Abstract
We investigated the treatment of experimental autoimmune encephalomyelitis (EAE) in mice with Niaspan, an agent used to elevate high density lipoprotein (HDL). EAE mice were treated with Niaspan starting on the immunization or clinical onset day. Neurological functional recovery was significantly increased in the Niaspan treated mice (100mg/kgbw) compared to the controls. Inflammatory infiltrates were significantly reduced in the Niaspan treatment group compared to the EAE controls. HDL level, intact myelin area, newly formed oligodendrocytes, regenerating axons, gene and protein levels of sonic hedgehog (Shh)/Gli1 were significantly increased in the Niaspan treated mice compared to EAE controls. These data indicate that Niaspan treatment improved functional recovery after EAE, possibly, via reducing inflammatory infiltrates and demyelination areas, and stimulating oligodendrogenesis and axonal regeneration. Niaspan mediated activation of Shh/Gli1 pathway may promote functional recovery post EAE.
Keywords: experimental autoimmune encephalomyelitis, Niaspan, Niacin, high-density lipoprotein, oligodendrocyte cell, demyelination, oligodendrogenesis, axon, sonic hedgehog
Introduction
The myelin sheath is formed by extensions of oligodendrocyte cell membranes that wrap around the axon. Seventy to 80% of the dry weight of myelin consists of lipids, a proportion that is significantly higher than in most other cell membranes(Hu et al., 2004). Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS)(Hemmer et al., 2002; Lucchinetti et al., 2000). Damage to the myelin sheath in MS occurs through membrane de-adhesion and swelling and ultimate vesiculation(Genain et al., 1999), and demyelinated axons are vulnerable to damage by inflammatory mediators, as proteolytic enzymes, cytokines, oxidative products, and free radicals are produced by activated immune and glial cells(Kuhlmann et al., 2002; Lassmann, 2003). EAE is an animal model of MS. Although much effort has been expended on gene, immune and cell therapy of MS/EAE(Gold et al., 2006), currently approved MS treatments, however, are only partly effective and are often limited by side-effects or toxicities. Generating safe and effective therapies remain a challenge.
In neurodegenerative diseases, large amounts of lipid are released from demyelination areas, and much of the cholesterol is stored by neural cells and reused during regeneration(Stewart et al., 1998). High-density lipoprotein (HDL) extracts cholesterol from cells containing excess cholesterol. Approximately, thirty percent of blood cholesterol is carried by HDL. HDL in vitro, forms myelin buds (liposomes)(Adams and Abdulla, 1978). HDL possesses anti-inflammatory properties and reduces the expression of adhesion molecules in endothelial cells(Barter et al., 2004), maintains endothelial integrity, and inhibits blood cell adhesion to vascular endothelium(Calabresi et al., 2003).
Niacin, also known as nicotinic acid or vitamin B3, lowers the concentration of all atherogenic plasma lipids/lipoproteins and raises levels of the protective HDL. Niacin is used to prevent and treat clinical atherosclerosis, and to promote the health of the myelin sheath(Adams and Abdulla, 1978; Nakashima and Suzue, 1982). Niaspan is a prolonged release formulation of Niacin, which has considerable advantages over both immediate release and slow release formulations of this drug (Carlson, 2004). The major early side effect of immediate release Niacin, flushing, is reduced with Niaspan, and the hepatotoxic effects with slow release Niacin are not present with Niaspan (Carlson, 2004; Guyton, 2004; Vogt et al., 2006). Niaspan has been clinically recommended to administer daily (Birjmohun et al., 2004; Capuzzi et al., 1998; Carlson, 2004; Goldberg, 1998; Guyton et al., 1998; Knopp et al., 1998; Morgan et al., 1998; Vogt et al., 2006).
It has long been thought that mature oligodendrocytes in the adult mammalian central nervous system (CNS) are post-mitotic and are unable to proliferate in response to injury (Ludwin, 1984). However, abundant oligodendrocyte progenitor cells exist in the white and gray matter of normal CNS, and are present in MS lesions, represent a viable target for therapies intended to enhance remyelination in MS patients (Chang et al., 2000). These proliferative oligodendrocyte progenitor cells contribute to remyelination (Althaus et al., 1992; Carroll and Jennings, 1994; Ffrench-Constant and Raff, 1986; Gensert and Goldman, 1996; Gensert and Goldman, 1997; Godfraind et al., 1989; Jiang et al., 2008; Keirstead et al., 1998; Ludwin, 1979; Ludwin, 1984; Prayoonwiwat and Rodriguez, 1993; Prineas et al., 1989; Raff et al., 1983; Raine et al., 1988; Raine et al., 1981; Rodriguez, 1991; Vick et al., 1992).
N20.1 cells are premature oligodendrocytes (Paez et al., 2004; Verity et al., 1993); they are widely used and useful cell models to study the cellular and molecular mechanisms involved in the development, maturation and possibly formation of myelin by oligodendrocytes in the mammalian brain (Allamargot and Gardinier, 2007; Boullerne et al., 2001; Campagnoni et al., 2001; Foster et al., 1995; Garcia et al., 2007; Newman et al., 1995; Paez et al., 2005; Paez et al., 2004; Paez et al., 2006; Studzinski and Benjamins, 2001; Studzinski et al., 1999; Zhang et al., 2008). In the present study, we will employ the N20.1 cells and focus on cell proliferation after Niacin treatment, and elucidate the underlying mechanisms.
Sonic hedgehog (Shh) is a member of the family of the hedgehog proteins. It is critical for oligodendrocyte development, including induction, survival, proliferation and migration of oligodendrocytes and control of axon growth(Dubois-Dalcq and Murray, 2000; Marti and Bovolenta, 2002; Merchan et al., 2007; Seifert et al., 2005; Sussman et al., 2002). Despite these various activities, it appears that the Shh signaling pathway is well conserved and that the same mechanisms are utilized to achieve a variety of cellular responses(Marti and Bovolenta, 2002). Shh binds to the transmembrane receptor protein, patched, to activate the transmembrane receptor, smoothened(Ingham and McMahon, 2001), and induces a complex series of intracellular reactions that target the Gli family of transcription factors(Ruiz i Altaba et al., 2002). Gli1 is the principal effector of Shh signaling in neural progenitor cells(Ahn and Joyner, 2005) (Wang et al., 2007).
In this manuscript, we demonstrate that Niaspan is an effective restorative treatment for EAE, enhances functional recovery and white-matter remodeling, and stimulates the Shh pathway.
