Abstract
Abiotic stresses such as drought, cold, and salinity affect normal growth and development in plants. The production and accumulation of reactive oxygen species (ROS) cause oxidative stress under these abiotic conditions. Recent research has elucidated the significant role of ethylene response factor (ERF) proteins in plant adaptation to abiotic stresses. Our earlier functional analysis of an ERF protein, JERF3, indicated that JERF3-expressing tobacco (Nicotiana tabacum) adapts better to salinity in vitro. This article extends that study by showing that transcriptional regulation of JERF3 in the oxidative stress response modulates the increased tolerance to abiotic stresses. First, we confirm that JERF3-expressing tobacco enhances adaptation to drought, freezing, and osmotic stress during germination and seedling development. Then we demonstrate that JERF3-expressing tobacco imparts not only higher expression of osmotic stress genes compared to wild-type tobacco, but also the activation of photosynthetic carbon assimilation/metabolism and oxidative genes. More importantly, this regulation of the expression of oxidative genes subsequently enhances the activities of superoxide dismutase but reduces the content of ROS in tobacco under drought, cold, salt, and abscisic acid treatments. This indicates that JERF3 also modulates the abiotic stress response via the regulation of the oxidative stress response. Further assays indicate that JERF3 activates the expression of reporter genes driven by the osmotic-responsive GCC box, DRE, and CE1 and by oxidative-responsive as-1 in transient assays, suggesting the transcriptional activation of JERF3 in the expression of genes involved in response to oxidative and osmotic stress. Our results therefore establish that JERF3 activates the expression of such genes through transcription, resulting in decreased accumulation of ROS and, in turn, enhanced adaptation to drought, freezing, and salt in tobacco.
Regulation of transcription has proved to be a vital aspect of the complex genetic and biochemical networks involved in plant responses to stresses. Understanding the interaction between stress-responsive transcription factors and their corresponding cis-acting elements in promoters of the downstream target genes is a prerequisite to dissecting the transcriptional regulatory network. For instance, investigation of the promoters of COR genes in Arabidopsis (Arabidopsis thaliana) indicated that they are regulated in an abscisic acid (ABA)-dependent or ABA-independent pathway under conditions of cold, dehydration, and high salinity (Liu et al., 1998; Yamaguchi-Shinozaki and Shinozaki, 2006). The subfamily of ethylene response factor (ERF) proteins belongs to the ERF/APETALA2 (AP2) superfamily, which contains one highly conserved AP2 or ERF DNA-binding domain (Kizis et al., 2001; Liu et al., 2006). ERF proteins were first isolated from tobacco (Nicotiana tabacum) as GCC-box binding proteins (Ohme-Takagi and Shinshi, 1995), and consequent investigations indicate that many ERF proteins found in other plants enhance resistance to pathogens by activating the expression of pathogenesis-related genes (Guo et al., 2002; Zhang et al., 2004). Evidence accumulated over the years has proved that ERF proteins bind not only to the GCC box but also to DRE/CRT, CE1, JERE, and CT-rich elements (Liu et al., 1998; Menke et al., 1999; Niu et al., 2002; Xue and Loveridge, 2004; Tang et al., 2005), thereby enhancing plant tolerance to multiple stresses, which suggests that ERF proteins are part of the plant's response to such stresses.
Accumulated evidence indicates that stress-stimulated physiological imbalance increases the level of reactive oxygen species (ROS) in plant cells. The steady-state levels of hydrogen peroxide (H2O2), singlet oxygen, the superoxide anion (O2−), and the hydroxyl radical depend on the balance between generation and removal, which is facilitated by the ROS-scavenging system of the cell (Bartoli et al., 2004; Gechev et al., 2006). In such a system, H2O2 plays a very important role in mediating signal transduction in response to stress in plant cells. H2O2 diffuses rapidly from its site of synthesis within subcellular microdomains, depending on its concentration, and can transmit intracellular signals to the nucleus by oxidizing various upstream components of the signaling pathway, including transcription factors that ultimately result in changes in gene expression (Mittler, 2002; Gadjev et al., 2006). Further, the expression of the ERF gene CaPF1 in Virginia pine (Pinus virginiana) enhances the level of antioxidant enzyme activities of ascorbate peroxidase, glutathione reductase, and superoxide dismutase (SOD). This results in the improved tolerance to stress (Tang et al., 2005), indicating that ERF proteins might be associated with the regulation of ROS pathway. Although many ERF proteins have been identified in the response of plants to abiotic stress, the details of involvement of an ROS-mediated regulatory module are yet to be established. An ERF protein, JERF3, was isolated from tomato (Solanum lycopersicum) using a yeast one-hybrid screen in our laboratory. It has been shown that JERF3 could be induced by ethylene, jasmonic acid, cold, salinity, and ABA, and that JERF3-expressing tobacco was better adapted to salinity in vitro (Wang et al., 2004); however, the molecular mechanism of that regulatory response remained to be elucidated. In this article, we now report that JERF3 transcriptionally regulates the expression of genes involved in plant responses to osmotic- and oxidative-related stresses; this results in decreased accumulation of ROS, thereby enhancing the adaptation to drought, salt, and freezing in tobacco.
RESULTS
Expression of JERF3 in Tobacco Increases Seed Germination and Root Elongation under Salt and Osmotic Stress, and Tolerance to Drought and Freezing in Seedling Stage
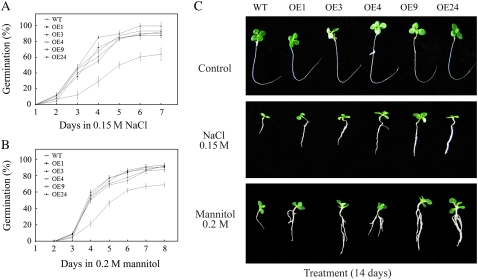
Salinity, drought, and low temperature result in osmotic stress. Our earlier experiments showed that after NaCl treatment, leaf discs from JERF3-overexpressing tobacco (OE) seedlings remained green, whereas those from wild type (wild-type tobacco) were bleached, indicating that JERF3 enhances tolerance to salt in vitro (Wang et al., 2004). This evidence prompted us to test whether JERF3 regulates tolerance to osmotic stress. Previously, we obtained nine independent OE lines, of which four were confirmed to contain one-copy insertions (Wang et al., 2004), and the other five lines two- to three-copy insertions. Three one-copy insertions (T3 generation, OE1, OE3, and OE9) and two two-copy insertions (T2 generation, OE4 and OE24) display distinctive expression of JERF3 (Supplemental Fig. S1A), yet show similar phenotypes during seedling (the controls of Figs. 1 and 2), mature (Supplemental Fig. S1B), and flowering developmental stages (Supplemental Fig. S1C). These were used in the following experiments. To exclude differences in seed germination between the wild-type and OE lines, we first checked the germination in water as control. Our results indicated that wild-type and OE lines showed an equal germination. Then we simulated osmotic stress by exposing wild-type and OE lines to NaCl and mannitol and studied the effects on germination and root elongation. As can be seen in Figure 1A, germination in OE lines was clearly higher than that in wild type. After 0.15 m NaCl treatment for 3 d, seed germination in wild type was about 10%, compared to over 40% in OE lines. Similarly, the germination percentage in OE lines was twice that in wild type after 0.2 mm mannitol treatment for 4 d (Fig. 1B).
