Abstract
Perennial species with the C4 pathway hold promise for biomass-based energy sources. We have explored the extent that CO2 uptake of such species may be limited by light in a temperate climate. One energetic cost of the C4 pathway is the leakiness (φ) of bundle sheath tissues, whereby a variable proportion of the CO2, concentrated in bundle sheath cells, retrodiffuses back to the mesophyll. In this study, we scale φ from leaf to canopy level of a Miscanthus crop (Miscanthus × giganteus hybrid) under field conditions and model the likely limitations to CO2 fixation. At the leaf level, measurements of photosynthesis coupled to online carbon isotope discrimination showed that leaves within a 3.3-m canopy (leaf area index = 8.3) show a progressive increase in both carbon isotope discrimination and φ as light decreases. A similar increase was observed at the ecosystem scale when we used eddy covariance net ecosystem CO2 fluxes, together with isotopic profiles, to partition photosynthetic and respiratory isotopic flux densities (isofluxes) and derive canopy carbon isotope discrimination as an integrated proxy for φ at the canopy level. Modeled values of canopy CO2 fixation using leaf-level measurements of φ suggest that around 32% of potential photosynthetic carbon gain is lost due to light limitation, whereas using φ determined independently from isofluxes at the canopy level the reduction in canopy CO2 uptake is estimated at 14%. Based on these results, we identify φ as an important limitation to CO2 uptake of crops with the C4 pathway.
Biomass production by perennial plant species can abate greenhouse gas emissions either by increased carbon sink activity and soil organic carbon sequestrations or by displacing fossil fuel emissions in the production of static energy (U.S. Department of Energy, 2006; Somerville, 2007). Perennial plant species with the C4 photosynthetic pathway combine high productivity and resource use efficiency with low requirements for agronomic inputs; thus, they seem well equipped for biomass-based energy production (Lobell et al., 2008). For instance, Miscanthus (Miscanthus × giganteus hybrid) is a perennial C4 grass with a recorded annual dry matter production up to 4 kg m−2 (Heaton et al., 2004a, 2004b).
Thus, attributes of the C4 pathway (potentially high productivity coupled with high nitrogen and water use efficiencies; Sage, 2004) can be coupled to the carbon sequestration potential of a rhizomatous perennial (Hansen et al., 2004; Clifton-Brown et al., 2007). Miscanthus has higher productivity than switchgrass (Panicum virgatum; Heaton et al., 2004a) and has sufficient cold tolerance (Beale et al., 1996; Naidu et al., 2003; Wang et al., 2008a, 2008b) for cultivation in temperate climatic conditions. However, we hypothesized that growth at low temperatures and low light in a temperate climate may reduce the conversion efficiencies of such a crop, and we set out to explore the specific limitations that may occur due to shading in a crop with a high leaf area index.
Our study focused on the leakiness (φ) of bundle sheath (BS) cells to CO2 as a potential bottleneck in the photosynthetic performance of a Miscanthus canopy. The carbon-concentrating mechanism inherent to the C4 pathway relies on spatial separation of carboxylases between mesophyll (M) cells (phosphoenolpyruvate carboxylase) and BS cells (Rubisco). Decarboxylation of organic acids within the BS generates high CO2 concentrations adjacent to Rubisco, thereby suppressing oxygenase activity, but some of this CO2 retrodiffuses and is refixed within the M cells. Such “leakage” in the system occurs through the BS cell walls, facilitated by the symplastic connections between M and BS, which are required for metabolite exchanges. Estimates for BS φ at the leaf level range between 10% and 40% (Osmond and Smith, 1976; Farquhar, 1983; Henderson et al., 1992; Hatch et al., 1995; Fravolini et al., 2002; Cousins et al., 2006, 2008; Tazoe et al., 2006, 2008; Kubasek et al., 2007). External factors associated with increasing φ are low temperature (Henderson et al., 1992; Kubasek et al., 2007), water stress (Farquhar, 1983; Bowman et al., 1989; Buchmann et al., 1996), low nitrogen nutrition (Meinzer and Zhu, 1998), and, most markedly, low light (Henderson et al., 1992; Buchmann et al., 1996; Cousins et al., 2006, 2008; Tazoe et al., 2006, 2008; Kubasek et al., 2007).
The loss of C4 efficiency under low photon flux density (PFD) was recently quantified in a series of laboratory measurements and theoretical modeling studies (Von Caemmerer, 2000; Cousins et al., 2006, 2008; Tazoe et al., 2006, 2008) using real-time carbon isotope discrimination (Δ13C). However, it is not clear if the increase in φ results initially from light limitation of Rubisco activity, relative to phosphoenolpyruvate carboxylase activity, or increased Rubisco oxygenase and photorespiratory costs, or the ATP requirement for phosphoenolpyruvate regeneration (Von Caemmerer and Furbank, 2003), and whether this phenomenon occurs transiently or permanently following the transfer from high-light to low-light conditions.
In this study, we set out to quantify the extent of φ during gas exchange at leaf and canopy scale for a 13-year-old Miscanthus stand at Teagasc Oak Park (Carlow, Ireland). Short-term field measurements of gas exchange and Δ13C at leaf level were used to compute φ and the corresponding loss of carbon assimilation under field conditions using the model for C4 photosynthesis by Von Caemmerer and Furbank (1999) and Von Caemmerer (2000). In parallel, canopy isotopic flux densities, or “isofluxes” (CO2 flux multiplied by isotopic signature), were constructed using eddy covariance data combined with [CO2] and δ13C (carbon isotope composition relative to Vienna Pee Dee Belemnite standard) diurnal vertical profile measurements within the canopy and used to derive canopy Δ13C and φ values. We infer that while the effect of φ is higher at leaf level when measured in a well-coupled cuvette, potential carbon uptake is still significantly reduced by φ when assessed at the canopy scale. We conclude that leakage represents a considerable constraint on C4 productivity, particularly in temperate climates, which is especially significant when the current and future importance of the C4 pathway for food production (e.g. maize [Zea mays], sorghum [Sorghum bicolor], sugarcane [Saccharum officinarum]) and bioenergy production and CO2 mitigation (Miscanthus, switchgrass) is taken into account.
RESULTS
Canopy Development
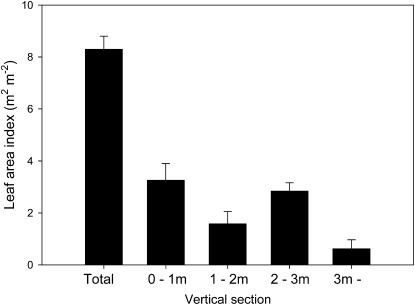
In Figure 1, we show the vertical distribution of leaf area through the canopy. The total leaf area index for the canopy was 8.3 ± 0.51 m2 m−2. A large proportion of this leaf area (2.8 ± 0.54 m2 m−2) was located between 2 and 3 m height, with a smaller proportion (1.4 ± 0.49 m2 m−2) located between 1 and 2 m. The section between 0 and 1 m also had a substantial fraction of the total leaf area (3.2 ± 0.80 m2 m−2).
Figure 1.
Total leaf area index (m2 m−2) and leaf area index separated into 1-m vertical sections. Error bars indicate 1 sd.
