Abstract
Photodynamic therapy (PDT) involves the administration of photosensitizer followed by local illumination with visible light of specific wavelength(s). In the presence of oxygen molecules, the light illumination of photosensitizer can lead to a series of photochemical reactions and consequently the generation of cytotoxic species. The quantity and location of PDT-induced cytotoxic species determine the nature and consequence of PDT. Much progress has been seen in both basic research and clinical application in recent years. Although the majority of approved PDT clinical protocols have primarily been used for the treatment of superficial lesions of both malignant and non-malignant diseases, interstitial PDT for the ablation of deep-seated solid tumors are now being investigated worldwide. The complexity of the geometry and non-homogeneity of solid tumor pose a great challenge on the implementation of minimally invasive interstitial PDT and the estimation of PDT dosimetry. This review will discuss the recent progress and technical challenges of various forms of interstitial PDT for the treatment of parenchymal and/or stromal tissues of solid tumors.
Keywords: photodynamic therapy, interstitial, dosimetry, solid tumor
Introduction
A solid tumor is an abnormal mass of body tissue other than blood, bone marrow, or the lymphatic system. It may be benign (not cancerous) or malignant (cancerous). Different types of solid tumors are named by their cell origin, for instance, sarcomas, carcinomas, and lymphomas. Treatment of solid tumors varies based on the type, location and stage of tumor. The term of solid tumor used in this article refers to the malignant parenchymal tumors.
Photodynamic therapy (PDT) is a disease site-specific treatment modality. It involves the local or systemic administration of a photosensitizer (i.e. photosensitizing or photoactive drug) followed by irradiating the targeted lesion site with non-thermal visible light of appropriate wavelength(s) (e.g. 500 – 800 nm). In the presence of molecular oxygen, the light irradiation of photosensitizer and energy transfer can lead to a series of photochemical reactions (i.e. Type I and Type II reaction) and generation of various cytotoxic species (e.g. singlet oxygen 1O2 and other reactive oxygen species, ROS), and consequently induce apoptosis and/or necrosis of targeted lesion. PDT can be performed in various forms in a non-invasive or minimally invasive fashion (1). While the majority of approved PDT protocols treat superficial lesions of skin and luminal organs, such as actinic keratosis and Barrett's esophagus, interstitial and intra-operative approaches have been investigated for the ablation of a broad range of superficial or bulky solid tumors located in the head and neck, brain, breast, lung, gastrointestinal, and genitourinary regions (2-5). The microenvironment and physiology of solid tumors are very different from those of normal tissue. The complexity of the geometry and the non-homogeneous nature of solid tumors still pose great technical challenges on the implementation of minimally invasive interstitial PDT. This review article will discuss recent progress in the technical development of interstitial PDT (IPDT) for treatment of parenchymal and/or stromal tissues of solid tumors.
Photosensitizer
The photosensitizer is considered to be a critical element. A major downside of PDT is the prolonged skin photosensitization after systemic administration of photosensitizer (e.g. Photofrin) and the need for patient avoidance of sunlight for several weeks. For the majority of cancer patients, however, the light avoidance is tolerable. Nonetheless, for localized tumors, there is still a need to explore drug delivery approaches to deliver photosensitizer locally, therefore to enhance therapeutic efficacy, shorten treatment time, and eliminate skin photosensitization completely. It can be predicted that the progress in intratumoral injection techniques, particularly in endoscopic needle injection, will renew the interest in developing truly localized interstitial PDT procedures – i.e. the combination of intratumoral drug injection and light irradiation. Inhalation of photosensitizer has been used to improve fluorescence endoscope examination and might provide a feasible drug delivery route for lung PDT (6,7).
The majority of photosensitizers possess a hetero-cyclic ring structure similar to that of chlorophyll or hematoporphyrin (8). Traditionally, the porphyrins and those photosensitizers developed in the 1970s and early 1980s are called the 1st generation photosensitizers (e.g. Photofrin). Porphyrin derivatives or synthetics of known chemical structures made since the late 1980s are called the 2nd generation photosensitizers (e.g. Foscan, Verteporfin). The length of light avoidance of some 2nd generation photosensitizers has been significantly reduced (e.g. <2 weeks). The 3rd generation photosensitizers generally refer to the modifications such as biologic conjugates (e.g. antibody conjugate, liposome conjugate) and built-in photo quenching or bleaching capability (9). Target-specific PDT uses the antibody- or antisense-conjugated photosensitizers which can combine the specificity to an over-expressed cellular marker with the phototoxic properties of the conjugated PDT photosensitizer. The targeted cellular marker can be a tumor-associated or non-tumor-associated marker. The conjugation may or may not necessarily enhance the internalization process, although the internalization might enhance PDT-induced cytotoxicity.
In general, for solid tumor PDT an ideal photosensitizer should meet at least some of the following criteria:
a commercially available pure chemical,
low dark toxicity but strong photocytotoxicity,
good selectivity towards tumor cells,
longer wavelength allowing deeper light penetration,
rapid removal from the body, and
multiple administration routes (oral, intravenous, intratumoral or inhalational).
These criteria provide a general guideline for comparison. Although some photosensitizers satisfy all of or some of these criteria, there are currently only a few PDT photosensitizers that have received official approval around the world. These include, but are not limited to:
Photofrin (630 nm, Axcan Pharma, Inc.)
Levulan (predrug of protoporphyrin IX; 630 nm, DUSA Pharmaceuticals, Inc.)
Metvix (predrug of protoporphyrin IX; 630 nm, PhotoCure ASA.)
Foscan (652 nm, Biolitec AG)
Laserphyrin (664 nm, Meiji Seika Kaisha, Ltd.)
Visudyne (693 nm, Novartis Pharmaceuticals)
Several 2nd generation photosensitizers (e.g. HPPH - 665 nm, SnET2 - 665 nm, Lu-Tex - 732 nm) have been investigated in many preclinical and clinical trials for various solid tumors (10-12). Although these photosensitizers show the selectivity towards tumor cells and are ideal for cellular-targeting PDT, vascular-targeting PDT is of growing interest.
Vascular-targeting PDT (VTP) is characterized by a very short drug to light interval (DLI), typically 0 – 30 minutes after the completion of IV injection of photosensitizer. The photosensitizer used in this type of PDT should have a fast clearance and therefore might have minimal selectivity toward tumor cells. Under this unique approach, light irradiation takes place while the photosensitizers are still circulating in the vascular compartment and therefore cause vascular damages through low-density lipoprotein receptor-mediated endocytosis pathways and lead to thrombosis and micro-vessel occlusion. Vascular-targeting PDT has been used primarily for the management of the neovascularization lesion (e.g. wet age-related macular degeneration, AMD) and cutaneous capillary malformations (e.g. port wine stain birthmarks, PWS) (13,14). Recently, vascular-targeting PDT-mediated by Pd-bacteriochlorophyll derivatives (e.g. WST09, also known as Tookad, and WST11) has been investigated for the curative or palliative treatment of solid tumors (e.g. prostate cancer) by targeting the tumor vasculature (15-17).
It has been hypothesized that the massive shutdown of pathological and normal vessels can deprive the supply of oxygen and nutrients and subsequently achieve tumor ablation. Antivascular PDT can also bypass the drug resistant nature of solid tumors (18). Although vascular-targeting PDT might change the traditional criteria of photosensitizer selection, longer wavelength and rapid clearance might be the key criteria for designing a photosensitizer for antivascular PDT. Ablating a bulky solid tumor might require a combination strategy, for instance, cellular-targeting plus vascular-targeting or systemic plus local drug delivery (see details in Biological Target).
Most of the approved photosensitizers have fluorescence emission and fluorescence emission spectra overlap with absorption spectra. In vivo measurement of fluorescence spectra, spectrofluorimetry and fluorescence microscopy are commonly used for the quantification of photosensitizer concentration in target tissue. For photosensitizers with weak or no fluorescence (e.g. WST09), diffuse reflectance spectroscopy is a useful tool to measure photosensitizer concentrations in vivo (19,20). Along with the detection of photosensitizer photobleaching during PDT, these measurements can be used for the estimation of PDT dose.