Figure 1.
Expression of JERF3 increases germination and root elongation in tobacco under salt and osmotic stress. A, Germination of tobacco seeds under 0.15 m NaCl. B, Germination of tobacco seeds under 0.2 mm mannitol. C, Elongation of tobacco roots under 0.15 m NaCl or 0.2 mm mannitol. About 80 seeds and 30 seedlings were used for each treatment. Results are the average of three replicates. Error bars indicate ±sd. [See online article for color version of this figure.]
Figure 2.
Expression of JERF3 enhances tolerance to drought and freezing in tobacco. A and B, JERF3 enhances the adaptation of tobacco to drought during 3- and 6-week-old seedlings, respectively. About 30 seedlings in triplicate were used for each line. C, Seedlings exposed to freezing (−2°C for 3 h). D, Ion leakage under −2°C for 3 h. Four-week-old seedlings were used for this assay. About 30 seedlings were used for each line. Results are the average of three replicates. Error bars indicate ±sd.
After 0.15 m NaCl treatment for 14 d, the seedling roots in the five independent OE lines were about twice as long than those in wild type (Fig. 1C). Interestingly, when subjected to higher concentrations of 0.2 mm mannitol for the same duration, roots of five independent OE lines were not only about 34% longer than those of wild type but also showed more lateral roots (Fig. 1C). Student's t tests indicate that the above data of seed germination or root elongation are not significantly different among the five independent OE lines at 95% probability, indicating that the distinctive transcript level of JERF3 (Supplemental Fig. S1A) did not correlate with the degree of tolerance in OE lines. However, there are differences (significant at 95% probability) in the seed germination or root elongation between OE lines and wild type. These results indicate that JERF3 increases the adaptation to salt and osmotic stress during seed germination and root elongation in tobacco.
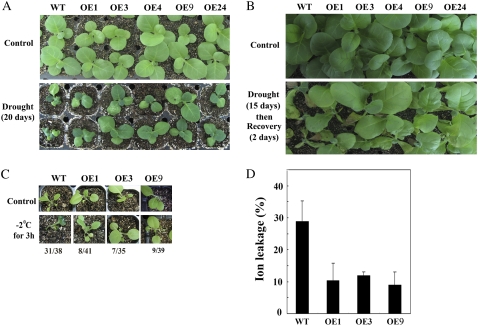
Next we tested the response of wild type and the above five independent OE seedlings to drought at different stages. We found that after 3-week-old seedlings were not watered for 20 d, more than 85% of wild-type seedlings had wilted whereas growth was almost normal in the five independent OE seedlings (Fig. 2A). Interestingly, when 6-week-old seedlings were exposed to drought for 15 d, the phenotypes of wilted leaves did not show obvious differences between wild type and five independent OE seedlings. However, after rewatering for another 2 d, more than 80% of wild-type seedlings did not recover from the wilted phenotype whereas growth was back to normal in the five independent OE seedlings (Fig. 2B), indicating that JERF3 increases tolerance to drought in tobacco seedlings.
We also examined the response of wild type and three one-copy insertion OE seedlings to freezing. Initially, we exposed seedlings to −10°C in the growth chamber but found no clear difference between OE lines and wild type. We then set the temperature to −2°C. As can be seen in Figure 2C, wild-type seedlings were injured after a 3-h exposure to −2°C but OE seedlings were not obviously affected. More interestingly, leakage of ions from wild-type seedlings after the treatment was 30% but that from OE seedlings was only 10% (Fig. 2D), which is consistent with the report that plasma membranes of plants are injured because of exposure to cold, resulting in leakage of ions from the cytoplasm (Gonzalez-Aguilar et al., 2000). These results suggest that JERF3 makes tobacco seedlings more tolerant to freezing by increasing the stability of the plasma membrane.
Expression of JERF3 in Tobacco Significantly Activates the Expression of Osmotic- and Oxidative-Related Genes
To investigate how JERF3 modulates plant response to drought, cold, and salt stresses, we compared the expression profiles in two independent OE seedlings with that in wild-type plants using cDNA-amplified fragment length polymorphism (AFLP; Vos et al., 1995). Using 240 primer pairs, we obtained 2,742 fragments, of which 526 were differentially expressed between OE lines and wild type (changes more than 2-fold). Compared to the transcript levels in wild type, there were 277 up-regulated genes and 249 down-regulated genes in OE lines, and approximately 23% of the up-regulated genes are believed to be involved in response to osmotic, photosynthetic carbon assimilation/metabolism, and oxidative stress.
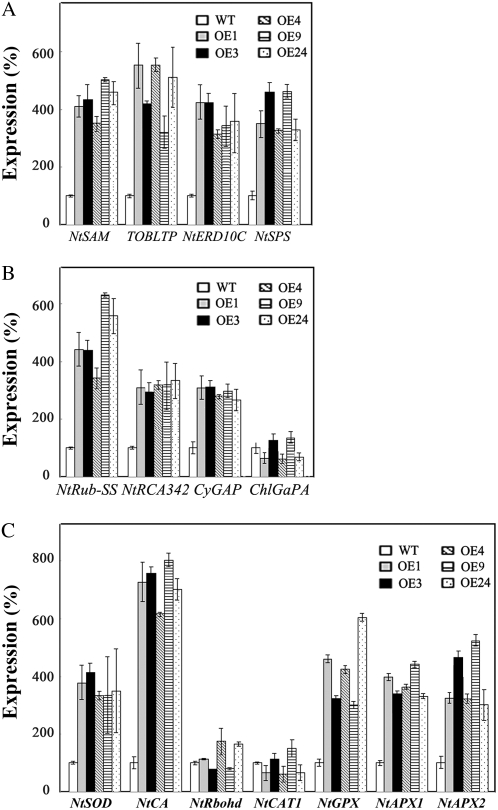
Among the up-regulated genes, we first confirmed the expression of four genes related to osmotic stress in five independent OE lines and wild type using quantitative real-time PCR (qPCR) amplifications, namely NtSAM1, TOBLTP, NtERD10C, and NtSPS. It is known that NtSAM1 encodes S-adenosyl-l-Met synthetase, which was reported to be inducible by ABA, mannitol, and NaCl (Espartero et al., 1994); TOBLTP encodes lipid transfer protein, which is inducible by ABA and drought (Masuta et al., 1992; Torres-Schumann et al., 1992; Trevino and O'Connell, 1998); NtERD10C is induced by cold and drought (Kasuga et al., 2004); and NtSPS encodes Suc-P synthase (Börnke, 2005), which was responsive to cold (Supplemental Fig. S2). The expression of the above four genes in OE seedlings was enhanced 3 to approximately 5 times that in wild type (Fig. 3A), suggesting that JERF3 constitutively activates the expression of genes responding to osmotic stress in tobacco.