Methods
In Vitro Oligodendrocyte Proliferation
We employed an oligodendrocyte cell line to measure cell proliferation. An immortalized mouse premature oligodendrocyte cell line (N20.1, generously provided by Dr. Anthony Campagnoni, University of California at Los Angeles) was obtained from mouse primary cultures of oligodendrocytes conditionally immortalized by transformation with a temperature-sensitive large T-antigen(Verity et al., 1993). N20.1 cells grow constantly in Dulbecco's modified Eagle's medium (DMEM)/F12 with 10% fetal bovine serum (FBS) and G418 (100 µg/ml) at 34°C (permissive temperature), and differentiate in DMEM/F12/1%FBS and G418 at 39°C (nonpermissive temperature)(Paez et al., 2004). Therefore, in the present experiments, the N20.1 cell lines were placed in DMEM/F12 high glucose (Invitrogen), with 3.6 g/L Dextrose anhydrous, 3.38 g/L HEPES, 2.16 g/L sodium bicarbonate, 90 mg/L Gentamicin, 1%FBS and 100 µg/ml G418 at 39°C (nonpermissive temperature) for 7 days.
Niacin (Sigma) was used in all the in vitro experiments. N20.1 cells were incubated in 4 groups (n=6/group, 6 wells were used in each group): (a) regular cell culture medium for control; (b) 10mM Niacin; (c) 10mM Niacin with 5uM cyclopamine (Calbiochem), which is a specific inhibitor of smoothened(Wang et al., 2007); (d) 80ug/ml HDL (Calbiochem). N20.1 cells were treated for 12h and 20ug/ml bromodeoxyuridine (BrdU, Sigma) was added to the cell cultures for 2h. Proliferation (BrdU immunostaining) of the oligodendrocytes was measured. Numbers of BrdU+ cells were calculated by counting 10 random fields in each well with 6 wells per group. The results are presented as a percentage (positive cells divided by total cells). An additional set of experimental groups were employed for mRNA analyses (n=3/group).
EAE Induction and Treatment
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
EAE was induced in female SJL/J mice (8–10 week old, the Jackson Laboratory) by subcutaneous injection with 100ug myelin proteolipid protein (PLP) (p139–151, HSLGKWLGHPDKF, SynPep Corporation), dissolved in complete Freund’s adjuvant (CFA, Difco Laboratories). On the day of immunization and 48 hours later, pertussis toxin (PT, List Biological laboratories, Inc) 200ng in phosphate buffered saline (PBS) was injected into the mouse tail vein(Zhang et al., 2005a). Mice were scored daily for clinical signs of EAE, as follows: 0, healthy; 1, loss of tail tone; 2, hind limb weakness; 3, hind limb paralysis; 4, hind limb and forelimb paralysis; 5, moribund or dead(Zhang et al., 2005a).
Mice were randomly divided into: 1). Niaspan treatment group: Niaspan (dissolved in saline; Kos Pharmaceuticals, Cranbury, NJ) at doses of 100mg, 200mg and 400mg/kgbw were gavaged once a day for 30 consecutive days starting on the day of clinical symptom onset (p.o., score ≥1). 2). Niaspan prevention group: the most beneficial dose determined from the Niaspan treatment groups, was gavaged daily starting on the day of immunization (p.i.) until 30 days after clinical onset. 3). EAE control group: EAE mice gavaged with the same volume of saline on the day of immunization until 30 days after clinical onset were used as a control group.
BrdU (100 mg/kgbw) was intraperitoneally injected once a day for 14 consecutive days into EAE mice starting on the day of clinical symptom onset.
Neurological functional tests were evaluated by an examiner blinded to the treatment status of each animal. Functional data were collected on a total of 47 mice in 5 treatment groups including mice receiving Niaspan 100 mg/kgbw p.i. (n=9), 100 mg/kgbw (n=10), 200 mg/kgbw (n=6), and 400 mg/kgbw (n=7) p.o., and EAE control (n=15). Neurological assessments were reported on a scale from 1 to 5, with 5 being the most severe neurological deficit. Scores were measured daily, for 30 days. Mice that died before 30 days received a score of 5 for each day following the death. Average scores and cumulative scores for each treatment group were compared to the EAE control group. Normality of the average and cumulative neurological scores were assessed, and data were not normal. Comparisons of average scores were made using Wilcoxon two-sample tests on data at days 7, 14, 21 and 30. For the cumulative scores, the Kolmogorov-Smirnov two-sample test was performed to calculate the percent neurological improvement in the treated group compared to the control group with the significant improvement detected at 0.05 level.
Tissue Preparation
For morphological study, EAE mice treated with or without Niaspan were euthanized at 30d p.o. Anesthetized mice were intracardiac perfused with saline and followed by 4% paraformaldehyde. The entire spinal cord was extracted from the vertebra and then immersed and fixed in 4% paraformaldehyde. The cervical, thoracic and lumbar spinal cord (C1–C4, T1–T6 and L1–L3) were embedded in paraffin and cut into serial 6-µm thick coronal slides. For gene and protein analysis, normal mice and EAE mice treated with or without Niaspan were euthanized at 15d p.o. The entire spinal cord was extracted quickly and kept in −80°C.
Histopathology and Quantification
Slides were stained with hematoxylin and eosin (HE) to detect inflammatory infiltrates(Zhang et al., 2005a; Zhang et al., 2005b).
Oligodendrocytes were identified by antibodies O4 (1:100, Chemicon) or MBP (1:50, Abcam, Cambridge, MA). GAP43 (1:100, Abcam) was employed to mark new sprouting axons. A mouse monoclonal antibody (mAb) against BrdU (1:100, Boehringer Mannheim) was employed to identify cell proliferation. Double immunostaining for MBP and GAP43 was used to demonstrate the relationship of myelin and regenerating axons. Double immunostaining O4 and BrdU was performed to identify oligodendrocyte proliferation.
Immunostaining was performed following standard protocols. Slides were treated first with the primary antibody, and then with the antibody conjugated to fluorescein isothiocyanate (FITC, Jackson ImmunoResearch). These slides were then treated with a second primary antibody, and then incubated with antibody conjugated to Cy3 (Vector). Negative control slides for each animal received identical preparations for immunostaining, except that primary antibodies were omitted.