Light Source and Light Delivery
The broad spectra of non-coherent and coherent light sources can be chosen to match the optimal absorption peak of a specific photosensitizer, although the wavelength is a determining factor of the tissue penetration. The choice of a specific light delivery mode (superficial or interstitial) in clinical settings is usually based on the nature and location of the disease. The optimal light dose can be obtained by adjusting the fluence rate and fluence.
The first light sources used in PDT were non-coherent light (e.g. conventional arc lamps). Non-coherent light sources are safer, easy to use and less expensive. They are mainly used for superficial lesions. The most commonly used PDT light sources are lasers. They produce high energy monochromatic light of a specific wavelength with a narrow bandwidth. The laser light can be focused, passed down an optical fiber through a conventional SMA connector and directly delivered to the target site through a specially designed illuminator tip (e.g. microlens, spherical diffuser, cylindrical diffuser or bare fiber). A microlens-tipped frontal illuminator can distribute the incident light uniformly to a flat surface. An isotropic spherical diffuser can be centered in a spherical cavity and deliver light to the cavity surface. A cylindrical diffuser of various lengths (e.g. 1 – 5 cm) can provide a cylindrical pattern of light emission which can be used to superficially irradiate a cylindrical lumen or interstitially irradiate a target tissue (Figure 1). All these light delivery modes can be easily applied during intraoperative PDT.
Figure 1.
Interstitial PDT using a cylindrical diffuser. (A) Implantation of a cylindrical diffuser with 2-cm active length. (B) Cylindrical pattern of light emission. (C) Zone of tumor necrosis.
It has been demonstrated that the longitudinal light emission profile of diffusers of different brand names might be different and therefore, a detailed analysis of the optical characteristics of the diffuser tip and target tissue might be needed for treatment planning of interstitial PDT (21). In intra-operative PDT procedures, light diffusing media and inflatable balloon have been used in conjunction with laser and optical fiber to provide uniform light distribution for treating a cavitary lesion (e.g. brain tumor) (22). A tailored, tapered or side-fire diffuser tip might be suitable for a special need of intraluminal and intratumoral irradiation (23,24). For a complicated anatomical structure and high risk location, a specially designed light applicator might be needed for conformal light delivery (25).
Argon dye, potassium-titanyl-phosphate (KTP) dye, metal vapor lasers and most recently diode lasers have been used for clinical PDT around the world. One advantage of the diode laser is that it can be engineered into a multi-channel unit to meet a highly sophisticated interstitial PDT procedure, which may require multi-channel diode lasers and each independent light output channel to simultaneously provide the light sources of variable power (26).
Two-photon excitation (TPE) of photosensitizer, i.e. the simultaneous absorption of two photons generated from ultrafast laser pulses of high flux in the near-infrared region (750 – 950 nm), has been investigated as an alternative method to improve conventional one-photon excitation PDT. The advantages of TPE PDT include the ability to treat deep-seated lesion and the precision to treat a small area (27,28).
The light emitting diode (LED) is another emerging PDT light applicator. LED can generate high energy light of desired wavelengths and can be assembled in a range of geometries and sizes (29,30). For intra-operative PDT of brain tumor, LED-probe may be arranged in a cylinder tip to fit into a balloon catheter (31), whereas, for interstitial PDT of liver cancer, a thin linear LED array can be implanted into the tumor percutaneously and powered by a battery (32,33).
Typically, achieving the total ablation of a massive solid tumor involves the implantation of multiple diffuser fibers or LED probes. The characterization of light penetration and distribution in solid tumors is important due to the significant inter- and intra-patient differences in the tissue optical properties. Several recent studies suggest that a real-time drug/light dosimetry measurement and feedback system for monitoring and adjusting drug concentration and light distribution during interstitial PDT should be considered (26,34-36). Furthermore, when using purely vascular targeting photosensitizer (e.g. Tookad), the interstitial PDT has a limitation of “treatment time window” since photosensitizer administration to light irradiation has to be carried out as a single session in a short period of time, which requires complicated dosimetry and treatment planning (26).
Tissue Oxygenation
The effectiveness of PDT is determined by many factors. Besides intrinsic target tissue sensitivity to ROS, the other important factors include location and concentration of the photosensitizer, absorption of light energy, and availability of molecular oxygen in the target tissue during light irradiation. The availability of molecular oxygen during irradiation has a profound effect on the treatment outcome. Without oxygen, PDT will have no antitumor effect. It is known that hypoxic tumor cells of solid tumor are generally resistant to PDT. There are two types of hypoxia: (i) pre-existing hypoxic cells, which are a result of tumor physiological development and exist in many solid tumors; (ii) the PDT itself can also induce acute hypoxia due to fast depletion of local oxygen supply. The generation of ROS and PDT efficacy can be affected by intra-tumor oxygen tension (pO2 in cellular-targeting PDT), hemoglobin oxygen saturation (SO2 in vascular-targeting PDT) and microenvironment of solid tumors (37-39). Therefore, monitoring tumor tissue oxygenation during PDT is important in understanding the basic physiological mechanisms and PDT dosimetry.
Tumor pO2 can be measured by oxygen sensitive electrodes, fluorescence quenching-based optical probes, non-invasive phosphorescence real time imaging techniques or other spectroscopic techniques (40). Hypoxia markers and hypoxia inducible factor (e.g. HIF-1a and HIF-2a) and expression of vascular endothelial growth factor (VEGF) have also been used as indicators of hypoxia (41). It has also been reported that positron emission tomography (PET) and magnetic resonance spectroscopy can also detect hypoxic cells in solid tumors (42,43). Intravascular production of ROS is associated with photodynamic consumption of O2 and consequent evolution of paramagnetic deoxyhemoglobin (an endogenous MRI contrast agent). Photosensitized blood oxygenation level-dependent (BOLD) contrast MRI can be used for real-time assessment of antivascular PDT efficacy and prediction of treatment outcomes (44,45).
Several techniques have been proposed to deal with tissue oxygen depletion during PDT, for instance, fractionating light irradiation into controlled light/dark periods or reducing the fluence rate (46-48). These techniques are to promote tissue oxygen re-perfusion to compensate for the oxygen depletion caused by the photochemical reactions. Although limited improvement of tumor response has been reported, there are some disadvantages associated with these techniques. Reducing fluence rate or fractionating a dose only affects oxygen depletion. The pre-existing hypoxic cells are not affected by these techniques. Furthermore, reducing irradiation rate or fractionation also significantly increases the time required for delivery of a specific light dose.
Clinical trials of supplementing hyper-oxygenation during radiation therapy have shown statistically significant enhancement of tumor response when combined with hyperbaric oxygen (HBO) and this has inspired an attempt of PDT-hyperoxygenation combination. For instance, one group performed PDT inside a hyperbaric chamber for various indications (49-52). Although the results are not conclusive, they have shown that hyperoxygenation has extended the survival period in patients with esophageal carcinomas. In comparison to combination therapy of radiotherapy and HBO, PDT combined with HBO poses far fewer technical difficulties. However, HBO requires a pressurized chamber which is not widely available.
Preclinical studies have shown that normobaric hyper-oxygenation is as effective as, if not more than, hyperbaric oxygenation (37,38). Hyperoxygenation can oxygenate pre-existing hypoxic cells to make them more susceptible to PDT treatment and compensate for oxygen depletion due to PDT photochemical reactions by boosting the oxygen availability. It has been shown that above certain levels of oxygen availability there is little gain in the PDT cell killing effect. However, the gain in the technical simplicity and statistically equivalency in the biological improvement clearly indicates that normobaric hyperoxygenation is a more feasible modality to be combined with PDT than HBO. Although certain PDT procedures (e.g. intra-operative PDT) are performed under normobaric hyperoxygenation (e.g. up to 100% oxygen breathing), the added effect of hyperoxygenation on the clinical outcomes has not been reported and certainly needs further investigation.
Biological Target
The choice of treatment depends on the clinical goal and the prognostic profile of the tumor. The latter consists of the stage of disease, tumor size, location and grade, and lymph node status. Tailored molecular chemotherapy might interact on various gene and protein markers of tumors. Antitumor PDT (either curative or palliative) used for the ablation of tumor mass is a site-specific treatment modality which generally acts on biological targets non-specifically.