Figure 3.
Expression of JERF3 in tobacco enhances the expression of osmotic- and oxidative stress-related genes derived from the analysis of cDNA-AFLP under normal growth conditions. A, Confirmation of the expression of genes responsive to osmotic stress in tobacco. B, Confirmation of expression of genes related to photosynthetic carbon assimilation/metabolism. C, Confirmation of expression of genes related to oxidative stress. Transcript levels of these genes in transgenic tobacco are indicated relative to the level of wild-type tobacco taken as 100, referring to the transcripts of actin in the same samples. Error bars are based on three independent experiments.
Next we analyzed the expression of four genes involved in photosynthetic carbon assimilation/metabolism in OE seedling. It is established that NtRub-SS encodes Rubisco small subunit (Mazur and Chui 1985); ChlGaPA encodes glyceraldehyde-3-P dehydrogenase A-subunit precursor; CyGAP encodes glyceraldehyde-3-P dehydrogenase; and NtRCA342 encodes Rubisco activase precursor (Shih et al., 1986). Our results showed that the expression of these genes except ChlGaPA was increased 3 to approximately 6 times that in wild type (Fig. 3B), indicating that JERF3 confers the expression of genes in the response to photosynthetic carbon assimilation/metabolism in tobacco.
Also we analyzed the expression of seven genes involved in the response to oxidative stress in OE seedling. It has been reported that NtCA encodes carbonic anhydrase, which displays antioxidant activity and functions in the hypersensitive defense response (Slaymaker et al., 2002); NtSOD encodes SOD, which was reported to enhance oxidative stress tolerance in tobacco (Slooten et al., 1995); NtRbohD, a tobacco respiratory burst oxidase homolog, encodes an enzyme similar to the mammalian NADPH oxidase, producing active oxygen species in elicited tobacco cells (Simon-Plas et al., 2002; Morel et al., 2004). And the genes NtCAT1, NtAPX1, NtAPX2, and NtGPX encode catalase, cytosolic ascorbate peroxidase, chloroplastic ascorbate peroxidase, and glutathione peroxidase, respectively, which all are considered to use H2O2 as an electron acceptor to catalyze a number of oxidative reactions (Orvar and Ellis, 1995; Pasqualini et al., 2007). Our results confirmed, with the exception of NtCAT1 and NtRbohD, that the expression of these up-regulated genes was increased 3 to approximately 8 times above controls (Fig. 3C). Moreover, the expression of down-regulated genes was consistent with the results of cDNA-AFLP (data not shown). Therefore JERF3 regulates the expression of photosynthetic carbon assimilation/metabolism and oxidative genes as well.
Expression of JERF3 in Tomato Is Responsive to Methyl Viologen But Not H2O2 and 3-Amintriazole
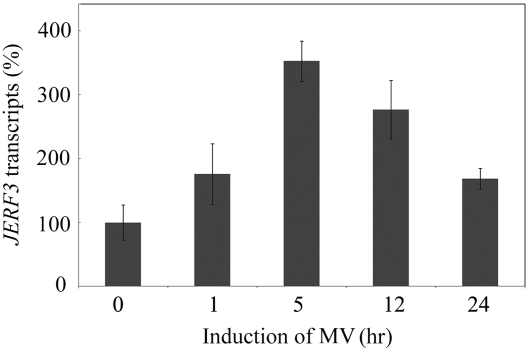
The finding that JERF3 regulates the expression of photosynthetic carbon assimilation/metabolism and oxidative genes indicates that ROS might be related to the enhanced tolerance to drought, salt, and freezing. The possible involvement of ERF factors in ROS transcriptional regulatory networks that govern plant stress responses has been analyzed in previous studies (Mittler, 2002; Gadjev et al., 2006), although detailed experimental data were not provided. To prove the involvement of ERF gene JERF3 in ROS transcriptional regulation we tested the response of JERF3 in its original source of tomato plants by treatment with H2O2 or ROS-generating agents such as methyl viologen (MV) and 3-amintriazole. qPCR results indicated that MV induced the expression of JERF3 in tomato seedlings (Fig. 4), but 3-amintriazole and H2O2 did not (data not shown), indicating that JERF3 might be associated with the ROS response.
Figure 4.
Expression of JERF3 in tomato is inducible by MV. The expression of JERF3 in tomato was detected by qPCR. The actin transcripts were used as internal control, and the expression level of JERF3 was standardized as 100 at time 0. The assay was repeated three times. The bars represent ±se.
Expression of JERF3 in Tobacco Increases the Activities of SOD But Decreases the Accumulation of ROS
The data so far indicate that plants accumulate osmotic solutes to regulate osmotic potential under drought, salt, and cold stress (Gilmour et al., 2000). To test whether the expression of JERF3 modulates the accumulation of osmotic solutes under drought, salt, and cold stresses, we checked the Pro content of wild-type and OE seedlings. As anticipated, the contents of osmotic solutes in OE seedlings were approximately 9% higher than those in wild-type seedlings under normal growth conditions, but the differences were not clearly correlated to the stresses of drought, salt, or cold (data not shown). This might suggest that osmotic regulation is not the exclusive regulatory pathway of JERF3 in adaptation to drought, salt, and cold in tobacco. In this case, there must be an alternative regulatory pathway involving JERF3 in the plant's response to abiotic stress.
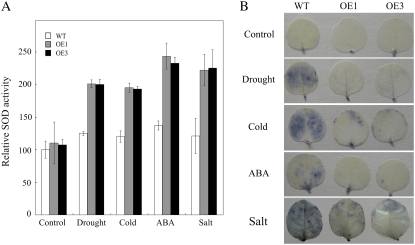
The fact that JERF3 increases the expression of genes related to oxidative stress suggests that JERF3 might modulate the levels of ROS. To evaluate the important ROS-scavenging pathways of SOD in cellular compartments, and the possible regulation of individual SOD genes at the transcriptional level, we further measured the activities of SOD in the oxidative response. Our results showed that the SOD activities were 5% to approximately 8% higher in OE seedlings than that in wild type under normal growth conditions, consistent with the significant expression of NtSOD in OE seedlings. SOD activities increased 160% to approximately 210% and 7% to approximately 12% in OE and wild-type seedlings, respectively, compared to that in wild type of control, after dehydration, cold, salt, and ABA treatments (Fig. 5A). Student's t tests indicate that the significant difference of SOD activities cannot be seen between the two independent OE lines, except between OE lines and wild type at 95% probability, indicating that JERF3 gives rise to the increase of SOD activities in tobacco under abiotic stresses and ABA treatment.
