolated from metastatic tumor sites and low or no α4 expression found in cells from local-regional or more differentiated tumors (Nai-Kong Cheung, unpublished results). NB8 cells with high α4, α5, and β1 expression, and SKNAS cells with high levels of β1, moderate levels of α4 and α5 expression were selected for further investigation (Fig. 1c).
Figure 1. α4 and α5 integrins are expressed in late stage NB and function to promote NB cell motility in vitro.
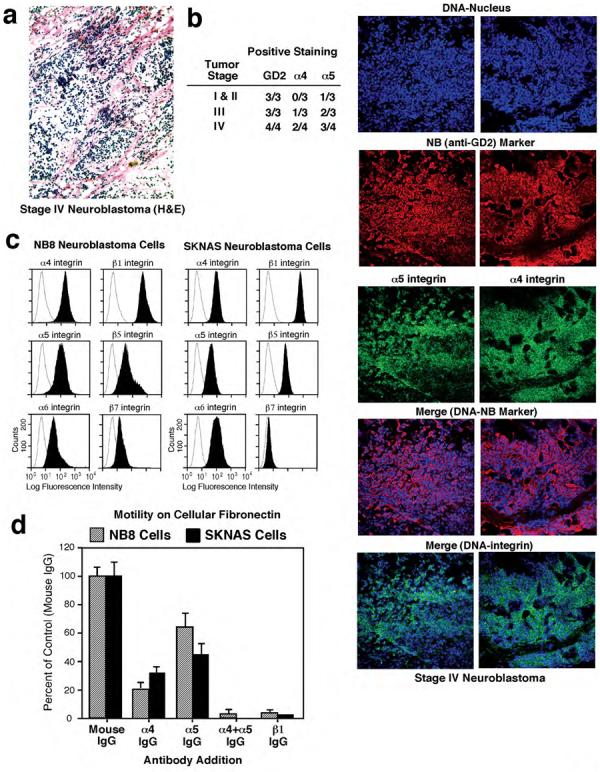
(a) Representative H&E staining of stage IV NB tumor sections reveals clusters of tumor cells throughout the stroma. Magnification 200X. (b) Chart summarizing GD2, α4, and α5 integrin staining results with stage I-IV tumor samples. Data are presented as positive staining per tumor. Representative co-staining of stage IV NB tumors with a DNA marker (blue), antibody to GD2 (red) and antibodies to either α5 or α4 (green) reveal the enrichment of integrin staining with the NB cells as shown by the merged images of DNA and NB marker (blue-red) and DNA-integrin (blue-green). Magnification 200X. (c) Flow cytometry analyses of endogenous α4, α5, α6, β1, β5, β7 expression in NB8 and SKNAS cells (shaded peaks). Staining with control mAb (open peaks). (d)* NB haptotaxis motility on cFN in the presence of anti-α4 mAb (HP2/1, 10 μg/ml), anti-α5 mAb (P1D6, 10 μg/ml), and anti-β1 mAb (P4C10, 10 μg/ml). *Values are means +/− SD of triplicates from two separate experiments.
Table I.
Integrin α4 expression on neuroblastoma cell lines was determined by FACS analysis using monoclonal antibody specific to the α4 subunit.
| Neuroblastoma Cells | Integrin α4 Expression |
|---|---|
| SKNJC2 | ++++ |
| NB8 | +++ |
| SKNLP | +++ |
| SKNJD | +++ |
| LA1-66N | +++ |
| SKNJC1 | +++ |
| SKNAS | ++ |
| IMR6 | ++ |
| SKNER | ++ |
| IMR32 | ++ |
| NB1691 | ++ |
| BE(2)N | ++ |
| BE(2)S | ++ |
| NB16 | ++ |
| NB10 | ++ |
| NB7 | ++ |
| SKNMM | + |
| LAN-1 | + |
| SH-SY5Y | + |
| NMB7 | + |
| LA1-15N | + |
| LA1-5S | +/− |
| BE(2)C | +/− |
| BE(1)N | +/− |
| BE(2)M17 | +/− |
| SKNCH | +/− |
| LA1−55N | +/− |
| LA1−6S | +/− |
| NB5 | − |
| SKNHM | − |
| CB-JMN | − |
Levels of α4 expression in different cell lines were defined according mean fluorescence intensity (MFI) values and designated : ++++, extremely high; +++, very high; ++, high; +, low; +/−, poor; −, not detected by FACS.
Haptotaxis motility assays of NB8 and SKNAS cells revealed that neutralizing antibodies to α4 or α5 inhibited cell migration 70-80% and 40-60% respectively, whereas a blocking antibody to β1 completely inhibited cFN-stimulated motility. Combined addition of anti-α4 and anti-α5 antibodies blocked cell migration (Fig. 1d) supporting the notion that α4β1 and α5β1 are the primary mediators of NB8 and SKNAS motility on cFN. Glutathione-S-transferase (GST) fusion proteins encompassing FN-(9-11) or FN-(CS-1) (Fig. 2a) can serve to selectively activate either α5β1 or α4β1, respectively (Hsia et al., 2005). NB8 and SKNAS motility on FN-(9-11) was blocked by addition of anti-α5 or anti-β1, but not anti-α4 antibodies (Fig. S1a-d), whereas cell motility on FN-(CS-1) was blocked by addition of anti-α4 or anti-β1, but not anti-α5 antibodies (Fig. S1a-c, S1e).
