Abstract
Guard cells, which form stoma in leaf epidermis, sense and integrate environmental signals to modulate stomatal aperture in response to diverse conditions. Under drought stress, plants synthesize abscisic acid (ABA), which in turn induces a rapid closing of stoma, to prevent water loss by transpiration. However, many aspects of the molecular mechanism for ABA-mediated stomatal closure are still not understood. Here, we report a novel negative regulator of guard cell ABA signaling, DOR, in Arabidopsis (Arabidopsis thaliana). The DOR gene encodes a putative F-box protein, a member of the S-locus F-box-like family related to AhSLF-S2 and specifically interacting with ASK14 and CUL1. A null mutation in DOR resulted in a hypersensitive ABA response of stomatal closing and a substantial increase of drought tolerance; in contrast, the transgenic plants overexpressing DOR were more susceptible to the drought stress. DOR is strongly expressed in guard cells and suppressed by ABA treatment, suggesting a negative feedback loop of DOR in ABA responses. Double-mutant analyses of dor with ABA-insensitive mutant abi1-1 showed that abi1-1 is epistatic to dor, but no apparent change of phospholipase Dα1 was detected between the wild type and dor. Affymetrix GeneChip analysis showed that DOR likely regulates ABA biosynthesis under drought stress. Taken together, our results demonstrate that DOR acts independent of phospholipase Dα1 in an ABA signaling pathway to inhibit the ABA-induced stomatal closure under drought stress.
Guard cells, which form stoma in leaf epidermis, control the uptake of CO2 for photosynthesis and the loss of water through transpiration. The closing and opening of the stomata is mediated by a turgor-driven volume change of the two surrounding guard cells. The guard cells sense and integrate environmental signals to modulate stomatal aperture in response to diverse conditions. Under drought stress, the endogenous level of abscisic acid (ABA) increases and, through its complex signaling cascade, results in stomatal closure to prevent transpirational water loss (Blatt, 2000). ABA acts directly on the guard cell and induces stomatal closure via the efflux of potassium and anions from guard cell and the removal of osmolytes (MacRobbie, 1998; Schroeder et al., 2001).
So far, many components involved in ABA signaling in Arabidopsis (Arabidopsis thaliana) guard cells have been characterized. The type 2C protein phosphatase ABI1 is a central regulator of ABA responses (Gosti et al., 1999; Merlot et al., 2001). The dominant abi1-1 mutations render Arabidopsis plants insensitive to ABA in seed germination, root growth, stomatal closure, and gene regulation (Koornneef et al., 1989; Leung et al., 1997). Intragenic loss-of-function revertants of the abi1-1 mutant and functional inactivation of the PP2C genes encoded by ABI1 and HAB1 by T-DNA insertions resulted in an ABA-hypersensitive phenotype (Gosti et al., 1999; Saez et al., 2004; Yoshida et al., 2006), indicating their functions as negative regulators of the ABA response. Subsequently, two new recessive loss-of-function alleles of ABI1, abi1-2 and abi1-3, were identified in Arabidopsis and showed enhanced responses to ABA both in seed and vegetative tissues (Saez et al., 2006), confirming that ABI1 functions as a negative regulator of ABA signaling. Recessive mutations leading to the ABA-hypersensitive stomatal closing have revealed additional negative regulators of ABA signaling in guard cells. These include the farnesyltransferase β-subunit ERA1 (Cutler et al., 1996; Pei et al., 1998), the mRNA cap-binding protein ABH1 (Hugouvieux et al., 2001), and the Sm-like snRNP protein SAD1 (Xiong et al., 2001). In contrast, the protein kinase OST1 (Mustilli et al., 2002), the heterotrimeric G protein (Wang et al., 2001), and the recessive gca2 mutation (Pei et al., 2000) are characterized as the positive regulators of ABA signaling in guard cells. In addition, recent studies have indicated that phospholipase D (PLD) acts as a positive regulator for ABA-mediated stomatal movement through the lipid metabolite phosphatidic acid (PA), binds to ABI1, and inhibits its activity (Zhang et al., 2004; Mishra et al., 2006). The plasma membrane protein SLOW ANION CHANNEL-ASSOCIATED1 (SLAC1) was identified as a guard cell plasma membrane protein that mediates anion channel activity, and the stomata of slac1 mutants showed a strong insensitivity to ABA (Negi et al., 2008; Vahisalu et al., 2008).
Although much progress has been made on the guard cell ABA signaling and a current working model suggests that more than 20 components have been shown to participate in ABA-induced stomatal closure (Li et al., 2006), many aspects of the molecular mechanism for ABA-mediated stomatal closure are still not understood. Recently, the identification of plant ABA-binding proteins marked a major advance in understanding ABA perception and ABA signaling in plants. The RNA-binding protein FCA, a homolog of an ABA-binding protein ABAP1, was identified as an ABA receptor in the regulation of flowering time (Razem et al., 2006). The Arabidopsis ABAR (for ABA-binding protein)/CHLH (for H subunit of Mg-chelatase), which specifically binds ABA and mediates ABA signaling as a positive regulator in seed germination, postgermination growth, and stomatal movement, was also identified as an ABA receptor (Shen et al., 2006). GCR2 (for G-protein-coupled receptor 2) also was identified as a putative plasma-membrane-localized ABA receptor (Liu et al., 2007), but its function remains a controversial issue (Gao et al., 2007; Johnston et al., 2007).