For each animal, 15 transverse sections (5 from cervical, 5 from thoracic and 5 from lumbar, each taken from every 20th slides) were obtained, which encompass the entire spinal cord. The numbers of vessels with inflammatory infiltrates, myelin area and the immunoreactive cells were measured in 10 fields in each 6-µm thick slide digitized under a 40x microscope (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970 MD) interfaced with Micro Computer Imaging Device (MCID) image analysis system (Imaging Research Inc.). The numbers of vessels were then divided by the total area of slides, and data are presented as numbers per mm2. Areas of myelin were determined as the measured MBP+ areas of all of the stained transverse slides, and are presented as the proportional area. The numbers of GAP43+ signals were calculated and divided by the measured areas, and presented as numbers (×102) per mm2.
Data are presented as mean ± SD. Significance between the two groups was examined by using ANOVA analysis. A value of p <0.05 was considered significant.
Real-time RT-PCR Analysis
Quantitative PCR was performed using the SYBR Green real-time PCR method. Total RNA was isolated from spinal cord or cell cultures using the TRIzol (Invitrogene). Quantitative RT-PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA) using three-stage program parameters provided by the manufacturer, as follows; 2 min at 50 °C, 10 min at 95 °C, and then 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Specificity of the produced amplification product was confirmed by examination of dissociation reaction plots. A distinct single peak indicated that a single DNA sequence was amplified during PCR. PCR products were run on 2% agarose gels to confirm that correct molecular sizes were present. Each sample was tested in triplicate, and samples obtained from three independent experiments were used for analysis of relative gene expression using the 2−ΔΔCT method(Livak and Schmittgen, 2001). The following primers for real-time RT-PCR were designed using Primer Express software (ABI): Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (FWD, AGAACATCATCCCTGCATCC; REV, CACATTGGGGGTAGGAACAC); Shh (FWD, CCTTTACCCTACAAGCAGTTTATTG C; REV, GTAATTGGGGGTGAGTTCCTTAAATC); patched (FWD, TAGCGCCTTCTTCTTTTGGA; REV, GTGGAAGTTGGTGGACGAGT); Gli1 (FWD, TCCACACGCCCCCTAGTG; REV, TGGCAACATTTTCGGTGATG); Mash1 (FWD, TCTCCTGGGAATGGACTTTG; REV, GGTTGGCTGTCTGGTTTGTT). One-way analysis of variance followed by Student-Newman-Keuls test was performed. The data are presented as means ± SD. A value of p<0.05 is considered significant.
Western Blot Analysis
Western blots were performed according to published methods(Wang et al., 2007). Protein was isolated from spinal cord or cell cultures using the TRIzol (Invitrogen). Protein concentration in the supernatants of tissue or cell extract was determined using a BCA protein assay kit (Pierce Biotechnology, Inc.). Equal amounts of proteins were loaded on 10% SDS-polyacrylamide gel. After electrophoresis, the proteins were transferred to nitrocellulose membranes, and the blots were subsequently probed with the following antibodies: Shh (N-19) (1:200; Santa Cruz Biotechnology), Gli1 (1:5000; Abcam Inc.), Mash1 (1:250; BD Biosciences), and MBP (1:2000, Chemicon). For detection, horseradish peroxidase-conjugated secondary antibodies were used (1:2000) followed by enhanced chemiluminescence development (Pierce). Normalization of results was ensured by running parallel Western blots with β-actin antibody. The optical density was quantified using an image processing and analysis program (Scion Image, Ederick, MA). One-way analysis of variance followed by Student-Newman-Keuls test was performed. The data are presented as means ± SD. A value of p<0.05 is considered significant.
High-Density Lipoprotein Cholesterol (HDL) Measurement
Whole blood HDL and total cholesterol were measured before treatment and 30 days after Niaspan treatment using CardioChek P•A analyzer and HDL and total Cholesterol check strips (Polymer technology System, Inc. Indianapolis, IN), according to the manufacturer’s instructions. 15ul of blood were collected from the tail vein. Measurement on each animal was repeated three times. The data are presented as mg/dl values.
Results
1. Niacin Treatment Promotes Oligodendrocyte Cell Culture Proliferation
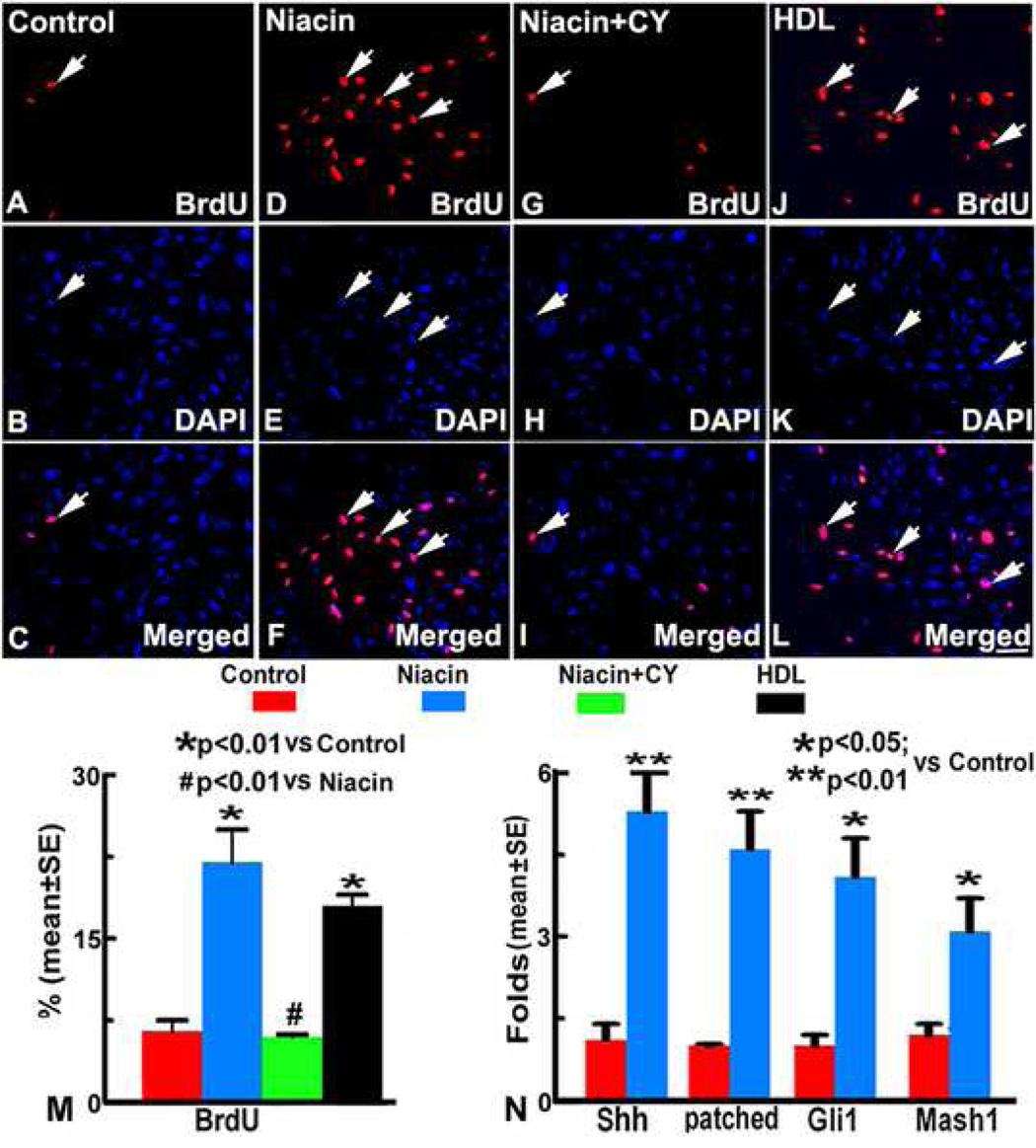
Under 1%FBS and 39°C conditions, most of N20.1 cells exhibited significantly decreased growth rate (Figure 1A–C). Both Niacin and HDL treatment significantly increased the N20.1 cell proliferation, as indicated by BrdU immunostaining, compared with the normal medium group (p<0.01, Figure 1D~F, J~L, M). Niacin induced N20.1 proliferation was significantly reduced by cyclopamine, a pharmacological inhibitor of the Shh signaling pathway (p<0.01, Figure 1G~I, M). RT-PCR derived that mRNA expression of Shh, patched, Gli1 and Mash1 significantly increased in Niacin treated N20.1 cells compared to non treated N20.1 cells (Figure 1N).