Direct cytotoxicity
The PDT induced cytotoxic species have a short lifetime and only act within a limited distance. Therefore, the subcellular localization of photosensitizer is critical in PDT-mediated cytotoxicities. The subcellular localization is determined by the photosensitizer's chemical properties, formulation, concentration and delivery route, the microenvironment of the lesion, and to certain degrees the phenotype of the target cells. The plasma membrane, intracellular membranes and organelles such as the endoplasmic reticulum (ER), Golgi apparatus, lysosomes, mitochondria and nucleus, have been identified as subcellular PDT targets for many photosensitizers. Some photosensitizers might distribute very broadly in these membranes and organelles, some specifically in lysosomes or mitochondria and some might redistribute during light irradiation.
The cell genotype, photosensitizer subcellular location and concentration, light dose and pO2 might determine whether cell death occurs through apoptosis or necrosis. In general, the mode of cell death switches from apoptosis to necrosis with the increase of the intensity of the insult. High-dose PDT induced apoptosis might be a stochastic rather than a threshold effect. Interestingly, some researchers show that subthreshold low-dose ALA-PDT can induce selective apoptosis of tumor cells without detectable (apoptotic or necrotic) damage to normal cells. This might offer the possibility of treating patients with low-dose ALA-PDT to minimize PDT damages to the surrounding normal tissue (53).
It has been proposed that apoptosis might play a role for disposing of dead cells and might not be a necessary step for PDT-mediated cell death (54). Nonetheless, in recent years there has been a steady increase in understanding the roles of the intracellular signaling machinery in cell response to PDT and, particularly, apoptotic pathways in PDT. Understanding PDT-induced unique changes and molecular events in signaling, transcription factors and regulating mechanisms may provide a means to modulate or enhance the cellular PDT effects at the molecular level. Two major apoptotic pathways have been characterized: (i) the death receptor-mediated, or extrinsic pathway, and (ii) the mitochondria-mediated apoptosis, or intrinsic pathway. The links between PDT effects and several apoptotic pathways have been clearly identified in numerous photosensitizers and cell lines in both in vitro and in vivo studies. In general, experimental evidences show a large heterogeneity in the mechanisms leading to cell death in cellular-targeted PDT. However, in addition to the activation of the molecular machinery leading directly to cell death, PDT may also initiate some metabolic reactions that could protect cells from oxidative damage. Therefore, it has been suggested that the control of these protective mechanisms is likely to enhance the cytotoxicity of cellular PDT on target cells (55,56).
Recently, it has been demonstrated that autophagy might be another cell death pathway since it can occur during in vitro tests involving photosensitizers that are located in the ER (57). Autophagy may be the dominant cell death pathway following PDT in cells that are incapable of undergoing normal apoptosis. It is still under investigation whether mitochondrial- and ER-associated Bcl-2 can protect against autophagic death (58).
There is no doubt that advances in cell and molecular biology allow for a better understanding of subcellular survival or pro-death pathways and consequences of PDT-induced direct cytotoxicity. Although such advances help the rational design and choice of photosensitizers, the gold standard of evaluation of the efficacy of clinical PDT still relies on the gross response of the lesion or disease to PDT. For instance, in tumor ablation, the typical clinically relevant response or biological endpoint would be acute tumor necrosis, the duration and rate of this response, and the length of the tumor-free period. These might be affected not only by the direct cytotoxicity but other PDT effects as well as the tumor sensitivity to various PDT effects.
Vascular effect
The growth of solid tumors is dependent on their capacity to acquire blood supply. Therefore, much effort has been directed towards the development of anti-angiogenic agents which inhibit the process of neovascularization in solid tumors. More recently, it has become apparent that the destruction of the established tumor vasculature represents a complementary avenue for developing new therapeutic opportunities.
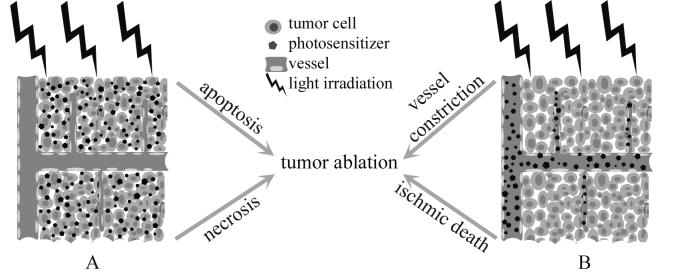
It has long been recognized since the early 1980s that an additional indirect mechanism, coexisting with the direct cytotoxicity of PDT, is the vascular effect in which vascular damage causes ischemic death and therefore provides another mechanism to treat solid tumors (Figure. 2) (59). Later, preclinical studies demonstrate that the mechanisms underlying the vascular effects differ greatly with photosensitizers, PDT approaches, and target tissues.
Figure 2.
Cellular PDT versus vascular PDT. (A) Long drug to light interval (DLI, > 24 h) allows the photosensitizers to be accumulated by target cells and therefore causes intracellular damages. (B) At short DLI (< 30 min), the light irradiation takes place while the photosensitizers are still circulating in the vascular compartment and therefore mainly causes direct vascular damages.
Irradiation of photosensitizers, either confined in the blood circulation, accumulated in endothelial cells or bound to the vessel walls, results in collateral damage to endothelial cells, which can be characterized by the loss of tight junctions between cells and exposure of vascular basement membranes. These primary damages within the vessel lumen lead to the formation of thrombogenic sites and initiation of a physiological cascade of reactions such as platelet aggregation, the release of vasoactive molecules, leukocyte adhesion, increase in vascular permeability and vessel constriction. Microvascular collapse, blood flow stasis and tissue hemorrhages can lead to persistent post-PDT tumor hypoxia and long-term tumor control (60-62).
Photosensitizers bound to carrier molecules, such as albumin, HDL or LDL, appear to have an active affinity to endothelial cells and tumor microvascular endothelium because of the existence of specific receptors in high numbers in their structure (63). This might be an additive factor in photosensitizer uptake and retention in the tumor tissue. Although one can expect that the localization of photosensitizer in both vascular and target cell compartment might produce a stronger combination effect, several investigations have also shown that tumor response to vascular-targeted PDT does not correlate with cellular concentration of photosensitizer in the treated tumor. In vascular-targeted PDT mode, therefore, the light irradiation should be applied during photosensitizer infusion and continued after the completion of infusion so as to center the light delivery period around the peak of the plasma concentration of photosensitizer and hence, putatively, maximizing the PDT effect (64).
Immune responses
PDT-induced immune responses and particularly antitumor-specific immunity have been studied in various animal models. PDT causes noticeable but short-term and reversible immune suppression under certain experimental conditions. However, unlike chemotherapy and irradiation therapy, PDT does not cause severe negative effects on the host immune system. Substantial evidences have demonstrated that antitumor-specific immune process and enhancement of host immune system might play important roles in secondary cytotoxicity, long-term tumor control and/or complete tumor response to PDT, although these effects are not necessarily lethal to all tumor cells or relevant to the initial tumor ablation (65-68).
Animal studies show that pro-inflammatory damages formed in cellular membranes and the blood vessel walls of treated sites start to recruit neutrophils, mast cells and monocytes/macrophages after PDT. These cells can also release more inflammatory mediators to enable massive recruitment of immune cells to tumor site. These immune cells and nonspecific immune effector cells have a profound impact on the destruction of tumors (69-71). PDT can also activate the expression and production of several cytokines, such as IL-1β, IL-2, IL-6, IL-10, TNF-α, and G-CSF. They can play important roles in regulating host immune response involving both lymphoid and non-lymphoid cells (72,73). Recent human trial suggests that PDT could enhance the uptake and presentation of tumor antigens by tumor-associated antigen presenting cells (APC) and could ensure lymphocyte involvement (74). Complement-activating agents may further enhance the antitumor effect of PDT (75). Recent studies also demonstrate that PDT-treated tumor cell lysates can be effective tumor vaccines, although the mechanism for enhancement of host anti-tumor immune responses to PDT vaccines is still unclear (76). These advances in understanding PDT-induced immune responses might lead to an attempt to optimize PDT-mediated antitumor modality through the modulation of important inflammatory/immune mediators (77).
