In this issue of the Annals, Verboon-Maciolek and coworkers show that human parechovirus (HPeV), specifically HPeV3, is an important cause of neonatal viral encephalitis.1 The six serotypes of HPeVs that are included in the genus Parechovirus are small single-stranded (ss) RNA viruses belonging to the family Picornaviridae.2, 3 These viruses bear many similarities with another and better-known Picornaviridae genus, Enterovirus (EV). Indeed, the genus Parechovirus began with the reclassification of echovirus 21 and 22 as HPeV1 and HPeV2 because of molecular and genetic differences from the remainder of EV. These differences are important because they explain, in part, why the usual polymerase chain reaction (PCR) testing for EV does not detect HPeV. Thus encephalitic infection by HPeV3 has been overlooked in the past, one important point of the current article. (Neonatal encephalitis by other HPeV subtypes is extremely rare and not discussed further.) The encephalitis caused by HPeV3 infection as well as by EV is associated with neonatal seizures and with apparent cerebral white matter injury.1, 4 The current report has important implications concerning the etiology of neonatal viral encephalitis, the differential diagnosis of neonatal seizures, and the pathology and pathophysiology of the white matter injury.
Concerning the etiology of neonatal encephalitis, the findings of Verboon-Maciolek et al.1 indicate that HPeV3 is a major cause. Indeed during the period of their study, neonatal encephalitis due to HPeV3 was three times more common than that due to EV and accounted for 64% of all encephalitic cases admitted to their NICU. Of the major viral infections of the developing nervous system (Table), i.e., rubella, cytomegalovirus (CMV), herpes simplex virus (HSV), varicella-zoster, human immunodeficiency virus (HIV), lymphocytic choriomeningitis virus, and EV/HPeV, most are acquired in utero and symptomatic neonatal encephalitis is uncommon (Table).5 HSV and EV/HPeV are associated most consistently with neonatal rather than fetal encephalitis.5 HSV encephalitis exhibits diffuse gray and white matter changes and is discussed elsewhere.5 Neonatal encephalitis caused by EV/HPeV has a distinctive clinical presentation and an apparent predilection for the white matter, as discussed next.
Table.
Major Viral Infections of the Developing Nervous System
| Primarily intrauterine (transplacental) infection |
| Rubella |
| Cytomegalovirusa |
| Varicella-Zoster |
| Lymphocytic choriomeningitis |
| Primarily parturitional or neonatal infection |
| Herpes simplex |
| Enteroviruses |
| Human parechovirus |
| Human immunodeficiency virusb |
Cytomegalovirus infection, although acquired relatively early in pregnancy, occasionally presents with symptomatic neonatal encephalitis.
Human immunodeficiency virus infection, although acquired primarily during the perinatal period, rarely causes symptomatic neonatal encephalitis.