It is known that cellular ABA levels fluctuate constantly to allow plants to adjust to the changing physiological and environmental condition. For example, endogenous ABA level can be rapidly increased when plants are subjected to osmotic stress and decreased when rescued from the stress condition. These physiological processes controlled by ABA are primarily regulated by bioactive ABA pool size, which is thought to be maintained through fine-tuning the rates of de novo biosynthesis and catabolism (Nambara and Marion-Poll, 2005). Previous studies showed that a number of steps may be regulated in the ABA biosynthesis pathway in higher plants. Among them, production of xanthoxin from epoxycarotenoids is a key step in ABA synthesis (Nambara and Marion-Poll, 2005), in which NCED3 (for 9-cis-epoxycarotenoid dioxygenase 3) is the key regulatory enzyme in the ABA biosynthesis pathway (Burbidge et al., 1999). Under drought stress, NCED3 is one of the most strongly induced seven NCED genes in Arabidopsis (Iuchi et al., 2001). On the other hand, ABA breakdown is mediated by the Cyt P450 named CYP707As, which is a key enzyme in the oxidative catabolism of ABA (Saito et al., 2004). Furthermore, recent research on ABA metabolism showed AtBG1 and the RING-H2 protein XERICO are involved in regulating ABA homeostasis in plant cells (Ko et al., 2006; Lee et al., 2006). Thus, the regulation of endogenous ABA level is crucial for plants to adapt environmental challenges (e.g. drought stress).
F-box proteins, as a subunit of SCF (Skp1/Cullin or CDC53/F-box protein) E3 ubiquitin ligases (Deshaies, 1999), have been shown to play essential roles in plant growth and development, including multiple phytohormone-signaling pathways, such as auxin, GA, and ethylene (Moon et al., 2004; Smalle and Vierstra, 2004).The Arabidopsis genome encodes more than 700 putative F-box proteins (Risseeuw et al., 2003; Gagne et al., 2004). SCFTIR1 was the first SCF complex identified in plants and has been shown to be involved in the auxin signaling. In addition, Arabidopsis F-box proteins COI1 and SLEEPY1 and the rice (Oryza sativa) GID2, EBF1, and EBF2 have been shown to exist as components of SCF E3 complexes involved in jasmonic acid, GA, and ethylene signaling pathways, respectively (Xu et al., 2002; Guo and Ecker, 2003; McGinnis et al., 2003; Potuschak et al., 2003; Sasaki et al., 2003). However, it is unclear if there is a direct link between an F-box protein and the guard cell ABA signaling. Here, we report a novel negative regulator of guard cell ABA signaling, DOR, in Arabidopsis. The DOR gene encodes a putative F-box protein, a member of the AtSFL (S-locus-F-box-like) family related to AhSLF-S2 (Wang et al., 2004) and specifically interacting with ASK14 and CUL1. A null mutation in DOR resulted in a hypersensitive ABA response of stomatal closing after drought stress. These findings suggest that DOR inhibits the ABA-induced stomatal closure under drought stress, revealing a novel mechanism in guard cell ABA signaling.
RESULTS
Identification of the dor Mutant
F-box proteins are known to play important roles in various aspects of plant growth and development via the ubiqutin/26S proteasome pathways (Vierstra, 2003). To examine possible functions of the Arabidopsis AtSFL gene family related to AhSLF-S2 (Wang et al., 2004), which controls the pollen function of S-RNase-based self-incompatibility (Lai et al.,2002; Qiao et al., 2004a, 2004b), we obtained a total of 61 T-DNA insertion lines corresponding to 40 individual genes (Wang et al., 2004). Most of the T-DNA mutations produced no obvious morphological phenotypes (Wang et al., 2004; Dong et al., 2006). We reasoned that some of them could produce conditional phenotypes under various environmental stresses given the fact that several of these genes were inducible under various abiotic stresses (data no shown). Thus, we first performed a drought treatment of these T-DNA mutants. Water was withheld from the T-DNA insertion mutant seedlings grown in a growth room for 2 weeks. Putative mutants with drought response phenotypes were scored by the degree of leaf wilting compared with that of wild-type plants. In this way, we identified a loss-of-function mutant that showed a significant increase in drought tolerance after the water withdrawal experiment; thus, the gene was subsequently named as DOR (for drought tolerance repressor; At2g31470).
