Abstract
Recent studies indicate that the infant rat has high affinity for ethanol ingestion and marked sensitivity to the drug’s reinforcing effects (Spear & Molina, 2005). A novel operant technique was developed to analyze reinforcing effects of ethanol delivery during the third postnatal week. The impact of this ethanol-reinforcement experience upon subsequent ethanol consumption during adolescence (postnatal weeks 5–6 was also examined. In Experiment 1, pups (postnatal days 14–17 were given an explicit contingency between nose-poking behavior and intraoral delivery of either water or 3.75% v/v ethanol (paired groups). Yoked controls (pups receiving either reinforcer independently of their behavior) were also included. Paired subjects reinforced with ethanol exhibited rapid and robust operant conditioning leading to blood ethanol concentrations in the 25–48 mg% range. In Experiment 2, a higher ethanol concentration (7.5% v/v) provided significant reinforcement. During adolescence, animals originally reinforced with 3.75% v/v ethanol exhibited greater ingestion of ethanol than control animals without prior ethanol reinforcement. These results indicate that, without extensive initiation to ethanol, infant rats rapidly learn to gain access to ethanol and that this experience has a significant impact upon later ethanol intake patterns.
Keywords: Ethanol, infant, adolescence, reinforcement, operant learning, voluntary intake
1. Introduction
Early ethanol exposure markedly determines or modulates subsequent ethanol seeking and consumption patterns as demonstrated in both preclinical and epidemiological research (for recent reviews see Chotro et al., 2007; Molina et al., 2007a; Spear & Molina, 2005). The developing organism becomes rapidly familiarized with ethanol’s chemosensory properties and easily acquires and retains information associated with the drug’s postabsorptive consequences.
Ethanol generally can exert positive, negative (anti-anxiety) or aversive effects when employed as an unconditional stimulus (US) in associative learning studies (Cunningham et al., 2000; Pautassi et al., 2007). The expression of these hedonic outcomes is regulated by several factors, including dose, postadministration time and characteristics of the evaluation procedure. Often overlooked, the method of ethanol delivery also can determine the motivational consequences of ethanol. Ciccocioppo et al. (1999a) found that intragastric (i.g.) ethanol administered through a surgically implanted catheter significantly increased time spent in an originally non-preferred context in genetically selected (Marchigian Sardinian) alcohol-preferring rats. On the other hand, no effect was found following conventional i.g. gavage or intraperitoneal (i.p.) administrations of ethanol. Method of drug administration also affects expression of ethanol-mediated conditioned taste aversions (Ciccocioppo et al., 1999b).
The pattern of ethanol consumption observed in humans is better modeled by using the intraoral (i.o.) route of administration than by i.g. or i.p. methods. Yet, the development of animal models showing substantial self-administration of i.o ethanol has proven difficult (Samson et al., 1988). Oral self-administration is regulated by several interacting factors including responsiveness to the sensory properties of the drug (taste, odor and trigeminal stimulation; Bachmanov et al., 2003), sensitivity to its pharmacological consequences and nature of previous experiences with ethanol (Samson & Czachowski, 2003). Palatability plays a key role in initial acceptance or rejection processes (Kiefer, 1989). Converging evidence indicates that both infant and adult rats assess the flavor of ethanol as aversive. For instance, naive rats exhibit substantial orofacial aversion reactions when intraorally stimulated with 10% v/v ethanol. These responses are markedly attenuated when organisms are repeatedly stimulated with i.o ethanol (Kiefer et al., 2005). Furthermore, heterogeneous, non-selected adolescent and adult rats are highly reluctant to consume ethanol when using one or two-bottle choice tests, particularly when ethanol concentrations are higher than 6 % v/v (Kiefer et al., 1987; Pepino et al., 2004; Ponce et al., 2004). Infant rats also avoid a tactile conditioned stimulus previously paired with intraoral ethanol infusion (Pautassi et al., 2008a).
The perceived aversiveness of ethanol taste limits use of intraoral ethanol as a reinforcer in operant conditioning procedures (Meisch & Thompson, 1974). In an operant conditioning procedure a given reinforcer, such as access to intraoral ethanol, is made contingent upon the execution of a target behavior. To overcome ethanol’s taste-related limitations, initiation techniques have been developed. In the sucrose-fading procedure (Samson, 1986) non-deprived animals are initially trained to lever-press in order to obtain 20% v/v sucrose. In subsequent training trials, ethanol is gradually added to the sweet solution while sucrose concentration is progressively decreased. After substantial training, rats show significant operant responding even for highly concentrated ethanol (e.g., 40% v/v). Operant self-administration of ethanol also increases following ethanol pre-exposure. Recently, passive pre-exposure to ethanol’s postabsorptive effects has been found to be associated with later self-administration of high ethanol doses (4–7 g/kg/day; Fidler et al., 2006). In this study, rats were allowed to self-administer the drug intragastrically by means of permanent implanted catheters. The results of Fidler et al. (2006) not only indicate that postingestive effects of ethanol are capable of supporting substantial operant ethanol-related responding. They also suggest that ethanol-mediated operant learning is more likely to emerge when the experimental procedures minimize direct exposure to ethanol’s chemosensory cues and are conducted with subjects which, due to prior experiences with ethanol, are likely to show tolerance to the drug’s aversive unconditioned effects (also see, Bienkowski et al., 1995; Reid et al., 1985).
Motivational properties of ethanol are easily detected in infant rats tested in Pavlovian learning paradigms. Fifteen-day-old pups rapidly acquire a conditioned taste aversion when saccharin is paired with i.g. ethanol (2.5 g/kg; Pautassi et al., 2002). Infants are also sensitive to ethanol’s appetitive properties, as demonstrated through the use of second-order conditioning techniques. Specifically, preweanlings express a conditioned preference to a tactile cue paired with intermittent intraoral infusions of sucrose or water, provided that the infusion had been paired with low-to-moderate ethanol doses (Molina et al., 2006); 2007b. Yet, positive reinforcing properties of the drug are not easily detected during early ontogeny in terms of operant conditioning. Among other factors, implementation of operant conditioning has proven troublesome due to the restricted behavioral repertoire of the infant rat and the lengthy training procedures often needed to shape and maintain operant responding. Moreover, it is likely that the aversiveness of the sensory attributes of ethanol compete with the drug’s reinforcing effects (Pautassi et al., 2008a). Domínguez et al. (1993) observed that 3–4, 9–10 and 15–16 day old pups readily learn to manipulate a lever that provided intraoral milk infusion, but this was less effective when the milk was supplemented with 6 % v/v ethanol.
We have recently tried to assess ethanol’s motivational effects by means of an operant task in which ethanol is self-administered by touching a sensor located in the floor of the experimental chamber (Pautassi et al., 2008b). Daily training sessions were executed in two-week old rats given 3 or 5 % v/v ethanol as a reinforcer. Relative to yoked controls, paired animals exhibited lesser probability of responding. Paired subjects also showed a progressive decrease in ethanol self-administration across training trials. Operant responding apparently was associated with aversive orosensory features of the drug.
In the present study we examined operant responding for i.o. ethanol in infant rats and tested the effect of this experience on ethanol intake during adolescence. Previous studies indicate that ethanol intake in adolescent and young adult rats is increased by ingestive experience with ethanol during infancy (Pepino et al., 2004; Ponce et al., 2004). In addition, exposure to ethanol’s olfactory cues during lactation seems sufficient to increase subsequent affinity for ethanol odor and ingestion (Bannoura et al., 1998; Molina et al., 1986).
The present study employed a novel operant preparation, different from that used by Domínguez et al. (1993) or Pautassi et al. (2008b). In the latter study, infants self-administered small volumes of ethanol (5 µl) by contacting a sensor located in the floor of the experimental chamber. In the present study, ethanol self-administration requires a discrete and distinctive target response: nose-poking. Each reinforced response results in the intraoral delivery of 25 µl of ethanol. When not under the control of operant contingencies, the probability of nose-poke is very low and stable. This preparation involves a behavior present throughout most of the ontogeny of the rat and, hence, more amenable to longitudinal studies than the more traditional lever-pressing models. In Experiment 1 we asked whether preweanling pups would be capable of acquiring operant responding supported by intraoral ethanol delivery. A second goal of this experiment was to assess if this learning is facilitated by prior brief experiences with ethanol’s sensory and postabsorptive attributes. Blood ethanol concentrations following drug pre-exposure (postnatal day 13, PD 13) or at termination of operant training procedures (PD 17) were also determined. Experiment 2 investigated long-term effects of infantile operant conditioning with ethanol upon adolescent ethanol consumption.
2. General Methods
2.1.1. Subjects
All subjects (Wistar-derived rats) employed in the present study were derived from litters born and raised at the vivarium of the Instituto de Investigación Médica M. y M. Ferreyra (INIMEC-CONICET, Córdoba, Argentina). The vivarium has controlled conditions of temperature and artificial light. A 12-h light-dark cycle was employed. Births were daily recorded and day of parturition was considered as postnatal day 0 (PD 0). Pups were housed with the dam in maternity cages with free access to water and lab chow (Cargill, Pilar, Argentina). Both experimental and maintenance procedures followed the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the Animal Care and use Committee at INIMEC-CONICET. Across experiments and to eliminate confounding of litter with treatment effects, no more than one subject from each sex was assigned to the same treatment condition in a given litter. Number of males and females in each group was balanced.