Potential Rate of Photosynthesis
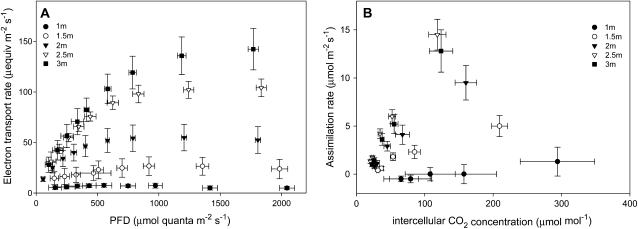
In Figure 2, photosynthetic CO2 uptake is depicted by electron transport capacity (J; determined using chlorophyll fluorescence; Fig. 2A) and CO2 response (determined from gas exchange; Fig. 2B) as a function of height within the canopy. Due to the CO2-concentrating mechanism of C4 photosynthesis, light saturation of J (maximum electron transport capacity; Jmax) typically occurred only at very high levels of PFD. At 3 m, Jmax reached 142.4 ± 20.5 μequiv m−2 s−1 at PFD of 1,769 ± 42 μmol quanta m−2 s−1, whereas at 2.5 m, Jmax was 104.1 ± 8.7 μequiv m−2 s−1 at PFD of 1,834 ± 41 μmol quanta m−2 s−1. At 2 m, Jmax and saturating PFD were lower (54.8 ± 13.1 μequiv m−2 s−1 at 1,205 ± 21.5 μmol quanta m−2 s−1), and they were even lower at 1.5 m (26.6 ± 9.1 μequiv m−2 s−1 at 919 ± 41.6 μmol quanta m−2 s−1). At 1 m, Jmax and saturating PFD were lowest (7.5 ± 1.8 μequiv m−2 s−1 at 553 ± 64.0 μmol quanta m−2 s−1), and there was very little response of J to light intensity. Mitochondrial (dark) respiration was also measured following 5 min in the dark, and averages yielded 0.89 ± 0.29 μmol m−2 s−1 for leaves at 3-m height, 0.71 ± 0.36 μmol m−2 s−1 for leaves at 2-m height, and 0.40 ± 0.25 μmol m−2 s−1 for leaves at 1-m height.
Figure 2.
A, Electron transport rate (J) at different levels of incident PFD for leaves at 1-, 1.5-, 2-, 2.5-, and 3-m height. Error bars indicate 1 sd. B, Light-saturated photosynthetic assimilation rates at different leaf intercellular CO2 concentrations for leaves at 1-, 1.5-, 2-, 2.5-, and 3-m height. Measurements were taken at 1,600 μmol m−2 s−1 PFD and 22°C, and vapor pressure deficit was kept below 1 kPa.
While Jmax at 2.5 and 2 m was reduced to 73% and 38% of values at 3 m, maximum photosynthetic capacity (Amax; Fig. 2B) values at 2.5 and 2 m were 113% and 74% of those at 3 m. Similarly, carboxylation efficiency (CE), calculated from the initial slopes of the CO2 response curves (Fig. 2B), was 0.13 μmol CO2 m−2 s−1 mol μmol−1 at 3 m, 0.15 at 2.5 m, and 0.08 at 2 m. Below 2 m, CE decreased sharply to 0.04 at 1.5 m and 0.007 at 1 m, with a clear separation in CO2 response between lower and higher leaf cohorts. Thus, whereas the light response of J had acclimated to a markedly lower capacity above 2 m, the transition in the decline of Amax and carboxylation efficiency occurred lower in the canopy.
Realized Rate of Photosynthesis
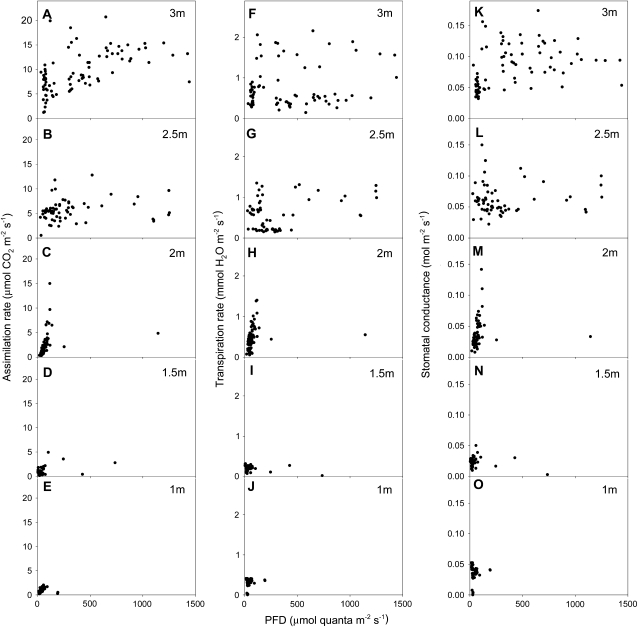
Vertical profiles of net CO2 assimilation (An; Fig. 3, A–E), transpiration (E; Fig. 3, F–J), and stomatal conductance (gs; Fig. 3, K–O), measured in situ on leaves throughout the canopy, are shown as a function of incident PFD. The observations reflected a steep light gradient within the canopy, with incident PFD below 2.5 m seldom higher than 50 μmol quanta m−2 s−1. High in the canopy at 3 m, An frequently was light saturated (10–12 μmol CO2 m−2 s−1; Fig. 3A). At 2.5 m, most CO2 fixation (3–8 μmol CO2 m−2 s−1) occurred at PFD lower than 500 μmol quanta m−2 s−1 (Fig. 3B). Leaves at 2 m (Fig. 3C) were subject to very low light levels (0–75 μmol quanta m−2 s−1), but reasonable values of An were still sustained (0–7 μmol CO2 m−2 s−1). At 1 and 1.5 m, the colimitation of light intensity and photosynthetic capacity clearly reduced An (0–2 μmol CO2 m−2 s−1; Fig. 3, D and E).
Figure 3.
Steady-state levels for photosynthetic assimilation rate (A–E), transpiration rate (F–J), and stomatal conductance (K–O) at different levels of incident PFD. Measurements were taken under field conditions for leaves at (bottom to top) 1-m (n = 53), 1.5-m (n = 59), 2-m (n = 91), 2.5-m (n = 64), and 3-m (n = 84) height.
At 2.5 and 3 m, gs ranged between 0.05 and 0.15 mol m−2 s−1 (Fig. 3, K and L), with generally higher conductances at 3 m than at 2.5 m for comparable light levels, which correspondingly led to higher rates of water loss in leaves at 3 m (Fig. 3, F and G). At 1 and 1.5 m, gs remained below 0.03 mol m−2 s−1 (Fig. 3, N and O), and it increased to just above 0.06 mol m−2 s−1 at 2 m (Fig. 3M), but only for light levels above 50 μmol quanta m−2 s−1. Corresponding transpiration rates were less than 0.4 mmol water m−2 s−1 for leaves at 1 and 1.5 m (Fig. 3, I and J) and increased with light intensity to 1.5 mmol water m−2 s−1 at 2 m (Fig. 3H).
In conclusion, leaves at 2.5 and 3 m receive higher incident PFD than leaves at lower locations and consequently exhibit much higher in situ rates of CO2 fixation and water loss than leaves at 1 and 1.5 m, while leaves at 2 m frequently achieve comparable assimilation and transpiration rates to leaves higher in the canopy.