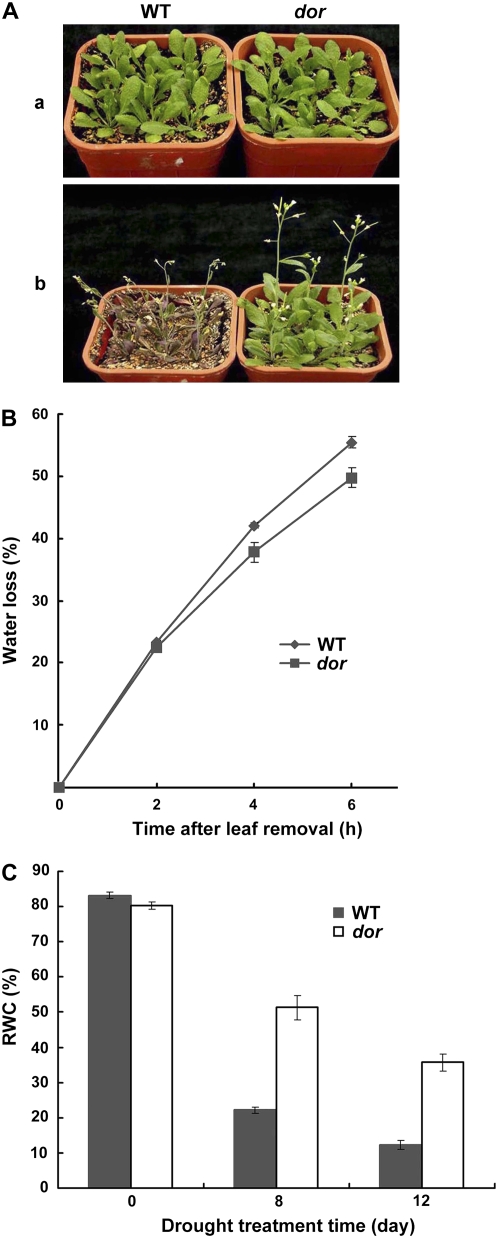
The dor mutant plants did not show any obvious morphological or developmental abnormalities under normal growth conditions (Fig. 1A). However, when subjected to the drought stress, as shown in Figure 1A, the dor plants were turgid and their leaves remained green, whereas the wild-type plants showed severe wilting and chlorosis of the rosette leaves, indicating the dor mutation enhanced the plant tolerance to drought stress.
Figure 1.
Increased tolerance of the dor plants under drought stress. A, Phenotype of the wild-type and dor plants after drought treatment. Both the wild-type and dor plants were grown under normal watering conditions for 24 d (a) and then stressed by completely depriving of irrigation for 11 d (b). B, Time courses of the water loss from the detached leaves of the dor and wild-type plants. The water loss is expressed as a percentage of the initial FW at indicated intervals. Each point indicates the mean of six measurements with ses. C, RWC in leaves from the wild-type and dor plants after drought stress. RWC was measured 0, 8, and 12 d after the drought stress. The leaves were detached and weighted for the initial FW, SW, and DW. RWC was expressed as (FW − DW)/(SW − DW) × 100%. Values are means ± sd (n = 3) from three independent experiments. [See online article for color version of this figure.]
To examine the physiological mechanism of the drought tolerance of the dor plants, we investigated the mutation on water loss and transpiration rate during drought stress. First, we measured the water loss from the wild-type and the dor rosette leaves after incubation at 22°C in the light. As shown in Figure 1B, detached leaves of the dor mutant lost water more slowly than did those of the wild-type plants. But the water loss showed no apparent difference between the wild type and dor in the first 2 h, owing to the fact that dor plants had a lower relative water content (RWC) level compared to wild-type plants prior the treatment, with the RWC of dor and wild-type leaves at approximately 82% and 84% (P < 0.05; Fig. 1C), respectively. To estimate whole-plant transpiration under drought stress, we examined the RWC level of wild-type and dor plants after the cessation of irrigation. Transpirational water loss, as determined by the RWC measurements after 8 and 12 d from the start of the drought treatment, was greatly reduced in the mutant compared to the wild type (P < 0.001; Fig. 1C). The higher RWC level in the dor plants during drought stress indicates that the dor plants could limit the water loss and maintain a higher RWC, resulting in an enhanced drought tolerance.
DOR Acts As a Negative Regulator of Drought Tolerance
DOR encodes a putative F-box protein, AtSFL35, a member of Class C of the AtSFL gene family (Wang et al., 2004). The dor mutant was found to have a T-DNA inserted 3 bp downstream of the translational start of the coding region (Supplemental Fig. S1A). DNA-blot hybridization showed that a single T-DNA copy was inserted into the genome (Supplemental Fig. S1B), and DOR is a single-copy gene in the Arabidopsis genome (Supplemental Fig. S1C). No DOR transcripts were detected in the homozygous dor plants, indicating a complete loss of gene function (Fig. 2A). To further confirm dor was indeed a null allele, we analyzed the cDNA fragment downstream of the insertion site, and the result showed that no DOR transcripts were detected in the homozygous dor plants (Supplemental Fig. S1D).
Figure 2.
Complementation of the dor drought tolerance phenotype by the wild-type DOR gene. A, RT-PCR analysis of DOR expression in the homozygous dor plants. No detectable DOR transcripts were found in the mutant. The expression of Tubulin is used as the control. “+” and “−” indicate whether moloney murine leukemia virus RT was present or absent in the process of RT reaction. B, Schematic presentation of the DOR:TAPa construct. DOR is driven by two copies of the cauliflower mosaic virus 35S promoter (2X35S) and a tobacco mosaic virus (TMV) U1 X translational enhancer. The TAPa tag consists of two copies of the protein A IgG-binding domain (2XIgG-BD), an eight-amino acid sequence corresponding to the 3C protease cleavage site (3C), a six-His stretch (6XHis), and nine repeats of the MYC epitope (9Xmyc). The vector contains the GATEWAY cloning sites (attR1∷Cmr∷ccdB∷attR2). A Nos terminator (Nos ter) sequence is located downstream of the expression cassette. C, Phenotypes of the transgenic Rescued dor-1S and Rescued dor-1E plants. The wild-type, Rescued dor-1S, Rescued dor-1E, and dor plants were grown under normal watering conditions for 24 d and then stressed by completely depriving of irrigation for 11 d. The top section shows the plants with regular watering and the bottom with the drought treatment. Rescued dor-1S and Rescued dor-1E indicate the dor plants harboring CaMV 35S-DOR:TAPa and DORp:DOR, respectively. D, qRT-PCR analysis of DOR expression in the seedlings from dor, the rescued transgenic lines, and the wild-type plants. The amplification of 18S rRNA was used as an internal control to normalize all data. [See online article for color version of this figure.]