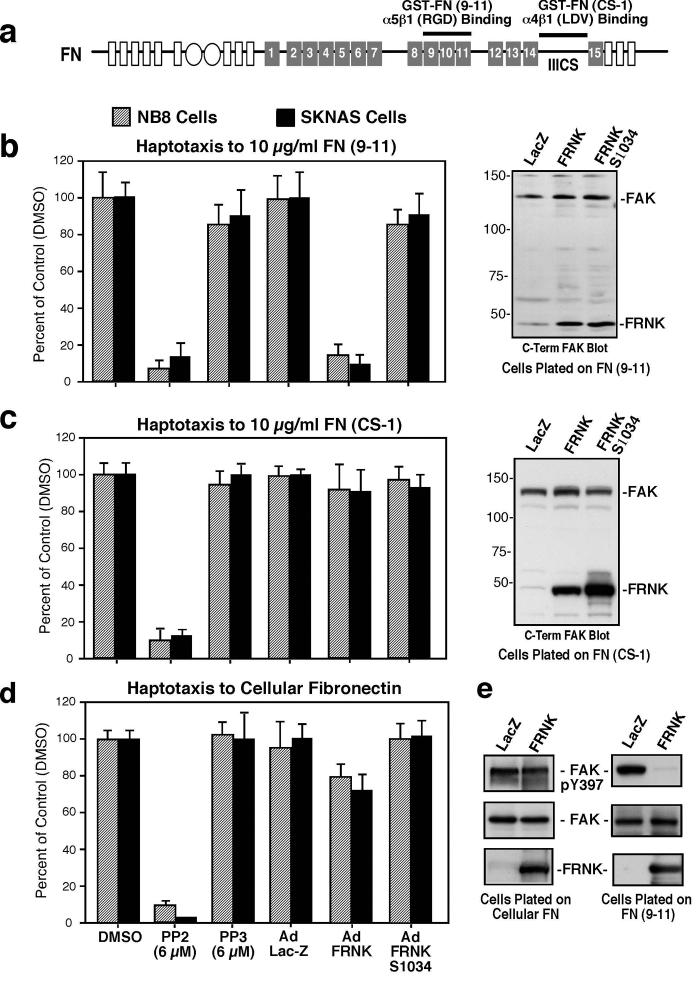
Figure 2. Importance of Src-family PTK but not FAK activity for α4β1 NB cell motility.
(a) Illustration of FN (type III repeats number 1-15) and recombinant GST-FN fusion proteins. GST-FN-(9-11) encompasses repeats 9-11 and contains α5β1 binding motifs. GST-FN-(CS-1) encompasses the IIICS region and contains the α4β1 binding motif. (b-d)* NB cell haptotaxis motility on FN-(9-11) (b, left panel), FN-(CS-1) (c, left panel), and cFN (d, left panel). Transient FRNK expression levels compared to endogenous FAK determined by anti-FAK (5592, C-term) blotting (b-c, right panel). (e) FAK IPs (5904, N-terminal) were made from Ad-LacZ and Ad-FRNK expressing NB8 cells plated on cFN or FN-(9-11) and analyzed by anti-pY397 phosphospecific and anti-FAK blotting. *See Fig.1
α5β1-stimulated NB motility on FN-(9-11) was reduced by an inhibitor to Src (PP2) and by expression of FRNK (Fig. 2b), a dominant-negative inhibitor of FAK (Parsons, 2003). No effects were observed with treatment of cells with the Src-inactive PP3 compound or by equal expression of FRNK S1034 (Hsia et al., 2005), which does not bind paxillin nor co-localize with integrins. Notably, FN-(CS-1) NB motility was inhibited by PP2 but not by FRNK expression (Fig. 2c). cFN-stimulated NB cell motility was inhibited by PP2 and only slightly reduced by FRNK expression (Fig. 2d). This lack of an effect of FRNK on cFN motility was associated with only minor inhibitory effects on FAK tyrosine phosphorylation (Fig. 2e, left panel). In contrast, FRNK potently blocked FN-(9-11)-stimulated FAK tyrosine phosphorylation (Fig. 2e, right panel), consistent with FRNK inhibition of FN-(9-11) motility. These results support the importance of Src but not necessarily FAK activity for α4β1-initiated NB cell motility and FAK-Src signaling for α5β1-mediated NB cell migration.
α4β1 promotes Src-dependent p130Cas tyrosine phosphorylation
To elucidate signaling changes associated with α4β1-mediated NB cell motility, NB8 cells were plated onto FN-(CS-1) and analyzed by anti-phosphotyrosine (pY) blotting (Fig. 3a). Strong phosphorylation of 120-130 kDa proteins was detected and this was blocked by PP2 addition but not by FRNK expression. Analysis of FAK showed no Tyr-397 phosphorylation differences between FRNK-expressing and control cells plated onto FN-(CS-1) (Fig. 3a). PP2 treatment inhibited Src activation as measured by phospho-specific blotting to Y418 and PP2 treatment also potently inhibited p130Cas adaptor protein tyrosine phosphorylation (Fig. 3b). FRNK over-expression did not effect Src pY418 or p130Cas tyrosine phosphorylation upon NB8 plating on FN-(CS-1). These results are consistent with α4β1-stimulated Src activation promoting p130Cas phosphorylation and motility in a FAK-independent manner.
Figure 3. Src inhibition reduces α4β1-stimulated p130Cas tyrosine phosphorylation.
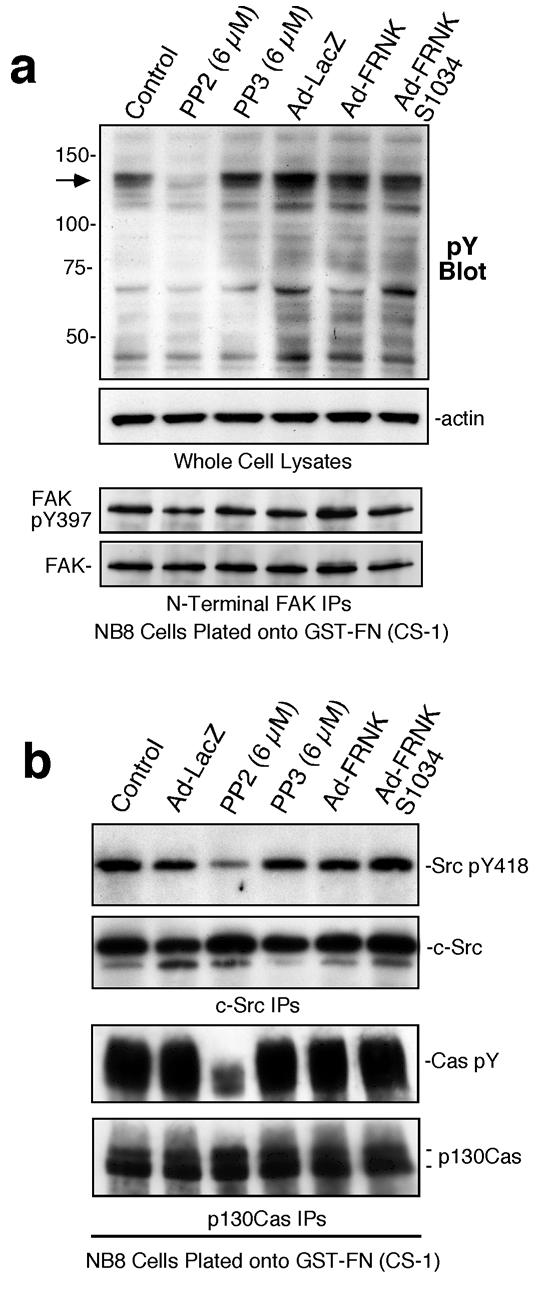
(a) Anti-phosphotyrosine (pY) blotting of whole cell lysates (WCLs) from NB8 cells plated onto FN-(CS-1) for 30 min. Actin blotting verified equal protein loading. Anti-N-terminal domain FAK IPs from lysates of NB8 cells plated onto FN-(CS-1) analyzed by anti-pY397 FAK and total FAK blotting showed no inhibition by FRNK expression. (b) Anti-phosphotyrosine (pY) blotting of Anti-Src IPs and anti-p130Cas IPs from lysates of NB8 cells plated onto FN-(CS-1). Equal levels of Src and p130Cas in the IPs were verified by anti-Src and anti-p130Cas blotting, respectively.