Figure 1.
BrdU immunostaining in normal N20.1 cells (A~C), Niacin treated N20.1 cells without and with cyclopamine (CY), (D~F, G~I) and HDL treated N20.1 cells (J~L). Quantitative data (M) show that the proliferation rate of N20.1 cells treated by Niacin or HDL significantly increased compared with normal cells (p<0.01), respectively. The N20.1 cell proliferation significantly decreased after Niacin plus cyclopamine treatment compared with Niacin treatment. RT-PCR analysis (N) shows the mRNA expression in N20.1 cells. mRNA expression of Shh, ptch1, Gli1 and Mash1 were significantly increased after Niacin treatment compared with the normal N20.1 cells (p<0.05). Scale bar in A~L=100µm.
2. Niaspan Treatment Improves Neurological Functional Recovery in EAE Mice
Given the significant oligodendrogenesis effect of Niacin in vitro, we sought to address whether Niacin treatment affects the EAE, which is the major demyelination disease in the CNS. First, we evaluated whether the administration of different doses of Niaspan, a prolonged release formulation of Niacin, reduces neurological deficits in EAE mice. Neurological function of EAE mice treated with or without Niaspan was tested daily until 30 days after clinical symptom onset (Figure 2A~B). Most of SJL/J mice developed a typical course of neurological disability at about 7d after PLP immunization which persisted for at least 30 days.
Figure 2.
The neurological response of EAE mice treated with or without Niaspan. A: Average clinical score shows that significant neurological improvement was present at all observed time points (day 7, 14, 21, 30) with 100mg/kgbw Niaspan treatment (p.i. and p.o.) compared with EAE control (p<0.05); Mice treated with Niaspan 200 or 400 mg/kgbw did not have significantly different average scores compared to controls at any of the observed time points. B: The cumulative clinical score results show a similar trend as that of average clinical score. Mice treated with Niaspan 100 mg/kgbw p.i. had 78% improvement on the cumulative neurological deficits up to 14, 21, and 30 days, compared to controls (p<0.01). Mice treated with Niaspan 100 mg/kgbw p.o. had functional improvement compared to controls at day 14, 21 (p<0.01), and day 30 (p<0.05). No significant differences were observed between Niaspan 200 or 400 mg/kgbw and controls.
Comparisons of average scores were made using Wilcoxon two-sample tests on data at days 7, 14, 21 and 30. Mice treated with Niaspan 100 mg/kgbw p.i. and p.o. had significantly decreased average scores compared to EAE control mice at all observed time points (p<0.05). Mice treated with Niaspan 200 or 400 mg/kgbw did not have significantly different average scores compared to controls at any of the observed time points (Figure 2A).
Results from the comparisons of cumulative scores show there were significant differences between Niaspan 100 mg/kgbw (p.i. and p.o.) and controls at days 14, 21 and 30 (Figure 2B). No differences were observed between Niaspan 200 or 400 mg/kgbw and controls. Mice treated with Niaspan 100 mg/kgbw p.i. had 78% improvement on the cumulative neurological deficits up to 14, 21, and 30 days, compared to controls (p<0.01). Mice treated with Niaspan 100 mg/kgbw p.o. had 77% improvement compared to controls at day 14 (p<0.01), 73% at day 21 (p<0.01), and 60% at day 30 (p<0.05).
We, therefore, chose 100mg/kgbw as the most beneficial dose treatment group for the prophylactic protocol and morphological studies. Niaspan (100mg/kgbw) administration initiated after PLP immunization, for the prophylactic protocol, significantly delayed the onset day (8.9±1.1 vs 7.1±0.7 days, p<0.01) and significantly attenuated the functional deficits of EAE (Figure 2A~B).
3. Niaspan Treatment Reduces Inflammatory Infiltrates in the Spinal Cord of EAE Mice
Using H&E staining, inflammatory infiltrates adjacent to vessels in the spinal cord of EAE mice were evident. However, the numbers of vessels containing inflammatory cell infiltration were significantly reduced in both 100mg/kgbw Niaspan treatment and prevention groups compared with the EAE control group (p<0.01, Figure 3A, B, I).
Figure 3.
H&E staining show inflammatory infiltrates adjacent to vessels (black arrows) in the spinal cord of EAE control mice (A) and Niaspan treated mice (B). Quantitative data show the numbers of vessels containing inflammatory infiltrates (I) were significantly reduced, and intact myelin area (J) and axonal regeneration (K) were significantly increased in the Niaspan (100mg/kgbw) treatment and prevention groups compared with the control group (p<0.05). The double immunostaining by MBP and GAP-43 shows the myelin and regenerating axons in the spinal cord of EAE mice treated with saline (C, E) or Niaspan (D, F). Double immunostaining shows regenerating axons in the demyelinated area (arrows, the merged G, H). Scale bars in D-I=25µm.