Figure 5.
Expression of JERF3 in tobacco enhances the activity of SOD and decreases the accumulation of ROS. A, Expression of JERF3 in tobacco enhances the activities of SOD. The SOD activities were measured based on the ability of SOD to inhibit the reduction of NBT by superoxide. And the data are shown compared to the wild type of control (taken as 100) after calculation of the SOD activities each sample. Results are the average of three replicates. B, Expression of JERF3 in tobacco decreases the accumulation of ROS. The contents of O2− in tobacco leaves were stained using NBT as substrate. Approximately 15 seedlings in triplicates were used for each line in this assay. Control indicates seedlings raised under normal conditions of growth, Drought indicates seedlings kept on tissue paper for 1 h, Cold represents seedlings exposed to 4°C for 2 d, and ABA and Salt indicate seedlings sprayed with ABA or NaCl, respectively.
Because H2O2, singlet oxygen, O2−, and the hydroxyl radical all belong to ROS (Bartoli et al., 2004; Gechev et al., 2006), we then checked the accumulation of O2− in OE and wild-type seedlings under dehydration, cold, salt, and ABA treatments. Under normal growth conditions, O2− accumulated at very low levels and showed no obvious difference between wild-type and OE seedlings. This reduced ROS was not detectable in OE seedlings, while wild type still can be observed along the main vein under normal growth conditions (Fig. 5B). After exposure to dehydration, cold, salt, or ABA treatments, O2− accumulated significantly greater quantities in wild-type plants whereas its levels in OE seedlings were lower (Fig. 5B), suggesting that JERF3 decreases the accumulation of ROS under dehydration, cold, salt, and ABA treatments.
JERF3 Transcriptionally Regulates Gene Expression through Interacting with Multiple Stress-Responsive cis-Acting Elements
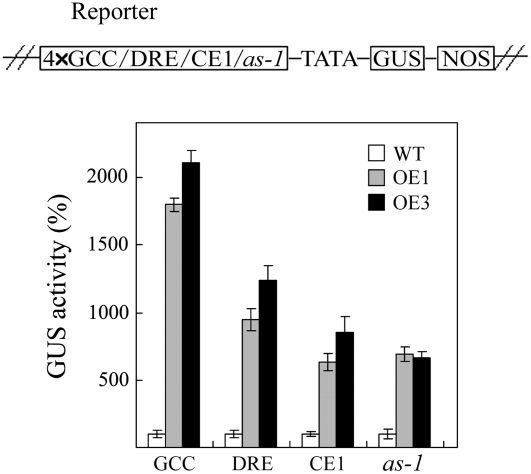
Our results indicate that JERF3 enhances tolerance to drought, cold, and osmotic stress. Moreover, it has been reported that ERF proteins interact with several elements such as GCC box (Ohme-Takagi and Shinshi, 1995), DRE (Narusaka et al., 2003), and CE1 (Niu et al., 2002) that are involved in plant response to stress. However, no ERF proteins were found to interact with as-1, which is related to oxidative stress and binds to bZIP transcription factors (Despres et al., 2003; Wormuth et al., 2007). Wang et al. (2004) established that JERF3 could bind to GCC box and DRE in vitro. In this work, GUS reporter vectors driven by GCC, DRE, CE1, or as-1 were constructed (see details in “Materials and Methods”) and Agrobacterium harboring these reporter vectors was infiltrated into the leaves of OE lines and wild type. The results showed that GUS activity in OE lines was about 19 to 21, 9 to 15, 6 to 8, and 7 times that in wild type when driven by GCC box, DRE, CE1, or as-1, respectively (Fig. 6). This result demonstrates that JERF3 may not only modulate with cis-acting elements involved in response to osmotic stress, but also with the elements involved in oxidative stress, thereby supporting the possibly transcriptional regulation of JERF3 in plant response to oxidative stress.
Figure 6.
Expression of JERF3 in tobacco activates the expression of the GUS gene controlled by GCC box, DRE, CE1, or as-1. The top section displays a schematic diagram of the reporter constructs used in Agrobacterium-mediated transient expression assay. Four-times cis-elements (GCC box, DRE, CE1, or as-1) are fused upstream of 35S minimal promoter in pBI121 as reporter. The bottom section shows relative GUS activity in leaves of wild-type and OE seedlings 48 h after Agrobacterium infiltration. The data for GUS activity in OE seedling leaves are relative to that in wild type (standardized to 100, inferred to the GUS activity driven by 35S minimal promoter in pBI121). GCC, DRE, CE1, and as-1 indicate that 4× cis-elements of GCC box, DRE, CE1, and as-1 are inserted upstream of the 35S minimal promoter in pBI121 as positive reporters, respectively. Error bars indicate ±sd.
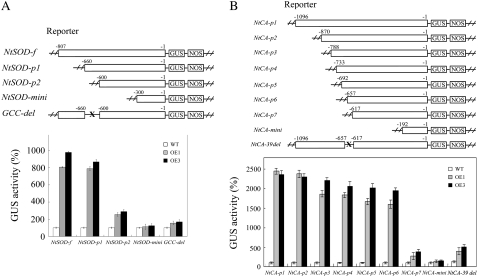
To further clarify the direct regulation of JERF3 in the expression of photosynthetic carbon assimilation/metabolism and oxidative genes, we cloned the promoters of Rubisco small subunit gene (the promoter sequence is reported to be 1,041 bp in length; Mazur and Chui 1985), NtCA (1,096 bp), and NtSOD (807 bp), from tobacco genomic DNA (NC89) using direct PCR amplification and thermal asymmetric interlaced (TAIL)-PCR, respectively (Liu et al., 1995). After analyzing the promoters using PLACE software, we found that there is a GCC box (AGCCGCC, −639 to −645) in the promoter of NtSOD, but no known cis-element that JERF3 interacts with in the promoters of NtCA and Rubisco small subunit gene. Then the promoters of the Rubisco small subunit gene, NtSOD and NtCA, were inserted into pCAMBIA1381Z upstream of GUS to test their possible interaction with JERF3. Our results indicate that JERF3 does not interact with the promoter of the Rubisco small subunit gene, indicating upstream regulation of JERF3 in the expression of this gene. However, JERF3 modulates the expression of GUS gene driven by the full-length promoter of NtSOD (−1 to −807 bp), but JERF3 does not interact with the promoter of NtSOD in which the GCC-box area (−625 to −658) was deleted (Fig. 7A), indicating that JERF3 activates the expression of reporter genes through interacting with the GCC box of NtSOD promoter. Previously, we proved the physical binding of JERF3 to GCC box (Wang et al., 2004), thus the direct transcriptional activation of JERF3 on the expression of oxidative stress gene NtSOD might contribute to the production of NtSOD activities in tobacco.