To further test whether the drought tolerance of dor was the result of DOR gene disruption, we introduced a 35S∷DOR wild-type genomic region construct (Fig. 2B) into the dor plants. Six independent transgenic lines were fully complemented showing the wild-type phenotype, indicating a full complementation of the null allele (one line, Rescued dor-1S, is shown in Fig. 2C). To further confirm the identity of DOR, the dor mutant was transformed with the DOR genomic region under its own promoter (Fig. 2C, Rescued dor-1E). There was no discernable difference observed between the two types of rescued plant phenotypes, and all the following analyses were thus performed with the dor plants rescued with DOR∷TAPa under the 35S promoter. In addition, the transgenic lines were analyzed by DNA blot to confirm their molecular phenotypes (Supplemental Fig. S2), and the results of quantitative real-time reverse transcription-PCR (qRT-PCR) indicated that DOR expression was markedly increased at the mRNA level in the rescued lines compared with the wild type (Fig. 2D). This latter finding was consistent with the fact that the overexpression lines were more susceptible to drought stress (Fig. 2C, Rescued dor-1S and Rescued dor-1E).
Infrared thermography can be used as a proxy indicator of stomatal function, because plants with stomata that are more closed lose less thermal energy by evaporative cooling and, therefore, register as being warmer by thermography. Conversely, plants whose stomata are more open should be cooler (Wang et al., 2004). Our infrared thermal imaging revealed that the leaf surface temperature of the overexpression line Rescued dor-1S was about 0.5°C cooler than that of the wild-type plants, whereas the temperature of the dor plants was approximately 0.5°C warmer than that of the wild type (Supplemental Fig. S3A). These results suggest that the Rescued dor-1S plants resulted in more open stomata, so as to lose more water by transpiration under drought stress. In addition, the results of the water loss for the Rescued dor-1S, wild-type, and dor plants also proved that the dor plant lost water more slowly during drought stress than Rescued dor-1S and wild-type plants (Supplemental Fig. S3B). Taken together, these results demonstrate that DOR encodes a negative regulator of drought tolerance.
The Stomata Closing in the dor Plant Is Hypersensitive to ABA
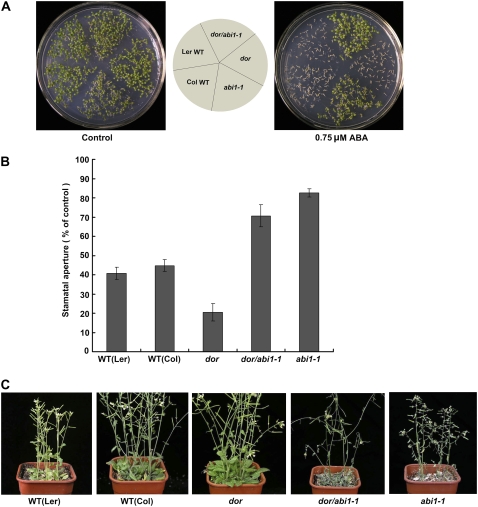
Stomata are known to close in response to drought stress to limit water loss by transpiration. During this process, ABA is synthesized and plays a role in closing stomata. To examine this possibility, we first measured the endogenous ABA in dor and the wild type using an immunoassay. The content of ABA in the wild type and dor did not show a significant difference in the absence of the stress treatment (Fig. 3). But after the drought treatment, the endogenous ABA was increased more in the dor mutant than that in the wild type (Fig. 3; P < 0.001), consistent with the higher RWC level in the dor plants (Supplemental Fig. S4), suggesting that the dor plant had an elevated endogenous ABA level under drought stress. To further investigate whether dor affected the sensitivity of guard cells to ABA, we measured the changes in stomatal aperture after ABA treatment. We exposed the wild-type and dor plants to strong light and high humidity to induce a full stomatal opening, and epidermal peels from these plants were used to analyze stomatal responses to ABA (Pei et al., 1997). In response to ABA treatment at different concentrations, stomata in the dor plants were almost completely closed with 5 μm ABA treatment for 2 h, but the stomata did not completely close even with 20 μm ABA in wild-type plants for the same treatment duration (Fig. 4, A and B). These results indicate that dor showed an ABA hypersensitivity of the stomatal closing compared with the wild type. However, other well-characterized responses to ABA, such as the inhibition of seed germination (Fig. 4C) and the reduction of vegetative growth (data not shown, but see Fig. 1A), were not significantly altered in the dor plants. These results show that DOR plays a role in controlling the ABA sensitivity of the guard cells.
Figure 3.
The dor plant has an increased cellular ABA level. ABA contents were measured from the leaves of the wild-type and dor plants grown for 21 d and then subjected to the drought treatment for 10 h (see “Materials and Methods”). The ABA amount is expressed as nanograms per gram FW leaf tissue. Error bars = mean ± sd from three independent experiments.
Figure 4.