4. Niaspan Treatment Increases Oligodendrogenesis and Axonal Regeneration in the Spinal Cord of EAE Mice
Given the significant and robust improvement in neurological outcome, we sought to address whether Niaspan treatment affects myelin protection, remyelination, and axonal regeneration. As indicated by MBP immunostaining, myelin damage and demyelination were obvious after EAE onset (Figure 3C). The proportional area of intact myelin (MBP+) in the spinal cord was significantly increased in the Niaspan treatment and prevention groups compared with the control group (Figure 3D, J). BrdU double immunostaining with the oligodendrocyte marker O4 shows that approximately 5.3±0.4% of oligodendrocytes proliferated in the spinal cord after EAE. Niaspan treatment significantly increased oligodendrocyte proliferation (15±2%, p<0.05). GAP43 is expressed by regenerating axons and is widely used to specifically label and score neuronal regeneration(Cafferty et al., 2004). GAP43+ signal is present in the spinal cord at 30d p.o., and significantly increased after Niaspan treatments (Figure 3E, F, K). Double immunostaining shows regenerating axons in the demyelinated area (Figure 3G, H). Western blots also show increased MBP protein level in the EAE spinal cord after Niaspan treatment (Figure 4B, C).
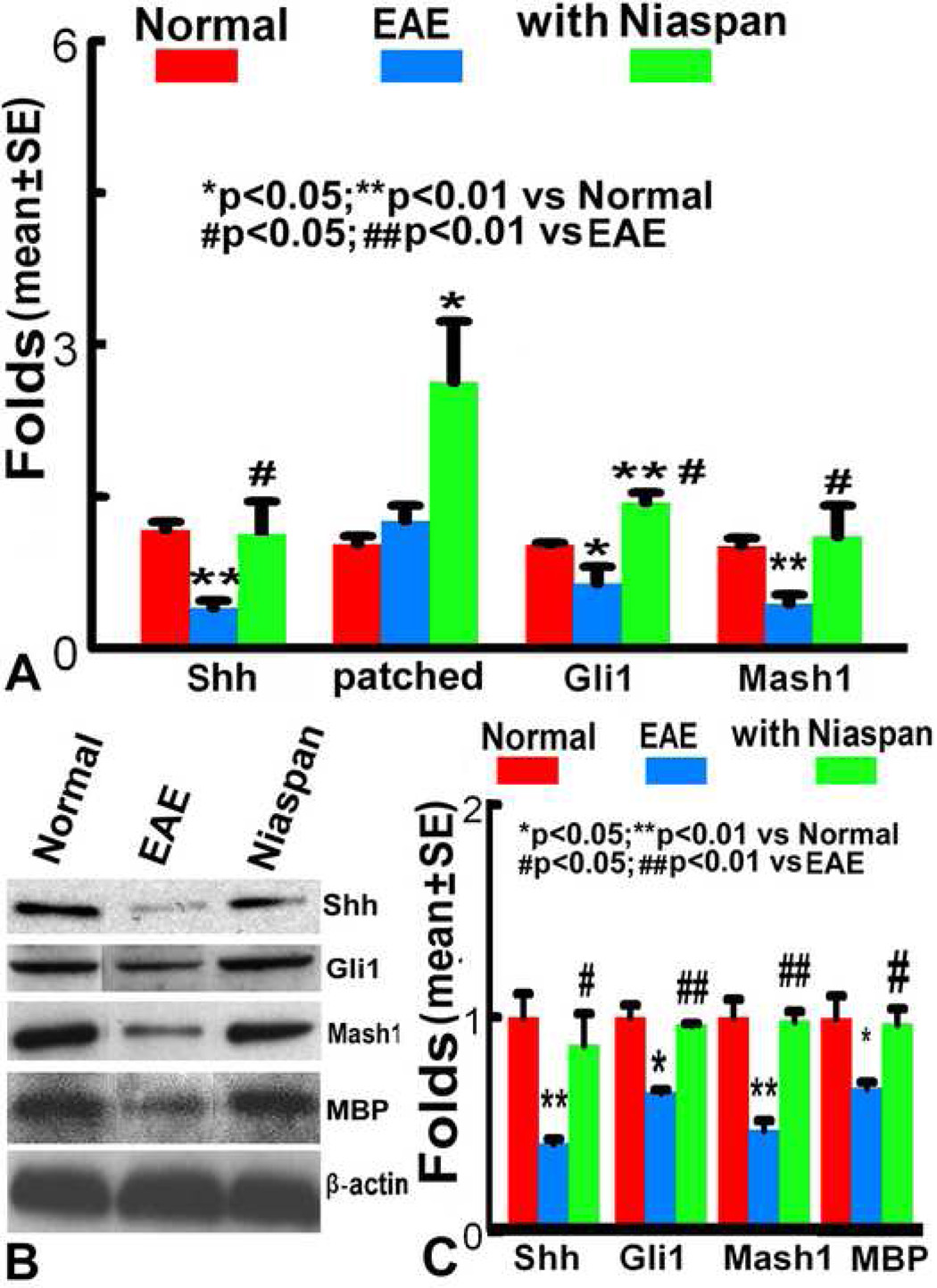
Figure 4.
A. RT-PCR analysis shows the mRNA expression of Shh, Gli1 and Mash1 were significantly decreased in the EAE mice compared with the normal mice, and increased after Niaspan treatment compared with the EAE mice (p<0.05). B~C. Western blot analysis shows Shh, Gli1, Mash1 and MBP protein levels were significantly decreased in spinal cord of the EAE mice compared with normal mice; however, Niaspan treatment increased these protein levels in spinal cord subjected to EAE.
5. Niaspan Promotes the Shh/Gli1 Signaling Pathway
The Shh/Gli1 signaling pathway regulates oligodendrogenesis in the adult rodent CNS(Wang et al., 2007). To determine whether Niaspan induces Shh/Gli1 signaling activation, we examined spinal cord expression of Shh, and its receptors patched and Gli1. Real-time RT-PCR analysis revealed that Shh, and Gli1 mRNA expression were significantly decreased in the EAE spinal cord compared with normal mice, and there is no significant change of patched mRNA; however, gene expression of Shh, patched and Gli1 significantly increased in the spinal cord of Niaspan treated mice (Figure 4A). Western blot analysis revealed that protein levels of Shh and Gli1 were significantly decreased in the EAE spinal cord, and significantly increased after Niaspan treatment (Figure 4B, C).