2.1.2. Cannulation Procedures
Across experiments, intraoral infusion was conducted by means of polyethylene tubing cannulae positioned in the pup’s cheek. These devices were employed during pre-exposure (PD 13) and operant training procedures (PDs 14–17). Intraoral cheek cannulation is minimally stressful in preweanlings (Spear et al., 1989) and has been shown to be a useful tool for the assessment of innate and learned patterns of responsiveness to tastants (e.g. Arias & Chotro, 2005; Pautassi et al., 2002). These cannulae were made from 6-cm sections of PE 10 polyethylene tubing (Clay-Adams, Parsippany, NJ). A small flange was created in one end of these devices. The unflanged end was attached to a curved 27-G ½ precision glide. The needle was pulled through the medial internal surface of the cheek of the subject. Consequently, the flanged end of the cannulae rested over the oral mucosae while the remainder exited from the mouth. When not in use, the remainder of the cannula was secured by means of a small cap made of PE 50 tubing.
2.1.3. Infantile Pre-exposure Procedures (PD 13)
Pups were removed from the maternal cage and placed in pairs in holding cages lined with clean pine shavings. These cages were kept warm (32–34 °C) through the use of heating pads. All pups were immediately implanted with an intraoral polyethylene cannula, as described in section 2.1.2. One hour after cannulation, pups’ bladders were voided by gently stroking the anogenital areas with cotton swabs. Animals were weighed to the nearest 0.01 g and randomly assigned to a pre-exposure group according to the experimental design of each experiment. Pups were then placed into individual boxes fitted with a cotton floor (15 × 7 × 14 cm). In these chambers animals received either intraoral infusions of ethanol (7.5% v/v, volume of each pulse: 25 µl; rate of delivery: 3 s on – 57 s off) or vehicle (distilled water). These solutions were delivered via an infusion pump (Manostat Cassette R Pump, N.Y) connected to the subject’s intraoral cannula. Two 15-min sessions were conducted. Hence, total amount of ethanol delivered throughout this stage was 750 µl. The interval between sessions was 30 min. Cannulae were removed following pre-exposure and pups were returned to the holding chambers where they remained for 120 min before being returned to their maternal chambers. This time interval was meant to allow for clearance of the drug before being reunited with the dam. Taking into account the mean weight of infants at this particular age, overall liquid infused, ethanol concentration and the drug’s specific weight, the overall amount of intraoral absolute ethanol available was 1.98 g/kg.
2.1.4. Infantile Operant Training Procedure (PDs 14 to 17)
Custom-made operant chambers (20 × 20 × 20 cm) constructed with black Plexiglas were employed. The chambers were also equipped with black Plexiglas floors. One of the lateral walls in these chambers had a hole in it (diameter: 1 cm, distance between the center of the circumference and the floor: 1.5 cm; distance from the adjacent wall: 0.8 cm). A single channel chargetransfer touch and proximity sensor chip (Model E11x; Evaluation Board; Quantum Research Group, Pittsburgh, PA) was located 1.5 cm away from the hole. The target behavior under training was nose-poke. Specifically, when the nose of a paired subject was proximal to the sensor chip (< 0.5 cm), an acoustic signal went off and a cassette infusion pump (Manostat Cassette R Pump, N.Y) was activated. This pump delivered the corresponding intraoral reinforcer to the paired animal as well as the yoked control.
On each training day pups were removed from their maternal cages. Two animals of the same litter, sex and pre-exposure treatment were placed in holding chambers kept warm (32–34° C) by means of heating pads. Eight hours later, they were intraorally cannulated as described in section 2.1.2. Operant training took place four hours after cannulation. First, the anogenital region of the preweanling was gently stroked with a cotton swab in order to stimulate defecation and void the subject’s bladders. Animal’s weights were then registered to the nearest 0.01 g (Ohaus dial-o-gram balance, Florham Park, NJ). Training began by individually placing a Paired and its corresponding Yoked control (P and Y, respectively) into the operant chambers. Duration of each training session was 20 min. In paired (P) pups, each nose-poke was reinforced with an intraoral infusion of a given reinforcer (volume: 25 µl, pulse duration: 1.5 s). Hence, the schedule of reinforcement can be described as a fixed ratio 1. Yoked controls (Y Group) received the infusion each time the paired animal did. In other words, Y controls received equivalent reinforcement as did P pups but had no control over the contingency between target operant behavior and liquid intraoral infusion. No attempt was made to shape the behavior of the animals. During each session, nose-poking frequency and latency to perform the first nose-poke were registered in real time by trained experimenters. A similar operant procedure has been previously conducted in our laboratory using sucrose as a reinforcer (6% w/v). Intraoral sucrose induced a progressive increase in nose-poking behavior in P animals. Yoked pups maintained a relatively low responding rate across training sessions (Ponce et al., 2006).
2.1.5. Determination of Blood Ethanol Concentration
Heparinized-sterilized syringes were used to collect the infantile blood samples. The procedure employed for blood collection was similar to that described in Pautassi et al., (2005) and Peana et al. (2007). Briefly, blood was collected from the right atrium in a procedure that took less than 5 sec, followed by immediate sacrifice of the animals by means of CO2 inhalation. Blood samples were fractionated to obtain two 100 µl samples, which in turn were placed in microvials containing 50 µl of a butanol solution (51 mg%). Butanol served as an internal standard. Microvials were hermetically sealed and incubated in a water bath at 60° C for 30 minutes. The volatile component of the samples was collected using gas-tight syringes (Hamilton, 10 ml) and injected into a gas chromatograph (Hewlett-Packard, Model 5890). Temperatures for the column (Carbowax 20M; 10 m × 0.53 mm × 1.33 mm film thickness), injector and detector were 60, 150, and 250 °C, respectively. Nitrogen was employed as the carrier gas (flow rate: 15 ml/min). All blood ethanol concentration (BECs) values were expressed as milligrams of ethanol per deciliter of body fluid (mg/dl).
2.1.6. Ethanol Intake Test during Adolescence (PDs 33–l36)
Subjects were weaned on PD 21 and housed in standard maternity cages (four rats of the same litter) until initiation of voluntary consumption tests (PD 33). Intake tests (2hr each) took place in standard individual wire mesh cages equipped with spring clamps that supported two graduated glass intake tubes (volume capacity: 25 ml; graduation: 0.1 ml) equipped with rubber stoppers. Testing was conducted during four consecutive days. Each intake session was preceded by 22 hr of liquid deprivation. During tests (PDs 33–36), adolescents had simultaneous availability of tap water and a given ethanol solution. On the first testing day, a 3 % v/v ethanol solution was available. This solution was increased by 1% v/v of ethanol concentration per day until reaching 6% v/v ethanol. The animals were returned to their standard maternity cages following each daily intake test. This is a standardized ethanol intake protocol that has proven sensitive to effects of several treatments upon ethanol consumption, including the effects of early exposure to ethanol during nursing (Ponce et al., 2004; Pepino et al., 2004). Under the effects of liquid deprivation adolescents are observed to readily ingest ethanol or water. In the case of ethanol ingestion, the deprivation schedule seems to favor rapid absorption and distribution of the drug and hence rapid perception of its postabsorptive effects (Molina et al., 1986; Ponce et al., 2004; Pepino et al., 2004). The rationale for employing ethanol concentrations up to 6% v/v is that preliminary studies conducted in Wistar rats as well as a recent study conducted with Long–Evans hooded rats (Youngentob et al., 2007) indicate that these animals drink very little ethanol when having access to 7% v/v or higher ethanol concentrations (also see Ponce et al., 2004; Pepino et al., 2004). To avoid place-preference effects, the position of the water and ethanol tubes was systematically varied across sessions. The main dependent variables under analysis were liquid ingestion (ml per 100 g of body weight) and ethanol intake scores in terms of grams of absolute ethanol per kilogram of body weight. An ethanol preference ratio [(consumption of ethanol/overall liquid ingestion) × 100] as well as maximum ethanol intake in a given day were also calculated. Maximum ethanol intake scores were obtained as follows. Each animal was given a single score indicating the highest level of ethanol consumption (g/kg) achieved across testing days. That is, this score is the highest of the 4 daily intake values registered in PDs 33, 34, 35 and 36. For example, an animal that consumed 0.33, 0.40, 1.20 and 0.23 g/kg across testing days would be given a score of 1.20 in terms of maximum amount of absolute ethanol intake. In previous reports, we found this index quite sensitive for detecting changes in ethanol intake as a function of several environmental variables, including previous exposure to ethanol or stressors such as shock (Ponce et al., 2004).