To evaluate the signaling roles of FAK, Src, p130Cas, and paxillin in α4β1-stimulated NB cell migration, lentiviral shRNA was used to stably knockdown these signaling proteins in NB8 cells (Fig. 4a). Greater than 95% reduction in FAK and paxillin expression and ∼80% reduction in p130Cas and Src expression was achieved in pooled populations of cells with no significant changes in cell shape (Fig. 4b). Whereas no compensatory increase in Pyk2 was detected in FAK shRNA NB8 cells, elevated levels of Hic5 were found in paxillin shRNA NB8 cells (Fig. 4c). No differences in either HEF1 or Fyn expression were detected in p130Cas or Src shRNA-expressing cells, respectively (data not shown). Notably, NB8 motility to FN-(CS-1) was inhibited by p130Cas and Src shRNA, while FAK or paxillin shRNA had no impact (Fig. 4d). NB8 motility to FN-(9-11) was inhibited by p130Cas, Src, and FAK shRNA expression (Fig. 4e).
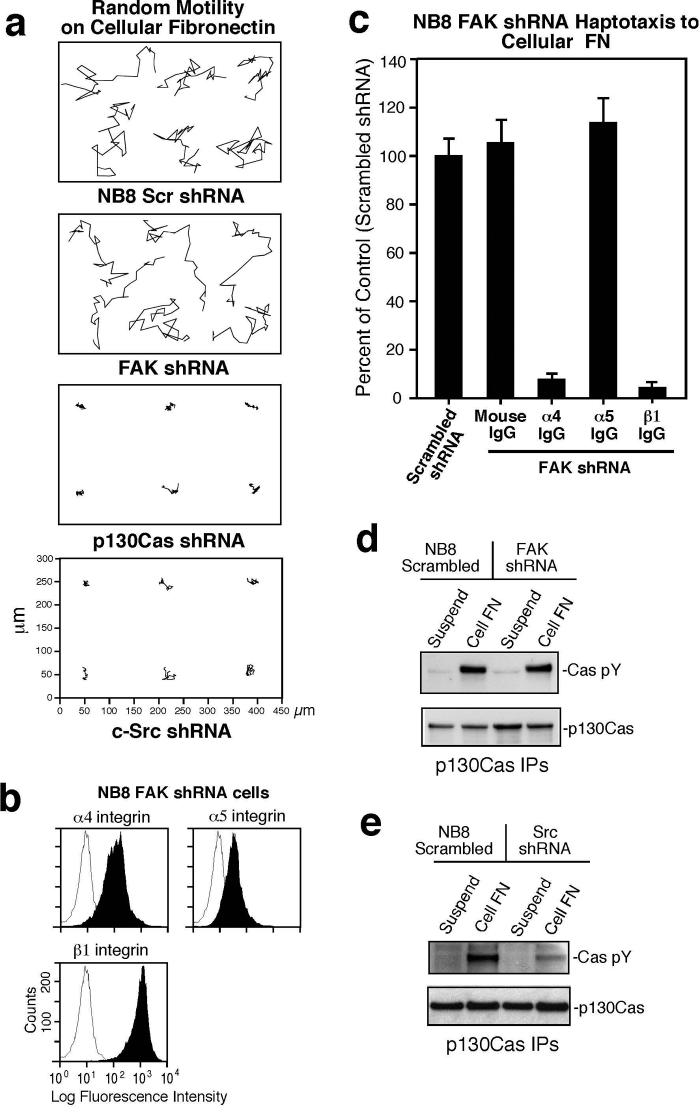
Figure 4. Src or p130Cas shRNA knockdown inhibits α4β 1 NB cell motility whereas anti-FAK shRNA selectively inhibits α5β 1 NB cell migration.
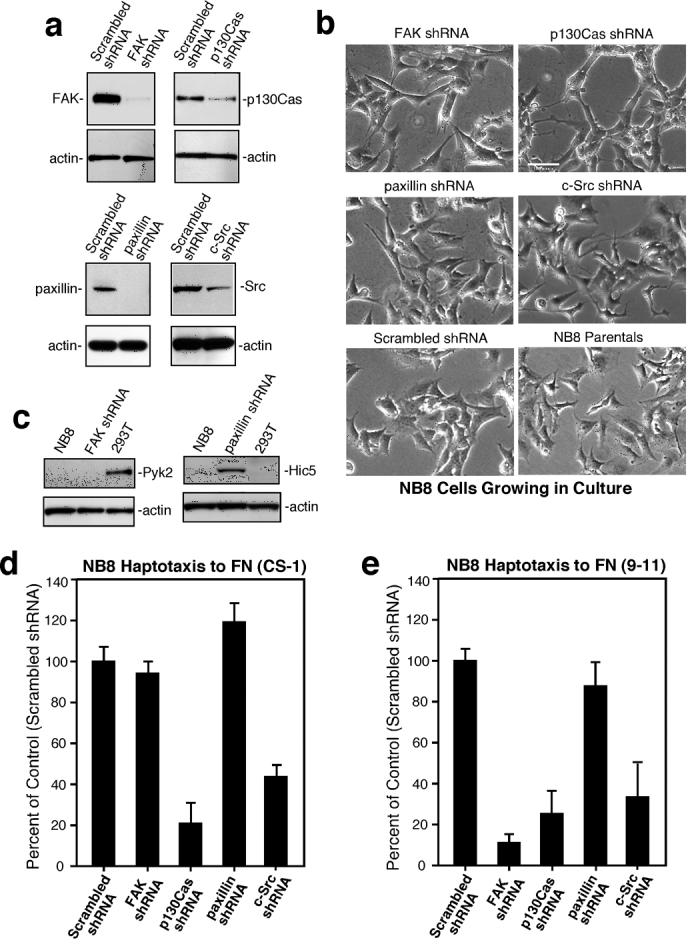
(a) Anti-FAK, p130Cas, paxillin, and Src immunoblots of NB cell lysates stably expressing scrambled or the indicated shRNAs. Actin blotting verified equal loading. (b) Phase contrast images of NB8 parental or the indicated shRNA-expressing cells growing in culture. Scale bar is 20 μm. (c) Expression levels of Pyk2 (left panel) or Hic5 (right panel) in NB8 parental or FAK-paxillin shRNA expressing cells. 293T cell lysates were used for Pyk2 positive or Hic5 negative expression control. NB8 cell haptotaxis motility on FN-(CS-1) (d)* and FN-(9-11) (e). *See Fig.1.