Stomatal behavior of the wild-type and dor plants in response to ABA. A and B, Stomatal closure in the dor plants is hypersensitive to ABA. Data represent the means ± se from 50 stomata measured for each data point, from three independent experiments. Stomatal aperture ABA (0) = (100%) corresponded to average stomatal apertures of 2.94 ± 0.12 μm (wild type) and 2.88 ± 0.18 μm in dor. Scale bars = 10 μm. Stomata were opened by exposing to light for 2 h and after 2 h of the treatment with different concentrations of ABA. C, Effect of ABA on the dor seed germination. Seeds of the wild type and dor were plated on agar plates with or without 0.75 μm ABA (top plate, control; bottom plate, 0.75 μm ABA). The germination was scored at day 7 after being incubated at 22°C. Seeds were incubated at 4°C for 3 d prior to incubation at 22°C for germination. [See online article for color version of this figure.]
DOR Is Preferentially Expressed in the Guard Cells
As a first step to look into the reason as why DOR specifically acts in the guard cells, we used qRT-PCR to analyze DOR expression in five major tissues, including silique, root, leaf, seedling, and flower. As shown in Figure 5A, DOR mRNA was detected in flower, seeding, and rosette leaves, but its expression in the root and silique was extremely low. In addition, DOR mRNA was generally of a very low abundance and hardly detectable in northern blots of these samples (data not shown). To gain more insight into the cell type expression pattern of DOR, we generated the transgenic plants harboring a transcriptional fusion of the GUS reporter gene and a putative DOR promoter. The result indicated that GUS activity was clearly visible in the stoma of the leaves (Fig. 5B). No GUS signals were detected in tissues devoid of stoma, suggesting that DOR expression is preferentially expressed in the guard cells. This was consistent with the finding that the DOR gene was expressed in the guard cells compared with the mesophyll cells by a previous microarray analysis (Leonhardt et al., 2004).
Figure 5.
DOR is preferentially expressed in the guard cells. A, qRT-PCR analysis of the expression of DOR in different tissues. Si, Siliques; R, roots; L, leaves; Se, seedling; F, flower. B, DOR promoter∷GUS expression in the guard cells. Blue color indicates the GUS expression. Scale bars = 10 μm. C, qRT-PCR analysis of DOR expression in response to 100 μm ABA in the wild-type plants. D, qRT-PCR analysis of the expression of DOR in response to desiccation in the wild-type plants. E, qRT-PCR analysis of DOR expression in response to 100 μm ABA in the abi1-1 mutant. The amplification of 18S rRNA was used as an internal control to normalize all data in the qRT-PCR analysis. [See online article for color version of this figure.]
Furthermore, we examined whether ABA treatment induced a change in the DOR transcript level. As shown in Figure 5C, DOR expression was rapidly reduced during early application of exogenous ABA in the wild-type plants. Similarly, DOR expression was reduced by drought stress (Fig. 5D). These results suggested that DOR acts as a negative regulator of guard cell ABA signaling, but its own expression is suppressed by ABA, revealing a negative feedback loop for ABA response.
ABA-Induced Stomatal Closure Is Defective in the dor/abi1-1 Double Mutant
As a guard cell-preferential gene, how is DOR involved in ABA signaling? We found that the ABA-induced reduction of DOR expression observed in the wild-type plants (see Fig. 5C) was completely abolished in an abi1-1 background (Fig. 5E), suggesting that ABI1 is essential for this ABA repression of DOR. In addition, DOR expression was elevated in abi1-1, suggesting that ABI1 negatively regulates DOR expression. To further examine whether DOR acts exclusively through the ABI1-mediated ABA signaling pathway, we generated a homozygous double mutant of dor/abi1-1. The dor/abi1-1 double mutant showed the ABA insensitivity in seed germination, the impairment in ABA-induced stomatal closing, and the wilty phenotype similar to those of the abi1-1 single mutant (Fig. 6, A–C; Supplemental Fig. S5), indicating that abi1-1 is epistatic to dor, the dor locus acts upstream of the abi1-1 locus, and, together with the increased expression of DOR in abi1-1, they also formed a negative regulatory feedback loop for ABA signaling.
Figure 6.
Analysis of dor/abi1-1 double mutant characteristics. A, Effect of ABA on dor/abi1-1 seed germination. Seeds of the wild type (ecotype Landsberg erecta [Ler]), wild type (Col), dor, dor/abi1-1, and abi1-1 were plated on agar plates with or without 0.75 μm ABA (left, control; right, 0.75 μm ABA). The germination was scored at day 7 after incubation at 22°C. Seeds were incubated at 4°C for 3 d prior to incubation at 22°C for germination. B, Comparison of the stomatal closing responses induced by ABA (10 μm) in the wild-type (Ler), wild-type (Col), dor, dor/abi1-1, and abi1-1 plants. Stomatal aperture ABA (0) = (100%) corresponded to average stomatal apertures of 2.96 ± 0.16 μm wild type (Ler), 2.98 ± 0.08 μm wild type (Col), and 2.80 ± 0.13 μm in dor, 3.07 ± 0.12 μm in dor/abi1-1, and 3.12 ± 0.05 μm in abi1-1. Data presented are the means of 50 stomatal apertures ± SEM. C, Phenotype of the dor/abi1-1 plants after drought treatment. The wild type (Ler), wild type (Col), dor, dor/abi1-1, and abi1-1 plants were grown under normal watering conditions for 24 d and then stressed by completely depriving them of irrigation for 11 d. [See online article for color version of this figure.]