Figure 7.
Expression of JERF3 in tobacco interacts with the promoters of NtSOD and NtCA in transient GUS assay. A and B, The top section displays a schematic diagram of the reporter constructs used in Agrobacterium-mediated transient expression assay. The promoter of NtSOD-f, NtSOD-p1, NtSOD-p2, NtSOD-mini, or removal of GCC box (GCC-del) is fused upstream of GUS gene in pCAMBIA1303. The promoter of NtCA-p1, NtCA-p2, NtCA-p3, NtCA-p4, NtCA-p5, NtCA-p6, NtCA-p7, NtCA-mini, or removal of 39-bp fragment (NtCA-39 del) is fused upstream of GUS gene in pCAMBIA1303. The chart shows relative GUS activity in leaves of wild-type and OE seedlings 48 h after Agrobacterium infiltration. The data for GUS activity in OE seedling leaves are relative to that in wild type (standardized to 100).
Interestingly, JERF3 also interacts with the full-length promoter (−1 to −1,096 bp) of NtCA (NtCA-p1). Concomitant with the deletion of the promoter, GUS activity weakened but not markedly until NtCA-p7 (−1 to −617 bp; Fig. 7B), suggesting that the deleted sequence between NtCA-p6 and NtCA-p7 might interact with JERF3. In fact, removal of the fragment in the promoter of NtCA (NtCA-39 del) significantly reduced the expression of the reporter gene (Fig. 7B). Therefore our results demonstrate that JERF3 might interact with a novel sequence of 39 bp (−617 to −657 bp, ATCTGTGATCAGCAATAATTGTTGAGTTGATTTGGAATT) located on the promoter of NtCA to activate gene expression in this manner.
DISCUSSION
It has been documented that the stress-induced increase of ROS in plant cells results from an imbalance between generation and removal. The production and accumulation of ROS cause oxidative stress under these abiotic stresses. Our earlier experiments (Wang et al., 2004) and this research with JERF3 have indicated that transcriptional regulation of JERF3 modulates the increased tolerance to drought, salt, and freezing in tobacco during germination and seedling development. Such regulation is not only through activating the expression of osmotic stress genes, but also through the activation of photosynthetic carbon assimilation/metabolism and oxidative genes. This establishes that the ERF protein JERF3 is involved in a ROS-mediated regulatory module in transcriptional networks that govern plant response to stress.
Chloroplast and mitochondria are the major sites of generation of ROS (Asada, 2006; Wormuth et al., 2007). The generation and removal of ROS is a homeostatic process during plant growth and development under normal conditions. However, this homeostasis is destroyed when plants are exposed to drought, cold, and salinity (Apel and Hirt, 2004; Bartoli et al., 2004). Cellular antioxidants, including enzymatic and nonenzymatic antioxidants, are the key to protecting the cells against damage due to ROS. Among enzymatic antioxidants, SOD playxs a pivotal role in clearing away ROS by catalyzing the highly damaging O2− into H2O2 (Apel and Hirt, 2004). The results we obtained are consistent with the view that expressing JERF3 in tobacco greatly increases the activities of SOD, resulting in the reduced accumulation of O2− under drought, cold, salt, and ABA treatments. To better understand the involvement of JERF3 in ROS transcriptional regulatory network, we found that the expression of JERF3 in its original tomato seedlings was inducible by the ROS-generating agent MV, suggesting that JERF3 might be associated with ROS responses. The enhanced expression of glutathione peroxidase NtGPX and cytosolic and chloroplastic ascorbate peroxidases (NtAPX1 and NtAPX2) in unstressed OE seedlings further supports the idea that JERF3 might transcriptionally regulate the expression of genes related to oxidative reactions. Furthermore, Rubisco, a key enzyme in photosynthesis, catalyzes ribulose-1,5-bisphosphate and assimilates CO2 and also catalyzes the oxygenase reaction of ribulose-1,5-bisphosphate into glycolate, which is metabolized in peroxisome (Ruuska et al., 2000; Marcus et al., 2005). This process helps scavenge ROS (Apel and Hirt, 2004). Carbonic anhydrase is important to CO2 transport because it catalyzes the reversible hydration of CO2 (Smith and Ferry, 2000) and has antioxidant activity (Slaymaker et al., 2002). In our earlier (Wang et al., 2004) and present research, we found that the ERF protein JERF3 significantly enhances tolerance to drought, freezing, and salinity in tobacco. Further research indicated that expression of photosynthetic carbon assimilation/metabolism and oxidative-related genes is up-regulated in JERF3-expressing tobacco, suggesting that JERF3 regulates photosynthesis and eliminates ROS. More importantly, we find that expressing JERF3 in tobacco activates the expression of NtSOD by interacting with the GCC box in the promoter of NtSOD, and NtCA via interaction with a 39-bp novel element, indicating that JERF3 regulates plant response to abiotic stresses mainly by modulating the expression of ROS-related genes.
After being detected by a receptor, ROS continues transferring the signal through two main pathways. In one, a group of transcription factors participating in different cellular pathways is activated by a mitogen-activated protein kinase cascade (Pitzschke and Hirt, 2006). In the other, the level of cellular calcium is changed after a receptor detects ROS and the pathway of calcium/calmodulin is activated (Mittler et al., 2004). For example, ROS may activate the Ca2+-permeable channels in plant membranes and induce stomatal closure by increasing the level of cellular calcium (Mori and Schroeder, 2004), which is further modulated by ABA (Mustilli et al., 2002; Apel and Hirt, 2004). The stability of the plasma membrane under cold conditions in JERF3-expressing tobacco indicates that regulation of JERF3 by oxidative stress might occur via a similar ROS-regulatory pathway. The involvement of JERF3 in ROS is also supported by the fact that JERF3 interacts with the ROS-responsive as-1 element in a transient assay, consistent with the report that a bZIP-type protein, which interacts with as1/ocs-like elements in the promoters of target genes, might be a component of the ROS-mediated pathway (Cheng et al., 2007). However, the predicted regulatory relationship between ROS-bZIP1 and the as1/ocs element-containing genes awaits further confirmation. Therefore, to our knowledge, it is a novel conclusion that the interaction of JERF3 with the promoters of genes related to oxidative stress regulates the expression of these genes. This results in enhanced SOD activities, consequently decreased accumulation of ROS and thereby enhancing tolerance to drought, salt, and freezing.