To verify the importance of p130Cas (Cas) in α4β1-stimulated cell migration, studies were performed with Cas-/- and reconstituted mouse embryonic fibroblasts (MEFs) expressing equivalent levels of α5 and β1 but no endogenous α4 (Fig. S2a, S2b). Adenoviral (Ad) human α4 infection resulted in high α4 surface expression (Fig. S2b) and facilitated MEF binding to FN-(CS-1) (Fig. S2c). At 60 min, Cas-reconstituted MEFs spread to a greater extent than Cas-/- MEFs (100 ± 22% versus 37 ± 15%) and this was associated with 2.7-fold elevated motility on FN-(CS-1) (Fig. S2d). Together with the NB8 shRNA data, these results support the notion that Src and Cas expression are important for both α4β1 and α5β1 motility-promoting signaling whereas FAK is required for α5β1 but not α4β1-stimulated NB8 cell migration.
FAK promotes α5β1-stimulated Src activation and Src Y418 phosphorylation
To confirm that FAK is selectively required for α5β1- but not α4β1-stimulated NB8 cell migration, murine WT or kinase-dead (KD) HA-tagged FAK were transiently-expressed in NB8 FAK shRNA cells (Fig. 5a). Surprisingly, both FAK constructs were phosphorylated at Y397 as detected by phospho-specific blotting and formed a complex with Src (Fig. 5a). In vitro kinase (IVK) assays (Fig. 5b) revealed that KD-FAK remained unphosphorylated at Y397 in the presence of ATP. However, addition of either purified recombinant FAK kinase domain (GST-FAK 411-686) or full length His-tagged Src (Fig. S3) promoted KD-FAK phosphorylation at Y397 (Fig. 5b). This result shows that KD-FAK can serve as a substrate for FAK and Src.
Figure 5. Intrinsic FAK kinase activity is required for α5β 1-stimulated NB8 cell motility and Src activation.
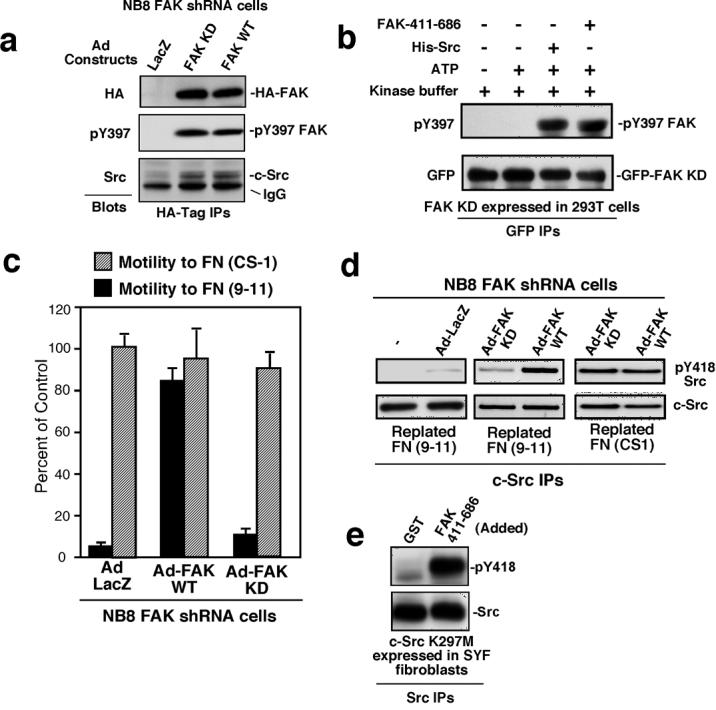
(a) Anti-HA tag IPs from NB8 FAK shRNA cells transiently-expressing Ad-LacZ, Ad-WT-HA-FAK, or Ad-KD-HA-FAK were analyzed by anti-HA, pY397 FAK, and anti-Src immunoblotting. IgG indicates HA-tag IgG cross-reactivity. (b) From cells held in suspension to promote FAK dephosphorylation (1 h) GFP-KD-FAK was isolated by anti-GFP IP, and incubated with or without ATP, His-Src, or FAK-411-686, and analyzed by anti-FAK pY397 and anti-GFP blotting. (c) NB8 FAK shRNA cell haptotaxis cell motility on FN-(9-11) was rescued by WT- but not KD-FAK re-expression. Cell motility on FN-(CS-1) was not affected by KD-FAK over-expression. Values presented are percent of FN-(CS-1)-stimulated cell motility and are means +/− SD of triplicates. (d) NB8 FAK shRNA cells were mock-treated or infected with Ad-LacZ, Ad-WT-FAK or Ad-KD-FAK, serum starved, held in suspension for 45 min, and then replated onto either FN-(9-11) or FN-(CS-1) coated dishes for 30 min. Protein lysates were prepared and Src IPs were sequentially evaluated by anti-pY418 Src and total Src blotting. (e) Src is directly phosphorylated at Y418 by FAK. KD-(K297M)-Src was transiently expressed in SYF fibroblasts, isolated by IP, and incubated in the presence of purified GST or GST-FAK 411-686 with ATP, washed, and analyzed by Src pY418 and total Src blotting.