DOR Acts Independently of the PLDα1 Pathway
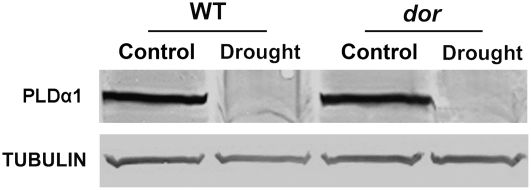
Recently, Mishra et al. (2006) reported PLDα1 and PA act upstream of GPA1 and ABI1 in a pathway regulating the stomatal responses to ABA signals. We next examined whether DOR and PLDα1 function in the same pathway by probing immunoblots with an antibody against PLDα1, and the results indicated that the PLDα1 protein did not show any obvious change in the wild-type and dor plants under the normal and drought stresses (Fig. 7). However, we found that PLDα1 protein in the wild type and dor was drastically decreased by drought treatment, and this may be related to other signal transduction pathway(s) that the PLDα1 protein involves. In addition, the ABA responses of the double mutant dor/pldα1 resembled the wild type (Supplemental Fig. S6), confirming that they function independently. These results suggested that DOR does not act through PLDα1 in its negative regulation of ABA responses in the guard cells and possibly represents a new pathway.
Figure 7.
PLDα1 levels in the wild-type and dor plants. Immunoblot analysis of PLDα1 was done with 10 μg protein of crude extracts of the wild type and dor with or without the drought stress. The proteins also were analyzed by immunoblot hybridization using an anti-tubulin antibody for loading control in both plants.
DOR Interacts with ASK14 and CUL1 Proteins
To confirm that DOR actually encodes an F-box protein (Fig. 8A), we examined whether DOR interacts with ASK proteins by a yeast two-hybrid analysis. We isolated 17 ASK genes from Arabidopsis and tested the interaction between them and DOR. The result indicated that DOR specifically interacts with the ASK14 protein (Fig. 8B). To further confirm that DOR forms the SCF complex, we performed a pull-down assay using an antibody against AtCUL1, which is a known subunit of several SCF complexes. CUL1 proteins were detected in the pull-down product of the TAPa-tagged DOR (DOR-TAPa) transgenic plants and the wild type, whereas no protein was detected in the negative control TAPa transgenic plants (Fig. 8C). In general, it was observed that conjugation of RUB1 occurred on wild-type CUL1 protein, but such a modification did not occur on the C-terminal DOR-TAPa, which could be explained by the finding that the N-terminal and C-terminal TAPa fusions could have different functional properties (Rubio et al., 2005). Taken together, these results indicate that DOR is associated with the two known subunits of the SCF complex and likely forms a bona fide SCFDOR complex in Arabidopsis.
Figure 8.
DOR interacts with ASK14 and CUL1 proteins. A, Schematic representation of the DOR domain structure. Besides the F-box motif, conserved domains in the C-terminal regions contain C1, C2, C3, and C4 domains (Wang et al., 2004). B, Yeast cells containing various combinations of BD and AD fusions were tested for their growth on SD/−Ade/−His/−Leu/−Trp media; pGBKT7-53 with pGADT7-RecT was used as positive control and pGBKT7 with pGADT7 as negative control (top). The strains were grown further to test for the expression of the β-galactosidase reporter gene (bottom). C, A pull-down assay for DOR and CUL1 proteins. Protein extracts from the DOR-TAPa and the negative control TAPa transgenic plants were subjected to the TAPa purification. Wild type was used as a positive control. Proteins corresponding to each fraction obtained were separated on a 12% SDS-PAGE gel. The Arabidopsis CUL1 antibody was used for immunoblotting. [See online article for color version of this figure.]
GeneChip Analysis of dor Plants
To find further functional clues to DOR, we performed an Affymetrix GeneChip analysis to investigate the effect of DOR gene disruption on global gene expression under drought stress. Subsequently, we found among the transcripts up-regulated in the dor mutant (Table I), the NCED3 gene encoding a key enzyme, 9-cis-epoxycarotenoid dioxygenase, in ABA biosynthesis (Qin and Zeevaart, 1999) was significantly enhanced in the dor plants after drought treatment, suggesting that more synthesis and accumulation of endogenous ABA occurred in the dor plant. Moreover, the ABA- and desiccation-inducible genes (i.e. RD29A, RD29B, XERO2, and RAB18; Lang and Palva, 1992; Yamaguchi-Shinozaki and Shinozaki, 1993; Welin et al., 1994) were also highly expressed in the dor plants (Table I). The high-level expression of ABA-biosynthetic and ABA-responsive genes in the dor plants is consistent with the fact that the disruption of the DOR gene slightly increased the cellular ABA levels but with the dramatic increase after the drought stress (see Fig. 3). In addition, we did drought treatment for the detached leaves from wild type and dor mutant and harvested the samples at two time points, 2 and 6 h, and then did qRT-PCR for the NCED3, RAB18, and RD29A expression profiles. The results showed that NCED3 expression was significantly enhanced in the dor plants after drought treatment in the first 2 h, and the RAB18 and RD29A were strongly enhanced at 6 h after drought treatment (Supplemental Fig. S8). Taken together, these results show that DOR, as an F-box protein, is likely involved in the regulation of ABA biosynthesis under drought stress, though other possible roles in regulating the ABA breakdown and/or signaling could not be completely excluded at this stage.
Table I.