Concerning the clinical features of EV/HPeV encephalitis, the usual presentation is fever, rash, irritability and seizures.6–8 Diarrhea is a common accompaniment, and occurrence in summer and fall, characteristic. Seizures are more common in HPeV3 encephalitis (90%)1 than in EV encephalitis (40%)8 and require more than one anticonvulsant drug for control in most cases. Importantly, the routine CSF examination is normal in 90% of HPeV3 cases and in the majority of EV cases, as well, and therefore on initial clinical evaluation, encephalitis could be easily overlooked. Thus, PCR analysis that includes analysis for HPeV3 as well as EV (and HSV) in CSF and blood is important in the assessment of newborns with unexplained seizures, especially with any of the other clinical features noted earlier or the distinctive imaging features or both.8, 9
Concerning the pathology of HPeV/EV encephalitis, the distinctive pattern of white matter involvement is noteworthy.1, 4, 10 At first glance the involvement of cerebral white matter suggests periventricular leukomalacia (PVL), as typically observed in premature infants.5 However, the 10 infants with HPeV3 infection were predominantly (70%) term infants from 6 – 14 days of age, and in the 3 premature infants, the onset of encephalitis was at 53 – 90 days of age (postconceptional age of 36 – 39 weeks). Moreover, unlike PVL the cerebral white matter abnormalities extend into the subcortical white matter and involve entire fiber tracts, such as corpus callosum, optic radiation, as well as gray matter regions, e.g., posterior thalamus. No neuropathological information is available. Among EV encephalitides, Coxsackie B encephalitis has been shown to exhibit the hallmarks of meningoencephalitis, i.e., infiltration of meninges with inflammatory cells, predominately mononuclear; perivascular cuffing with inflammatory cells; and neuronal necrosis with collections of microglia, macrophages and, later, astrocytes, although notably the findings generally are not marked.5, 11 (Poliovirus, of course, produces a myelitis, also with clear neuronal tropism.) Indeed, the clinical presentation in HPeV/EV encephalitis, with seizures predominating, suggests neuronal involvement, and neurological sequelae, present in the minority of cases, include cognitive deficits and epilepsy more than cerebral palsy, again most consistent with neuronal/axonal disease. The apparent white matter involvement by MRI of course does not prove an exclusive disorder of the premyelinating oligodendrocyte, the principal oligodendrocyte form in the newborn,12, 13 and indeed the involvement of fiber tracts and the diffuse distribution of the white matter abnormality are consistent with axonal involvement. Diffuse axonal injury has been delineated recently in cerebral white matter in premature infants with PVL.14
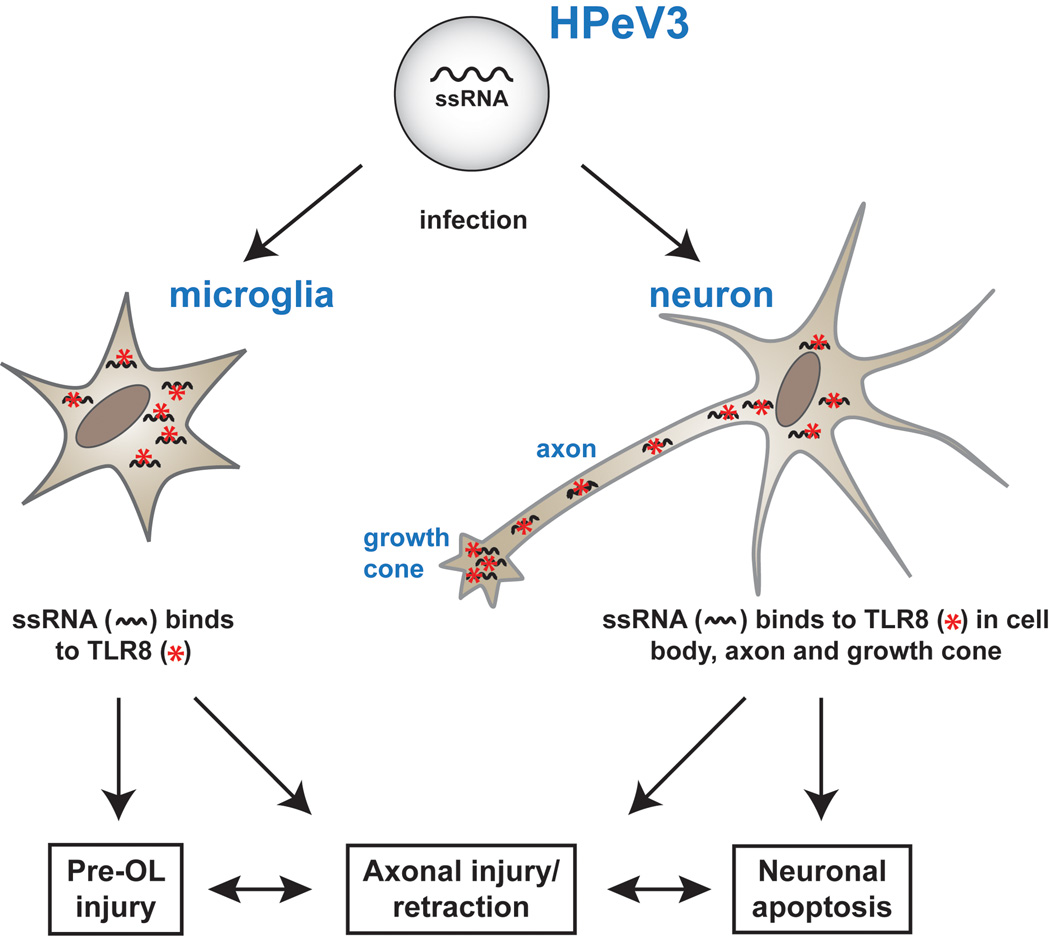
Concerning the pathophysiology of the white matter abnormality, although the similarity to PVL is not perfect, the two major initiating mechanisms for PVL, ischemia and systemic inflammation, deserve consideration.5, 15 Ischemia is not likely in these infants since only 4 of the 10 subjects in the report of Verboon-Maciolek et al.1 exhibited “hypotension”, which additionally was only “mild”. Systemic inflammation seems unlikely to be involved, since even with proven EV sepsis biomarkers of inflammation are only marginally elevated.16 The cause of the injury in the cerebral white matter in EV/HPeV3 encephalitis likely involves activation of microglia, resulting, at least in part, from activation of the intracellular toll-like receptors (TLRs) 7 and 8 by the ssRNA of HPeV or EV (Figure). TLRs are the mediators of the innate