Expression of WT-FAK in NB8 FAK shRNA cells promoted >8-fold increased motility to FN-(9-11) compared with Lac-Z-expressing cells whereas KD-FAK-expressing cells remained non-motile (Fig. 5c). Neither WT-FAK nor KD-FAK significantly affect NB8 motility on FN-(CS-1). When plated on FN (9-11), only low levels of Src Y418 phosphorylation were detected in NB8 FAK shRNA cells (Fig. 5d). WT- but not KD-FAK promoted Src Y418 phosphorylation (Fig. 5d) and Src IVK activation (data not shown) on FN-(9-11) (Fig. 6d). Alternately, Src was equally activated upon FAK shRNA NB8 cells plating on FN-(CS-1) with WT- or KD-FAK re-expression (Fig. 5d). These results show that FAK catalytic activity is required for α5β1-stimulated Src activation and motility whereas FAK is not required for α4β1 connections to Src and migration.
Figure 6. NB8 FAK shRNA cell motility on cFN is mediated by α4 and β1 but not by α5.
(a) Random motility tracks of NB8 cells expressing scrambled, FAK, p130Cas, or Src shRNA. Time-lapse images were collected every 4 min for 14 hrs and the scale is in μm. (b) Flow cytometry analyses of α4, α5, and β1 integrin expression (shaded peaks) in NB8 FAK shRNA cells. Open peaks show staining with control mAb. (c)* Haptotaxis motility of NB8 FAK shRNA expressing cells on cFN is in the presence of anti-α4 mAb (HP2/1, 10 μg/ml), anti-β1 mAb (P4C10, 10 μg/ml), and anti-α5 mAb (P1D6, 10 μg/ml). (d-e) Anti-p130Cas IPs from NB8 Scrambled or FAK shRNA cells (d) and Src shRNA cells (e) held in suspension (30 min) or plated on cFN (45 min) were analyzed by anti-phosphotyrosine (pY) followed by anti-p130Cas blotting. *See Fig.1.
As both WT- and KD-FAK can form a complex with Src, but only WT-FAK functions to promote Src activation upon FN-(9-11) binding, the FAK catalytic domain was used to determine whether it could directly phosphorylate Src (Fig. 5e). To avoid complications of intrinsic Src phosphorylation, kinase-inactive (K297M) Src was expressed in SYF fibroblasts. As analyzed by anti-pY418 Src blotting after an IVK assay, the FAK kinase domain strongly promoted Src Y418 phosphorylation (Fig. 5e). Thus, FAK-Src activation following α5β1-mediated cell binding may be initiated in part by FAK phosphorylation of and enhancement of Src catalytic activity.
Compensatory motility mechanisms for FAK shRNA cells on cFN
To evaluate the motility responses of shRNA-expressing NB8 cells on a natural ligand, random motility analyses and time-lapse imaging were performed with cells plated on cFN (Fig. 6a). Cell tracking analyses confirmed that NB8 motility on cFN was inhibited by p130Cas and Src shRNA but not by FAK shRNA expression (Fig. 6a). The lack of FAK shRNA effects on cFN motility contrasts with the partial inhibitory effects of α5 antibody addition for cFN NB8 motility (Fig. 1d). Compared to parental NB8 cells (Fig. 1a), FAK shRNA cells expressed lower levels of α5 but similar levels of α4 and β1 (Fig. 6b). Notably, FAK shRNA cell motility was blocked by anti-α4 and anti-β1 but not by ant-α5 antibodies (Fig. 6c), indicating that FAK shRNA-expressing NB8 cell motility on cFN was mediated by α4β1. Reduced FAK expression had no effect on cFN-stimulated Cas tyrosine phosphorylation (Fig. 6d) whereas in Src shRNA NB8 cells, Cas phosphorylation upon cFN binding was dramatically reduced (Fig. 6e). Thus, α5 expression may be regulated by FAK and NB8 FAK shRNA motility on cFN is associated with α4β1-stimulated and Src-associated Cas tyrosine phosphorylation.
The α4 cytoplasmic domain promotes α4β1-stimulated NB8 motility and Src activation
To identify whether the α4 cytoplasmic domain is required for cell motility and PTK activation, NB8 cells were sorted to create a α4-null cell population and in parallel, α4 expression was reduced by lentiviral shRNA (Fig. S4a). Loss of α4 expression in NB8 cells impaired motility to cFN, prevented motility to FN-(CS-1), but did not affect motility on FN-(9-11) (Fig. S4b). Decreased cFN-stimulated motility was not associated with altered binding and NB8 cells lacking α4 did not bind to FN-(CS-1) (Fig. S4c).
To determine whether the motility-defective phenotype of α4-null and α4 shRNA cells on FN-(CS-1) was specifically due to α4 loss, wild type human α4 (Ad-α4 WT) was transiently re-expressed (Fig. 7a). Re-expression of α4 WT rescued cell motility on FN-(CS-1) (Fig. 7b), whereas equivalent expression of cytoplasmic domain-truncated α4 (Ad-α4 ΔCyto) promoted adhesion to FN-(CS-1) (data not shown) but did not promote cell motility to FN-(CS-1) (Fig. 7b). When NB8 cells were plated onto FN-(CS-1), re-expression of α4 WT promoted Src Y418 phosphorylation equal to control NB8 cells whereas α4 ΔCyto only weakly activated Src (Fig. 7c). Thus, the α4 cytoplasmic domain is required to activate Src and plays a critical role in promoting α4β1-mediated NB motility.
Figure 7. α4 cytoplasmic domain and PTPα are important forα4β 1-stimulated Src activation and NB8 cell motility on FN-(CS-1).