3. Experiment 1
The goal of the present experiment was to assess whether infants would learn to perform a low-probability target behavior (nose-poke) to gain access to intraoral ethanol. As mentioned, ethanol’s chemosensory attributes have seemed to act as a barrier that competes with the drug’s motivational effects. Hence, in this experiment we exposed infants to intraoral ethanol administration prior to operant training. This strategy has proven to be successful for reducing aversive taste reactivity induced by ethanol intraoral stimulation (Kiefer et al., 2005) as well as detection of ethanol’s positive reinforcing effects (Pautassi et al., 2008a). Preliminary research (Ponce et al., 2006) was conducted to determine ethanol concentrations that could serve to reinforce operant conditioning as well as to effectively reduce chemosensory aversiveness through prior passive exposure to ethanol. In these studies, a relatively low ethanol concentration (3–5% v/v) promoted ethanol self-administration, a phenomenon also observed in studies conducted with neonatal rats (Bordner et al., 2008). It also appeared that prior exposure to an ethanol concentration (7–10% v/v) higher than that employed during operant conditioning facilitated responding during this learning phase. Taking these observations into account, pups were pre-exposed to intraoral infusions of either water or 7.5% v/v ethanol and subsequently trained using an operant conditioning preparation defined by the contingency between nose-poke behavior and reinforcement delivery (ethanol: 3.75% v/v or its vehicle: tap water). At the end of operant training (PD 17) BECs were determined in both paired and yoked pups reinforced with 3.75% v/v ethanol. In a separate group of animals we also determined BECs following ethanol pre-exposure (PD 13).
3.1. Methods
3.1.1. Subjects and Procedures
A total of 72 infants representative of 8 litters were employed for the analysis of operant conditioning. Groups were defined by the following factors: pre-exposure treatment (Water or 7.5 % v/v Ethanol), conditioning (Paired or Yoked controls; P or Y, respectively) and intraoral reinforcer (Water or 3.75% v/v Ethanol). Each group was composed of 9 infants. Pre-exposure treatments took place on PD 13 while operant training procedures were conducted during PDs 14–17 (see section 2, “General Methods”). The dependent variables under consideration were total frequency of nose poking and latency to perform the first nose-poke during each particular training session. At the end of the last training session, P and Y rats reinforced with ethanol were sacrificed and BECs were determined. Data corresponding to three pairs of P and Y pups were not taken into consideration due to technical problems during blood ethanol determination.
Twenty-two additional pups representative of 5 litters were exposed to ethanol intraoral delivery only during PD 13. Half of these animals were sacrificed following the first pre-exposure trial, in which they passively received an intraoral infusion of 7.5 % v/v ethanol (see section 2, General Methods). The remaining half was sacrificed following the second (last) pre-exposure trial. BECs were determined in both groups. This was performed to determine the level of intoxication, operationalized through metabolic parameters, associated with pre-exposure treatment.
3.2. Results
Preliminary Considerations
The results of the present experiment have been depicted in Figure 1 (nose-poke frequency) and Figure 2 (latency to perform the first nose-poke). Four-way mixed analyses of variance (ANOVAs) were employed to process nose-poke frequency as well as latency to perform the first nose-poke. In these ANOVAs pre-exposure treatment (Water or Ethanol) and reinforcer (Water or Ethanol) served as between-group variables. Conditioning (Paired or Yoked) and training days (sessions 1–4, PDs 14–17) served as within factors. BECs attained during the last operant conditioning day (PD 17) were analyzed by means of a 2 × 2 mixed ANOVA. Pre-exposure treatment (Water or 7.5 % v/v Ethanol) served as a between factor while conditioning (Paired or Yoked) served as a within variable. In the current and subsequent experiment, the loci of significant main effects or interactions were further examined through post-hoc comparisons (Newman-Keuls with an initial alpha level set at 0.05). Given the multiplicity of comparisons and in order to avoid spurious positives, the alpha value of post-hoc tests was lowered by the Bonferoni correction.
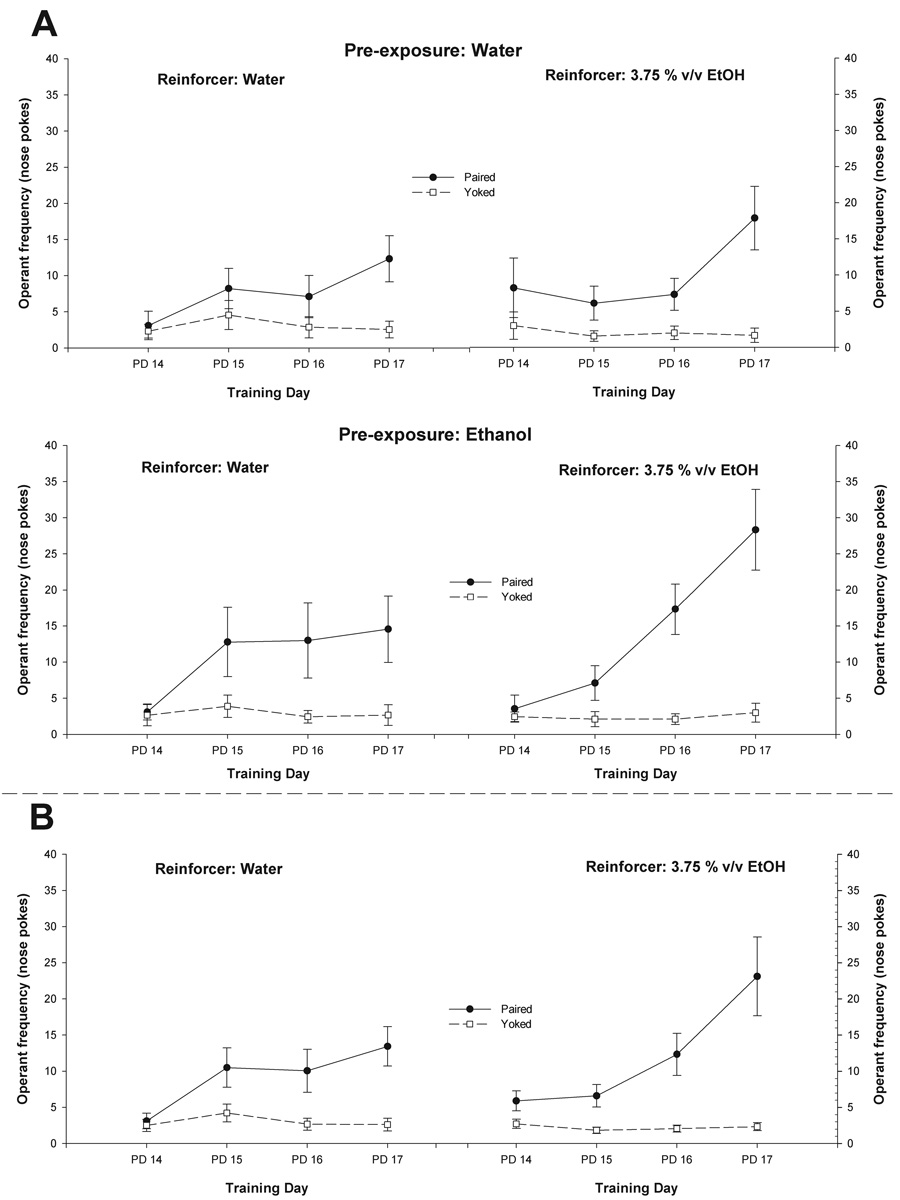
Figure 1.
A) Mean number of nose-pokes as a function of pre-exposure treatment (Water or Ethanol), reinforcer (Water or 3.75% v/v Ethanol), contingency between behavioral emission and intraoral reinforcement delivery (Paired or Yoked) and training day (Postnatal days 14–17, PD 14–17). B) The illustration depicts the significant interaction comprising contingency, nature of the reinforcer and training day. In both figures vertical lines represent standard errors of the mean.
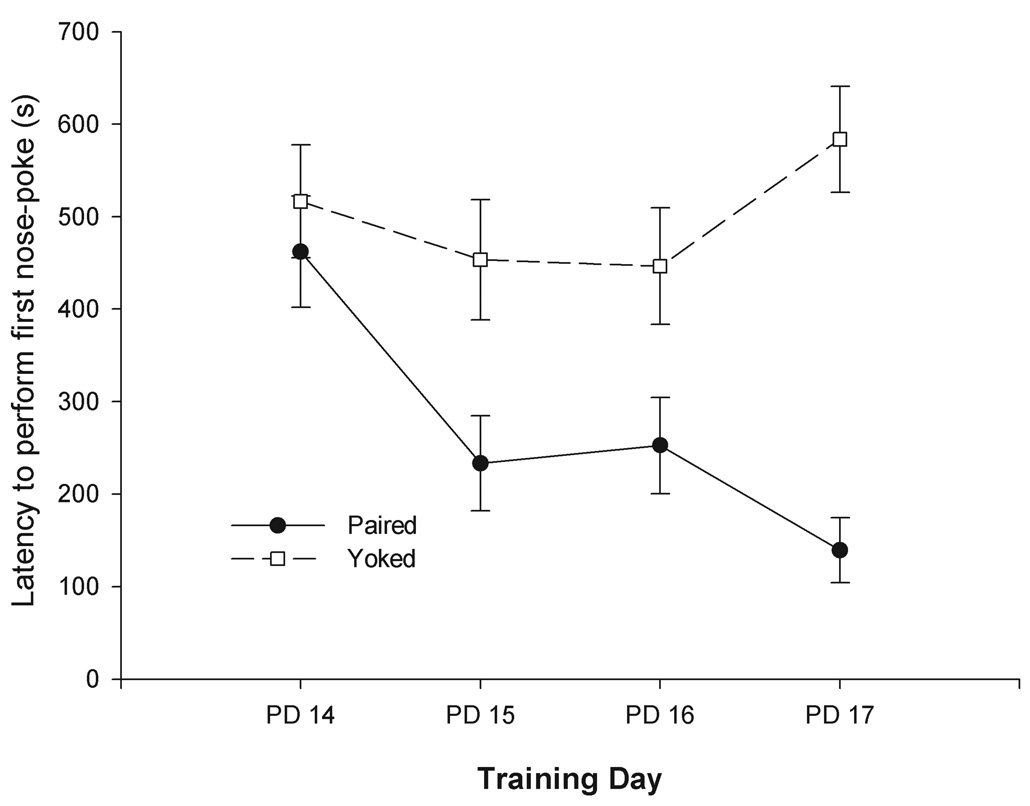
Figure 2.