Top candidate genes differentially expressed between the wild-type and dor leaves under drought stress using ATH1 GeneChip
| Affy Probe Set ID | Description | AGI No. | dor-dr/ck Log2 Ratioa | P Valueb | Fold-Changec | Wild Type-dr/ck Log2 Ratioa | P Valueb | Fold-Changec |
|---|---|---|---|---|---|---|---|---|
| 257280_at | NCED3de | At3g14440 | 5.15 | 0.00002 | 35.5 | 2.2 | 0.000836 | 4.6 |
| 263570_at | AAO3e | At2g27150 | 3.05 | 0.000025 | 8.3 | 1.4 | 0.002744 | 2.6 |
| 247957_at | ABI2e | At5g57050 | 2.9 | 0.000194 | 7.5 | 1.75 | 0.000366 | 3.4 |
| 247095_at | RAB18d | At5g66400 | 6.1 | 0.00002 | 68.6 | 4.65 | 0.00002 | 25.1 |
| 248337_at | RD29Ad | At5g52310 | 5 | 0.00002 | 32.0 | 1 | 0.165514 | 2.0 |
| 248352_at | RD29B | At5g52300 | 6.5 | 0.00002 | 90.5 | 3.95 | 0.001028 | 15.5 |
| 252102_at | XERO2 | At3g50970 | 6.7 | 0.00002 | 104.0 | 3.05 | 0.00002 | 8.3 |
| 259231_at | AtPP2CA | At3g11410 | 2.85 | 0.00002 | 7.2 | 1.9 | 0.00002 | 3.7 |
| 259570_at | COR47 | At1g20440 | 4.65 | 0.00002 | 25.1 | 2.3 | 0.000025 | 4.9 |
| 262128_at | LEA | At1g52690 | 7.7 | 0.00002 | 207.9 | 4.7 | 0.000044 | 26.0 |
| 258347_at | LEA-like | At3g17520 | 10.35 | 0.00002 | 1,305.2 | 6.05 | 0.00002 | 66.3 |
| 265216_at | MAP kinase | At1g05100 | 3.9 | 0.00002 | 14.9 | 2.45 | 0.201916 | 5.5 |
| 264005_at | AGP | At2g22470 | 1.95 | 0.000229 | 3.9 | 0.5 | 0.631018 | 1.4 |
| 267080_at | Unknown | At2g41190 | 5.5 | 0.00002 | 45.3 | 4.4 | 0.00002 | 21.1 |
| 263881_at | Unknown | At2g21820 | 6.3 | 0.00002 | 78.8 | 5.7 | 0.00002 | 52.0 |
| 264524_at | tat-binding | At1g10070 | 4.95 | 2.15E-05 | 30.9 | 3.2 | 2.85E-05 | 9.2 |
Log2 ratio measures the change in expression level for each probe set between drought treatment (dr) versus control (ck). This change is expressed as the log2 ratio. A log2 ratio of 1 is the same as a fold-change of 2. Here, we averaged the log2 ratio for two biological replicates.
Change P value, which measures the probability that the expression levels of each probe set between drought treatment versus control are the same. Here, we averaged the change P value for two biological replicates.
Fold-change is calculated using the signal log ratio.
Indicates that the gene expression level was independently validated by qRT-PCR, shown in Supplemental Figure S7.
Indicates some key genes for ABA biosynthesis and signal transduction pathway, but was not shown in the Affymetrix 8k chip used previously (Leonhardt et al., 2004).
DISCUSSION
In this study, we have identified an F-box protein DOR that is important for drought response and provided evidence for a connection between ABA guard cell signaling and the ubiquitin/26S-mediated proteolysis pathway, revealing a novel inhibitory pathway for modulating the ABA-induced stomatal closure under the drought condition in Arabidopsis.
DOR Acts As a Novel Negative Regulator of the Guard Cell ABA Response in Arabidopsis
ABA signal transduction in guard cells is a highly complex process (Li et al., 2006). The type 2C protein phosphatase ABI1 acts as an important regulator of ABA responses in guard cells (Leung et al., 1994; Meyer et al., 1994; Nilson and Assmann, 2007) and plays a negative role in the ABA-induced stomatal closure. Some of the signaling components functioning upstream and downstream of ABI1 have been identified. To our knowledge, most of them function downstream of ABI1, such as OST1 and ERA1. OST1 kinase activation in response to ABA is suppressed in the dominant abi1-1 mutant (Mustilli et al., 2002), indicating that ABI1 negatively regulates the ABA signal transduction upstream of OST1. Partial suppression of the ABA-insensitive phenotypes of the abi1 and abi2 mutants by ERA1 deletion suggests that the target of the ERA1 FTase may function downstream or in parallel to these ABI protein phosphatases (Pei et al., 1998).
However, the early events of ABA signal transduction remain largely unknown, especially those acting upstream of ABI1. At the present time, very few factors functioning upstream of ABI1, such as PLD, have been identified in Arabidopsis. The PLD generates PA, which is reported to bind to ABI1 and thereby abolishes ABI1 inhibition of the ABA-induced stomatal closing (Mishra et al., 2006). In this study, we have found the F-box protein DOR plays a negative role in the ABA-induced stomatal closing (Fig. 4, A and B). The dor/abi1-1 double mutant analysis indicated that abi1-1 is epistatic to dor, and the dor locus acts upstream of the abi1-1 locus. Although PLDα1 functions upstream of ABI1, our results suggest that DOR does not act through PLDα1 in its negative regulation of ABA responses in the guard cells. So we deduce that DOR represents a novel regulator implicated in the ABA signaling and is negatively regulated by ABI1 in the guard cells.