Mash1, basic helix-loop-helix protein transcription factor, a downstream target of the Shh signaling pathway, plays an important role in oligodendrogenesis(Battiste et al., 2007; Parras et al., 2004). To examine whether Mash1 is a potential target for Niaspan-induced oligodendrogenesis, we examined Mash1 gene expression and protein level. After EAE, mRNA and protein level were significantly decreased in the spinal cord. Niaspan treatment significantly increased Mash1 mRNA expression and protein level in the spinal cord, respectively, compared with that in the EAE control group (p<0.05, Figure 4A–C). These data suggest that Niaspan activates the Shh/Gli1 pathway and Mash1 in the EAE spinal cord, and affects oligodendrogenesis.
6. Niaspan Treatment Increases HDL Level
To test whether Niaspan regulates serum HDL level, we measured HDL before treatment and 30 days after Niaspan treatment. The data show that for all three groups, HDL levels were not significantly different before treatment (p>0.05); however, HDL level significantly increased at 30 days in Niaspan treated and prophylactic groups compared with EAE controls (p<0.05, Figure 5). Total cholesterol level did not change among the 3 groups (data not shown).
Figure 5.
Quantitative data show HDL levels were not significantly different before treatment (p>0.05); however, HDL levels were significantly increased at 30 days in Niaspan treated and prophylactic groups compared with EAE control (p<0.05).
Discussion
In this study, we demonstrate for the first time that Niaspan treatment of EAE starting before or after clinical onset significantly improves neurological functional recovery. Concomitant with this neurological benefit are significant increases of serum HDL level, reduction of inflammation, protection of myelin and induction of oligodendrogenesis and axonal sprouting. These data suggest that Niaspan treatment is an effective pharmacological therapy for EAE mice.
In the present study, we performed a dose-response experiment. The 200 and 400mg dose groups did not provide a superior therapeutic response compared to the 100mg group. The optimal dose for treatment, i.e. best effect at the lowest dose, was therefore selected for the present study. The reason why 100mg group appears superior to the high dose groups is not known. However, these data are consistent with a similar dose-response observed in the Niaspan treatment in the stroke rats (Chen et al., 2007).
Since EAE is an immune-mediated response that recognizes myelin peptide determinants and initiates attacks directly against myelin constituents, and causes myelin destruction and axonal loss(Hemmer et al., 2002; Lucchinetti et al., 2000), the reasonable first step in combating EAE is to suppress the immune onslaught. Our study demonstrated that the inflammatory infiltration was significantly reduced in the Niaspan treatment group compared with the EAE control group. Niaspan treatment increases HDL level, consistent with our previous and other’s studies(Birjmohun et al., 2004) (Chen et al., 2007). HDL possesses anti-inflammatory properties and reduces the expression of adhesion molecules in endothelial cells(Barter et al., 2004), maintains endothelial integrity(Chen et al., 2007), and inhibits blood cell adhesion to vascular endothelium(Calabresi et al., 2003). Therefore, the Niaspan mediated increase in HDL level may contribute to the reduction of inflammatory cell infiltration after EAE.
HDL can not reach the CNS either through fenestrations in the capillary membranes or through paracellular diffusion. However, apolipoprotein A-I (apoA-I), the primary protein constituent of HDL, can be bound by the HDL receptor scavenger receptor class B type I (SR-BI) which is expressed in brain capillary endothelial cells and contributes to selective uptake of HDL-C movement across the BBB (Goti et al., 2001). HDL-C is taken up by distal axons and transported to cell bodies for use in axonal regeneration (Posse De Chaves et al., 2000). These studies suggest that HDL can be increased in the CNS through an intact blood brain barrier. However, we note, that in the present study, HDL-C or apoA-I were not measured in the CNS of EAE mice. We investigated the effects of Niaspan on myelin repair/remyelination. Widespread death of oligodendrocytes occurs in MS/EAE leading to demyelination(Gold et al., 2006). BrdU and O4 (an oligodendrocyte marker) double immunostaining demonstrated oligodendrocyte proliferation in the spinal cord of EAE mice; however, this process is largely insufficient to restore neurological function in the adult CNS. Niaspan significantly increased myelin area, BrdU+−O4+ cells, and MBP protein level in the spinal cord of EAE mice, suggesting that Niaspan treatment protects oligodendrocytes and promotes oligodendrogenesis and promotes their differentiation into myelinated oligodendrocytes. This hypothesis is also supported by our in vitro data that Niacin or HDL significantly increased oligodendrocyte proliferation.
We also investigated the effects of Niaspan on axon regeneration. Lipoproteins act to deliver lipids from degenerating cells to cells for new membrane synthesis or storage(Stewart et al., 1998) (Boyles et al., 1989). Axonal regeneration requires the expansion of axonal membranes by the addition of new membrane materials (proteins and lipids) to the growing axon(Posse De Chaves et al., 2000). Lipoproteins in the vicinity of CNS neurons are HDL-sized(Posse De Chaves et al., 2000). Therefore, the Niaspan-mediated increase in HDL level may be a factor contributing to axon regeneration after EAE.
The in vitro study demonstrates that blockage of the Shh pathway with the Shh antagonist cyclopamine abolished the effect of Niacin on oligodendrocyte proliferation. These data suggest that activation of Shh/Gli1 pathway underlies Niacin or Niaspan induced oligodendrogenesis. In MS victims, the total amount of Shh in white matter homogenates was less than in normals (Mastronardi et al., 2003). Previous studies found Shh contributes to oligodendrocyte progenitor cell generation, proliferation and migration(Dubois-Dalcq and Murray, 2000) (Merchan et al., 2007; Murray et al., 2002; Nery et al., 2001; Orentas et al., 1999), facilitates remyelination(Seifert et al., 2005), and controls axon growth(Marti and Bovolenta, 2002). Mash1, a bHLH transcription factor, is a downstream target of the Shh signaling pathway, and may regulate cell survival, proliferation, and differentiation in the oligodendrocyte lineage(Wang et al., 2007) (Battiste et al., 2007; Parras et al., 2004). Our data indicate that Niacin treatment increased Shh, patched, Gli1 and Mash1 mRNA expression in vitro and in vivo after Niacin treatment, and in vivo protein data revealed that Shh, Gli1 and Mash1 levels increased in the spinal cord compared with non Niacin treated animal, implying Niacin enhances oligodendrogenesis which may be mediated by the Shh signaling pathway and its downstream target, Mash1.