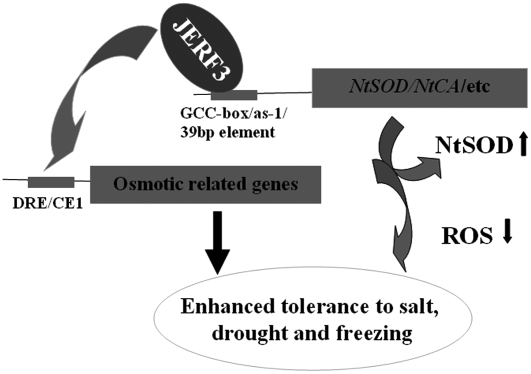
In Arabidopsis, ethylene receptors including ETR1, ETR2, EIN4, ERS1, and ERS2 (Potuschak et al., 2003) play a negative role in the ethylene pathway (Guo and Ecker, 2004). CTR1, a Ser/Thr kinase downstream of receptors, is also a negative regulator of the pathway (Kieber et al., 1993; Bishopp et al., 2006). Downstream of CTR1 is EIN2, which further regulates EIN3 (Chao et al., 1997; Solano et al., 1998). Although ERF1 is known to be a downstream component of EIN3 in Arabidopsis (Guo and Ecker, 2004) it was unclear whether the ERF protein, JERF3, is modulated by EIN3/EILs. To answer this question, we cloned a 1,595-bp upstream fragment of JERF3 from genomic DNA of tomato (Lichun) using TAIL-PCR. Analysis of the promoter using PLACE software indicates that there is no PERE element that EIN3 interacts with (Guo and Ecker, 2004). However, we observed that tomato LeEIL1, an Arabidopsis EIN3 homolog (Tieman et al., 2001), activates the expression of GUS reporter driven by JERF3 promoter in an Agrobacterium-mediated transient expression assay (Supplemental Fig. S3). The facts that the promoter of JERF3 could be transcriptionally modulated by LeEIL1, that JERF3 is inducible by ethylene, and that JERF3 interacts with GCC box (Wang et al., 2004) suggest that JERF3 may be concerned with ethylene pathway. Furthermore, JERF3 could bind to DRE, CE1, and as-1 or to other elements such as the 39-bp fragment in the promoter of NtCA to directly regulate the expression of oxidative stress genes. Such genes include NtSOD and NtCA, which would decrease the content of ROS. Lastly, JERF3 enhances adaptation to drought, salt, and cold in tobacco not only through binding to the promoters of oxidative stress genes, resulting in the expression of these genes and decreasing the accumulation of ROS, but also through activating the expression of genes responsive to osmotic stress. Therefore, based on our previous report (Wang et al., 2004) and present study, we propose a regulatory model for JERF3 in tobacco abiotic stresses (Fig. 8). In such a model, JERF3, dominantly binding to GCC box, transcriptionally activates the expression of genes related to both osmotic and oxidative stresses. The expression of these genes subsequently results in the decreased accumulation of ROS, thereby enhancing adaptation to drought, freezing, and salt in tobacco.
Figure 8.
Transcriptional regulatory module of JERF3 in ROS-mediated stress responses.
MATERIALS AND METHODS
Plant Material and Growth Conditions
JERF3-expressing tobacco (Nicotiana tabacum; Wang et al., 2004) and wild-type tobacco (cv NC89) were grown in a growth chamber at 25°C under a 16-h photoperiod. T2 (OE4 and OE24 with two-copy insertions) and T3 (OE1, OE3, and OE9 with one-copy insertion) transgenic tobacco plants were used for the experiments. JERF3-expressing tobacco and wild-type tobacco are referred to as OE and wild type, respectively.
Salt, Drought, and Freezing Stress Assays
All seeds in the following assays were surface sterilized and kept at 4°C for 2 d to break dormancy (Mukhopadhyay et al., 2004) before subjecting them to any of the experimental treatments. For the germination assay, the tobacco seeds were plated in one-half Murashige and Skoog medium (0.6% agar) plus mannitol or NaCl, and the number of germinated seeds was counted every day. For root elongation assay, the germinated seeds were first placed upright in Murashige and Skoog plates until the roots were about 0.5 cm and the seedlings then transferred to the same medium supplemented with mannitol or NaCl and placed upright in the growth chamber. The root lengths were recorded before and after the treatments.
For drought assays, both 3-week-old and 6-week-old OE and wild-type seedlings in pots were adequately watered and then water was withheld for 15 to approximately 20 d. For freezing assays, 3-week-old OE lines and wild type in pots were placed in a growth chamber at −2°C for 3 h. For electrolyte leakage assay, seedlings of 3-week-old NC89 wild-type and OE seedlings were placed at −2°C in a growth chamber for 0, 2, 4, 8, and 12 h to determine electrolyte leakage as described by Guo et al. (2002). The percentage of electrolyte leakage was calculated as the ratio of the conductivity before autoclaving to that after autoclaving.
cDNA-AFLP Assay
Total RNA was extracted from unstressed 3-week-old tobacco plants using Trizol (Invitrogen) according to the manufacturer's recommendations. cDNA was synthesized from 1 μg of total RNA using 200 units of moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega) following the manufacturer's instructions. For synthesis of the second strand, M-MLV RTase cDNA synthesis kit (TaKaRa) was used. cDNA-AFLP assay was performed as described by Vos et al. (1995).
Confirmation of Gene Expression Derived from cDNA-AFLP Using qPCR Amplifications
Total RNA was extracted from unstressed 3-week-old tobacco plants using Trizol (Invitrogen) according to the manufacturer's recommendations. cDNA from unstressed 3-week-old seedlings was synthesized from 1 μg of total RNA using 200 units of M-MLV reverse transcriptase (Promega) following the manufacturer's instructions. After reverse transcription, qPCRs were performed using an ABI Prism 7000 system (Applied Biosystems). The relative transcript abundance for some genes derived from cDNA-AFLP analysis is relative to the actin transcript levels measured in the same sample. The primers used in this article for qPCR are listed in Supplemental Table S1.
Inducible Expression of JERF3 in Tomato in Response to H2O2, MV, and 3-Amintriazole
Three-week-old tomato plants (Lycopersicon esculentum ‘Lichun’) were grown in growth chambers at 25°C with a 16-h light regime with illumination from cool-white fluorescent lights of about 150 μmol m−2 s−1. For the H2O2 and MV treatments, 100 μm H2O2 or 50 μm MV in 0.1% Tween 20 was used to spray tomato seedling leaves (Nishizawa et al., 2008). A total of 0.1% Tween 20 solution was used to spray tomato seedling leaves as control treatment. After treatments, tomato plants were used to detect expression of JERF3 using qPCR.