Latency to perform the first nose-poke behavior (s) as a function of contingency (Paired or Yoked) and training day (PDs 14–17). To facilitate data visualization, data has been collapsed across the nature of the reinforcer (Water or 3.75 % v/v EtOH). The latter factor did not affect latencies nor significantly interacted with the remaining factors. Vertical lines represent standard errors of the mean.
Nose-poke frequency
This variable was significantly affected by the nature of the contingency existing between the target behavior and reinforcement delivery [conditioning main effect: F (1, 32) = 40.59, p < 0.001]. Training days also exerted a significant main effect, F (3, 96) = 12.61, p < 0.001. The following two-way interactions attained significance: reinforcer × day [F (3, 96) = 4.04, p < 0.01] and conditioning × day [F (3, 96) = 17.28, p < 0.001]. Finally, the three-way interaction comprising reinforcer, conditioning and days was found to exert significant effects, F (3, 96) = 2.79, p < 0.05. Newman Keuls post-hoc tests indicated that paired pups exhibited higher frequencies of responding than yoked counterparts, particularly after the first training session. By the end of training (PD 17) both paired groups (reinforced with either water or ethanol) exhibited significantly higher nose-poking frequencies than pertinent yoked controls. Interestingly, paired pups reinforced with 3.75 % v/v ethanol at PD17 also had significantly higher levels of responding than paired pups reinforced with water. Nose-poke frequency was not significantly affected by pre-exposure manipulations. The statistical analysis also indicated that pre-exposure failed to significantly interact with conditioning and day of assessment. Overall nose poke frequency can be observed in Figure 1. Figure 1B shows the significant three-way interaction comprising contingency, nature of the reinforcer and training day.
Latency to perform the first nose-poke behavior
The statistical analysis of this variable also confirmed the emergence of associative learning. The ANOVA revealed significant main effects of conditioning and day of training [F(1, 32) = 24.54 and F(3, 96) = 3.24, both p’s < 0.05; respectively] as well as a significant interaction between these factors [F(3, 96) = 4.73, p< 0.05]. Pre-exposure treatment did not exert a significant main effect upon latency to perform the first nose-poke and did not significantly interact with the remaining variables. Post-hoc tests showed that yoked pups exhibited similar latencies across sessions. That was not the case in paired pups. After the first training session these pups exhibited a sharp decrease in latency to execute the first nose-poke. Furthermore, latencies of paired and yoked controls were significantly different during the last three training sessions.
Blood ethanol Concentrations
BECs corresponding to the pre-exposure phase of the experiment were found to differ significantly as a function of number of trials. As could be expected, BECs after two intraoral infusion trials were significantly higher than those encountered after a single trial (t = 5.46; df = 20; p < 0.001). BECs during the pre-exposure phase of this experiment were: One trial, 69.4 +/− 7.2 mg/dl; Two trials, 150.0 +/− 12.9 mg/dl (values represent mean +/− standard error).
The ANOVA for BECs achieved at termination of operant training (PD 17) indicated that neither pre-exposure nor conditioning exerted significant main effects. The interaction between these factors also failed to achieve significance. BEC values across groups ranged between 25 and 48 mg/dl (overall mean +/− SEM: 25.2 +/− 6.6 mg/dl).
The present results indicate that the present experimental preparation is suitable for analysis of early operant conditioning. Infants rapidly learned the association between nose-poking behavior and intraoral delivery of fluids. Both water and a low-concentrated ethanol solution (3.75% v/v) appeared to act as effective reinforcers. Yet, when focusing on the frequency of responding at the end of training, it appears that ethanol was more effective than water in terms of positive reinforcing effects. Ethanol pre-exposure resulted in a pharmacologically relevant amount of the drug in blood (approximately 150 mg/dl after two pre-exposure trials). However, this ethanol pre-exposure did not significantly affect later ethanol- or water-mediated operant responding.
4. Experiment 2
The present experiment was executed to replicate what probably constitutes the most relevant result of Experiment 1, i.e., ethanol’s capability to serve as a reinforcer in an operant task. In Experiment 1 pre-exposure did not exert a significant influence upon operant conditioning. However, familiarization with ethanol’s sensory cues has been shown to facilitate, under certain circumstances, appetitive learning induced by intraoral delivery of ethanol (Pautassi et al., 2008a). Hence, in Experiment 2 we utilized only a brief non-reinforced exposure to ethanol prior to operant training. By maintaining a pre-exposure phase we minimized procedural changes across experiments. Operant conditioning was defined not only through the use of water or a low-concentrated ethanol solution (3.75% v/v) but also through inclusion of a higher ethanol concentration (7.5 % v/v). This latter concentration was the same as that during pre-exposure. A second goal, and perhaps the most important feature of Experiment 2, was to examine the effects of the contingency between operant behavior and these reinforcers upon adolescent affinity for ethanol ingestion. Early exposure to ethanol’s sensory or postabsorptive effects has been found to modulate later acceptance of the drug (Bannoura et al., 1998; Molina et al., 1986; Pepino et al., 2004; Ponce et al., 2004). Consumption patterns during adolescence (PDs 33–36) were assessed by means of voluntary intake tests.
4.1. Methods
4.1.1. Subjects and Procedures
Sixty infants representative of 8 litters were employed. These animals were randomly assigned to one of six groups defined by conditioning procedure (paired or yoked) and reinforcer (water, 3.75 or 7.5 % v/v ethanol). All groups were composed of 10 infants. On PD 13, pups were pre-exposed to intraoral infusions of a 7.5% v/v ethanol solution, following procedures described in section 2.1.3. Operant training procedures occurred during PD14 –17 (one daily 20-min session, see General Methods). Following termination of operant training, pups were housed with their mothers until PD 21, when they were weaned. Weanlings of the same sex were then housed in groups of 4 in standard maternity cages partially filled with wood shavings and continuous access to lab chow and water. During PDs 33–36, subjects were assessed in terms of voluntary ethanol intake as described in General Methods and prior studies (Ponce et al., 2004; Pepino et al., 2004). Due to technical problems 4 pairs of paired and yoked animals were not tested during adolescence. Two of these pairs were originally reinforced with water while the remaining two pairs were given either 3.75 or 7.5% v/v ethanol during operant training.
4.2. Results
Preliminary considerations
The results derived from Experiment 2 are depicted in Figure 3 (nose poke frequency), Figure 4 (latency to execute the first target operant behavior), Figure 5 (absolute and percent ethanol intake in adolescence, PD33–36)) and Figure 6 (maximal absolute ethanol intake). Nose poke frequency and latency to perform the first nose-poke were analyzed by means of three-way mixed ANOVAs. In these analyses, reinforcer (3.75 ethanol, 7.5% v/v ethanol or water) was the between-group variable whereas conditioning (paired or yoked) and training day (sessions 1–4, PD14–17) were within factors. Similar 3 × 2 × 4 ANOVAs (reinforcer × conditioning × day) were employed to analyze the dependent variables related to ethanol intake patterns during adolescence.