Leaf Δ13C and φ
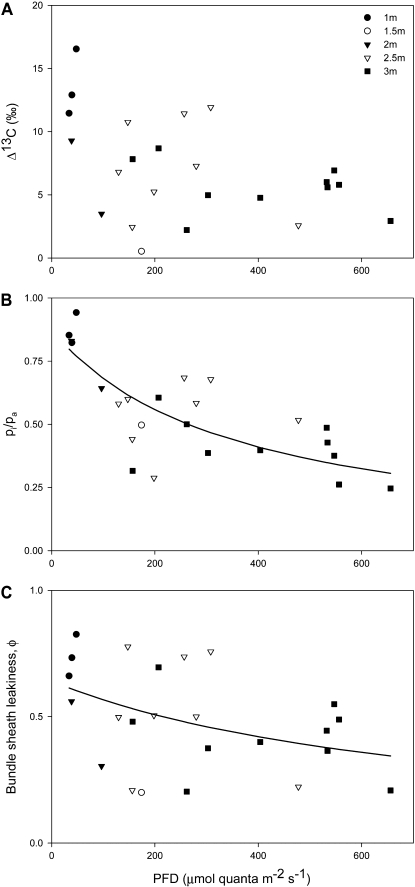
In Figure 4, we show the results for instantaneous Δ13C and corresponding ratios of intercellular CO2 and ambient CO2 partial pressures (pi/pa) from gas exchange. Also, corresponding derived values of φ are shown (derived from Eq. 3 below). Δ13C observations for individual leaves covered a wide range of values. Δ13C above 350 μmol quanta m−2 s−1 PFD showed some variation (Fig. 4A), ranging between 2.6‰ and 6.9‰. At lower PFD, Δ13C measurements were much more variable, ranging from 0.5‰ (1.5 m) to 16.5‰ (1 m). There was also a wide range of pi/pa values derived from gas exchange during the isotopic determinations, ranging from 0.25 (high PFD, high in the canopy) to 0.8 (shaded leaves, low in the canopy). BS φ to CO2, derived from pi/pa and Δ13C using Equation 3 below, showed a similar trend (Fig. 4C), with relatively lower values of φ above 350 μmol quanta m−2 s−1 PFD higher in the canopy and a wide range of values below this threshold light intensity lower in the canopy. At higher light intensity, φ was mostly between 0.2 and 0.5 for leaves at 3 m. Below 350 μmol quanta m−2 s−1 PFD, the range for φ increased with values up to 0.8 (specifically found at 1 m and 48 μmol quanta m−2 s−1).
Figure 4.
A, Short-term Δ13C measured concurrently with gas exchange at natural light intensity for leaves at 1-m (n = 3), 1.5-m (n = 1), 2-m (n = 2), 2.5-m (n = 8), and 3-m (n = 9) height. B, Ratio between internal and ambient CO2 mole fractions. The line plot represents nonlinear regression [pi/pa = (a × b)/(b + PFD), where a = 0.8738, b = 353.2301, r2 = 0.55] used in Figure 6 to calculate canopy φ. C, φ calculated with Equation 1 using measurements of Δ13C (A) and the ratio between internal and ambient CO2 mole fractions (B). The line plot shows the best fit of φ [(a × b)/(b + PFD), where a = 0.6409, b = 763.0974, r2 = 0.16] used in Figure 6 to calculate An.
Canopy CO2 Uptake, Δ13C, and φ
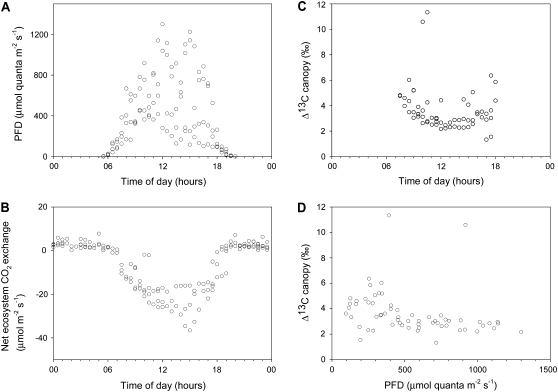
In Figure 5, we present eddy covariance flux measurements of ecosystem CO2 exchange with corresponding levels of incident PFD as well as canopy Δ13C (derived with the eddy covariance-flask method, as described by Bowling et al. [2003]). Except for measurements in the early morning and late afternoon, the canopy was thoroughly mixed and storage flux below the eddy covariance measurements was insignificant (determined by vertical profile measurements of CO2 concentrations within the canopy), apart from measurements between 6:00 and 7:00 am and between 6:00 and 7:00 pm, which were not used for canopy Δ13C determinations.
Figure 5.
Accumulated data for August 28 to September 1, 2007. A, Incident PFD. B, Eddy covariance measurements of net ecosystem CO2 exchange. Direction of net flux is indicated by plus/minus sign, and negative (positive) flux indicates net CO2 uptake (net respiration). C, Canopy Δ13C calculated by assigning isotopic signatures (from regressions in Table I) to gross CO2 uptake derived from daytime net CO2 fluxes (B) and ecosystem respiration based on nighttime regression with soil temperature (y = 0.5505e0.0748T; r2 = 0.887). D, Canopy Δ13C (C) as a function of incident PFD.
Ecosystem net CO2 exchange (Fig. 5B) showed a clear diurnal pattern, with nocturnal respiration mainly correlated with temperature (1°C bin-averaged r2 = 0.887) and net uptake mostly controlled by incident PFD (Fig. 5A). The δ13C of ecosystem respiration was determined by taking the y intercept of a Keeling plot (linear geometric mean regression of 1/[CO2] versus δ13C) assuming a two-source mixing model (Keeling, 1958, 1961). For our 13-year-old stand (Table I), δ13C of respiration was still very much influenced by a C3 carbon isotope signal from the previous cropping system, which is consistent with the low turnover of soil organic carbon in Miscanthus ecosystems (Hansen et al., 2004; Clifton-Brown et al., 2007).
Table I.
Regressions to derive ecosystem gross fluxes (values of parameters ± se)
| Regression | Intercept | Slope | r2 | No. |
|---|---|---|---|---|
| Nocturnal Keeling plot | −25.885 ± 0.801 | 6,334 ± 318 | 0.942 | 25 |
| Daytime [CO2] versus δ13C | 13.519 ± 2.441 | −0.06031 ± 0.00673 | 0.776 | 20 |
Canopy Δ13C followed a diurnal trend, with lower values around midday and slightly increased values in morning and afternoon (Fig. 5C). Figure 5D shows the canopy Δ13C determinations as a function of incident PFD, revealing a similar pattern as the measurements of leaf Δ13C (Fig. 4A). Except for two outliers, canopy Δ13C remained between 1.3‰ and 4.3‰ above 350 μmol m−2 s−1 PFD. Below this light intensity, the range of canopy Δ13C values steadily expanded, with values between 1.5‰ and 6.3‰.
Losses of Potential CO2 Fixation Due to φ
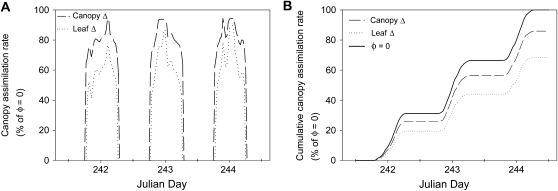
Canopy An was modeled (according to the model by Von Caemmerer and Furbank [1999] and Von Caemmerer [2000], with some adjustments; see Supplemental Appendix S1) for 3 d to show how φ would reduce net carbon gain on the basis of either the leaf or canopy data presented above (Figs. 4 and 5, respectively; hereafter referred to as “leaf level” and “canopy level”). Measured vertical canopy profiles of temperature, PFD, and photosynthetic capacity were used to compute a vertical profile of An, which was then multiplied by leaf area at each height and summed to obtain canopy An. Expression of individual leaves in canopy An, therefore, was weighted by their assimilation rate. In order to estimate the impact of φ, we calculated theoretical values for canopy An without leakage (φ = 0) and compared these values with canopy An obtained with our estimates for φ at leaf or canopy level (Fig. 6A).
Figure 6.
Results for August 29 to August 31, 2007 (Julian days 242–244). A, Modeled canopy An for two scenarios: φ based on leaf level measurements of Δ13C and on canopy Δ13C (rates are depicted as percentages of φ = 0). φ is taken from regression φ = a × b/(b + PFD), where a = 0.6409 and b = 763.0974 for leaf level (Figure 4C) and a = 0.4014 and b = 470.342 for canopy level (based on nonlinear fit of the canopy model in Figure 5C, using Eq. 3). B, Cumulative modeled canopy An for φ = 0, based on leaf level measurements of Δ13C and on canopy Δ13C. Scenarios are compared with total cumulative canopy An with φ = 0.