Detection of SOD Activities and O2− in Tobacco Leaves
The stress treatments were applied as described below. For the drought treatment, OE and wild-type seedlings were removed from Murashige and Skoog medium, washed with water, dried with tissue paper, and then placed on tissue paper for 1 h. For the low-temperature treatment, OE and wild-type seedlings in plates were placed at 4°C in a growth chamber for 2 d. For the ABA and salt treatments, OE and wild-type seedlings in plates were sprayed with 100 μm ABA or 200 mm NaCl and kept under high humidity for 3 h. Activity of SOD was determined using the method of Winterbourn et al. (1975) that is based on the ability of SOD to inhibit the reduction of nitro-blue tetrazolium (NBT) by superoxide. In our assays, 0.3 g leaves from 3-week-old tobacco seedlings each sample cultured in Murashige and Skoog medium were used. Each sample of the supernatants was placed in two 1.5-mL tubes, one was in the dark as control, another under light (150 μmol m−2 s−1). After incubation at 30°C for 20 min, the tubes were placed on ice to stop the reaction. The inhibition of NBT reduction was determined at A560. Leaves of 3-week-old seedlings treated with stresses were also used to determine superoxide using NBT staining as described by Lee et al. (2002).
TAIL-PCR Assay
TAIL-PCR was performed as described by Liu et al. (1995). The genomic DNA of NC89 was used as a template. AD primers were the same as those used by Liu et al. (1995). The sequences of specific primers for NtSOD, NtCA, and JERF3 were given in Supplemental Table S1.
Agrobacterium-Mediated GUS Transient Assay
For constructing the reporter vectors, four-times-repeated sequences of cis-acting elements GCC (AGCCGCC), DRE (TACCGACAT), CE1 (TGCCACCGG), or as-1 (TGACG) were inserted upstream of the minimal TATA box (−46 to +10) to replace the cauliflower mosaic virus 35S promoter in pBI121 (CLONTECH). The plasmids were then introduced into the Agrobacterium tumefaciens strain LBA4404. Agrobacterium-mediated transient assay was performed on the leaves of 4-week-old wild-type and OE seedlings as described by Yang et al. (2000). The GUS activity was measured about 48 h later.
To ascertain whether JERF3 interacts with the upstream sequence of the ATG start code of NtCA, DNA fragments 1,096 bp (NtCA-p1), 870 bp (NtCA-p2), 788 bp (NtCA-p3), 733 bp (NtCA-p4), 692 bp (NtCA-p5), 657 bp (NtCA-p6), 617 bp (NtCA-p7), and 192 bp (NtCA-mini as a minimal promoter) upstream of the ATG start code of NtCA were inserted separately into pCAMBIA1303 using the enzymes PstI and BglII.
Similarly, a DNA fragment 807 bp (NtSOD-f), 660 bp (NtSOD-p1), 600 bp (NtSOD-p2), and 300 bp (NtSOD-mini as a minimal promoter) upstream of the ATG start code of NtSOD was inserted separately into pCAMBIA1303 using the enzymes PstI and BglII. Sequences of the primers of NtCA and NtSOD promoters were listed in Supplemental Table S1. To generate the removal of GCC box or 39-bp fragment from NtSOD-f or NtCA-p1, respectively, PCR amplifications were used with special primers listed in Supplemental Table S1 using pCAMBIA1303-NtCA-p1 or pCAMBIA1303-NtSOD-f as a template. Then PCR production was self linked with T4 DNA ligase and was followed by transformation into Escherichia coli. After sequencing confirmation, the above-constructed plasmids were then introduced separately into the A. tumefaciens strain LBA4404. Agrobacterium-mediated transient assay was performed and the GUS activity measured about 48 h later.
The GenBank accession numbers for the sequences used as materials in this article are: AY383630 (JERF3), X63603 (actin), AF127243 (NtSAM1), D13952 (TOBLT), AB049337 (NtERD10C), AF194022 (NtSPS), X02353 (NtRub-SS), M14417 (ChlGaPA), M14419 (CyGAP), U35111 (NtRCA342), AB093097 (NtSOD), AF454759 (NtCA), AJ309006 (NtRbohD), U93244 (NtCAT1), AB041518 (NtGPX), AU15933 (NtAPX1), D85912 (NtAPX2), EU342357 (NtCA promoter), EU342358 (NtSOD promoter), AF328784 (LeEIL1), and EU910896 (JERF3 promoter).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression of JERF3 in tobacco has no effect on the growth at normal growth conditions.
Supplemental Figure S2. Expression of NtSPS in response to cold.
Supplemental Figure S3. LeEIL1 interacts with the promoter of JERF3 in transient expression assay.
Supplemental Table S1. The primers used in this article.
Supplementary Material
Acknowledgments
The authors thank Professor Clive W. Lloyd and International Science Editing for improving the text.
This work was supported by the National Science Foundation of China (grant nos. 30525034 and 30671135) and the National Basic Research Program of China (grant nos. 2006CB100102 and 2007CB108801).