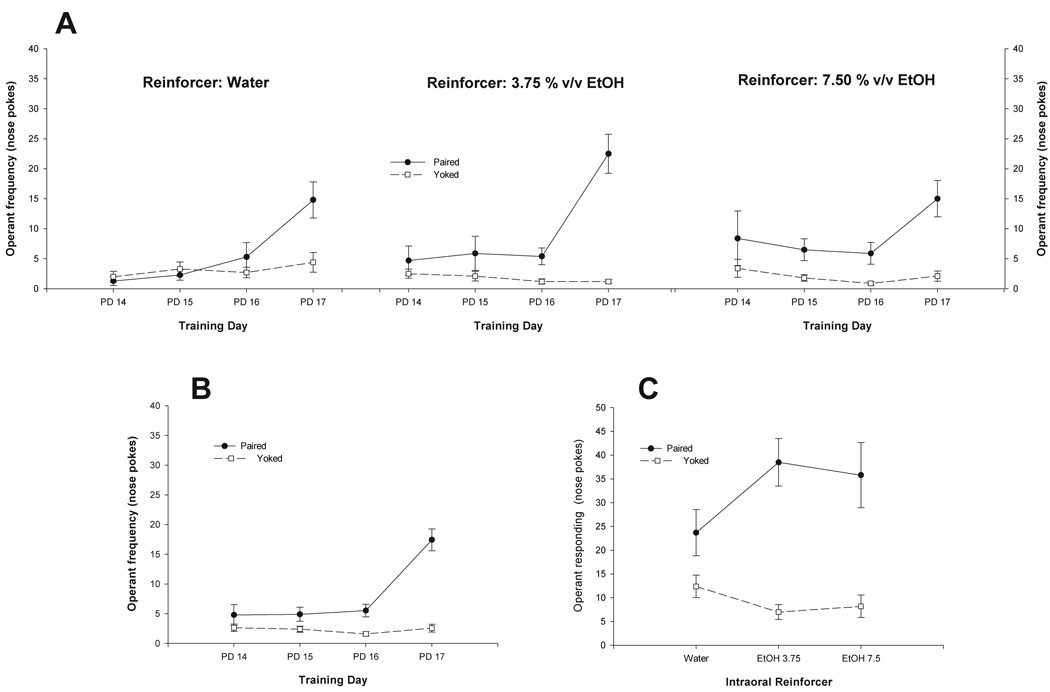
Figure 3.
A) Mean number of infantile nose-pokes as a function of reinforcer (Water, 3.75 or 7.5% v/v Ethanol), contingency between behavioral emission and intraoral reinforcement delivery (Paired or Yoked) and training day (Postnatal days 14–17, PD 14–17). B) Significant interaction comprising Contingency and training day. C) Significant interaction comprising Contingency and Reinforcer. In all figures vertical lines represent standard errors of the mean.
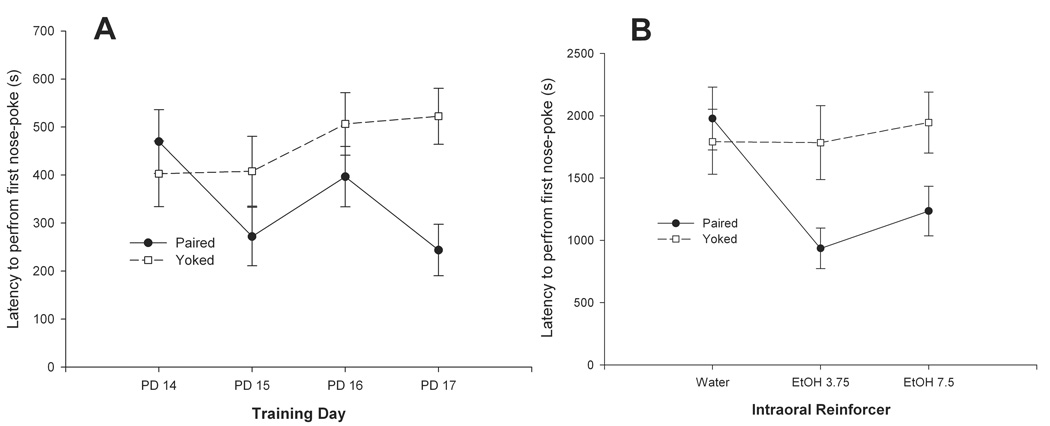
Figure 4.
A) Significant interaction between contingency (Paired or Yoked) and training day (PDs 14–17) affecting latency (s) to perform the first nose-poke behavior. B) Significant interaction between Contingency and Reinforcer affecting latency. In both figures vertical lines represent standard errors of the mean.
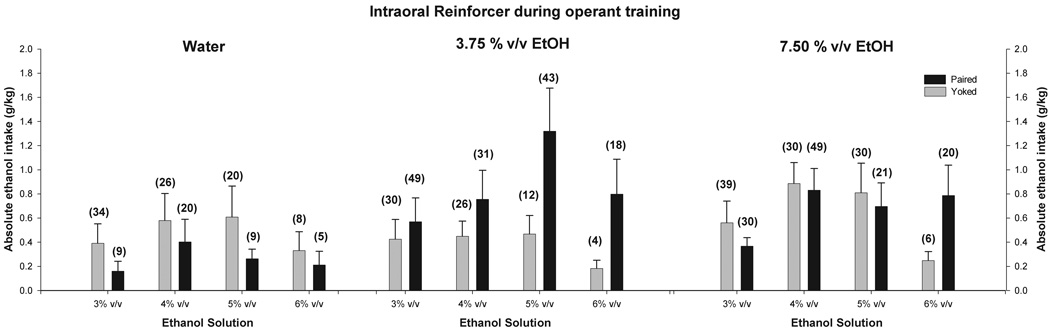
Figure 5.
Absolute ethanol intake (g/kg) during adolescence as a function of the ethanol solution available in each test session (3, 4, 5 and 6% v/v) and infantile operant training defined by contingency (Paired or Yoked) and reinforcer (Water, 3.75 or 7.5% v/v Ethanol). Numbers between parentheses illustrate mean percent ethanol preference scores. Vertical lines represent standard errors of the mean.
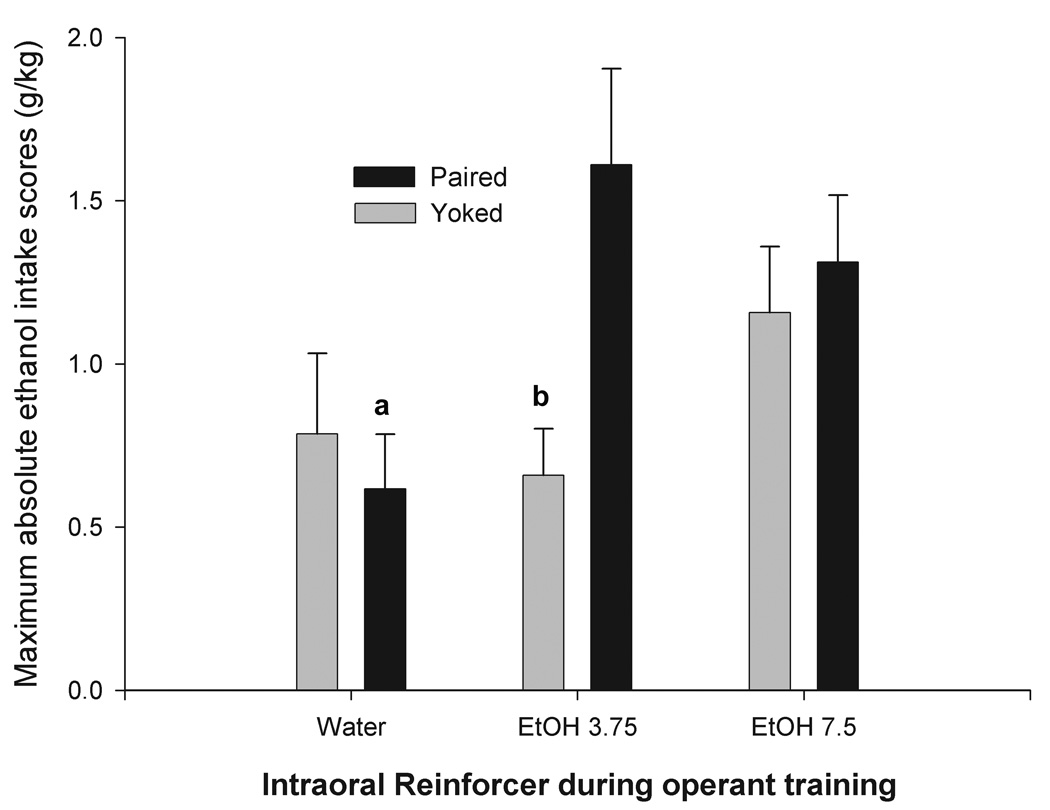
Figure 6.
Maximum absolute ethanol intake scores (g/kg) as measured during assessment days as a function of prior infantile operant training defined by contingency (Paired or Yoked) and reinforcer (Water, 3.75 or 7.5% v/v Ethanol). Letters above the bars indicate a significant difference between a given group and the P-3.75 group. Vertical lines represent standard errors of the mean.
Nose poke frequency
As can be observed in Figure 3A, P pups had higher levels of responding than did Y controls. This difference is particularly noticeable at the end of training (PD17). Figure 3A also indicates higher frequency of responding in paired pups reinforced with ethanol than in yoked controls. The ANOVA confirmed these impressions. Significant main effects of conditioning and training day were found: F(1, 27) = 62.08 and F(3, 81) = 14.66, both p’s < 0.001. The following two-way interactions also achieved significance: conditioning × reinforcer [F(2, 27) = 4.31, p < 0.05] and conditioning × day [F(3, 81) = 19.74, p < 0.001]. Newman Keuls’ post-hoc tests indicated that regardless of the nature of the reinforcer, paired pups during the third and fourth training day exhibited significantly higher rates of nose poking than did Yoked controls. This difference approached significance during the second training day. In addition, post-hoc tests showed that across training days, Paired pups reinforced with either ethanol solution (3.75 or 7.5% v/v) had higher levels of operant responding than did Paired infants reinforced with water. The significant interactions indicated by the ANOVA are also illustrated in Figure 3B and 3C.