In Figure 6A, the 100% datum represents canopy An with φ set to zero. Both leaf- and canopy-level modeled values of canopy An were reduced because of φ. When canopy An was calculated with the use of φ based on canopy-level determinations, the reduction due to leakage was lower than when canopy An was derived from leaf-level measurements. The decrease in canopy An would be most severe when light was limiting early in the morning or in the evening, when canopy An was predicted to be only 43% (leaf level) or 62% (canopy level) of canopy An without leakage of CO2. Canopy An at midday was predicted to range from 85% (leaf level) to 94% (canopy level) of canopy An without leakage.
In order to assess the cumulative effect of φ, An was also summed over 3 d. Cumulative canopy An was 68% (leaf level) to 86% (canopy level) of canopy An without CO2 leakage (Fig. 6B), indicating that cumulative losses of canopy CO2 fixation due to φ over 3 d were between 14% and 32%.
DISCUSSION
This study makes a major contribution to understanding the constraints to CO2 uptake within Miscanthus, an important biomass crop. We have evaluated the impact of φ, an intriguing physiological correlate of the C4 pathway, which has previously been investigated largely under laboratory conditions. The data have demonstrated that φ provides a significant constraint to carbon assimilation at leaf and canopy level under field conditions when estimated using real-time Δ13C techniques. We now evaluate the implications of our findings for integrating leaf-level and canopy-level gas-exchange processes and scaling likely physiological constraints to Miscanthus productivity in a temperate climate.
Gas Exchange and Fluorescence
Measurements of assimilation rate under controlled conditions comply well with previous findings. The high-light saturation points in Figure 2A are consistent with reported values for C4 photosynthesis (Usuda, 1987; Beale et al., 1996, 1997). Carboxylation efficiency under saturating light (initial slope; Fig. 2B) as well as gas exchange under field conditions (Fig. 3) at 3 and 2.5 m agreed with findings by Beale et al. (1996, 1997) for the third leaf of Miscanthus (top down) during the second half of the growing season. Below 2 m, An decreased rapidly, which can be explained by leaf age and associated lower enzymatic activity (Usuda, 1984) as well as by early stages of leaf senescence at 1-m height. At canopy level, half-hourly averages of net ecosystem exchange were consistent with long-term measurements above Miscanthus (F. Carroll and M.B. Jones, unpublished data).
In the absence of simultaneous measurements of An and J under field conditions, we are not able to make any direct comparison of how An responded to changes in light use efficiency caused by φ. Also, measurements of the quantum yield of PSII are known to be influenced by quantitative and qualitative light history of the leaf as well as leaf temperature (Edwards and Baker, 1993). Figure 2B was determined under steady-state laboratory conditions, and so interpolating the ratio of J versus An, which should increase at low PFD based on our φ determinations (assuming that cyclic electron flow occurs at a constant fraction of J and absence of other significant electron sinks), could only be inferred on a qualitative basis. However, we note that for a fast-growing crop, leaves that were previously fully exposed were subsequently shaded by the developing canopy. For our observations of Miscanthus, it was evident that Jmax was down-regulated higher in the canopy (2.5 m), while photosynthetic capacity (CE, Amax) was sustained lower in the canopy (2 m). The implications for the effect of φ on the ratio between J and An, therefore, need to be addressed in a more detailed study.
Δ13C
At high PFD (above 350 μmol quanta m−2 s−1), Δ13C for leaves at different heights matched well with values generally assigned to C4 photosynthesis (for reviews, see Farquhar, 1983; O'Leary, 1988). Δ13C at lower PFD tended to be much more variable, ranging from 0.5‰ (1.5 m) to 16.5‰ (1 m), which is consistent with likely changes in the coupling of C4 and C3 cycles, variations in photosynthetic capacity, local microenvironment, and associated enzyme activity between leaves. Also, Δ13C for individual leaves, when calculated using Equation 4 below, becomes increasingly less precise when CO2 depletion is low relative to the CO2 concentration in air. The observation that higher values of Δ13C seemed to occur more often at low PFD deep in the canopy is consistent with previously reported values under low PFD for Amaranthus cruentus (Tazoe et al., 2006, 2008) and Flaveria bidentis (Cousins et al., 2006).
Remarkably, the increase in Δ13C at low PFD was also observed when derived independently at canopy level. Canopy Δ13C mostly reflected Δ13C processes by leaves high in the canopy. Since Δ13C at leaf level is weighted in canopy Δ13C according to the rate of CO2 uptake relative to other leaves, predictably leaves high in the canopy with higher assimilation rates influence canopy An and Δ13C more than leaves at lower locations. The method used to determine canopy Δ13C (Bowling et al., 2003) depended on measurements of ecosystem CO2 exchange together with isotopic compositions of ecosystem respiration and daytime canopy air. A single isotopic respiratory signal had to be assumed for the complete ecosystem, which is likely to be an oversimplification, given the difference between current (C4) and previous (C3) vegetation on soil respiration (Buchmann and Ehleringer, 1998) and light effects on respiration of aboveground biomass (Atkin et al., 1998).
The ecosystem respiratory flux was defined by an exponential regression between soil temperature and nocturnal eddy covariance measurements of CO2 efflux (Lloyd and Taylor, 1994), which could only be firmly established by bin averaging the ecosystem respiration measurements in 1°C increments. A similar problem was found by Bowling et al. (2003) and appears to be quite common in previous reports of net ecosystem partitioning into gross assimilation and respiration fluxes (Reichstein et al., 2005; Knohl et al., 2008). Bowling et al. (2003) explained part of the variability by spatial variation of soil moisture content, and Knohl et al. (2008) implied nonhomogeneity of the soil profile. Both factors may have contributed in our study, especially since diurnal measurements of soil respiration as well as soil temperature and moisture content were also spatially variable (data not shown).
Increased Δ13C at Low Light Intensity
Our results support previous observations of an increase in Δ13C at low light intensity. The underlying mechanisms have been analyzed extensively. Based on studies of photosynthetic behavior in C4 plants, Leegood et al. (1989) argued that the energy supply for the assimilatory pathway (production of ATP and NADPH) declines drastically following a transition from high to low PFD. Stimulation of cyclic electron flow and Q-cycle activity (Furbank et al., 1990) might make up for part of the ATP shortage (by cyclic partitioning of electrons around the PSI complex and altering the ATP-NADPH production ratio). However, we can find no evidence in the literature for increased activity of these alternative electron cycling processes as a result of lower PFD; so ultimately, photosynthesis remains limited by the energy supply.
Energy limitation could increase measured Δ13C in several ways. First, an increase in the relative contribution from mitochondrial respiration under low light could vary Δ13C (Duranceau et al., 2001), particularly if respiratory CO2 is enriched in 13C (Ghashghaie et al., 2003). Alternatively, Henderson et al. (1992) stated that with reduced phosphoenolpyruvate regeneration slowing C4 cycle activity under low light, a decrease in the BS CO2 concentration or CO2-O2 ratio would allow the relative contribution of photorespiration to increase. The extra energy needed for the photorespiratory cycle would limit C3 cycle activity compared with C4 cycle activity, which would lead to an increase in leakage of CO2 (Von Caemmerer et al., 1997a, 1997b) from BS cells, thereby allowing more of the isotopic fractionation by Rubisco (30‰) to be expressed. Recently, Tazoe et al. (2008) may have provided evidence for an increase in φ as a result of energy limitation under low PFD, since the increase in Δ13C and, hence, φ, following a transfer to low PFD, was lower in Amaranthus cruentus plants grown under low light intensity compared with high-light-grown plants. This may have been caused by a slightly altered relative composition (within the limits of C4 pathway plasticity) of the photosynthetic apparatus in the plants grown under low PFD, resulting in an improved ability to sustain carboxylation efficiency in BS cells at low light. The relative changes in light use and carboxylation efficiency within the canopy (Fig. 2; see discussion above) certainly suggest that differing degrees of acclimation occur at reduced light intensity, once fully expanded leaves are overtopped by the newly growing canopy.