In summary, our data indicate that Niaspan treatment improves neurological functional recovery, after EAE in mice, possibly via, reducing inflammatory infiltrates and demyelination, and by increasing oligodendrogenesis and axonal regeneration. Activation of the Shh/Gli1 pathway may underlie Niaspan’s restorative effects on EAE in mice.
Acknowledgements
This work was supported by the Benson Ford Foundation, NIH grants PO1 NS42345 and RO1 NS45041.
The authors thank Mr. Alex Zacharek, Dr. Mark Katakowski and Qinge Lu for their technical assistance and Deborah Jewell for secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Adams CW, Abdulla YH. The action of human high density lipoprotein on cholesterol crystals. Part 1. Light-microscopic observations. Atherosclerosis. 1978;31:465–471. doi: 10.1016/0021-9150(78)90142-9. [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Allamargot C, Gardinier MV. Alternative isoforms of myelin/oligodendrocyte glycoprotein with variable cytoplasmic domains are expressed in human brain. J Neurochem. 2007;101:298–312. doi: 10.1111/j.1471-4159.2006.04296.x. [DOI] [PubMed] [Google Scholar]
- Althaus HH, et al. Nerve growth factor induces proliferation and enhances fiber regeneration in oligodendrocytes isolated from adult pig brain. Neurosci Lett. 1992;135:219–223. doi: 10.1016/0304-3940(92)90440-i. [DOI] [PubMed] [Google Scholar]
- Barter PJ, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- Battiste J, et al. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–293. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Birjmohun RS, et al. Increasing HDL cholesterol with extended-release nicotinic acid: from promise to practice. Neth J Med. 2004;62:229–234. [PubMed] [Google Scholar]
- Boullerne AI, et al. Role of calcium in nitric oxide-induced cytotoxicity: EGTA protects mouse oligodendrocytes. J Neurosci Res. 2001;63:124–135. doi: 10.1002/1097-4547(20010115)63:2<124::AID-JNR1004>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Boyles JK, et al. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest. 1989;83:1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, et al. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci. 2004;24:4432–4443. doi: 10.1523/JNEUROSCI.2245-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi L, et al. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler Thromb Vasc Biol. 2003;23:1724–1731. doi: 10.1161/01.ATV.0000094961.74697.54. [DOI] [PubMed] [Google Scholar]
- Campagnoni CW, et al. Identification of genes in the oligodendrocyte lineage through the analysis of conditionally immortalized cell lines. Dev Neurosci. 2001;23:452–463. doi: 10.1159/000048732. [DOI] [PubMed] [Google Scholar]
- Capuzzi DM, et al. Efficacy and safety of an extended-release niacin (Niaspan): a longterm study. Am J Cardiol. 1998;82:74U–81U. doi: 10.1016/s0002-9149(98)00731-0. discussion 85U–86U. [DOI] [PubMed] [Google Scholar]
- Carlson LA. Niaspan, the prolonged release preparation of nicotinic acid (niacin), the broad-spectrum lipid drug. Int J Clin Pract. 2004;58:706–713. doi: 10.1111/j.1368-5031.2004.00233.x. [DOI] [PubMed] [Google Scholar]
- Carroll WM, Jennings AR. Early recruitment of oligodendrocyte precursors in CNS demyelination. Brain. 1994;117(Pt 3):563–578. doi: 10.1093/brain/117.3.563. [DOI] [PubMed] [Google Scholar]
- Chang A, et al. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Murray K. Why are growth factors important in oligodendrocyte physiology? Pathol Biol (Paris) 2000;48:80–86. [PubMed] [Google Scholar]
- Ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- Foster LM, et al. Conditionally immortalized oligodendrocyte cell lines migrate to different brain regions and elaborate 'myelin-like' membranes after transplantation into neonatal shiverer mouse brains. Dev Neurosci. 1995;17:160–170. doi: 10.1159/000111284. [DOI] [PubMed] [Google Scholar]
- Garcia CI, et al. Differential gene expression during development in two oligodendroglial cell lines overexpressing transferrin: a cDNA array analysis. Dev Neurosci. 2007;29:413–426. doi: 10.1159/000097317. [DOI] [PubMed] [Google Scholar]
- Genain CP, et al. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. In vivo characterization of endogenous proliferating cells in adult rat subcortical white matter. Glia. 1996;17:39–51. doi: 10.1002/(SICI)1098-1136(199605)17:1<39::AID-GLIA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Godfraind C, et al. In vivo analysis of glial cell phenotypes during a viral demyelinating disease in mice. J Cell Biol. 1989;109:2405–2416. doi: 10.1083/jcb.109.5.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R, et al. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- Goldberg AC. Clinical trial experience with extended-release niacin (Niaspan): dose-escalation study. Am J Cardiol. 1998;82:35U–38U. doi: 10.1016/s0002-9149(98)00952-7. discussion 39U–41U. [DOI] [PubMed] [Google Scholar]
- Goti D, et al. Scavenger receptor class B, type I is expressed in porcine brain capillary endothelial cells and contributes to selective uptake of HDL-associated vitamin E. J Neurochem. 2001;76:498–508. doi: 10.1046/j.1471-4159.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- Guyton JR. Extended-release niacin for modifying the lipoprotein profile. Expert Opin Pharmacother. 2004;5:1385–1398. doi: 10.1517/14656566.5.6.1385. [DOI] [PubMed] [Google Scholar]
- Guyton JR, et al. Effectiveness of once-nightly dosing of extended-release niacin alone and in combination for hypercholesterolemia. Am J Cardiol. 1998;82:737–743. doi: 10.1016/s0002-9149(98)00448-2. [DOI] [PubMed] [Google Scholar]
- Hemmer B, et al. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci. 2002;3:291–301. doi: 10.1038/nrn784. [DOI] [PubMed] [Google Scholar]
- Hu Y, et al. Synergistic interactions of lipids and myelin basic protein. Proc Natl Acad Sci U S A. 2004;101:13466–13471. doi: 10.1073/pnas.0405665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jiang S, et al. Remyelination after chronic spinal cord injury is associated with proliferation of endogenous adult progenitor cells after systemic administration of guanosine. Purinergic Signal. 2008;4:61–71. doi: 10.1007/s11302-007-9093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, et al. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Knopp RH, et al. Equivalent efficacy of a time-release form of niacin (Niaspan) given once-a-night versus plain niacin in the management of hyperlipidemia. Metabolism. 1998;47:1097–1104. doi: 10.1016/s0026-0495(98)90284-0. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T, et al. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–2212. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Axonal injury in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2003;74:695–697. doi: 10.1136/jnnp.74.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ludwin SK. An autoradiographic study of cellular proliferation in remyelination of the central nervous system. Am J Pathol. 1979;95:683–696. [PMC free article] [PubMed] [Google Scholar]