Latency to perform the first nose-poke
The corresponding ANOVA revealed a significant main effect of conditioning [F (1, 27) = 6.37, p < 0.025] and of the interaction comprising conditioning and training day [F (3, 81) = 2.97, p < 0.05]. The interaction between conditioning and reinforcer approached significance [F(2, 27) = 3.20, p = 0.056]. Further examination of the data by means of post-hoc tests indicated that, at PD 17, paired pups exhibited significantly lower latency to first nose-poke than Y controls (see Figure 4A). Furthermore, across days, paired animals trained with 3.75 or 7.5 % v/v ethanol showed lower latencies than did P pups reinforced with only vehicle. P pups trained with ethanol (3.75 or 7.5 %) also exhibited lower latencies than any of the Yoked conditions (Figure 4B).
Adolescent ethanol intake
Overall liquid intake (ml/100g) was found to progressively and significantly increase during the course of the testing procedure, F(3, 66) = 56.30, p < 0.001. The ANOVA for water consumption scores (ml) indicated a significant main effect of day [F(3, 66) = 39.40, p < 0.005] as well as a significant interaction between infantile conditioning and reinforcer available during operant training [F(2, 22) = 7.94, p < 0.001]. Post-hoc analyses revealed similar water consumption scores in yoked control pups regardless of the solution ingested in their tests at PD14–17. On the other hand, paired animals that had nose-poked for water during infancy consumed more water as adolescents than did counterparts trained with ethanol (either 3.75 or 7.5 % v/v). Overall intake values (ml/100g) and water consumption scores can be observed in Table 1 and Table 2, respectively.
Table 1.
Overall intake values (ml/100g) during adolescence as a function of day of intake assessment (PDs 33, 34, 35 and 36) and nature of the contigency during infantile operant training (Paired or Yoked). Values represent mean +/− SEM.
| Day of Intake Assessment | ||||
|---|---|---|---|---|
| Conditioning Treatment | Postnatal day 33 | Postnatal day 34 | Postnatal day 35 | Postnatal day 36 |
| Paired | 6.00 +/− 0.32 | 7.60 +/− 0.30 | 8.68 +/− 0.32 | 9.32 +/− 0.42 |
| Yoked | 5.79 +/− 0.32 | 7.62 +/− 0.32 | 8.60 +/− 0.35 | 9.78 +/− 0.46 |
Table 2.
Water intake (ml) during adolescence as a function of day of intake assessment (PDs 33, 34, 35 and 36) and prior infantile operant training defined by contingency (Paired or Yoked) and reinforcer (Water, 3.75 or 7.5% v/v Ethanol). Values represent mean +/−SEM.
| Day of Intake Assessment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reinforcer at Infancy | Postnatal day 33 | Postnatal day 34 | Postnatal day 35 | Postnatal day 36 | ||||
| Paired | Yoked | Paired | Yoked | Paired | Yoked | Paired | Yoked | |
| Ethanol (3.75 % v/v) | 2.50 +/− 0.69 | 3.03 +/− 0.57 | 4.00 +/− 0.61 | 4.55 +/− 0.45 | 4.15 +/− 1.03 | 5.93 +/− 0.45 | 4.95 +/− 0.47 | 7.22 +/− 0.60 |
| Ethanol (7. 5 % v/v) | 3.15 +/− 0.46 | 2.87 +/− 0.56 | 3.27 +/− 0.65 | 3.42 +/− 0.67 | 5.09 +/− 0.40 | 4.80 +/− 0.83 | 5.69 +/− 0.75 | 7.38 +/− 0.61 |
| Water | 4.82 +/− 0.38 | 2.55 +/− 0.60 | 5.25 +/− 0.82 | 4.18 +/− 0.66 | 6.05 +/− 0.39 | 4.70 +/− 0.56 | 7.63 +/− 0.44 | 5.97 +/− 0.35 |
In terms of absolute ethanol ingestion (g/kg) the ANOVA showed a significant main effect of day [F(3, 66) = 5.02, p < 0.005] and a significant interaction between infantile conditioning and nature of the reinforcer during operant training [F(2, 22) = 3.60, p < 0.05]. Post-hoc tests indicated that absolute ethanol consumption was significantly higher for 4 and 5% v/v ethanol than for the lowest and highest ethanol concentrations (3 and 6% v/v). These tests also indicated that the nature of the experience during infancy affected ethanol intake: adolescents that had learned to gain access to 3.75% v/v ethanol through emission of operant behaviors (P rats) ingested more ethanol than counterparts previously reinforced with water. Post-hoc tests also revealed a significant difference between the former group and its corresponding yoked control group. Paired animals originally reinforced with 7.5% v/v ethanol exhibited intermediate ethanol intake values relative to pups reinforced with the lower ethanol concentration or with water. These latter differences did not achieve significance. Figure 5 depicts these results as well as percent ethanol preference scores, which yielded very similar profiles. Percent ethanol preference scores are indicated in Figure 5 by the numbers between parentheses above each bar.
When ethanol and water intake patterns are considered together, it seems evident that operant training at infancy affected voluntary intake in adolescence. Paired animals reinforced with ethanol in infancy showed greater consumption of ethanol later in life than those reinforced with water. On the other hand, pups that had learned to nose-poke for water showed greater predisposition to ingest water at adolescence than animals previously trained with ethanol as the reinforcer. The effect of the operant training with ethanol upon adolescent ethanol intake can be seen more clearly in terms of maximum daily intake of absolute ethanol intake, i.e., the highest level of ethanol intake (g/kg) achieved by each animal across tests. The pertinent ANOVA for this dependent variable indicated a significant interaction between prior operant training and reinforcement, F(2, 22) = 3.65, p < 0.05. In this case, post-hoc comparisons indicated that P pups reinforced with 3.75% v/v ethanol had higher levels of ethanol ingestion in adolescence than did P pups reinforced with water or Y controls given 3.75% v/v ethanol (Figure 6).
In summary, frequency of nose-pokes and latency to perform this target response indicated that both water and ethanol (3.75 or 7.5 % v/v) served as effective reinforcers in the present operant task. Ethanol was a more effective reinforcer than water. Infantile operant learning with ethanol affected ethanol intake at adolescence. Animals that had originally learned to respond for 3.75% v/v ethanol drank more ethanol in adolescence than did animals given similar experience with this ethanol concentration but unrelated with their behavior.
General Discussion
The common denominator between the two current experiments was the observation that without the use of extensive initiation procedures, infants learn to gain access to intraoral delivery of ethanol. In both experiments it was clear that, after three training trials, pups given the explicit association between nose-poke behavior and ethanol exhibited greater frequency of responding than infants reinforced with water or controls given similar infusions of 3.75% ethanol but unrelated to their behavioral performance. Operant conditioning was also seen with a higher ethanol concentration (7.5% v/v). There were no statistically significant indications that ethanol pre-exposure facilitated ethanol-mediated operant responding.
It was also observed that infantile learning mediated by ethanol had a significant impact on intake of ethanol in adolescence. Specifically, pups trained to obtain a low-ethanol concentration (3.75% v/v) subsequently consumed more ethanol than controls reinforced with water or controls given similar infantile ethanol exposure independent of their behavior.
The present test of operant conditioning revealed ethanol’s reinforcing effects in two-week old pups when intraoral administration of the drug was made contingent upon a specific behavior, in this case nose-poking. Previous studies conducted with a similar age group have suggested that the sensory features of ethanol act as a barrier precluding substantial responding for the drug (Domínguez et al., 1993; Pautassi et al., 2008b). These studies have common denominators. First, infants were reinforced with a low volume of ethanol following execution of each target behavior (3–5 µl of 5–6% v/v ethanol, with milk or water as vehicles). Second, in both Domínguez et al. (1993) and Pautassi et al. (2008b), the probability of the target response was relatively high from the beginning of training. These two factors probably provide optimal contiguity between the response under analysis and the sensory attributes of the drug. Under similar experimental circumstances pups seem to encode these chemosensory properties as aversive (Pautassi et al., 2008a).
Previous studies of ethanol ingestion or sensitivity to the drug’s motivational effects during early ontogeny suggest that rapid accumulation of ethanol in blood or brain is conducive to high levels of ethanol ingestion or preference for stimuli that predict ethanol’s postabsorptive effects (e.g. see: Molina et al., 2006; 2007b; Nizhnikov et al. 2006; Pautassi et al., 2008a; Sanders & Spear 2007). In other words, and in accordance with recent studies conducted in adult rats (Fidler et al., 2006; Kiefer et al., 2005; Samson et al., 2003), it appears that acceptance of ethanol requires a balance between the sensory and postabsorptive effects of the drug. In the present study, infants gradually learned the contingency between a low-probability target behavior and relatively low-concentrated ethanol solutions (3.75 or 7.5% v/v). Furthermore, the volume of ethanol provided after each target behavior (25 µl) was higher than previously employed by Domínguez et al. (1993) and Pautassi et al. (2008b). When taking these considerations into account it appears that operant conditioning in the present study was successful due to the relatively low sensory intensity of the reinforcer and the progressive exposure to ethanol’s postabsorptive effects. Further studies will be required to dissect the specific weights of these factors or the interaction between them. Despite this need for experimental clarification, it should be noted that a recent study conducted in our laboratory using neonatal rats also indicated that low concentrations of ethanol and rapid accumulation of ethanol in blood facilitate the establishment of operant conditioning (Bordner et al., 2008).