BS φ
At high light intensity, φ was similar to reported values in NADP-malic enzyme C4 photosynthesis of 0.2 to 0.3 in maize (Henderson et al., 1992) and 0.3 to 0.4 in sugarcane (Meinzer et al., 1994; Meinzer and Saliendra, 1997). The maximum values for φ at low light intensity (below a threshold PFD of 350 μmol quanta m−2 s−1) were comparable to reported values of 0.65 at 150 μmol quanta m−2 s−1 for F. bidentis by Cousins et al. (2006) and of 0.76 at 40 μmol quanta m−2 s−1 for A. cruentus by Tazoe et al. (2008). Other contributions to the measured leaf-level variability of Δ13C and φ could be related to the input parameters (pi/pa, b4, b3, s, and a) and simplifying assumptions for Equation 3 below. The assigned parameter value of −6‰ (at 22°C) to b4 is based on the assumption that carbonic anhydrase (CA) is abundant enough to maintain isotopic equilibrium between CO2 and HCO3−. However, if CA activity is too low to maintain equilibrium, b4 becomes less negative and Δ13C increases (leading to an overestimation of φ with Eq. 3), which was shown by Cousins et al. (2006) with F. bidentis RNA antisense CA plants. Von Caemmerer et al. (2004) argued that CA activity was too high to maintain steady-state assimilation rates in F. bidentis; however, Gillon and Yakir (2000, 2001) have reported that CA activity in C4 monocots could limit photosynthetic rates. Equation 3 requires high CO2 concentration in BS cells relative to that in M cells. If this requirement was not met, φ might have been overestimated by 10% or more for very low BS CO2 concentrations (Tazoe et al., 2008). Also, the gradient between intercellular and M CO2 concentrations was assumed to be negligible in Equation 3. Results of a sensitivity analysis with the extended model by Farquhar (1983) showed that a gradient of 30 μmol mol−1 would cause a mean absolute difference in calculated φ of 0.026.
Furthermore, there might be additional fractionations associated with photorespiration (Gillon and Griffiths, 1997; Lanigan et al., 2008), variable mitochondrial respiration between old and young leaves (Villar et al., 1995), or different carbon sources for respiration (Ghashghaie et al., 2003). A sensitivity analysis (Table II) was undertaken, using the extended model by Farquhar (1983), with various combinations of values for mitochondrial respiration, the fractionation factor assigned to respiration (e), and carboxylation rate in BS cells (Vc). The results were relatively insensitive to most of the modeled combinations, and only when Vc was low (3 μmol m−2 s−1) and mitochondrial respiration was high (1.2 μmol m−2 s−1) did mean absolute differences in φ range from −0.06 to 0.08 (depending on the value of e; Table II). The observed dark respiration rate in leaves at 1, 2, and 3 m in our study (0.40, 0.71, and 0.89 μmol m−2 s−1, respectively) suggest that such errors would not occur, due to the relative reduction in both assimilation and respiration rates low in the canopy.
Table II.
Sensitivity of leaf level calculations of φ to different values of mitochondrial respiration rate (Rd), fractionation factor associated with mitochondrial respiration (e), and carboxylation rate in BS cells (Vc)
The extended model by Farquhar (1983) was used to calculate the mean absolute difference of φ between the extended model and the simplified version without respiratory fractionation (Eq. 3). Phosphoenolpyruvate carboxylation and photorespiration rates were set at 1.5 and 0.03 times Vc, respectively. A value of 11.5‰ was used for the fractionation factor associated with photorespiration, based on recent estimates by Lanigan et al. (2008).
| Rd | e | Vc | Mean Difference in φ |
|---|---|---|---|
| μmol m−2 s−1 | ‰ | μmol m−2 s−1 | |
| 0.4 | 6 | 30 | 0.0080 |
| 0.4 | −6 | 30 | 0.0032 |
| 0.4 | 6 | 3 | 0.0306 |
| 0.4 | −6 | 3 | −0.018 |
| 1.2 | 6 | 30 | 0.0129 |
| 1.2 | −6 | 30 | −0.0016 |
| 1.2 | 6 | 3 | 0.0852 |
| 1.2 | −6 | 3 | −0.0614 |
Implications of Results for Canopy CO2 Fixation
Assuming that φ is the major correlate of variations in leaf and canopy Δ13C, we suggest that φ significantly reduces canopy net assimilation rate. Having calculated φ from leaf-level Δ13C and simultaneous gas exchange, and also independently from canopy Δ13C and PFD and temperature measurements within the canopy, there were relative reductions in cumulative canopy An ranging from 14% to 32%. There are implications for losses of carbon-fixing capacity in densely planted C4 crops. Screening for varieties with low Δ13C under a range of limiting light conditions might be a means to increase the productivity of Miscanthus (or other C4 crops), especially in the temperate climate conditions of northwestern Europe. Additionally, the observed variations in the canopy profile of Jmax and Amax may provide a mechanistic understanding of the energetic factors controlling φ.
In conclusion, our results have established that retrodiffusion or leakage of CO2 from BS cells is a major limitation to carbon gain at leaf and canopy scales under field conditions. While additional laboratory studies are required to evaluate the exact nature of this energetic inefficiency at low light (perhaps through the analysis of transient responses of φ to light in sun- and shade-acclimated plants), there was an agreeable consistency in response to incident PFD measured independently for individual leaves and their cumulative light limitation within the upper part of a full C4 crop canopy. Second, we have been able to demonstrate how φ, as a function of leaf area index and incident PFD within the canopy, can be translated into canopy-scale carbon partitioning and potential productivity. Additional work is required to confirm the consistency of these responses during canopy development and to allow more precise partitioning between respiratory components and canopy net assimilation. However, carbon isotopes have provided a powerful means to analyze instantaneous photosynthetic limitation at leaf and canopy scale and allowed us to derive estimates of the likely reduction due to φ in CO2 uptake for a C4 crop limited by light and temperature in a northern temperate environment. The good agreement between previous measurements of φ versus PFD from controlled environment studies (Henderson et al., 1992; Meinzer et al., 1994; Meinzer and Saliendra, 1997; Cousins et al., 2006; Tazoe et al., 2008) and our observations under field conditions might also suggest that within-canopy measurements of PFD can be used as a rough proxy for φ losses due to light limitation arising from self shading.
MATERIALS AND METHODS
Site Description
Measurements were carried out at Teagasc Agricultural Research Centre (Oak Park, Carlow, Ireland) on a 13-year-old stand of Miscanthus (Miscanthus × giganteus) in the summer of 2007. The plot was rainfed and unfertilized except for one application of 50 kg ha−1 nitrogen fertilizer in 2006. Harvest of previous-year stems took place in early April 2007.
Potential Rate of Photosynthesis
Five leaves were randomly selected from 1-, 1.5-, 2-, 2.5-, and 3-m height within the canopy to assess electron transport rate (J; μmol m−2 s−1) as a function of incident PFD. All measurements were done just above the end of the visible mid rib. First, the quantum yield of PSII (ΦPSII) was measured using pulse amplitude-modulated (PAM) chlorophyll fluorescence at a range of light intensities with a mini-PAM photosynthetic yield analyzer (Heinz Walz) according to Genty et al. (1989):
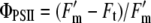 |
(1) |
where Fm′ and Ft refer to maximum fluorescence at saturating light pulse and steady-state fluorescence immediately prior to the pulse. J could then be calculated at each light level with the use of Equation 2:
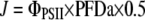 |
(2) |
where PFD represents the absorbed PFD, and 0.5 is a factor that accounts for the partitioning of energy between PSI and PSII. Leaf absorptance was set at 0.84, which has been shown to be valid for a wide range of growth conditions in Miscanthus (Farage et al., 2006). As a control, we also measured the chlorophyll content of different leaves (n = 5) from each height using the protocol by Lichtenthaler (1987), but no significant differences were found.