Dosimetry
Acceptable clinical PDT, either as an alternative or an adjuvant, needs accurate prediction of the biological effect (78). In contrast to the well-established treatment-planning system in radiation therapy, where absorbed dose not only has an unambiguous definition but also correlates with the resultant biological effect, there is a lack of well-accepted dose definition and calculation in PDT (79). Current recommendation for clinical PDT dosimetry can be found in the American Association of Physicists in Medicine (AAPM) report No. 88 (80). In simplified words, clinicians should ensure that the product of irradiance (or fluence rate) and exposure time should exceed a certain threshold that is required to initiate tumor kill effect. Over- and under-treatment may occur using this clinical protocol given that PDT relies on three factors as described above: light, drug, and oxygen. Although over-treatment may cause relatively minor adverse consequences compared with radiation therapy, the threshold property of the PDT treatment response means that under-treatment that does not reach the threshold dose will result in treatment failure (81). The aim of the dosimetry is that the combination of light fluence distribution, drug concentration and distribution, and tissue oxygenation results in maximum tumor ablation while adjacent normal tissue can be spared to an acceptable degree. To reach this goal entails a full understanding of the distribution of light, drug, and oxygen in the target tissue.
Classes of PDT dosimetry
PDT dosimetry presently under active research can generally be categorized in four classes – direct dosimetry, biological dosimetry, implicit dosimetry and explicit dosimetry (81). Each class has its own intrinsic merits and limitations. At present, there is no single dose metric that can address all dosimetry problems. Choice of dosimetry depends on the type and location of tumor, type and characteristic of drug and light, desired accuracy and instrumentation complexity, etc.
(I) Direct dosimetry uses singlet oxygen (1O2), the putative toxic agent, as PDT dose metric. It is found that cell death correlated strongly with the cumulative 1O2 luminescence (at wavelength of 1270 nm) and allowed for direct estimation of the 1O2 per cell required to achieve a specific cell damage (82). However, unless there is a substantial reduction in cost and/or complexity of the instrumentation required, direct 1O2 monitoring is unlikely to become a routine tool in clinical or even preclinical PDT (83). Hence, its most important roles may be as a gold standard for other simpler dosimetry techniques and to answer critical photobiological questions. The usefulness of indirect 1O2 monitoring still needs to be validated.
(II) Biological dosimetry is a true photobiological dosimetry in that it uses measurable change(s) in tissue that is correlated to the direct result of PDT treatment. Methods such as CT or MRI, BOLD MRI, laser Doppler, PET, electrical impedance spectroscopy, and bioluminescence imaging have been used to investigate their potential roles in assessing PDT-induced biological effects (44,45,84-88). It is still unclear whether any of these specific techniques could be used to predict tissue responses and their outcome.
(III) Implicit dosimetry uses an implicit surrogate which is indicative of response of PDT treatment. This method is appealing since only one quantity needs to be measured. Fluorescence photobleaching and NADH autofluoresence are convenient quantities to measure (89). Although the biological effects of Foscan-mediated PDT can well be predicted by photobleaching (90), correlation of the implicit surrogate with PDT response using other PDT drugs may not be good. For example, Photofrin can bleach through non singlet oxygen mediated mechanism (91). Photobleaching is not a reliable dose metric for ALA/PpIX-mediated PDT (92).
(IV) Explicit dosimetry involves the measurements of fluence, drug concentration and tissue oxygenation. It is proposed that the energy absorbed by the drug per unit tissue volume would be a predictor of biological response (93). Cumulative PDT dose, activated at a particular wavelength for the drug, can be expressed as the time integral of light fluence rate φ(q,t), and absorption coefficient μap(q,t) of the photosensitizer:
| [1] |
where T is the total light irradiation time, q is the generalized spatial coordinate. Individual measurement of light distribution, drug concentration and pO2 level is nontrivial since each varies dynamically and inter-dependently.
Interstitial PDT is feasible for treating deeply-seated solid tumors, in which light delivery and detection are most efficiently accomplished through implanted optical fibers. The commonly encountered sites of parenchymal tumors include but are not limited to the head and neck (5,94), brain (3,22,95), liver (4,96), lung (97), pancreas (98) and prostate (2,15,28,99).
Generally, in treating bulky solid tumors, light with longer wavelengths is preferred due to its capability of deeper penetration depth, resulting in more access to the tumor (100). Even with the longer wavelength light, the size of the tissue such as the prostate gland cannot be covered by a sufficient light intensity using a single transurethral source. The commonly used light sources are cylindrical diffuse fibers, resembling the radioactive seeds used in brachytherapy. It is also possible that multiple locations of the cylindrical fibers are needed in order to cover the whole prostate. The knowledge of light distribution in the target tissue is important because the generation of cytotoxic species depends on how much available light can interact with the photosensitiser. Quantification of the light distribution requires the accurate characterization of optical properties of target tissue. It is found that both the distributions of absorption and scattering coefficients (e.g. μa and μs) in the human prostate at typical prostate PDT wavelengths (e.g. 665, 732 and 760 nm) are not uniform and their distributions also change during the treatment (12,26,101). Clearly, this is the evidence that pre-set values of fluence before the treatment is not sufficient for predicting the subsequent fluence distribution. The knowledge of optical properties of targeted tissue is needed and they might change even during the treatment.
Another factor is the drug concentration and distribution. Current clinical prescription only specifies this quantity in terms of the amount of drug given to a patient per body weight (102). The simplest method to measure the drug concentration is to detect the fluorescence emitted by the drug, using a single optical fiber as both a source and a detector (103). The drug concentration can also be measured with spatially-resolved detection technique exploiting the absorption of the drug with multiple implanted interstitial optical fibers (101). In those methods, light distribution was calculated using an appropriate light propagation model with prior known optical properties. The assumption was made that the optical properties of tissue and drug concentration distribution were homogenous (or partially homogenous), which is not necessarily true.
As discussed earlier, the role of oxygen in PDT has been well recognized since as early as the late 1980's (60). It was found that hypoxic tumor cells were protected from PDT damage. Therefore, complete explicit dosimetry clearly needs a term describing the oxygen consumption during the PDT session. This is particularly important in the situation where high light fluence and high drug concentration are used. Measurement of oxygen level can be accomplished either using Eppendorf or spectroscopic measurement of absorption coefficient, from which oxy- and deoxy-hemoglobin concentration and drug concentration can be extracted using a spectral deconvolution method such as the singular value decomposition method. Zhu et al. showed that oxygen level depends on location (102). Weersink et al. observed significant blood oxygenation changes during vascular PDT (26). They also indicated that the assumption was made that blood was uniformly distributed. Wang et al. recently developed a mathematical model describing spatial and temporal dynamics of oxygen consumption and transport in PDT in vivo (104). Their calculations demonstrate that intercapillary heterogeneity of drug contributes significantly to the distribution of photodynamic dose.
The above discussion indicates that there exists a gap between the current clinical protocols and the actual response. Hence, it is not surprising that it has drawn criticism that clinical PDT is still largely based upon empirical dose escalation trials without much consideration of the individual variations amongst patients. The challenge in explicit dosimetry is to find a proper method and procedure that allows us to accurately measure the light, drug and oxygen level in real-time (or nearly real-time) such that local PDT dose can be calculated as per Eq. [1]. The adjustment of light distribution and/or drug concentration can be made accordingly depending on whether the PDT dose exceeds the threshold dose or not. An attempt has been made to develop a clinical system for interstitial ALA-PDT with on-line dosimetry measurement (105). Light fluence is modeled with finite-element method using the previously determined optical properties of tumor and normal tissue. Treatment monitoring parameter measurements are performed for drug concentration from the fluorescence intensity and oxygen saturation level from the hemoglobin spectral measurements. They observed major treatment-induced light absorption increase, rapid drug photobleaching and relatively constant global tissue oxygen saturation.