In Experiment 2, animals trained to nose-poke for ethanol (3.75 or 7.5 % v/v) exhibited lower latency to perform the target behavior than either yoked controls or paired animals reinforced with water. Yet, in Experiment 1 all paired pups showed lower latencies than control animals. In other words, in Experiment 1 latency did not differentiate the reinforcing capability of ethanol and water, although overall frequency of responding did. These findings may indicate that, in the context of the present experimental preparation, latency to perform the first target behavior is a less sensitive or reliable dependent variable than frequency of operant behavior.
At the end of the training phase, operant self-administration of ethanol resulted in BECs of 25 – 48 mg/dl. These BECs have been found in previous studies to exert pharmacologically significant effects during early development. Indeed, preweanlings appear to be highly sensitive to the positive reinforcing effects of such low doses of ethanol. For instance, neonate rats express ethanol-mediated appetitive learning as revealed by enhanced attachment to a surrogate nipple (conditioned stimulus, CS) following explicit pairings of the nipple and postabsorptive effects of low ethanol doses (e.g. 0.25 g/kg; Petrov et al., 2003; Cheslock et al., 2001). In a follow up study, neonates were given central administration of ethanol while exposed to a distinctive olfactory conditioned stimulus (lemon odor, CS). Central doses as low as 25 mg % promoted subsequent conditioned preference to the CS (Nizhnikov et al., 2006); 2007. Evidence for reinforcement effects of low ethanol doses also has been found during the second postnatal week (Pautassi et al., 2006); 2007. Specifically, ethanol doses of 17–60 mg% BECs are capable of devaluing a US consisting of an innate aversive tastant (citric acid), an effect probably mediated by appetitive or anti-anxiety effects of the drug. In a more recent study (Pautassi et al., 2008a), fourteen-day old rats with or without a history of ethanol exposure were given i.o. ethanol resulting in doses between 0.33 and 0.66 g/kg. Soon after ingestion of this ethanol, the pups were exposed to a salient texture (sandpaper, CS) in combination with the postabsorptive effects of the ethanol. Subsequently the previously ethanol-experienced animals given pairings of sandpaper and postabsorptive ethanol exhibited heightened preference for sandpaper. Finally, reinforcement effects associated with low blood ethanol concentrations (20 – 70 mg/dl) have been also found in infant rats in terms of second-order conditioning procedures (Molina et al., 2006); 2007b. Taken together, these studies indicate that infancy in the rat is characterized by high sensitivity to the motivational effects of low doses of ethanol. Within this framework, the present experimental strategy seems useful for analysis of the reinforcement effects of low-to-moderate ethanol doses.
EtOH enhances GABA transmission and also interacts with other neurotransmitter systems such as glutamate and dopamine to influence ethanol reinforcement (Gonzalez & Jaworski, 1997; Manto et al., 2005). Levels of dopamine in the CNS increase under the effects of ethanol. Dopamine is a neurotransmitter known to be involved in appetitive reinforcement (Diana et al., 1993). Adult rats will learn to press a lever in order to receive ethanol directly into the posterior ventral tegmental area (VTA), a region rich in dopaminergic neurons (Gatto et al., 1994). Furthermore, ethanol-mediated operant responding is associated with activation of dopaminergic neurons in the VTA (Rodd et al., 2005). These studies indicate that ethanol activation of VTA dopaminergic neurons might underlie the reinforcing capabilities of ethanol found in the present set of experiments.
In Experiment 2, there were clear indications that the nature of ethanol-mediated learning during infancy had an impact upon later ethanol ingestion. During adolescence, heightened voluntary ethanol consumption was encountered in subjects that previously experienced the explicit contingency between their behavior and intraoral delivery of low-concentrated ethanol (3.75% v/v). There are numerous preclinical and epidemiological reports linking early experiences with ethanol and subsequent ethanol intake patterns (for recent reviews see Chotro et al., 2007; Molina et al., 2007a; Spear & Molina, 2005). One likely mechanism underlying this association is a simple familiarization effect: the mere exposure to ethanol’s sensory cues may be sufficient to promote later ethanol acceptance (Bannoura et al., 1998; Molina et al., 1986; Pepino et al., 2004; Ponce et al., 2004; Spear & Molina, 2005). However, the involvement of associative learning mediated by appetitive ethanol reinforcement cannot be disregarded. In the present study familiarization with ethanol is clearly insufficient to explain the present effects of increased ethanol ingestion during adolescence, which occurred only for Paired pups reinforced with 3.75 % v/v ethanol as a consequence of their operant behavior and not if the ethanol were delivered regardless of their behavior. It is possible that these animals not only learn about the sensory features of the drug but also about the association existing between such cues and ethanol’s postabsorptive effects. Hence, this associative memory may be reactivated during the process of voluntary ethanol consumption during adolescence. (for a comprehensive review see Spear & Molina, 2005).
Enhancement of adolescent intake of ethanol by ethanol-mediated operant learning during infancy occurred only when the operant was paired with 3.75% v/v ethanol. This effect did not emerge when 7.5% v/v ethanol served as the reinforcer. It is difficult to determine why this was the case, especially when considering that these solutions seemed to share equivalent reinforcement capabilities during infancy. Examination of ethanol intake patterns during adolescence (see Figure 5 and Figure 6) suggests that pups exposed to the higher ethanol solution (7.5%) did ingest more ethanol than those originally reinforced with water, although contingency appeared not to be a factor. Perhaps when infants experience relatively concentrated ethanol solutions, familiarity with the drug overrides the contribution of associative mechanisms that affect later ethanol affinity. Also, it may be important to consider that rats in Experiment 2 were water deprived for 22 hours prior to ethanol intake assessments. Liquid deprivation is a stressor (Caplan & Puglisi, 1986). This raises the possibility that ethanol intake in Experiment 2 was driven by anti-anxiety effects of the drug. Empirical evidence for the existence of anxiolytic effects of ethanol in rats of this age has recently been provided (Pautassi et al., 2006); 2007.
It was observed recently that the level of operant learning in the infant rat mediated by bovine milk is positively correlated with subsequent intake of this particular reinforcer (Arias et al., 2007). It was suggested that self-administration processes regulated through operant learning accentuated the inherent value of the reinforcer. In the present study we cannot disregard a similar interpretation. It is also possible that the intake test performed during adolescence involves operant processes acting on the contingency between emission of particular behaviors (e.g., licking) and ingestion of ethanol. Under this assumption, animals that had originally learned to operate for the drug during infancy might be more likely to engage in self-administration of the drug during adolescence. Nevertheless, it is necessary to temper this speculation by the fact that numerous reports indicate a dissociation between ethanol consummatory processes and learning regulated by the drug’s reinforcing effects (e.g. see Files et al., 1997; Ritz et al., 1994; Samson et al., 1998). Specifically, these reports indicate that level of ethanol consumption in two-bottle choice tests similar to the one employed in the present work do not necessarily predict performance in behavioral tasks indicative of ethanol’s motivational properties (i.e., conditioned place preference or operant self-administration), although a recent review has reached a different conclusion (Green & Grahame, 2008).
Without the use of extensive initiation procedures it has been difficult to demonstrate ethanol’s reinforcing capabilities in genetically heterogeneous adult rats, particularly when employing the oral route of administration (Fidler et al., 2006; Samson, 1986). This seems not to be the case very early in postnatal ontogeny (e.g., Nizhnikov et al. 2006; Pautassi et al., 2008a; Spear & Molina, 2005). The present study in conjunction with that of Bordner et al. (2008) with newborn rats appears to strengthen the notion that the developing rat is highly sensitive to appetitive motivational effects of ethanol, as demonstrated through rapid and robust instrumental learning. The memory of such early learning experiences seems sufficient to significantly determine or modulate patterns of voluntary ethanol consumption during adolescence. This set of results endorses the hypothesis that early onset of ethanol-related experiences markedly affects the predisposition of the organism to later use or abuse the drug and indicates that associative learning mechanisms may play a crucial role in the establishment of such predisposition.