CO2 responses of net assimilation rate were analyzed on three leaves that were cut at the ligule, with the cut end placed in water and recut under water, 3 cm from the base (Beale et al., 1996). The leaves were positioned in the leaf cuvette (area = 6 cm2) of a LI6400 portable, open gas-exchange system (Li-Cor Biosciences) illuminated by a Walz Fiber Illuminator FL440 with controllable intensity, with a model 400-F fiberglass connection and projector to filter heat, and left for 30 min to acclimatize to saturating light (1,600 μmol quanta m−2 s−1) at ambient CO2. Leaf temperature was set at 22°C, while the vapor pressure deficit was kept below 1 kPa. After reaching steady state, the photosynthetic assimilation rate as a function of internal CO2 concentration was determined from high to low external CO2 concentrations (380, 150, 100, 50, and 40 μmol mol−1).
Mitochondrial respiration not associated with photorespiration (Rd) was measured on different leaves from 1, 2, and 3 m (n = 5) by gas exchange with the LI6400 (leaf temperature set at 22°C), with readings taken after 5 min in the darkened cuvette.
Realized Rate of Photosynthesis
Leaves were randomly selected at 1-, 1.5-, 2-, 2.5-, and 3-m height within the canopy, and (still attached) photosynthetic assimilation rate, transpiration rate, and stomatal conductance were measured under natural light and temperature by placing them in the leaf cuvette of the LI6400 portable, open gas-exchange system, while being careful not to change the angle and orientation of the leaf. A reading was taken after steady state was reached (approximately 5 min).
Use of Δ13C to Calculate BS φ
Hattersley (1976) first suggested that variation in δ13C of C4 species may reflect variations in the amount of leakage. An increase in BS φ results in higher Δ13C, because fractionation by Rubisco becomes more expressed. This relationship is used in Equation 3, developed by Farquhar (1983) and Henderson et al. (1992), which allows calculation of BS φ if Δ13C and pi/pa are known.
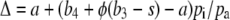 |
(3) |
where a is the fractionation during diffusion of CO2 in air (4.4‰), b4 is the combined fractionation of phosphoenolpyruvate carboxylation (2.2‰) and the isotopic equilibrium during dissolution of CO2 and conversion to bicarbonate, yielding a net value of −6‰ (at 22°C; Mook et al., 1974), b3 is the fractionation by Rubisco (30‰), and s (1.8‰) is the fractionation during leakage.
Δ13C Measured Concurrently with Gas Exchange
To measure Δ13C during gas exchange, the exhaust tube of a LI6400 portable photosynthesis system was connected to a cryogenic water and CO2 trapping-purification line as described by Griffiths et al. (1990). CO2 was trapped and purified for 15 min. The obtained CO2 samples were stored in sealed glass vials and analyzed afterward with a VG SIRA dual-inlet isotope ratio mass spectrometer (modified and maintained by Pro-Vac Services), and values were corrected for the presence of N2O and 17O. The δ13C of CO2 measured before and after photosynthetic depletion yields the net discrimination using the following equation by Evans et al. (1986):
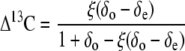 |
(4) |
where
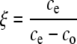 |
(5) |
where δe and δo represent the isotopic compositions of CO2 relative to the PDB standard, and ce and co represent the CO2 mole fractions in air entering and leaving the cuvette, respectively. The Δ13C values obtained in this way thus reflect average values for 15 min of continuous photosynthetic discrimination.
Canopy Microclimate Record
A 3.5-m-tall pole with cross bars at 1-, 2-, and 3-m height was erected within the canopy with the cross bars facing north/south. PFD quantum sensors (SKP-215; Skye Instruments) were mounted on the cross bars facing south and thermocouples (Cu-Co) were mounted facing north. Also, a thermocouple and a soil moisture probe (SM200; Delta-T Devices) were inserted 0.02 m below the soil surface. A data-logger (CR21X; Campbell Scientific) was used to record and store average values every 10 min.
Another pole with air inlets on the cross bars was connected to the air inlet of a LI6400 portable, open gas-exchange system via 4-mm-diameter Teflon tubing and a switching manifold to measure and record within-canopy CO2 concentrations at 1-, 2-, 3-, and 3.5-m height. During the recordings, the exhaust tube of the LI6400 leaf cuvette was connected to the trapping-purification line, and CO2 was trapped and purified for 5 min and stored in sealed glass vials. The isotopic ratio of purified CO2 was determined with a VG SIRA dual-inlet isotope ratio mass spectrometer and corrected for the presence of 17O and N2O.
Canopy Characterization
Leaf area index was measured with a Sunscan System SS-1 (Delta-T Devices). Measurements were made with the sunprobe at 0-, 1-, 2-, and 3-m height to analyze the specific leaf area index of vertical sections within the canopy as well as the total leaf area of the stand (0-m measurements).
Ecosystem CO2 Exchange
Net ecosystem exchange (NEE) of CO2 was measured using an eddy covariance system consisting of an open-path infrared gas analyzer (Li-Cor LI-7500) coupled to a three-dimensional sonic anemometer (Soluent R3; Gill Instruments). The sonic anemometer and air intake were positioned 1 m above the canopy. CO2 concentrations were measured with the open-path LI-7500 infrared gas analyzer. Measurements were recorded at 20 Hz on a CR23X datalogger (Campbell Scientific), and 30-min average fluxes were processed using the EdiRe software version 1.4.3.987 (Edinburgh University). Data quality and stability were tested using the methods of Foken and Wichura (1995), and data were discarded based on a lower u* threshold of 0.1 (m s−1), high sd in measured CO2 concentration, and CO2 concentration and flux values outside of approved bandwidths. The footprint model of Kormann and Meixner (2001) was used to determine the percentage of measured flux originating from within the measurement plot, when less than 70% of the data were discarded.
Canopy CO2 Uptake and Isotope Discrimination
Data from diurnal measurements (August 28–29 and August 31–September 1, 2007) as well as eddy covariance flux measurements (August 28–September 1, 2007) were used to reconstruct canopy CO2 uptake and short-term Δ13C using the method described in detail by Bowling et al. (2003).
A regression (exponential, ordinary least squares) was made of nocturnal eddy covariance measurements of ecosystem respiratory fluxes against soil temperature (bin averaged per 1°C). This regression was extrapolated over daytime to partition daytime gross ecosystem respiratory flux. The daytime gross ecosystem respiratory flux was then subtracted from the daytime total net flux, measured with eddy covariance, which yielded the canopy CO2 uptake gross flux.
We assumed that daytime net ecosystem CO2 fluxes were composed of gross fluxes of ecosystem respiration (FR) and canopy uptake (FA; Eq. 6), and net ecosystem uptake fluxes were assigned a negative sign by micrometeorological convention.
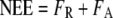 |
(6) |
Analogously, net isofluxes were also assumed to consist of gross respiration and photosynthetic isofluxes (Eq. 7).
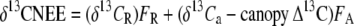 |
(7) |
The isoflux of ecosystem respiration [(δ13CR)FR] was determined by multiplying the daytime gross respiratory flux with the intercept of the nocturnal Keeling plot (geometric mean regression of 1/[CO2] versus δ13C; Keeling, 1958, 1961). Net daytime isofluxes (δ13CNEE) were calculated by multiplying the daytime net ecosystem exchange fluxes with corresponding isotopic signatures using daytime geometric mean regression of CO2 concentrations versus δ13C. Since (δ13CR)FR, δ13CNEE, ambient isotopic signature of CO2 (δ13Ca), and gross fluxes of canopy CO2 uptake (FA) were identified, based on mass balance and negligible storage flux in NEE measurements (checked with diurnal vertical CO2 concentration profiles), the isotopic contribution of canopy CO2 uptake (δA) became the only unknown variable and could be solved.