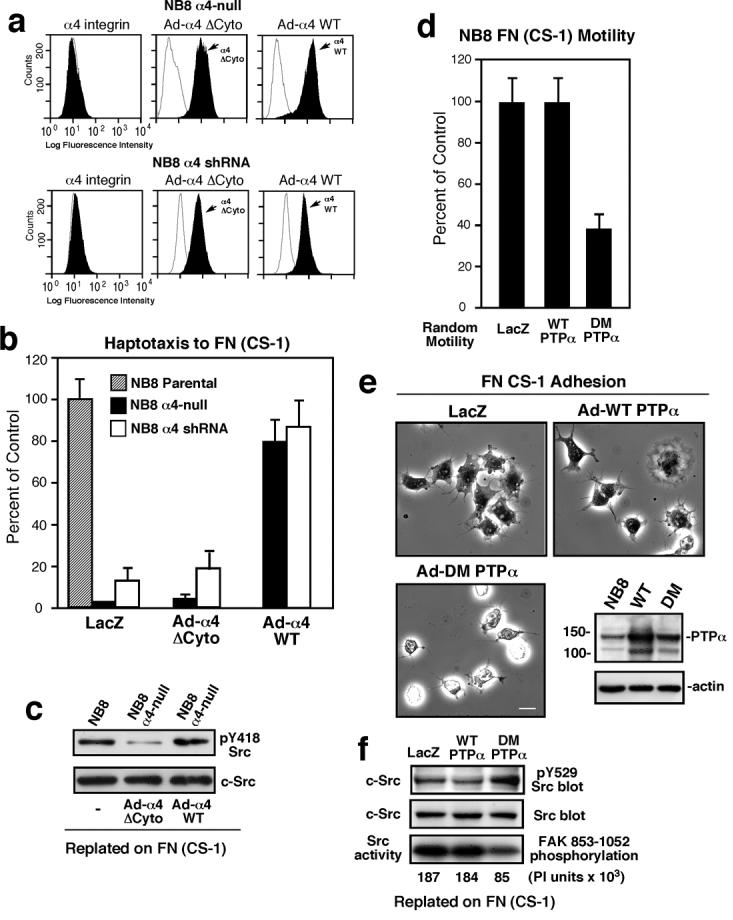
(a) α4 flow cytometry analyses of NB8 α4-null or NB8 α4 shRNA-expressing cells transiently reconstituted Ad-α4 WT or Ad-α4 ΔCyto (shaded peaks). Open peaks show control mAb staining. (b) *Haptotaxis motility assays on FN-(CS-1). Values are percent of NB8 parental motility (Control). (c) Src IPs from protein lysates from NB8 parental or NB8 α4-null cells transiently re-expressing α4 ΔCyto or α4 WT and plated on FN-(CS-1) for 45 min. Src IPs were sequentially evaluated by pY418 Src and total Src blotting. (d) *NB8 cells were transduced with Ad-LacZ, Ad-WT-PTPα, or a catalytically-inactive mutant PTPα (DM) and assessed for random motility on FN-(CS-1). Values are percent of Ad-LacZ transduced NB8 cells (Control). (e) Phase contrast images showing adhesion and spreading on FN-(CS-1) of NB8 cells transduced with Ad-LacZ, Ad-WT-PTPα, or Ad-DM-PTPα at 15 min after plating. Scale bar is 10 μm. Inset, protein lysates of Ad-transduced NB8 cells reveals moderate WT-PTPα and DM-PTPα over-expression compared with endogenous PTPα expression. Actin blotting verified equal loading. (f) NB8 cells were transduced with the indicated Ad constructs, plated onto FN-(CS-1) for 15 min, and Src IPs analyzed for pY529 Src phosphorylation and expression. In parallel, Src IPs were evaluated for IVK activity using GST-FAK 853-1052 as a substrate. Phosphoimager (PI) units are an average from two experiments. *See Fig.1.
PTPα facilitates α4β1-stimulated Src activation, cell spreading, and motility
As Src activation is accompanied by dephosphorylation of the C-terminal regulatory Tyr-529 site, and Src Tyr-529 is dephosphorylated by PTPα during FN-stimulated fibroblast migration (Zeng et al., 2003), we tested whether over-expression of WT- or a catalytically-defective (C433S/C723S) double mutant (DM) of PTPα could influence α4β1-stimulated NB8 cell motility (Fig. 7d). DM-PTPα inhibited FN-(CS-1)-stimulated NB8 migration >50% with decreased cell spreading (52 ±11% of LacZ cell area) (Fig. 7e). DM-PTPα expression resulted in elevated Src pY529 phosphorylation and decreased Src IVK activity compared to control and WT-PTPα-over-expressing cells (Fig. 7f).
In contrast, WT-PTPα over-expression decreased Src pY529 phosphorylation but did not significantly affect Src catalytic activation (Fig. 7f) or FN-(CS-1) cell motility (Fig. 7d). As NB8 cells express moderate levels of endogenous PTPα, PTPα-/- MEFs and Ad-mediated expression of WT- or DM-PTPα was used to test the importance of PTPα in promoting α4β1-stimulated Src activation and motility (Fig. S5a and b). When PTPα-/- cells expressing α4 were plated onto FN-(CS-1), WT- but not DM-PTPα increased Src Y418 phosphorylation by 38% and decreased Src Y529 phosphorylation by 32% (Fig. S5c). Further, WT- but not DM-PTPα promoted enhanced cell spreading on FN-(CS-1) (Fig. S5d and e) and only WT-PTPα functioned to promote efficient α4β1-stimulated fibroblast motility (Fig. S5f). These results suggest that PTPα is involved in promoting α4-mediated Src activation through enhanced Src Y529 dephosphorylation and contributes to α4β1-stimulated motility.
Discussion
Here, we analyzed the signaling properties of endogenous α4β1 and α5β1 integrins on NB cells using recombinant ligands to selectively activate α4β1 or α5β1 and find that α5β1-mediated NB8 cell migration required both Src and FAK PTK activities whereas α4β1-stimulated NB8 cell motility required Src but not FAK. Moreover, we identified a novel and overlooked mechanism of direct FAK-mediated Src activation through FAK phosphorylation of Src within the catalytic region at Y418.
Current models of α5β1-stimulated FAK-Src signaling place FAK activation and autophosphorylation at Y397 as a receptor-proximal event (Mitra and Schlaepfer, 2006). FAK Y397 phosphorylation promotes Src SH2 domain binding to FAK, presumably leading to conformational Src activation with a FAK-Src complex. Alternatively, integrin-regulated dephosphorylation of Src at Y529 can enhance Src activation (Zeng et al., 2003). In both models, it remains undetermined how maximal Src activation occurs via kinase domain Y418 phosphorylation. Although this may occur via Src auto-phosphorylation (Roskoski, 2005), we found that Src Y418 phosphorylation only weakly occurred in FAK shRNA NB8 cells after α5β1 stimulation. Re-expression of WT- and KD-FAK formed a complex with Src, but only WT-FAK functioned to promote α5β1-stimulated Src Y418 phosphorylation and cell motility. As recombinant FAK kinase domain could directly phosphorylate Src Y418 in vitro, we conclude that α5β1-stimulated Src activation occurs via direct FAK phosphorylation of Src. As Src has been shown to phosphorylate FAK kinase domain residues Y576/Y577 leading to maximal FAK activation (Calalb et al., 1995), our results suggest that the formation of a FAK-Src complex after α5β1-stimulation leads to mutual PTK activation through corresponding trans-phosphorylation events.