immune response, via recognition of specific molecular motifs (so-called pathogen-associated molecular patterns) shared by whole classes of microbials.17 Indeed, TLRs 7 and, especially 8, are involved in the host’s immune response to HPeV.18 Activation of microglia would lead to release of reactive oxygen and nitrogen species and pro-inflammatory cytokines, especially TNFα and IL1-β, toxic to premyelinating oligodendrocytes5, 19 and, likely, to developing axons.20–22 By analogy, the role of innate immunity, mediated by TLRs on microglia, in the genesis of white matter injury is exemplified by the demonstrations that activation on microglia of TLR 4 by lipopolysaccharide or of TLR 2 by group B β-streptococcus results in toxicity to premyelinating oligodendrocytes or to developing axons or both.23–27 Nevertheless, in view of the very uncommon finding of CSF pleocytosis in PCR-positive HPeV3 CNS infection with white matter injury,1 it seems unlikely that CNS inflammation is the dominant or at least exclusive feature of the process. Of particular interest in this context, recent work shows that TLR 8 also is localized to neurons and axons (Figure).22, 28 Strikingly, this TLR is distributed especially in growth cones and axonal fiber tracts and only in the developing nervous system. Indeed the axonal expression of TLR 8 correlates closely with that of growth associated protein (GAP)-43, a marker of axonal growth.22 Notably, recent study of GAP-43 immunostaining in the developing human brain shows that axonal growth and development is very active in the perinatal period.29 Activation of TLR 8 results in growth cone collapse, inhibition of axonal outgrowth and neuronal apoptosis.22, 28 Thus, it appears possible not only that activation of TLR 8 in microglia could lead to premyelinating oligodendrocyte and axonal injury but also that activation of TLR 8 in developing neurons/axons could result in disturbed axonal development, with axonal retraction, and neuronal apoptosis (Figure). The axonal disturbance could be exacerbated by loss of trophic interactions with the developing oligodendrocyte and in turn could itself lead to impaired oligodendrocyte development.30–35
Figure.
Potential role of toll-like receptors (TLR) in pathogenesis of white matter injury with HPeV3 encephalitis. HPeV3, a small single-stranded (ss) RNA virus, is taken up by brain microglia and the organism’s released ssRNA binds to intracellular TLR 8 to initiate the innate immune response. The resulting release of reactive oxygen and nitrogen species and proinflammatory cytokines would lead to pre-oligodendrocyte (Pre-OL) and axonal injury. Additionally the ssRNA of HPeV3, after infecting the neuron, could activate TLR 8 in the cell body but especially in the developing axon and growth cone (where TLR 8 is most abundant). The result would be axonal retraction and neuronal apoptosis (see text for details).
Thus, this interesting paper by Verboon-Maciolek et al.1 is important for several reasons. The work highlights a previously unrecognized and, likely, common type of neonatal viral encephalitis, provides new information concerning the etiologic evaluation of neonatal seizures, and suggests the broader possibility that viral encephalitis may co-opt the innate immune system to produce both destructive and dysgenetic effects.