Challenges of vascular PDT dosimetry
Vascular PDT presents particular challenges compared to the conventional cellular PDT in its dosimetry. Explicit dosimetry becomes a good practical candidate if the drug (such as Tookad) is not fluorescent. As mentioned earlier in this article, vascular PDT drugs typically are confined in blood vessels and diminish to an undetectable level within a couple of hours after the completion of IV injection of photosensitizer. Hence spatial and temporal delivery of light fluence is critical in order to achieve satisfactory treatment outcome. Generally, there exist two types of vascular PDT dosimetry recently published: (i) determination of optimal drug to light interval and (ii) real-time adjustment of light fluence based on the feedback measurement.
Short DLI in Tookad-PDT has been well documented in several studies (26,106-108). Huang et al. observed that drug concentration peak occurred in less than 10 minutes via IV injection using spontaneous and normal canine models. Light irradiation can be applied in three distinct modes: simultaneous mode, peak mode or drug concentration peak and light irradiation overlapping mode, and post-infusion mode (108). Borle et al., using a hamster cheek pouch model, reported a decreased tissue response with increasing DLI, and the highest response at the shortest DLI (107). Woodhams et al. showed that, in an animal colon model, DLI of around 5 minutes resulted in a maximum lesion for normal colon and around 5-15 minutes resulted in a maximum PDT necrosis in colon tumor (106). Another concern raised by them is that there exists a trade-off between tumor selectivity and maintenance of high level drug in blood vessels, since drug bolus injection would give more drug to the tumor (better selectivity) while IV injection would give high drug level. This particular challenge means that light fluence should be delivered in such a way that light threshold fluence is conformal to the tumor-normal tissue demarcation. A related preliminary study of the prostate cancer and its normal adjacent tissue and organs was performed, which indicated that the protection of the adjacent tissues should be taken into consideration during the total prostate ablation process due to their sensitivity to PDT (108). Conformal light delivery may need specialized optical fibers. Rendon et al. reported some attainable light iso-dose profile resulting from diffusers with tailored longitudinal emission profiles (24,109).
An alternative method for vascular-PDT is to perform mathematic modeling and real-time measurements of light fluence, drug concentration, and oxygen level (110). With this real-time information, light fluence can be adjusted accordingly during the treatment session such that the time integral of the product of fluence and drug concentration exceeds the required threshold PDT dose in order to elicit tumor ablation. Johansson et al. developed a real-time software tool for interstitial photodynamic therapy of the human prostate (35). Irradiation time of multiple implanted fibers is optimized during the treatment session based on the continuous monitoring of the change in the detected light attenuation. However, this method performed in the steady state without absolute light fluence measurement could not determine drug concentration variation and possible oxygen depletion, which may not completely reflect the true PDT process. Determination of absorption and reduced scattering coefficients has been shown to be possible in frequency domain in a phantom study. Xu and Patterson demonstrated that both coefficients can be determined within 10% for a wide range of optical properties using a fast and accurate forward model (111). This opens the possibility that both light fluence (from μa and μs') and drug concentration (from μa) can be determined from the combined steady state and frequency domain measurement and adjust the light fluence accordingly.
Future Prospects
There is a strong and increasing interest and research effort internationally focused on developing new photosensitizers, exploring PDT mechanisms at the molecular level, enhancing PDT efficacy with combined modality and evaluating potential clinical indications (112). Although regulatory approvals for the clinical use of PDT photosensitizers and light applicators now exist in many countries around the world, the total number of approved clinical indications is still limited. It is expected that the involvement of the pharmaceutical industry and research institutes will continue to launch numerous clinical trials to evaluate applications of PDT in conjunction with or as a replacement for traditional methods for treating solid tumors. Optical methods and nanotechnology will continue to play a wide range of roles in characterizing target tissue, determining PDT dose and assessing treatment outcome. Combined modality, individualized treatment planning and real-time dosimetry will likely become an essential component of interstitial PDT for treatment of parenchymal and/or stromal tissues of solid tumor over the next decade.
Acknowledgement
This work is supported by a NIH Grant (CA43892). Authors thank Sue Huang for editorial assistance.
References
- 1.Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat. 2005;4:283–294. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selman SH. Photodynamic therapy for prostate cancer: One urologist's perspective. Photodiag Photodyn Ther. 2007;4:26–30. doi: 10.1016/j.pdpdt.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Muller PJ, Wilson BC. Photodynamic therapy of brain tumors - a work in progress. Lasers Surg Med. 2006;38:384–389. doi: 10.1002/lsm.20338. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z. Photodynamic therapy in China: 25 years of unique history – Part two: clinical experience. Photodiag Photodyn Ther. 2006;3:71–84. doi: 10.1016/j.pdpdt.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Biel M. Advances in photodynamic therapy for the treatment of head and neck cancers. Lasers Surg Med. 2006;38:349–355. doi: 10.1002/lsm.20368. [DOI] [PubMed] [Google Scholar]
- 6.Hautmann H, Pichler JP, Stepp H, et al. In-vivo kinetics of inhaled 5-aminolevulinic acid-induced protoporphyrin IX fluorescence in bronchial tissue. Respir Res. 2007;8:33. doi: 10.1186/1465-9921-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arens C, Reussner D, Woenkhaus J, et al. Indirect fluorescence laryngoscopy in the diagnosis of precancerous and cancerous laryngeal lesions. Eur Arch Otorhinolaryngol. 2007;264:621–626. doi: 10.1007/s00405-007-0251-y. [DOI] [PubMed] [Google Scholar]
- 8.Allison RR, Downie GH, Cuenca R, et al. Photosensitizers in clinical PDT. Photodiag Photodyna Ther. 2004;1:27–42. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- 9.Moser JG. Definitions and general properties of 2nd & 3rd generation photosensitizers. In: Moser JG, editor. Photodynamic Tumor Therapy - 2nd & 3rd Generation Photosensitizers. Harwood Academic Publishers; London: 1997. pp. 3–8. [Google Scholar]
- 10.Loewen GM, Pandey R, Bellnier D, Henderson B, Dougherty T. Endobronchial photodynamic therapy for lung cancer. Lasers Surg Med. 2006;38:364–370. doi: 10.1002/lsm.20354. [DOI] [PubMed] [Google Scholar]
- 11.Jankun J, Lilge L, Douplik A, et al. Optical characteristics of the canine prostate at 665 nm sensitized with tin etiopurpurin dichloride: need for real-time monitoring of photodynamic therapy. J Urol. 2004;172:739–743. doi: 10.1097/01.ju.0000135304.96496.20. [DOI] [PubMed] [Google Scholar]
- 12.Du KL, Mick R, Busch TM, et al. Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg Med. 2006;38:427–434. doi: 10.1002/lsm.20341. [DOI] [PubMed] [Google Scholar]