Although previous studies have implicated integrin β1 in mediating FAK-Src activation after α5β1-stimulation of cells (Mitra and Schlaepfer, 2006), we find that it is the α4 cytoplasmic domain in combination with β1 which facilitates Src PTK activation in a FAK-independent manner to promote NB8 cell motility. Accordingly, abolishing α4 integrin expression in NB8 cells inhibited motility on cFN, and prevented binding to FN-(CS-1), but did not significantly impact migration on an α5β1-selective ligand FN-(9-11). This suggests that minimal cross-talk exists between α4β1 and α5β1 receptors on NB8 cells. Additionally, knockdown of FAK blocked only α5β1-stimulated NB8 migration whereas knockdown of Src or p130Cas prevented both α4β1- and α5β1-initiated cell motility. Fibroblast reconstitution experiments showed that p130Cas, which binds to both FAK and Src, is needed for both α5β1 and α4β1 cell motility. These NB studies extend results obtained using FAK-/- fibroblasts where exogenous human α4 expression was sufficient to form a functional α4β1 receptor that rescued FAK−/− cell motility defects (Hsia et al., 2005), and support the existence of a conserved α4-specific signaling linkage promoting Src activation and cell motility.
It is known that integrins can generate intracellular signals through the lateral association with other receptors or the clustering of signaling proteins with integrin cytoplasmic domains (Ruoslahti, 1999). It is interesting that α4-associated signaling is dependent on the integrity of the α4 cytoplasmic domain. This differs from α5β1-stimulated signaling that is generated through protein binding interactions with the β1 cytoplasmic domain. In α4-null or α4-shRNA NB8 cells, α4β1-stimulated motility and Src activation were rescued by re-expression of wild type but not a cytoplasmic domain-truncated mutant of α4 (α4 ΔCyto). Our finding that α4 ΔCyto re-expression in NB8 cells promoted adhesion but not Src activation or motility do not support a dominant role for the β1 subunit within an α4β1 signaling complex.
The most simplistic mechanism for α4 to activate Src is via a direct binding interaction. Nonetheless, pull down assays using a recombinant α4 cytoplasmic domain revealed only a weak binding between α4 and Src (data not shown). However, Src can become partially activated by integrins through the dephosphorylation of the C-terminal regulatory Y529 site mediated via the protein tyrosine phosphatases such as PTPα (Zeng et al., 2003). The cytoplasmic tails of α integrins, α1 and αL, can associate with T-cell PTP in HeLa cells and with the CD45 PTP in leucocytes, respectively (Geng et al., 2005; Mattila et al., 2005). We found that over-expression of a catalytically-inactive mutant of PTPα inhibited α4-associated Src activation and NB8 cell motility. In complimentary studies, WT PTPα re-expression in PTPα−/− MEFs (but not catalytically-inactive PTPα) enhanced α4β1-stimulated Src activation, spreading, and motility. However, as α4-expressing PTPα−/− MEFs partially spread and migrated on FN-(CS-1), PTPα is likely important but not essential for α4-Src-associated cell motility. It remains unclear how α4 facilitates Src Y418 phosphorylation in a FAK-independent manner.
Distinct from the widely-expressed α5β1 pair, α4β1 is more selectively expressed in inflammatory, endothelial progenitor, and in a subset of tumor cells (Rose et al., 2002). In addition to cFN, α4β1 binds to VCAM-1 that is up-regulated at sites of inflammation and present within the bone marrow; a common site of NB tumor metastasis (Brodeur, 2003). α4β1 engagement with ligand stimulates cell spreading but does not readily promote cell contraction and mature focal adhesion formation (Chan et al., 1992; Pinco et al., 2002); thus potentially permitting rapid changes in cell movement. This differs from α5β1 that promotes both cell spreading and focal contact maturation into stable adhesion structures. Moreover, α5β1 but not α4β1 signaling has been linked to enhanced FN matrix assembly (Chan et al., 1992; Na et al., 2003; Wu et al., 1995). As FAK has been shown to be a critical signaling component associated with FN matrix assembly (Ilic et al., 2004), the differential activation of FAK by α5β1 may be a key discriminatory event distinguishing the biological outcomes of α4β1 and α5β1-initiated signaling events.
Methods
Tumor Immunostaining
NB tumors were obtained at the time of surgery with informed patient/guardian consent and according to the regulations of the Institutional Review Board at Memorial Sloan-Kettering Cancer Center. Tumors were embedded in OCT and cryo-preserved. Frozen tissue sections were fixed in 3% paraformaldehyde and processed for mouse IgG-specific staining to co-detect either α4 integrin (IgG1, Chemicon MAB1383) or α5 integrin (IgG1, Chemicon MAB1969) with anti-GD2 NB marker (IgG3, PHB781) followed by secondary IgG-specific antibodies (flourescein goat anti-mouse IgG1 and rhodamine goat anti-mouse IgG3 from Jackson Immuno Research). Draq5 (Alexis Biochemicals) was used for nuclear staining. Confocal images were captured using Nikon Eclipse C1 microscope and background fluorescence was determined with tissues stained with only secondary antibodies. Three images were assessed for intensity in the RGB channels using Image J software, and the average intensity was calculated. The ratio of staining in the red and green channels was assessed relative to controls, normalized to blue channel (nuclei), and scored positive based upon a threshold at twice of the sampled background.
Adenovirus production and infection
Recombinant Ad for Lac Z, human α4 integrin, cytoplasmic domain truncated α4 integrin were used as described (Hsia et al., 2005). FAK WT, FAK R454 KD, FRNK, and FRNK S1034 are HA-tagged, cloned into pADtet7, and protein expression was performed as described (Hsia et al., 2005). Ad-PTPα-WT and Ad-PTPα-DM (C4333S/C723S) were used as described (Chen et al., 2006). In Ad-LacZ transduced cells, staining for β-gal activity using X-gal as a substrate revealed LacZ expression in >85% of cells.
Short hairpin RNAs, lentivirus production, and infection
The sequences (see supplemental information) used to inhibit human FAK, p130Cas, Src, or paxillin expression were cloned into pLentiLox3.7. For induction of lentiviruses, 293T cells were transfected with plentilox3.7 shRNA construct, CMV-VSVG envelope vector, pMD.G, RSV-Rev, and pMDL g/p RRE as described (Hsia et al., 2005). Integrin α4 shRNA lentivirus was from Sigma. Lentivirus-containing media was used to infect NB8 cells in the presence of polybrene (5 μg/ml) for 2 days. Infected cells expressing GFP were obtained by FACS and maintained as a pooled population. α4 shRNA infected cells were selected with 2 μg/ml puromycin for 5 days and maintained as a pooled population.