Referemces
- 1.Verboon-Maciolek MA, Groenendaal F, Hahn CD, et al. Human parechovirus causes encephalitis with white matter injury in neonates. Ann Neurol. 2008 doi: 10.1002/ana.21445. in press. [DOI] [PubMed] [Google Scholar]
- 2.Joki-Korpela P, Hyypia T. Parechoviruses, a novel group of human picornaviruses. Ann Med. 2001;33:466–471. doi: 10.3109/07853890109002095. [DOI] [PubMed] [Google Scholar]
- 3.Baumgarte S, de Souza Luna LK, Grywna K, et al. Prevalence, types, and RNA concentrations of human parechoviruses, including a sixth parechovirus type, in stool samples from patients with acute enteritis. J Clin Microbiol. 2008;46:242–248. doi: 10.1128/JCM.01468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verboon-Maciolek MA, Groenendaal F, Cowan F, et al. White matter damage in neonatal enterovirus meningoencephalitis. Neurology. 2006;66:1267–1269. doi: 10.1212/01.wnl.0000208429.69676.23. [DOI] [PubMed] [Google Scholar]
- 5.Volpe JJ. Neurology of the newborn. 5th ed. Philadelphia: Elsevier; 2008. [Google Scholar]
- 6.Verboon-Maciolek MA, Krediet TG, Gerards LJ, et al. Clinical and epidemiologic characteristics of viral infections in a neonatal intensive care unit during a 12-year period. Pediatr Infect Dis J. 2005;24:901–904. doi: 10.1097/01.inf.0000180471.03702.7f. [DOI] [PubMed] [Google Scholar]
- 7.Benschop KS, Schinkel J, Minnaar RP, et al. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin Infect Dis. 2006;42:204–210. doi: 10.1086/498905. [DOI] [PubMed] [Google Scholar]
- 8.Verboon-Maciolek MA, Krediet TG, Gerards LJ, et al. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J. 2008;27:241–245. doi: 10.1097/INF.0b013e31815c1b07. [DOI] [PubMed] [Google Scholar]
- 9.Noordhoek GT, Weel JF, Poelstra E, et al. Clinical validation of a new real-time PCR assay for detection of enteroviruses and parechoviruses, and implications for diagnostic procedures. J Clin Virol. 2008;41:75–80. doi: 10.1016/j.jcv.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.de Vries LS, Verboon-Maciolek MA, Cowan FM, Groenendaal F. The role of cranial ultrasound and magnetic resonance imaging in the diagnosis of infections of the central nervous system. Early Hum Dev. 2006;82:819–825. doi: 10.1016/j.earlhumdev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Friede RL. Developmental Neuropathology. 2nd ed. NY: Springer-Verlag: 1989. [Google Scholar]
- 12.Back SA, Luo NL, Borenstein NS, et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Back SA, Luo NL, Borenstein NS, et al. Arrested oligodendrocyte lineage progression during human cerebral white matter development: Dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol. 2002;61:197–211. doi: 10.1093/jnen/61.2.197. [DOI] [PubMed] [Google Scholar]
- 14.Haynes RL, Billiards SS, Borenstein NS, et al. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 2008;63:656–661. doi: 10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volpe JJ. Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J Pediatr. 2008 doi: 10.1016/j.jpeds.2008.04.057. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verboon-Maciolek MA, Thijsen SF, Hemels MA, et al. Inflammatory mediators for the diagnosis and treatment of sepsis in early infancy. Pediatr Res. 2006;59:457–461. doi: 10.1203/01.pdr.0000200808.35368.57. [DOI] [PubMed] [Google Scholar]
- 17.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 18.Triantafilou K, Vakakis E, Orthopoulos G, et al. TLR8 and TLR7 are involved in the host's immune response to human parechovirus 1. Eur J Immunol. 2005;35:2416–2423. doi: 10.1002/eji.200526149. [DOI] [PubMed] [Google Scholar]
- 19.Kadhim HJ, Tabarki B, De Prez C, et al. Interleukin-2 in the pathogenesis of perinatal white matter damage. Neurology. 2002;58:1125–1128. doi: 10.1212/wnl.58.7.1125. [DOI] [PubMed] [Google Scholar]
- 20.McCarran WJ, Goldberg MP. White matter axon vulnerability to AMPA/kainate receptor-mediated ischemic injury is developmentally regulated. J Neurosci. 2007;27:4220–4229. doi: 10.1523/JNEUROSCI.5542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68:S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Haynes RL, Sidman RL, Vartanian T. TLR8: an innate immune receptor in brain, neurons and axons. Cell Cycle. 2007;6:2859–2868. doi: 10.4161/cc.6.23.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehnardt S, Lachance C, Patrizi S, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehnardt S, Massillon L, Follet P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Baud O, Vartanian T, et al. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehnardt S, Henneke P, Lien E, et al. A mechanism for neurodegeneration induced by Group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177:583–592. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Ramenaden R, Peng J, et al. TNFα mediates LPS-induced microglial toxicity to developing oligodendrocytes when astrocytes are present. J Neurosci. 2008 doi: 10.1523/JNEUROSCI.3995-07.2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Y, Li J, Chiu I, et al. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haynes RL, Borenstein NS, DeSilva TM, et al. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484:156–167. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- 30.Roy K, Murtie JC, El-Khodor BF, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci USA. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakazawa T, Nakazawa C, Matsubara A, et al. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26:12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkins A, Majed H, Layfield R, et al. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: A novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lappe-Siefke C, Goebbels S, Gravel M, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 34.Dai X, Lercher LD, Clinton PM, et al. The trophic role of oligodendrocytes in the basal forebrain. J Neurosci. 2003;23:5846–5853. doi: 10.1523/JNEUROSCI.23-13-05846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du YZ, Dreyfus CF. Oligodendrocytes as providers of growth factors. J Neurosci. 2002;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]