Acknowledgements
This work was supported by Supported by grants from the NIAAA (AA11960, AA013098, AA15992) and the NIMH (MH035219) to NES and the Agencia Nacional de Promocion Cientifica y Tecnologica, Argentina, to JCM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arias C, Chotro MG. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharm Biochem Behav. 2005;82:434–442. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Arias C, Spear NE, Molina JC, Molina A, Molina JC. Rapid acquisition of operant conditioning in 5-day-old rat pups: a new technique articulating suckling-related motor activity and milk reinforcement. Dev Psychobiol. 2007;49:576–588. doi: 10.1002/dev.20236. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Kiefer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk LM, Mennella JA. Chemosensory factors influencing alcohol perception, preferences, and consumption. Alcohol Clin Exp Res. 2003;27:220–231. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannoura MD, Kraebel KS, Spear LP, Spear NE. Effects of preweanling ethanol odor exposure on ethanol preference. Alcohol. 1998;15:213–217. doi: 10.1016/s0741-8329(97)00122-5. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kuka P, Kowstowski W. Conditioned place preference after prolonged preexposure to ethanol. Polish J Pharmachol. 1995;47:185–187. [PubMed] [Google Scholar]
- Bordner KA, Molina JC, Spear NE. Analysis of ethanol reinforcement in 1-day-old rats: Assessment through a brief and novel operant procedure. Alcohol Clin Exp Res. 2008;32:580–592. doi: 10.1111/j.1530-0277.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Caplan MA, Puglisi K. Stress and conflict conditions leading to and maintaining voluntary alcohol consumption in rats. Pharmacol Biochem Behav. 1986;24:271–280. doi: 10.1016/0091-3057(86)90350-3. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Silveri MM, Spear LP, Spear NE. Ethanol as a reinforcer in the newborn's first suckling experience. Alcohol Clin Exp Res. 2001;25:391–402. [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci Biobehav Rev. 2007;31:181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Quitadamo E, Massi M. Ethanol induces conditioned place preference in genetically selected alcohol-preferring rats. Psychopharmacology. 1999a;141:235–241. doi: 10.1007/s002130050830. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Chhada M, Perfumi M, Froldi R, Massi M. Conditioned taste aversion induced by ethanol in alcohol-preferring rats: Influence of the method of ethanol administration. Pharmacol Biochem Behav. 1999b;64:563–566. doi: 10.1016/s0091-3057(99)00104-5. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol's motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- Diana M, Rossetti ZL, Gessa G. Rewarding and aversive effects of ethanol: interplay of GABA, glutamate and dopamine. Alcohol Alcohol Suppl. 1993;2:315–319. [PubMed] [Google Scholar]
- Domínguez HD, Bocco G, Chotro MG, Spear NE, Molina JC. Operant responding controlled by milk or milk contaminated with alcohol as positive reinforcers in infant rats. Pharmacol Biochem Behav. 1993;44:403–409. doi: 10.1016/0091-3057(93)90482-9. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcohol Clin Exp Res. 2006;30:414–428. doi: 10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- Files FJ, Denning CE, Hyytia P, Kiianmaa K, Samson HH. Ethanol-reinforced responding by AA and ANA rats following the sucrose-substitution initiation procedure. Alcohol Clin Exp Res. 1997;21:749–753. [PubMed] [Google Scholar]
- Gonzalez RA, Jaworski JN. Acohol and Glutamate. Alcohol Res Health. 1997;21:120–127. [PMC free article] [PubMed] [Google Scholar]
- Gatto GJ, Mcbride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. Commission on Life Sciences. [Google Scholar]
- Kiefer SW, Lawrence GJ, Metzler CW. Alcohol preference in rats lacking gustatory neocortex. Alcohol. 1987;4:37–43. doi: 10.1016/0741-8329(87)90058-9. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Dopp JM. Taste reactivity to alcohol in rats. Behav Neurosci. 1989;103:1318–1326. doi: 10.1037//0735-7044.103.6.1318. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Hill KG, Coonfield DL, Ferraro FA. Ethanol familiarity and naltrexone treatment affect ethanol responses in rats. Alcohol. 2005;37:167–172. doi: 10.1016/j.alcohol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Manto M, Laute MA, Pandolfo M. Depression of extracellular GABA and increase of NDMA-induced nitric oxide following acute intra-nucelar administration of alcohol in the cerebellar nuclei of the rat. The Cerebellum. 2005;4:230–238. doi: 10.1080/14734220500243835. [DOI] [PubMed] [Google Scholar]
- Meisch RA, Thompson T. Rapid establishment of ethanol as a reinforcer for rats. Psychopharmacologia. 1974;37:311–321. doi: 10.1007/BF00428917. [DOI] [PubMed] [Google Scholar]
- Molina JC, Serwatka J, Spear NE. Alcohol drinking patterns of young adult rats as a function of infantile aversive experiences with alcohol odor. Behav Neural Biol. 1986;46:257–271. doi: 10.1016/s0163-1047(86)90191-3. [DOI] [PubMed] [Google Scholar]
- Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol's motivational effects: Ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30:1506–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- Molina JC, Spear NE, Spear LP, Mennella JA, Lewis MJ. The International Society for Developmental Psychobiology 39th Annual Meeting Symposium: Alcohol and development: Beyond fetal alcohol syndrome. Dev Psychobiol. 2007a;49:227–242. doi: 10.1002/dev.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007b;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Molina JC, Spear NE. Central reinforcing effects of ethanol are blocked by catalase inhibition. Alcohol. 2007;41:525–534. doi: 10.1016/j.alcohol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing properties of ethanol in neonatal rats: Involvement of the opioid system. Behav Neurosci. 2006;120:267–280. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Godoy JC, Spear NE, Molina JC. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002;26:644–654. [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov M, Molina JC, Boehm SL, Spear N. Differential effects of ethanol and midazolam upon the devaluation of an aversive memory in infant rats. Alcohol. 2007;41:421–431. doi: 10.1016/j.alcohol.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Molina JC, Spear N. Infant rats exhibit aversive learning mediated by ethanol's orosensory effects but are positively reinforced by ethanol's post-ingestive effects. Pharmacol Biochem Behav. 2008a;88:393–402. doi: 10.1016/j.pbb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Sanders S, Miller S, Spear NE, Molina JC. Early ethanol's anxiolytic effects assessed through an unconditional stimulus revaluation procedure. Alcohol Clin Exp Res. 2006;30:448–459. doi: 10.1111/j.1530-0277.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Truxell E, Molina JC, Spear N. Motivational effects of intraorally-infused ethanol in rat pups in an operant self-administration task. Physiol Behav. 2008b;93:118–129. doi: 10.1016/j.physbeh.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Melloni C, Ponce LF, Molina JC. Acute ethanol counteracts the acquisition of aversive olfactory learning in infant rats. Alcohol. 2005;36:99–105. doi: 10.1016/j.alcohol.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Peana AT, Enrico P, Assaretti AR, Pulighe E, Muggironi G, Nieddu M, Piga A, Lintas A, Diana M. Key role of ethanol-derived acetaldehyde in the motivational properties induced by intragastric ethanol: a conditioned place preference study in the rat. Alcohol Clin Exp Res. 2007;32:249–258. doi: 10.1111/j.1530-0277.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Abate P, Spear NE, Molina JC. Heightened ethanol intake in infant and adolescent rats after nursing experiences with an ethanol-intoxicated dam. Alcohol Clin Exp Res. 2004;28:895–905. doi: 10.1097/01.alc.0000128223.95184.c9. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin Exp Res. 2003;27:1583–1591. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Ethanol reinforcing properties during infancy in the rat: operant self-administration of the drug (Abstract) Alcohol Clin Exp Res. 2006;30 Supplement to June:186A. [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Nursing from an ethanol-intoxicated dam induces short- and long-term disruptions in motor performance and enhances later self-administration of the drug. Alcohol Clin Exp Res. 2004;28:1039–1050. doi: 10.1097/01.alc.0000131298.32045.96. [DOI] [PubMed] [Google Scholar]
- Reid LD, Hunter GA, Beaman CM, Hubbell CL. Toward understanding ethanols capacity to be reinforcing - a conditioned place preference following injections of ethanol. Pharm Biochem Behav. 1985;22:483–487. doi: 10.1016/0091-3057(85)90051-6. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Garcia JM, Protz D, George FR. Operant ethanol-reinforced behavior in P, NP, HAD, and LAD rats bred for high versus low ethanol preference. Alcohol Clin Exp Res. 1994;18:1406–1415. doi: 10.1111/j.1530-0277.1994.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of cocaine within the posterior ventral tegmental area of Wistar rats: evidence for involvement of serotonin-3 receptors and dopamine neurons. J Pharmacol Exp Ther. 2005;313:134–145. doi: 10.1124/jpet.104.075952. [DOI] [PubMed] [Google Scholar]
- Samson HH. initiation of ethanol reinforcement using a sucrose-substitution procedure in food-sated and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Pfeffer AO, Tolliver GA. oral ethanol self-administration in rats - models of alcohol-seeking behavior. Alcohol Clin Exp Res. 1988;12:591–598. doi: 10.1111/j.1530-0277.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: A measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22:1783–1787. [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Chappell A, Legg B. Measuring the appetitive strength of ethanol: use of an extinction trial procedure. Alcohol. 2003;31:77–86. doi: 10.1016/j.alcohol.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: Rodent models. Internat Review Neurobiol. 2003;54:109–145. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31:1148–1158. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Spear LP, Specht SM, Kirstein CL, Kuhn CM. Anterior and posterior, but not cheek, intraoral cannulation procedures elevate serum corticosterone levels in neonatal rat pups. Dev Psychobiol. 1989;22:401–411. doi: 10.1002/dev.420220407. [DOI] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Molina JC, Spear NE, Youngentob LM. The effect of gestational ethanol exposure on voluntary ethanol intake in early postnatal and adult rats. Behav Neurosci. 2007;121:1306–1315. doi: 10.1037/0735-7044.121.6.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]