- 13.Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularisation in age-related macular degeneration with verteporfin. Two year results of two randomized clinical trials – TAP report 2. Arch Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- 14.Yuan KH, Li Q, Yu WL, et al. Comparison of photodynamic therapy and pulsed dye laser in patients with port wine stain birthmarks: A retrospective analysis. Photodiagn Photodyn Ther. 2008;5:50–57. doi: 10.1016/j.pdpdt.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Huang Z, Chen Q, Luck D, et al. Studies of a vascular-acting photosensitizer, Pd-bacteriopheophorbide (Tookad), in normal canine prostate and spontaneous canine prostate cancer. Lasers Surg Med. 2005;36:390–397. doi: 10.1002/lsm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trachtenberg J, Bogaards A, Weersink RA, et al. Vascular Targeted Photodynamic Therapy With Palladium-Bacteriopheophorbide Photosensitizer for Recurrent Prostate Cancer Following Definitive Radiation Therapy: Assessment of Safety and Treatment Response. J Urol. 2007;178:1974–1979. doi: 10.1016/j.juro.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 17.Brandis A, Mazor O, Neumark E, et al. Novel water-soluble bacteriochlorophyll derivatives for vascular-targeted photodynamic therapy: synthesis, solubility, phototoxicity and the effect of serum proteins. Photochem Photobiol. 2005;81:983–993. doi: 10.1562/2004-12-01-RA-389. [DOI] [PubMed] [Google Scholar]
- 18.Preise D, Mazor O, Koudinova N, et al. Bypass of tumor drug resistance by antivascular therapy. Neoplasia. 2003;5:475–480. doi: 10.1016/s1476-5586(03)80031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson BC, Weersink RA, Lilge L. Fluorescence in Photodynamic Therapy Dosimetry. In: Mycek M, Pogue BW, editors. Handbook of Biomedical Fluorescence. Marcel Dekker, Inc.; New York: 2003. pp. 529–561. [Google Scholar]
- 20.Tagg R, Asadi-Zeydabadi M, Meyers AD. Biophotonic and other physical methods for characterizing oral mucosa. Otolaryngol Clin North Am. 2005;38:215–240. doi: 10.1016/j.otc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Jankun J, Keck RW, Skrzypczak-Jankun E, Lilge L, Selman SH. Diverse optical characteristic of the prostate and light delivery system: implications for computer modelling of prostatic photodynamic therapy. BJU Int. 2005;95:1237–1244. doi: 10.1111/j.1464-410X.2005.05512.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilson BC, Muller PJ, Yanch JC. Instrumentation and light dosimetry for intra-operative photodynamic therapy (PDT) of malignant brain tumours. Phys Med Biol. 1986;31:125–133. doi: 10.1088/0031-9155/31/2/002. [DOI] [PubMed] [Google Scholar]
- 23.Roche JVE, Whitehurst C, Watt P, Moore JV, Krasner N. Photodynamic Therapy (PDT) of Gastrointestinal Tumours: A New Light Delivery System. Lasers Med Sci. 1998;13:137–142. [Google Scholar]
- 24.Rendon A, Beck JC, Lilge L. Treatment planning using tailored and standard cylindrical light diffusers for photodynamic therapy of the prostate. Phys Med Biol. 2008;53:1131–1149. doi: 10.1088/0031-9155/53/4/021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyst HJ, van, Veen RL, Tan IB, et al. Performance of a dedicated light delivery and dosimetry device for photodynamic therapy of nasopharyngeal carcinoma: Phantom and volunteer experiments. Lasers Surg Med. 2007;39:647–653. doi: 10.1002/lsm.20536. [DOI] [PubMed] [Google Scholar]
- 26.Weersink RA, Bogaards A, Gertner M, et al. Techniques for delivery and monitoring of TOOKAD (WST09)-mediated photodynamic therapy of the prostate: clinical experience and practicalities. J Photochem Photobiol B. 2005;79:211–222. doi: 10.1016/j.jphotobiol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Samkoe KS, Clancy AA, Karotki A, Wilson BC, Cramb DT. Complete blood vessel occlusion in the chick chorioallantoic membrane using two-photon excitation photodynamic therapy: implications for treatment of wet age-related macular degeneration. J Biomed Opt. 2007;12:034025. doi: 10.1117/1.2750663. [DOI] [PubMed] [Google Scholar]
- 28.Drobizhev M, Makarov NS, Stepanenko Y, Rebane A. Near-infrared two-photon absorption in phthalocyanines: enhancement of lowest gerade-gerade transition by symmetrical electron-accepting substitution. J Chem Phys. 2006;124:224701. doi: 10.1063/1.2200355. [DOI] [PubMed] [Google Scholar]
- 29.Juzeniene A, Juzenas P, Ma LW, Iani V, Moan J. Effectiveness of different light sources for 5-aminolevulinic acid photodynamic therapy. Lasers Med Sci. 2004;19:139–149. doi: 10.1007/s10103-004-0314-x. [DOI] [PubMed] [Google Scholar]
- 30.Mang TS. Lasers and light sources for PDT: past, present and future. Photodiag Photodyn Ther. 2004;1:43–48. doi: 10.1016/S1572-1000(04)00012-2. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt MH, Bajic DM, Reichert KW, II., et al. Light-emitting diodes as a light source for intraoperative photodynamic therapy. Neurosurgery. 1996;38:552–556. doi: 10.1097/00006123-199603000-00025. [DOI] [PubMed] [Google Scholar]
- 32.Lustig RA, Vogl TJ, Fromm D, et al. A multicenter Phase I safety study of intratumoral photoactivation of talaporfin sodium in patients with refractory solid tumors. Cancer. 2003;98:1767–1771. doi: 10.1002/cncr.11708. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Keltner L, Christophersen J, et al. New technology for deep light distribution in tissue for phototherapy. Cancer J. 2002;8:154–163. doi: 10.1097/00130404-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Martin NE, Hahn SM. Interstitial photodynamic therapy for prostate cancer: a developing modality. Photodiag Photodyn Ther. 2004;1:123–136. doi: 10.1016/S1572-1000(04)00037-7. [DOI] [PubMed] [Google Scholar]
- 35.Johansson A, Axelsson J, Andersson-Engels S, Swartling J. Realtime light dosimetry software tools for interstitial photodynamic therapy of the human prostate. Med Phys. 2007;34:4309–4321. doi: 10.1118/1.2790585. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Zhu TC. Determination of in vivo light fluence distribution in a heterogeneous prostate during photodynamic therapy. Phys Med Biol. 2008;53:2103–2114. doi: 10.1088/0031-9155/53/8/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Z, Chen Q, Shakil A, et al. Hyperoxygenation enhances the tumor cell killing of Photofrin-mediated photodynamic therapy. Photochem Photobiol. 2003;78:496–502. doi: 10.1562/0031-8655(2003)078<0496:hettck>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Chen Q, Huang Z, Chen H, Beckers J, Hetzel FW. Improvement of tumor response by manipulation of tumor oxygenation during photodynamic therapy. Photochem Photobiol. 2002;76:197–203. doi: 10.1562/0031-8655(2002)076<0197:iotrbm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Vakrat-Haglili Y, Weiner L, Brumfeld V, et al. The microenvironment effect on the generation of reactive oxygen species by Pd-bacteriopheophorbide. J Am Chem Soc. 2005;127:6487–6497. doi: 10.1021/ja046210j. [DOI] [PubMed] [Google Scholar]
- 40.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 41.Hutchison GJ, Valentine HR, Loncaster JA, et al. Hypoxia-inducible factor 1alpha expression as an intrinsic marker of hypoxia: correlation with tumor oxygen, pimonidazole measurements, and outcome in locally advanced carcinoma of the cervix. Clin Cancer Res. 2004;10:8405–8412. doi: 10.1158/1078-0432.CCR-03-0135. [DOI] [PubMed] [Google Scholar]
- 42.Woodhams JH, Macrobert AJ, Bown SG. The role of oxygen monitoring during photodynamic therapy and its potential for treatment dosimetry. Photochem Photobiol Sci. 2007;6:1246–1256. doi: 10.1039/b709644e. [DOI] [PubMed] [Google Scholar]
- 43.Vaupel P, Höckel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9:1221–1235. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 44.Tempel-Brami C, Pinkas I, Scherz A, Salomon Y. Detection of light images by simple tissues as visualized by photosensitized magnetic resonance imaging. PLoS ONE. 2007;2:e1191. doi: 10.1371/journal.pone.0001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross S, Gilead A, Scherz A, Neeman M, Salomon Y. Monitoring photodynamic therapy of solid tumors online by BOLD-contrast MRI. Nat Med. 2003;9:1327–1331. doi: 10.1038/nm940. [DOI] [PubMed] [Google Scholar]