Losses of Potential CO2 Fixation Due to φ
We used the following approach to calculate the implications of φ at leaf level on canopy CO2 uptake (An). Based on the leaf-level determinations, a function for hyperbolic decay [φ = (a × b)/(b + PFD)] was fitted to define φ as a function of incident PFD (Fig. 4C). Measured incident PFD and temperature at 1, 2, and 3 m from August 29 to August 31 were used to represent the range of conditions defining CO2 fixation. A vertical profile of An was calculated using the model for C4 photosynthesis by Von Caemmerer and Furbank (1999) and Von Caemmerer (2000) with some adaptations (see Supplemental Appendix S1), which was multiplied by a profile of leaf area index and summed to yield canopy An. We calculated potential canopy An without CO2 leakage (φ = 0) and compared it with canopy An computed with φ determined by the fitted relationship between φ and PFD in Figure 4C.
A similar approach was used in combination with Equation 3 to translate measurements of canopy Δ13C into φ for each height. First, leaf-level data were used to derive a regression between incident PFD and pi/pa (Fig. 4B). Based on previous results (Cousins et al., 2006; Tazoe et al., 2006, 2008) and the model by Von Caemmerer and Furbank (1999) and Von Caemmerer (2000), we used a descriptive function for hyperbolic decay [φ = (a × b)/(b + PFD)] to describe φ as a function of incident PFD. The measured incident PFD within the canopy, together with Equation 3, then allowed the calculation of An and Δ13C for each layer. Δ13C weighted by An was used to calculate canopy Δ13C, which was compared with measured canopy Δ13C. This comparison allowed fitting parameters a and b by means of nonlinear regression, and the best fit was used to produce results for canopy An based on canopy-level determinations of φ (as described in the previous paragraph).
The Ventana Simulation Environment Vensim DSS for Windows, version 5.6a Double Precision (Ventana Systems), was used for all calculations and nonlinear regression.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Appendix S1. Modeling An for C4 photosynthesis.
Supplementary Material
Acknowledgments
We thank Dr. Julian Hibberd and Moritz Meyer for discussions and comments on early versions of the manuscript.
This work was supported by the Alexander James Keith Studentship and the European Framework Program Infrastructure for Measurement of the European Carbon Cycle through Trinity College Dublin, Department of Botany (to J.K.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Johannes Kromdijk (wk229@cam.ac.uk).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Atkin OK, Evans JR, Siebke K (1998) Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Aust J Plant Physiol 25 437–443 [Google Scholar]
- Beale CV, Baker MG, Farage PK, Humphries SW, Long SP (1997) Final Progress Report and Final Report of the European Miscanthus Network. University of Essex, Essex, UK, pp 1–55
- Beale CV, Bint DA, Long SP (1996) Leaf photosynthesis in the C4-grass Miscanthus × giganteus, growing in the cool temperate climate of southern England. J Exp Bot 47 257–273 [Google Scholar]
- Bowling DR, Pataki DE, Ehleringer JR (2003) Critical evaluation of micrometeorological methods for measuring ecosystem-atmosphere isotopic exchange of CO2. Agric For Meteorol 116 159–179 [Google Scholar]
- Bowman WD, Hubick KT, Von Caemmerer S, Farquhar GD (1989) Short term changes in leaf carbon isotope discrimination in salt- and water-stressed C4 grasses. Plant Physiol 90 162–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann N, Brooks JR, Rapp KD, Ehleringer JR (1996) Carbon isotope composition of C4 grasses is influenced by light and water supply. Plant Cell Environ 19 392–402 [Google Scholar]
- Buchmann N, Ehleringer JR (1998) CO2 concentration profiles, and carbon and oxygen isotopes in C3 and C4 crop canopies. Agric For Meteorol 89 45–58 [Google Scholar]
- Clifton-Brown JC, Breuer J, Jones MB (2007) Carbon mitigation by the energy crop, Miscanthus. Glob Change Biol 13 2296–2307 [Google Scholar]
- Cousins AB, Badger MR, Von Caemmerer S (2006) Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis: insights from antisense RNA in Flaveria bidentis. Plant Physiol 141 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, Von Caemmerer S (2008) C4 photosynthetic isotope exchange in NAD-ME- and NADP-ME-type grasses. J Exp Bot 59 1695–1703 [DOI] [PubMed] [Google Scholar]
- Duranceau M, Ghashghaie J, Brugnoli E (2001) Carbon isotope discrimination during photosynthesis and dark respiration in intact leaves of Nicotiana sylvestris: comparisons between wild type and mitochondrial mutant plants. Aust J Plant Physiol 28 65–71 [Google Scholar]
- Edwards GE, Baker NR (1993) Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth Res 37 89–102 [DOI] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13 281–292 [Google Scholar]
- Farage PK, Blowers D, Long SP, Baker NR (2006) Low growth temperatures modify the efficiency of light use by photosystem II for CO2 assimilation in leaves of two chilling-tolerant C4 species, Cyperus longus L. and Miscanthus x giganteus. Plant Cell Environ 29 720–728 [DOI] [PubMed] [Google Scholar]
- Farquhar GD (1983) On the nature of carbon isotope discrimination in C4 species. Aust J Plant Physiol 10 205–226 [Google Scholar]
- Fravolini A, Williams DG, Thompson TL (2002) Carbon isotope discrimination and bundle sheath leakiness in three C4 subtypes grown under variable nitrogen, water and atmospheric CO2 supply. J Exp Bot 53 2261–2269 [DOI] [PubMed] [Google Scholar]
- Foken T, Wichura B (1995) Tools for quality assessment of surface-based flux measurements. Agric For Meteorol 78 83–105 [Google Scholar]
- Furbank RT, Jenkins CLD, Hatch MD (1990) C4 photosynthesis: quantum requirement, C4 acid overcycling and Q-cycle involvement. Aust J Plant Physiol 17 1–7 [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990 87–92 [Google Scholar]
- Ghashghaie J, Badeck FW, Lanigan G, Nogues S, Tcherkez G, Deleens E, Cornic G, Griffiths H (2003) Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochem Rev 2 145–161 [Google Scholar]
- Gillon J, Yakir D (2001) Influence of carbonic anhydrase activity in terrestrial vegetation on the O-18 content of atmospheric CO2. Science 291 2584–2587 [DOI] [PubMed] [Google Scholar]
- Gillon JS, Griffiths H (1997) The influence of (photo)respiration on carbon isotope discrimination in plants. Plant Cell Environ 20 1217–1230 [Google Scholar]
- Gillon JS, Yakir D (2000) Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against CO18O during photosynthesis. Plant Cell Environ 23 903–915 [Google Scholar]
- Griffiths H, Broadmeadow MSJ, Borland AM, Hetherington CS (1990) Short-term changes in carbon-isotope discrimination identify transitions between C3 and C4 carboxylation during Crassulacean acid metabolism. Planta 181 604–610 [DOI] [PubMed] [Google Scholar]
- Hansen EM, Christensen BT, Jensen LS, Kristensen K (2004) Carbon sequestration in soil beneath long-term Miscanthus plantations as determined by 13C abundance. Biomass and Bioenergy 26 97–105 [Google Scholar]
- Hatch MD, Agostino A, Jenkins CLD (1995) Measurements of leakage of CO2 from bundle sheath cells of leaves during C4 photosynthesis. Plant Physiol 108 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley PW (1976) Speciation and functional significance of the leaf anatomy of C4 plants. PhD thesis. Australian National University, Canberra, Australia