Cell migration
Haptotaxis motility and random motility were performed as described using MilliCell chambers (Millipore, 8 μm pores for fibroblasts and 3 μm pores for NB cells) (Hsia et al., 2005).
Supplementary Material
Acknowledgments
We thank the laboratory of Mark Ginsberg for various α4 fusion proteins and we greatly appreciate the administrative assistance provided by Theresa Villalpando. Y. Lim was supported in part by Korea Research Foundation Grant (M01-2005-000-10071-0). This work was supported by grants from the NIH to David Schlaepfer (CA75240, CA87038, CA102310), to Dwayne Stupack (CA107263), and from the Canadian Institutes of Health Research to Catherine Pallen (MOP-49410). Nai-Kong Cheung is supported by CA106450 and the Robert Steel Foundation. D. Schlaepfer is an American Heart Association Established Investigator (0540115N). This manuscript is dedicated to the memory of Jaewon Han Ph.D. who's work stimulated our interest into the novel aspects of α4 integrin signaling.
Abbreviations List
Abbreviations used in this paper:
- Ad
adenovirus
- BSA
bovine serum albumin
- cFN
cellular fibronectin
- CS-1
connecting segment-1
- DM
double mutant
- FACS
fluorescence activated cell sorting
- FAK
focal adhesion kinase
- FBS
fetal bovine serum
- FN
fibronectin
- GFP
green fluorescent protein
- GST
glutathione-S-transferase
- IP
immunoprecipitation
- KD
kinase dead
- Lac Z
β-galactosidase
- mAb
monoclonal antibody
- moi
multiplicity of infection
- MEFs
mouse embryonic fibroblasts
- NB
neuroblastoma
- PTK
protein-tyrosine kinase
- pTyr
phosphotyrosine
- PTPα
receptor protein-tyrosine phosphatase α
- VCAM-1
vascular cell adhesion molecular 1
- WCL
whole cell lysate
- WT
wildtype
Footnotes
Additional Methods available online at:
Supplemental Figures available online at:
References
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- Calalb M, Polte T, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for the Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BM, Kassner PD, Schiro JA, Byers HR, Kupper TS, Hemler ME. Distinct cellular functions mediated by different VLA integrin alpha subunit cytoplasmic domains. Cell. 1992;68:1051–60. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Chen M, Chen SC, Pallen CJ. Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-alpha is required for cytoskeletal reorganization and cell migration. J Biol Chem. 2006;281:11972–80. doi: 10.1074/jbc.M600561200. [DOI] [PubMed] [Google Scholar]
- Geng X, Tang RH, Law SK, Tan SM. Integrin CD11a cytoplasmic tail interacts with the CD45 membrane-proximal protein tyrosine phosphatase domain 1. Immunology. 2005;115:347–57. doi: 10.1111/j.1365-2567.2005.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–26. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Hsia DA, Lim ST, Bernard-Trifilo JA, Mitra SK, Tanaka S, den Hertog J, et al. Integrin α4β1 Promotes Focal Adhesion Kinase-Independent Cell Motility via α4 Cytoplasmic Domain-Specific Activation of c-Src. Mol Cell Biol. 2005;25:9700–12. doi: 10.1128/MCB.25.21.9700-9712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A. Cell polarization mechanisms during directed cell migration. Nat Cell Biol. 2005;7:336–7. doi: 10.1038/ncb0405-336. [DOI] [PubMed] [Google Scholar]
- Ilic D, Kovacic B, Johkura K, Schlaepfer DD, Tomasevic N, Han Q, et al. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci. 2004;117:177–87. doi: 10.1242/jcs.00845. [DOI] [PubMed] [Google Scholar]
- Kil SH, Krull CE, Cann G, Clegg D, Bronner-Fraser M. The α4 subunit of integrin is important for neural crest cell migration. Dev Biol. 1998;202:29–42. doi: 10.1006/dbio.1998.8985. [DOI] [PubMed] [Google Scholar]
- Mattila E, Pellinen T, Nevo J, Vuoriluoto K, Arjonen A, Ivaska J. Negative regulation of EGFR signalling through integrin-alpha1beta1-mediated activation of protein tyrosine phosphatase TCPTP. Nat Cell Biol. 2005;7:78–85. doi: 10.1038/ncb1209. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Mostafavi-Pour Z, Askari JA, Parkinson SJ, Parker PJ, Ng TT, Humphries MJ. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J Cell Biol. 2003;161:155–67. doi: 10.1083/jcb.200210176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Marsden M, DeSimone DW. Differential regulation of cell adhesive functions by integrin alpha subunit cytoplasmic tails in vivo. J Cell Sci. 2003;116:2333–43. doi: 10.1242/jcs.00445. [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J. Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pinco KA, He W, Yang JT. α4β1 integrin regulates lamellipodia protrusion via a focal complex/focal adhesion-independent mechanism. Mol Biol Cell. 2002;13:3203–17. doi: 10.1091/mbc.02-05-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DM, Han J, Ginsberg MH. Alpha4 integrins and the immune response. Immunol Rev. 2002;186:118–24. doi: 10.1034/j.1600-065x.2002.18611.x. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr. Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Teitz T, Potter MD, Mikolon D, Houghton PJ, Kidd VJ, et al. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature. 2006;439:95–9. doi: 10.1038/nature04323. [DOI] [PubMed] [Google Scholar]
- Testaz S, Duband JL. Central role of the α4β1 integrin in the coordination of avian truncal neural crest cell adhesion, migration, and survival. Dev Dyn. 2001;222:127–40. doi: 10.1002/dvdy.1181. [DOI] [PubMed] [Google Scholar]
- Wu C, Fields AJ, Kapteijn BA, McDonald JA. The role of α4β1 integrin in cell motility and fibronectin matrix assembly. J Cell Sci. 1995;108(Pt 2):821–9. doi: 10.1242/jcs.108.2.821. [DOI] [PubMed] [Google Scholar]
- Zeng L, Si X, Yu WP, Le HT, Ng KP, Teng RM, et al. PTPα regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration. J Cell Biol. 2003;160:137–46. doi: 10.1083/jcb.200206049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.