- 46.Sitnik TM, Hampton JA, Henderson BW. Reduction of tumour oxygenation during and after photodynamic therapy in vivo: effects of fluence rate. Br J Cancer. 1998;77:1386–1394. doi: 10.1038/bjc.1998.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henderson BW, Busch TW, Vaughan LA, et al. Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate. Cancer Res. 2000;60:525–529. [PubMed] [Google Scholar]
- 48.Curnow A, Haller JC, Bown SG. Oxygen monitoring during 5-aminolaevulinic acid induced photodynamic therapy in normal rat colon. Comparison of continuous and fractionated light regimes. Photochem Photbiol B. 2000;58:149–155. doi: 10.1016/s1011-1344(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 49.Maier A, Anegg U, Fell B, et al. Effect of photodynamic therapy in a multimodal approach for advanced carcinoma of the gastro-esophageal junction. Lasers Surg Med. 2000;26:461–466. doi: 10.1002/1096-9101(2000)26:5<461::aid-lsm5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 50.Maier A, Tomaselli F, Anegg U, et al. Combined photodynamic therapy and hyperbaric oxygenation in carcinoma of the esophagus and the esophago-gastric junction. Eur J Cardiothorac Surg. 2000;18:649–654. doi: 10.1016/s1010-7940(00)00592-3. [DOI] [PubMed] [Google Scholar]
- 51.Tomaselli F, Maier A, Sankin O, et al. Acute effects of combined photodynamic therapy and hyperbaric oxygenation in lung cancer - a clinical pilot study. Lasers Surg Med. 2001;28:399–403. doi: 10.1002/lsm.1067. [DOI] [PubMed] [Google Scholar]
- 52.Tomaselli F, Maier A, Pinter H, Stranzl H, Smolle-Juttner FM. Photodynamic therapy enhanced by hyperbaric oxygen in acute endoluminal palliation of malignant bronchial stenosis (clinical pilot study in 40 patients) Eur J Cardiothorac Surg. 2001;5:549–554. doi: 10.1016/s1010-7940(01)00635-2. [DOI] [PubMed] [Google Scholar]
- 53.Bisland SK, Lilge L, Lin A, Rusnov R, Wilson BC. Metronomic photodynamic therapy as a new paradigm for photodynamic therapy: rationale and preclinical evaluation of technical feasibility for treating malignant brain tumors. Photochem Photobiol. 2004;80:22–30. doi: 10.1562/2004-03-05-RA-100.1. [DOI] [PubMed] [Google Scholar]
- 54.Chiu SM, Xue LY, Azizuddin K, Oleinick NL. Photodynamic therapy-induced death of HCT 116 cells: Apoptosis with or without Bax expression. Apoptosis. 2005;10:1357–1368. doi: 10.1007/s10495-005-2217-0. [DOI] [PubMed] [Google Scholar]
- 55.Gomer CJ, Luna M, Ferrario A, et al. Cellular targets and molecular responses associated with photodynamic therapy. J Clin Laser Med Surg. 1996;14:315–321. doi: 10.1089/clm.1996.14.315. [DOI] [PubMed] [Google Scholar]
- 56.Verma S, Watt GM, Mai Z, Hasan T. Strategies for enhanced photodynamic therapy effects. Photochem Photobiol. 2007;83:996–1005. doi: 10.1111/j.1751-1097.2007.00166.x. [DOI] [PubMed] [Google Scholar]
- 57.Kessel D, Reiners JJ., Jr. Apoptosis and autophagy after mitochondrial or endoplasmic reticulum photodamage. Photochem Photobiol. 2007;83:1024–1028. doi: 10.1111/j.1751-1097.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue LY, Chiu SM, Azizuddin K, Joseph S, Oleinick NL. Protection by Bcl-2 against apoptotic but not autophagic cell death after photodynamic therapy. Autophagy. 2008;4:125–127. doi: 10.4161/auto.5287. [DOI] [PubMed] [Google Scholar]
- 59.Star WM, Marijnissen JP, van, den, Berg-Blok AE, Reinhold HS. Destructive effect of photoradiation on the microcirculation of a rat mammary tumor growing in “sandwich” observation chambers. Prog Clin Biol Res. 1984;170:637–645. [PubMed] [Google Scholar]
- 60.Henderson BW, Fingar VH. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer Res. 1987;47:3110–3114. [PubMed] [Google Scholar]
- 61.Fingar VH. Vascular effects of photodynamic therapy. J. Clin. Laser Med Surg. 1996;14:323–328. doi: 10.1089/clm.1996.14.323. [DOI] [PubMed] [Google Scholar]
- 62.Abels C. Targeting of the vascular system of solid tumours by photodynamic therapy (PDT) Photochem Photobiol Sci. 2004;3:765–771. doi: 10.1039/b314241h. [DOI] [PubMed] [Google Scholar]
- 63.Chen B, Pogue BW, Hoopes PJ, Hasan T. Vascular and cellular targeting for photodynamic therapy. Crit Rev Eukaryot Gene Expr. 2006;16:279–305. doi: 10.1615/critreveukargeneexpr.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- 64.Huang Z, Chen Q, Luck D, et al. Studies of a vascular-acting photosensitizer, Pd-bacteriopheophorbide (Tookad), in normal canine prostate and spontaneous canine prostate cancer. Lasers Surg Med. 2005;36:390–397. doi: 10.1002/lsm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korbelik M. Induction of tumor immunity by photodynamic therapy. J Clin La ser Med Surg. 1996;14:329–334. doi: 10.1089/clm.1996.14.329. [DOI] [PubMed] [Google Scholar]
- 66.Canti G, De Simone A, Korbelik M. Photodynamic therapy and the immune system in experimental oncology. Photochem Photobiol Sci. 2002;1:79–80. doi: 10.1039/b109007k. [DOI] [PubMed] [Google Scholar]
- 67.van Duijnhoven FH, Aalbers RIJM, Rovers JP, Terpstra OT, Kuppen PJK. The immunological consequences of photodynamic treatment of cancer, a literature review. Immunobiol. 2003;207:105–113. doi: 10.1078/0171-2985-00221. [DOI] [PubMed] [Google Scholar]
- 68.Nowis D, Stokłosa T, Legat M, et al. The influence of photodynamic therapy on the immune response. Photodiag Photodyn Ther. 2005;2:283–298. doi: 10.1016/S1572-1000(05)00098-0. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto N, Homma S, Sery TW, Donoso TW, Hoober JK. Photodynamic immunopotentiation: in vitro activation of macrophages by treatment of mouse peritoneal cells with haematoporphyrin derivative and light. Eur J Cancer. 1991;27:467–471. doi: 10.1016/0277-5379(91)90388-t. [DOI] [PubMed] [Google Scholar]
- 70.Krosl G, Korbelik M, Dougherty GJ. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br J Cancer. 1995;71:549–555. doi: 10.1038/bjc.1995.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korbelik M, Krosl G, Krosl J, Dougherty GJ. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996;15:5647–5652. [PubMed] [Google Scholar]
- 72.Gollnick SO, Evans SS, Baumann H, et al. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br J Cancer. 2003;42:1772–1779. doi: 10.1038/sj.bjc.6600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–45. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001;61:192–196. [PubMed] [Google Scholar]
- 75.Korbelik M, Sun J, Cecic I, Serrano K. Adjuvant treatment for complement activation increases the effectiveness of photodynamic therapy of solid tumors. Photochem Photobiol Sci. 2004;3:812–816. doi: 10.1039/b315663J. [DOI] [PubMed] [Google Scholar]
- 76.Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002;62:1604–1608. [PubMed] [Google Scholar]
- 77.Qiang YG, Yow CMN, Huang Z. Combination of photodynamic therapy and immunomodulation - current status and future trends. Med Res Rev. 2008;28:632–644. doi: 10.1002/med.20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Star WM. Light dosimetry in vivo. Phys Med Biol. 1997;42:763–787. doi: 10.1088/0031-9155/42/5/003. [DOI] [PubMed] [Google Scholar]
- 79.Patterson MS, Wilson BC. Photodynamic therapy. In: Van Dyk J, editor. The modern technology of radiation oncology. Medical Physics Publishing; Madison, Wisconsin: 1999. Chap. 23. [Google Scholar]
- 80.AAPM task group report of the general medical physics committee of the science council. Medical Physics Publishing; 2005. AAPM Report No. 88, Photodynamic therapy dosimetry. [Google Scholar]
- 81.Wilson BC, Patterson MS, Lilge L. Implicit and explicit dosimetry in photodynamic theory: a new paradigm. Lasers Med Sci. 1997;12:182–199. doi: 10.1007/BF02765099. [DOI] [PubMed] [Google Scholar]