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Rongfeng Huang (rongfeng@public3.bta.net.cn).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Apel K, Hirt H (2004) REACTIVE OXYGEN SPECIES: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373–399 [DOI] [PubMed] [Google Scholar]
- Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Gomez F, Martinez DE, Guiamet JJ (2004) Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot 55 1663–1669 [DOI] [PubMed] [Google Scholar]
- Bishopp A, Mahonen AP, Helariutta Y (2006) Signs of change: hormone receptors that regulate plant development. Development 133 1857–1869 [DOI] [PubMed] [Google Scholar]
- Börnke F (2005) The variable C-terminus of 14-3-3 proteins mediates isoform-specific interaction with sucrose-phosphate synthase in the yeast two-hybrid system. J Plant Physiol 162 161–168 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89 1133–1144 [DOI] [PubMed] [Google Scholar]
- Cheng C, Yun KY, Ressom HW, Mohanty B, Bajic VB, Jia Y, Yun SJ, Reyes BG (2007) An early response regulatory cluster induced by low temperature and hydrogen peroxide in seedlings of chilling-tolerant japonica rice. BMC Genomics 8 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR (2003) The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15 2181–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espartero J, Pintor-Toro JA, Pardo JM (1994) Differential accumulation of S-adenosylmethionine synthetase transcripts in response to salt stress. Plant Mol Biol 25 217–227 [DOI] [PubMed] [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inze D, Mittler R, Breusegem FV (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Breusegem FV, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28 1091–1101 [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124 1854–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Aguilar GA, Fortiz J, Cruz R, Baez R, Wang CY (2000) Methyl jasmonate reduces chilling injury and maintains postharvest quality of mango fruit. J Agric Food Chem 48 515–519 [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2004) The ethylene signaling pathway: new insights. Curr Opin Plant Biol 7 40–49 [DOI] [PubMed] [Google Scholar]
- Guo Y, Xiong L, Ishitani M, Zhu JK (2002) An Arabidopsis mutation in translation elongation factor 2 causes superinduction of CBF/DREB1 transcription factor genes but blocks the induction of their downstream targets under low temperatures. Proc Natl Acad Sci USA 99 7786–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2004) A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol 45 346–350 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72 427–441 [DOI] [PubMed] [Google Scholar]
- Kizis D, Lumbreras V, Pages M (2001) Role of AP2/EREBP transcription factors in gene regulation during abiotic stress. FEBS Lett 498 187–189 [DOI] [PubMed] [Google Scholar]
- Lee BH, Lee H, Xiong L, Zhu JK (2002) A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 14 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10 1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhao TJ, Liu JM, Liu WQ, Liu Q, Yan YB, Zhou HM (2006) The conserved Ala37 in the ERF/AP2 domain is essential for binding with the DRE element and the GCC box. FEBS Lett 580 1303–1308 [DOI] [PubMed] [Google Scholar]
- Liu YG, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8 457–463 [DOI] [PubMed] [Google Scholar]
- Marcus Y, Altman-Gueta H, Finkler A, Gurevitz M (2005) Mutagenesis at two distinct phosphate-binding sites unravels their differential roles in regulation of Rubisco activation and catalysis. J Bacteriol 187 4222–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuta C, Furuno M, Tanaka H, Yamada M, Koiwai A (1992) Molecular cloning of a cDNA clone for tobacco lipid transfer protein and expression of the functional protein in Escherichia coli. FEBS Lett 311 119–123 [DOI] [PubMed] [Google Scholar]
- Mazur BJ, Chui CF (1985) Sequence of a genomic DNA clone for the small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Res 13 2373–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke FLH, Champion A, Kijne JW, Memelink J (1999) A novel jasmonate- and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor, ORCA2. EMBO J 18 4455–4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Breusegem FV (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9 490–498 [DOI] [PubMed] [Google Scholar]
- Morel J, Fromentin J, Blein JP, Simon-Plas F, Elmayan T (2004) Rac regulation of NtrbohD, the oxidase responsible for the oxidative burst in elicited tobacco cell. Plant J 37 282–293 [DOI] [PubMed] [Google Scholar]
- Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca2+ channels: a signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 135 702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Vij S, Tyagi AK (2004) Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc Natl Acad Sci USA 101 6309–6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusaka Y, Nakashima K, Shinwari ZK, Sakuma Y, Furihata T, Abe H, Narusaka M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J 34 137–148 [DOI] [PubMed] [Google Scholar]
- Nishizawa A, Yabuta Y, Shigeoka S (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol 147 1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Helentjaris T, Bate NJ (2002) Maize ABI4 binds coupling element1 in abscisic acid and sugar response genes. Plant Cell 14 2565–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme-Takagi M, Shinshi H (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvar BL, Ellis BE (1995) Isolation of a cDNA encoding cytosolic ascorbate peroxidase in tobacco. Plant Physiol 108 839–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini S, Paolocci F, Borgogni A, Morettini R, Ederli L (2007) The overexpression of an alternative oxidase gene triggers ozone sensitivity in tobacco plants. Plant Cell Environ 30 1545–1556 [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Hirt H (2006) Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiol 141 351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115 679–689 [DOI] [PubMed] [Google Scholar]
- Ruuska SA, Andrews TJ, Badger MR, Price GD, von Caemmerer S (2000) The role of chloroplast electron transport and metabolites in modulating Rubisco activity in tobacco: insights from transgenic plants with reduced amounts of cytochrome b/f complex or glyceraldehyde 3-phosphate dehydrogenase. Plant Physiol 122 491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih MC, Lazar G, Goodman HM (1986) Evidence in favor of the symbiotic origin of chloroplasts: primary structure and evolution of tobacco glyceraldehyde-3- phosphate dehydrogenases. Cell 47 73–80 [DOI] [PubMed] [Google Scholar]
- Simon-Plas F, Elmayan T, Blein JP (2002) The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J 31 137–147 [DOI] [PubMed] [Google Scholar]
- Slaymaker DH, Navarre DA, Clark D, del Pozo O, Martin GB, Klessig DF (2002) The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc Natl Acad Sci USA 99 11640–11645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slooten L, Capiau K, Van Camp W, Van Montagu M, Sybesma C, Inze D (1995) Factors affecting the enhancement of oxidative stress tolerance in transgenic tobacco overexpressing manganese superoxide dismutase in the chloroplasts. Plant Physiol 107 737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Ferry JG (2000) Prokaryotic carbonic anhydrases. FEMS Microbiol Rev. 24 335–366 [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Charles TM, Newton RJ (2005) Overexpression of the pepper transcription factor CaPF1 in transgenic Virginia pine (Pinus Virginiana Mill.) confers multiple stress tolerance and enhances organ growth. Plant Mol Biol 59 603–617 [DOI] [PubMed] [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ (2001) Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J 26 47–58 [DOI] [PubMed] [Google Scholar]
- Torres-Schumann S, Godoy JA, Pintor-Toro JA (1992) A probable lipid transfer protein gene is induced by NaCl in stems of tomato plants. Plant Mol Biol 18 749–757 [DOI] [PubMed] [Google Scholar]
- Trevino MB, O'Connell MA (1998) Three drought-responsive members of the nonspecific lipid-transfer protein gene family in Lycopersicon pennellii show different developmental patterns of expression. Plant Physiol 116 1461–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23 4407–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Huang Z, Chen Q, Zhang Z, Zhang H, Wu Y, Huang D, Huang R (2004) Ectopic overexpression of tomato JERF3 in tobacco activates downstream gene expression and enhances salt tolerance. Plant Mol Biol 55 183–192 [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Hawkins RE, Brian M, Carrell RW (1975) The estimation of red cell superoxide dismutase activity. J Lab Clin Med 85 337–341 [PubMed] [Google Scholar]
- Wormuth D, Heiber I, Shaikali J, Kandlbinder A, Baier M, Dietz KJ (2007) Redox regulation and antioxidative defence in Arabidopsis leaves viewed from a systems biology perspective. J Biotechnol 129 229–248 [DOI] [PubMed] [Google Scholar]
- Xue GP, Loveridge CW (2004) HvDRF1 is involved in abscisic acid-mediated gene regulation in barley and produces two forms of AP2 transcriptional activators, interacting preferably with a CT-rich element. Plant J 37 326–339 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57 781–803 [DOI] [PubMed] [Google Scholar]
- Yang Y, Li R, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22 543–551 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang D, Chen J, Yang Y, Huang Z, Huang D, Wang XC, Huang R (2004) Tomato stress-responsive factor TSRF1 interacts with ethylene responsive element GCC box and regulates pathogen resistance to Ralstonia solanacearum. Plant Mol Biol 55 825–834 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.