- Heaton EA, Long SP, Voigt TB, Jones MB, Clifton-Brown J (2004. a) Miscanthus for renewable energy generation: European Union experience and projections for Illinois. Mitig Adapt Strategies Glob Change 9 433–451 [Google Scholar]
- Heaton EA, Voigt T, Long SP (2004. b) A quantitative review comparing the yields of two candidate C4 perennial biomass crops in relation to nitrogen, temperature and water. Biomass and Bioenergy 27 21–30 [Google Scholar]
- Henderson SA, Von Caemmerer S, Farquhar GD (1992) Short-term measurements of carbon isotope discrimination in several C4 species. Aust J Plant Physiol 19 263–285 [Google Scholar]
- Keeling CD (1958) The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochim Cosmochim Acta 13 322–334 [Google Scholar]
- Keeling CD (1961) The concentration and isotopic abundance of carbon dioxide in rural and marine air. Geochim Cosmochim Acta 24 277–298 [Google Scholar]
- Knohl A, Søe ARB, Kutsch WL, Göckede M, Buchmann N (2008) Representative estimates of soil and ecosystem respiration in an old beech forest. Plant Soil 302 189–202 [Google Scholar]
- Kormann R, Meixner FX (2001) An analytical footprint model for non-neutral stratification. Boundary-Layer Meteorol 99 207–224 [Google Scholar]
- Kubasek J, Setlik J, Dwyer S, Santrucek J (2007) Light and growth temperature alter carbon isotope discrimination and estimated bundle sheath leakiness in C4 grasses and dicots. Photosynth Res 91 47–58 [DOI] [PubMed] [Google Scholar]
- Lanigan GJ, Betson N, Griffiths H, Seibt U (2008) Carbon isotope fractionation during photorespiration and carboxylation in Senecio. Plant Physiol 148 2013–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood RC, Adcock MD, Doncaster HD, Hill R (1989) Analysis of the control of photosynthesis in C4 plants by changes in light and carbon dioxide. Philos Trans R Soc Lond B Biol Sci 323 339–355 [Google Scholar]
- Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148 350–382 [Google Scholar]
- Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8 315–323 [Google Scholar]
- Lobell DB, Burke MB, Tebaldi C, Mastrandrea MD, Falcon WP, Naylor RL (2008) Prioritizing climate change adaptation needs for food security in 2030. Science 319 607–610 [DOI] [PubMed] [Google Scholar]
- Meinzer F, Plaut Z, Saliendra N (1994) Carbon isotope discrimination, gas exchange and growth of sugarcane cultivars under salinity. Plant Physiol 104 521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer F, Saliendra N (1997) Spatial patterns of carbon isotope discrimination and allocation of photosynthetic activity in sugarcane leaves. Aust J Plant Physiol 24 769–775 [Google Scholar]
- Meinzer F, Zhu J (1998) Nitrogen stress reduces the efficiency of the C4 CO2 concentrating mechanism, and therefore quantum yield, in Saccharum (sugarcane) species. J Exp Bot 49 1227–1234 [Google Scholar]
- Mook WG, Bommerson JC, Staverman WH (1974) Carbon isotope fractionations between dissolved bicarbonate and gaseous carbon dioxide. Earth Planet Sci Lett 22 169–176 [Google Scholar]
- Naidu SL, Moose SP, Al-Shoabi AK, Raines CA, Long SP (2003) Cold-tolerance of C4 photosynthesis in Miscanthus x giganteus: adaptation in amounts and sequence of C4 photosynthetic enzymes. Plant Physiol 132 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary MH (1988) Carbon isotopes in photosynthesis. Bioscience 38 328–336 [Google Scholar]
- Osmond CB, Smith FA (1976) Symplastic transport of metabolites during C4 photosynthesis. In BES Gunning, AW Robards, eds, Intercellular Communication in Plants: Studies on Plasmodesmata. Springer-Verlag, Berlin, pp 229–241
- Reichstein M, Falge E, Baldocchi D, Papale D, Aubinet M, Berbigier P, Bernhofer C, Buchmann N, Gilmanov T, Granier A, et al (2005) On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob Change Biol 11 1–16 [Google Scholar]
- Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161 341–370 [DOI] [PubMed] [Google Scholar]
- Somerville C (2007) Biofuels. Curr Biol 17 115–119 [DOI] [PubMed] [Google Scholar]
- Tazoe Y, Hanba YT, Furumoto T, Noguchi K, Terashima I (2008) Relationships between quantum yield for CO2 assimilation, activity of key enzymes and CO2 leakiness in Amaranthus cruentus, a C4 dicot, grown in high or low light. Plant Cell Physiol 49 19–29 [DOI] [PubMed] [Google Scholar]
- Tazoe Y, Noguchi K, Terashima I (2006) Effects of growth light and nitrogen nutrition on the organization of the photosynthetic apparatus in leaves of a C4 plant, Amaranthus cruentus. Plant Cell Environ 29 691–700 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Energy (2006) Breaking the Biological Barriers to Cellulosic Ethanol: A Joint Research Agenda. DOE/SC-0095. U.S. Department of Energy, Washington, DC, www.doegenomestolife.org/biofuels/
- Usuda H (1984) Variations in the photosynthesis rate and activity of photosynthetic enzymes in maize leaf tissue of different ages. Plant Cell Physiol 25 1297–1301 [Google Scholar]
- Usuda H (1987) Changes in levels of intermediates of the C4 cycle and reductive pentose phosphate pathway under various light intensities in maize leaves. Plant Physiol 84 549–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar R, Held AA, Merino J (1995) Dark leaf respiration in light and darkness of an evergreen and a deciduous plant species. Plant Physiol 107 412–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Caemmerer S (2000) Modelling C4 photosynthesis. In Biochemical Models of Leaf Photosynthesis: Techniques in Plant Sciences, 2. CSIRO Publishing, Collingwood, Australia, pp 91–122
- Von Caemmerer S, Furbank RT (1999) Modelling C4 photosynthesis. In The Biology of C4 Photosynthesis. Physiological Ecology Series. Academic Press, New York, pp 173–211
- Von Caemmerer S, Furbank RT (2003) The C4 pathway: an efficient CO2 pump. Photosynth Res 77 191–207 [DOI] [PubMed] [Google Scholar]
- Von Caemmerer S, Ludwig M, Millgate A, Farquhar GD, Price D, Badger M, Furbank RT (1997. a) Carbon isotope discrimination during C-4 photosynthesis: insights from transgenic plants. Aust J Plant Physiol 24 487–494 [Google Scholar]
- Von Caemmerer S, Millgate A, Farquhar GD, Furbank RT (1997. b) Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase by antisense RNA in the C4 plant Flaveria bidentis leads to reduced assimilation rates and increased carbon isotope discrimination. Plant Physiol 113 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Caemmerer S, Quinn V, Hancock NC, Price GD, Furbank RT, Ludwig M (2004) Carbonic anhydrase and C4 photosynthesis: a transgenic analysis. Plant Cell Environ 27 697–703 [Google Scholar]
- Wang D, Naidu SL, Portis AR Jr, Moose SP, Long SP (2008. a) Can the cold-tolerance of C4 photosynthesis in Miscanthus x giganteus relative to Zea mays be explained by differences in activities and thermal properties of Rubisco? J Exp Bot 59 1779–1787 [DOI] [PubMed] [Google Scholar]
- Wang D, Portis AR Jr, Moose SP, Long SP (2008. b) Cool C4 photosynthesis: Pyruvate Pi dikinase expression and activity corresponds to the exceptional cold tolerance of carbon assimilation in Miscanthus × giganteus. Plant Physiol 148 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.