- 82.Niedre MJ, Secord AJ, Patterson MS, Wilson BC. In vitro tests of the validity of singlet oxygen luminescence measurements as a dose metric in photodynamic therapy. Cancer Res. 2003;63:7986–7994. [PubMed] [Google Scholar]
- 83.Davis SJ, Zhu L, Minhaj A, et al. Ultra-senstitive, diode laser based monitor for singlet oxygen. Proc SPIE. 2003;4952:140–148. [Google Scholar]
- 84.Yeung WT, Lee TY, Del Maestro RF, Kozak R, Brown T. In vivo CT measurement of blood-brain transfer constant of iopamidol in human brain tumors. J Neurooncol. 1992;14:177–187. doi: 10.1007/BF00177622. [DOI] [PubMed] [Google Scholar]
- 85.Herman MA, Fromm D, Kessel D. Tumor blood-flow changes following protoporphyrin IX-based photodynamic therapy in mice and humans. J Photochem Photobiol. 1999;52:99–104. doi: 10.1016/s1011-1344(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 86.Berard V, Lecomte R, van Lier JE. Positron emission tomography imaging of tumor response after photodynamic therapy. J Environ Pathol Toxicol Oncol. 2006;25:239–250. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.150. [DOI] [PubMed] [Google Scholar]
- 87.Molckovsky A, Wilson BC. Monitoring of cell and tissue responses to photodynamic therapy by electrical impedance spectroscopy. Phys Med Biol. 2001;46:983–1002. doi: 10.1088/0031-9155/46/4/306. [DOI] [PubMed] [Google Scholar]
- 88.Moriyama EH, Bisland SK, Lilge L, Wilson BC. Bioluminescence imaging of the response of rat gliosarcoma to ALA-PpIX-mediated photodynamic therapy. Photochem Photobiol. 2004;80:242–249. doi: 10.1562/2004-02-20-RA-088. [DOI] [PubMed] [Google Scholar]
- 89.Pogue BW, Pitts JD, Mycek MA, et al. In vivo NADH fluorescence monitoring as an assay for cellular damage in photodynamic therapy. Photochem Photobiol. 2001;74:817–824. doi: 10.1562/0031-8655(2001)074<0817:ivnfma>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 90.Dysart JS, Singh G, Patterson MS. Calculation of singlet oxygen dose from photosensitizer fluorescence emission and photobleaching during mTHPC photodynamic therapy of MLL cells. Photochem Photobiol. 2005;81:196–205. doi: 10.1562/2004-07-23-RA-244. [DOI] [PubMed] [Google Scholar]
- 91.Finlay JC, Mitra S, Patterson MS, Foster TH. Photobleaching kinetics of Photofrin in vivo and in multicell tumor spheroids indicate two simultaneous bleaching mechanisms. Phys Med Biol. 2004;49:4837–4860. doi: 10.1088/0031-9155/49/21/001. [DOI] [PubMed] [Google Scholar]
- 92.Dysart JS, Patterson MS. Photobleaching kinetics, photoproduct formation and dose estimation during ALA induced PpIX PDT of MLL cells under well oxygenated and hypoxic conditions. Photochemical and Photobiological Sciences. 2006;5:73–81. doi: 10.1039/b511807g. [DOI] [PubMed] [Google Scholar]
- 93.Svaasand LO, Potter WR. The implications of photobleaching for photodynamic therapy. In: Henderson BW, Dougherty TJ, editors. Photodynamic therapy: Basic principles and clinical applications. Marcel Dekker; New York: 1992. pp. 369–385. [Google Scholar]
- 94.Tan IB, Oppelaar H, Ruevekamp MC, et al. The importance of in situ light dosimetry for photodynamic therapy of oral cavity tumors. Head Neck. 1999;21:434–441. doi: 10.1002/(sici)1097-0347(199908)21:5<434::aid-hed9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 95.Eljamel MS. New light on the brain: The role of photosensitizing agents and laser light in the management of invasive intracranial tumors. Technol Cancer Res Treat. 2003;2:303–309. doi: 10.1177/153303460300200404. [DOI] [PubMed] [Google Scholar]
- 96.Vogl TJ, Eichler K, Mack MG, Zangos S, Herzog C, Thalhammer A, Engelmann K. Interstitial photodynamic laser therapy in interventional oncology. Eur Radiol. 2004;14:1063–1073. doi: 10.1007/s00330-004-2290-8. [DOI] [PubMed] [Google Scholar]
- 97.Fielding DI, Buonaccorsi GA, MacRobert AJ, et al. Fine-needle interstitial photodynamic therapy of the lung parenchyma: photosensitizer distribution and morphologic effects of treatment. Chest. 1999;115:502–510. doi: 10.1378/chest.115.2.502. [DOI] [PubMed] [Google Scholar]
- 98.Bown SG, Rogowska AZ, Whitelaw DE, et al. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549–557. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu TC, Finlay JC, Hhan SM. Determination of the distribution of light, optical properties, drug concentration, and tissue oxygenation in-vivo in human prostate during motexafin lutetium-mediated photodynamic therapy. J Photochem Photobiol B. 2005;79:231–241. doi: 10.1016/j.jphotobiol.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arnfield MR, Chapman JD, Tulip J, Fenning MC, McPhee MS. Optical properties of experimental prostate tumors in vivo. Photochem Photobiol. 1993;57:306–311. doi: 10.1111/j.1751-1097.1993.tb02292.x. [DOI] [PubMed] [Google Scholar]
- 101.Zhu TC, Hahn SM, Kapatkin AS, et al. In vivo optical properties of normal canine prostate at 732 nm using motexafin lutetium-mediated photodynamic therapy. Photochem Photobiol. 2003;77:81–88. doi: 10.1562/0031-8655(2003)077<0081:ivopon>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 102.Zhu TC, Finlay JC. Prostate PDT dosimetry. Photodiag Photodyn Ther. 2006;3:234–246. doi: 10.1016/j.pdpdt.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Finlay JC, Zhu TC, Dimofte A, et al. Interstitial fluorescence spectroscopy in the human prostate during motexafin lutetium-mediated photodynamic therapy. Photochem Photobiol. 2006;82:1270–1278. doi: 10.1562/2005-10-04-RA-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang KK, Mitra S, Foster TH. A comprehensive mathematical model of microscopic dose deposition in photodynamic therapy. Med Phys. 2007;34:282–293. doi: 10.1118/1.2401041. [DOI] [PubMed] [Google Scholar]
- 105.Thompson MS, Johansson A, Johansson T, et al. Clinical system for interstitial photodynamic therapy with combined on-line dosimetry measurements. Appl Opt. 2005;44:4023–4031. doi: 10.1364/ao.44.004023. [DOI] [PubMed] [Google Scholar]
- 106.Woodhams JH, MacRobert AJ, Novelli M, Bown SG. Photodynamic therapy with WST09 (Tookad): Quantitative studies in normal colon and transplanted tumours. Int J Cancer. 2006;118:477–482. doi: 10.1002/ijc.21335. [DOI] [PubMed] [Google Scholar]
- 107.Borle F, Radu A, Monnier P, van den Bergh H, Wagnieres G. Evaluation of the photosensitizer Tookad® for photodynamic therapy on the Syrian golden hamster cheek pouch model: light dose, drug dose and drug light interval effects. Photchem Photbiol. 2003;78:377–383. doi: 10.1562/0031-8655(2003)078<0377:eotptf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 108.Huang Z, Chen Q, Dole KC, et al. The effect of Tookad-mediated photodynamic ablation of the prostate gland on adjacent tissues - in vivo study in a canine model. Photochem Photobiol Sci. 2007;6:1318–1324. doi: 10.1039/b705984a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rendon A, Weersink R, Lothar L. Towards conformal light delivery using tailored cylindrical diffusers: attainable light dose distributions. Phys Med Biol. 2006;51:5967–5975. doi: 10.1088/0031-9155/51/23/001. [DOI] [PubMed] [Google Scholar]
- 110.Huang N, Cheng G, Li X, et al. Influence of drug-light-interval on photodynamic therapy of port wine stains - Simulation and validation of mathematic models. Photodiag Photodyn Ther. 2008;5:120–126. doi: 10.1016/j.pdpdt.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 111.Xu H, Patterson MS. Determination of the optical properties of tissue-simulating phantoms from interstitial frequency domain measurements of relative fluence and phase difference. Optics Express. 2006;14:6485–6501. doi: 10.1364/oe.14.006485. [DOI] [PubMed] [Google Scholar]
- 112.Allison RR, Bagnato VS, Cuenca R, Downie GH, Sibata CH. The future of photodynamic therapy in oncology. Future Oncol. 2006;2:53–71. doi: 10.2217/14796694.2.1.53. [DOI] [PubMed] [Google Scholar]