- Ludwin SK. Proliferation of mature oligodendrocytes after trauma to the central nervous system. Nature. 1984;308:274–275. doi: 10.1038/308274a0. [DOI] [PubMed] [Google Scholar]
- Marti E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- Mastronardi FG, et al. The amount of sonic hedgehog in multiple sclerosis white matter is decreased and cleavage to the signaling peptide is deficient. Mult Scler. 2003;9:362–371. doi: 10.1191/1352458503ms924oa. [DOI] [PubMed] [Google Scholar]
- Merchan P, et al. Sonic hedgehog promotes the migration and proliferation of optic nerve oligodendrocyte precursors. Mol Cell Neurosci. 2007;36:355–368. doi: 10.1016/j.mcn.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Morgan JM, et al. A new extended-release niacin (Niaspan): efficacy, tolerability, and safety in hypercholesterolemic patients. Am J Cardiol. 1998;82:29U–34U. doi: 10.1016/s0002-9149(98)00732-2. discussion 39U–41U. [DOI] [PubMed] [Google Scholar]
- Murray K, et al. Sonic hedgehog is a potent inducer of rat oligodendrocyte development from cortical precursors in vitro. Mol Cell Neurosci. 2002;19:320–332. doi: 10.1006/mcne.2001.1079. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Suzue R. Effect of nicotinic acid on myelin lipids in brain of developing rat. J Nutr Sci Vitaminol (Tokyo) 1982;28:491–500. doi: 10.3177/jnsv.28.491. [DOI] [PubMed] [Google Scholar]
- Nery S, et al. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;128:527–540. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- Newman SL, et al. Myelinogenic potential of an immortalized oligodendrocyte cell line. J Neurosci Res. 1995;40:680–693. doi: 10.1002/jnr.490400514. [DOI] [PubMed] [Google Scholar]
- Orentas DM, et al. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419–2429. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- Paez PM, et al. Overexpression of human transferrin in two oligodendroglial cell lines enhances their differentiation. Glia. 2005;52:1–15. doi: 10.1002/glia.20214. [DOI] [PubMed] [Google Scholar]
- Paez PM, et al. Apotransferrin promotes the differentiation of two oligodendroglial cell lines. Glia. 2004;46:207–217. doi: 10.1002/glia.20001. [DOI] [PubMed] [Google Scholar]
- Paez PM, et al. Expression of myelin basic protein in two oligodendroglial cell lines is modulated by apotransferrin through different transcription factors. J Neurosci Res. 2006;83:606–618. doi: 10.1002/jnr.20750. [DOI] [PubMed] [Google Scholar]
- Parras CM, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse De Chaves EI, et al. Uptake of lipoproteins for axonal growth of sympathetic neurons. J Biol Chem. 2000;275:19883–19890. doi: 10.1074/jbc.275.26.19883. [DOI] [PubMed] [Google Scholar]
- Prayoonwiwat N, Rodriguez M. The potential for oligodendrocyte proliferation during demyelinating disease. J Neuropathol Exp Neurol. 1993;52:55–63. doi: 10.1097/00005072-199301000-00007. [DOI] [PubMed] [Google Scholar]
- Prineas JW, et al. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989;61:489–503. [PubMed] [Google Scholar]
- Raff MC, et al. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raine CS, et al. Oligodendrocyte proliferation and enhanced CNS remyelination after therapeutic manipulation of chronic relapsing EAE. Ann N Y Acad Sci. 1988;540:712–714. doi: 10.1111/j.1749-6632.1988.tb27222.x. [DOI] [PubMed] [Google Scholar]
- Raine CS, et al. Multiple sclerosis. Oligodendrocyte survival and proliferation in an active established lesion. Lab Invest. 1981;45:534–546. [PubMed] [Google Scholar]
- Rodriguez M. Immunoglobulins stimulate central nervous system remyelination: electron microscopic and morphometric analysis of proliferating cells. Lab Invest. 1991;64:358–370. [PubMed] [Google Scholar]
- Ruiz i Altaba A, et al. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- Seifert T, et al. Differential expression of sonic hedgehog immunoreactivity during lesion evolution in autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2005;64:404–411. doi: 10.1093/jnen/64.5.404. [DOI] [PubMed] [Google Scholar]
- Stewart JE, et al. Receptor binding of an apolipoprotein E-rich subfraction of high density lipoprotein to rat and human brain membranes. Int J Biochem Cell Biol. 1998;30:407–415. doi: 10.1016/s1357-2725(97)00151-9. [DOI] [PubMed] [Google Scholar]
- Studzinski DM, Benjamins JA. Cyclic AMP differentiation of the oligodendroglial cell line N20.1 switches staurosporine-induced cell death from necrosis to apoptosis. J Neurosci Res. 2001;66:691–697. doi: 10.1002/jnr.10003. [DOI] [PubMed] [Google Scholar]
- Studzinski DM, et al. Increased intracellular calcium alters myelin gene expression in the N20.1 oligodendroglial cell line. J Neurosci Res. 1999;57:633–642. [PubMed] [Google Scholar]
- Sussman CR, et al. Extracellular and intracellular regulation of oligodendrocyte development: roles of Sonic hedgehog and expression of E proteins. Glia. 2002;40:55–64. doi: 10.1002/glia.10114. [DOI] [PubMed] [Google Scholar]
- Verity AN, et al. Expression of myelin protein genes and other myelin components in an oligodendrocytic cell line conditionally immortalized with a temperature-sensitive retrovirus. J Neurochem. 1993;60:577–587. doi: 10.1111/j.1471-4159.1993.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Vick RS, et al. Role of adult oligodendrocytes in remyelination after neural injury. J Neurotrauma. 1992;9 Suppl 1:S93–S103. [PubMed] [Google Scholar]
- Vogt A, et al. Evaluation of the safety and tolerability of prolonged-release nicotinic acid in a usual care setting: the NAUTILUS study. Curr Med Res Opin. 2006;22:417–425. doi: 10.1185/030079906x89766. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. The Sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J Biol Chem. 2007;282:32462–32470. doi: 10.1074/jbc.M706880200. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005a;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005b;1034:34–39. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. Bone marrow stromal cells protect oligodendrocytes from oxygen-glucose deprivation injury. J Neurosci Res. 2008;86:1501–1510. doi: 10.1002/jnr.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]