More recently, ABAR was identified as an ABA receptor to regulate a series of the components involved in the ABA signaling (Shen et al., 2006). Interestingly, we found that ABAR expression was down-regulated in the dor mutant under drought stress (data not shown), suggesting a positive regulation of DOR by ABAR. Further investigation of the relationship between them could provide a clue to the function of DOR. In addition, the role of DOR in the guard cell ABA responses is consistent with the fact that DOR is mainly expressed in the guard cells, because we did not find any other well-characterized responses to ABA, such as inhibition of seed germination and reduction of vegetative growth, in the dor plants. Furthermore, DOR may also play a role in regulating pollen development and/or pollination, because its expression also was detected in pollen (Supplemental Fig. S9).
DOR Is Likely Involved in the Regulation of ABA Biosynthesis under Drought Stress
Plants are subjected to constant environmental and physiological changes and accordingly need to fine-tune the ABA level in relation to the severity and duration of the stresses. The current model indicates that an increase in ABA levels occurs primarily through de novo biosynthesis under various abiotic stress conditions (Seo and Koshiba, 2002; Nambara and Marion-Poll, 2005). ZEP (ABA1), NCED3, ABA3, and AAO3 act as key enzymes regulating the ABA biosynthesis pathway in Arabidopsis and other plant species, whose expression is generally up-regulated by the physiological needs or environmental stresses (Audran et al., 1998; Cutler and Krochko, 1999; Iuchi et al., 2000; Nambara and Marion-Poll, 2005). The increased transcription of these genes might lead to an increase in ABA levels, which, in turn, would induce a positive feedback regulation of NCED3, AAO3, and ABA1 gene expression (Xiong et al., 2002). In this scenario, NCED3 is considered the most responsive gene to the newly synthesized ABA. Our GeneChip analysis also confirmed that NCED3 is the ABA biosynthetic gene most responsive to the drought stress in the dor plants (Table I). However, the transcriptional regulatory mechanism did not appear to be the only one to regulate the ABA biosynthesis. The activation of inactive ABA pools by polymerized AtBG1 is a mechanism by which plants rapidly adjust ABA levels and respond to changing environmental conditions (Lee et al., 2006). In addition, Ko et al. (2006) also reported the up-regulation of XERICO, a RING-H2-type zinc-finger protein, substantially increased cellular ABA levels. In this study, we found that the stress-induced ABA reduces DOR expression and could in turn enhance the ABA biosynthesis. Thus, they could form a negative feedback regulatory loop for ABA in the guard cells under drought stress. In this way, the ABA production by its biosynthesis regulated by DOR could be controlled precisely and rapidly to meet the plant requirements under water stress. Indeed, the content of ABA in the wild type and dor did not show a significant difference in the absence of the stress. But after the drought treatment, dor had an elevated endogenous ABA level higher than that detected in the wild type (Fig. 3). The enhanced ABA content in the dor plants correlated directly with the rapidly induced stomatal closing and thus increases the plant tolerance to the drought stress. These results highlight the importance of the F-box protein DOR in providing a requisite ABA level for the cellular responses to drought stress. Further examination of dor with mutants involved in the ABA synthesis as well as using its inhibitor could help reveal how these factors are related to each other.
Previous studies have shown that most F-box proteins are associated with the ubiquitin/26S proteasome-mediated protein degradation. DOR encodes one of the AtSFL proteins and specifically interacts with ASK14 and CUL1 based on our yeast two-hybrid and pull-down assays (Fig. 8), indicating that it may function as a subunit of SCF E3 ligase in the ubiquitin/26S-mediated proteolysis pathway. Incidentally, we detected a similar expression pattern of ASK14 to DOR (data not shown). Functional analysis of ASK14 could provide further insights into the DOR action in the guard cells.
In conclusion, we have shown that DOR is perceived by the guard cell as a signal that triggers a response to inhibit the ABA-induced stomatal closure under drought stress. However, it is not clear how exactly DOR is involved in regulating the guard cell ABA signaling. As an F-box protein, we propose that DOR could target a factor(s) directly involved in the ABA signaling for degradation via the ubiquitin/26S-mediated proteolysis pathway under drought stress. Modulation of DOR or its target(s), specifically in the guard cells, could provide an avenue to genetically modify the plant tolerance to drought stress.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia was used. The dor mutant was obtained from the SALK T-DNA insertion collection (http://signal.salk.edu). The original line was Salk-050074. The abi1-1 mutant seeds were a gift from Xiangdong Fu (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences). Arabidopsis plants were grown on Murashige and Skoog medium containing 2% (w/v) Suc and 0.8% (w/v) agar or grown in soil under a long-day condition (16 h light, 8 h dark) at 22°C, except where otherwise stated.
RT-PCR Analysis
Total RNA was prepared as previously described (Lai et al., 2002) and was digested with DNase I (TaKaRa). Reverse transcriptase (Invitrogen) was used to synthesize the first strand cDNA. Specific primers of DOR used for RT-PCR were as follows: 5′-ACCCTATTCTTATATCGTTCC-3′ (forward) and 5′-AGGGTTACATATCGCCGAGAC-3′ (reverse). Specific primers of DOR used for RT-PCR of the downstream sequence of the T-DNA insertion site were as follows: 5′-CCAGAATTTGTCTCGGCGAT-3′ (forward) and 5′-CCTCTTCCTTGTCTTAGGTTTTGG-3′ (reverse). Specific primers of TUBULIN used for RT-PCR were as follows: 5′-TTTGGAGCCTGGGACTATGGAT-3′ (forward) and 5′-ACGGGGGAATGGGATGAGAT-3′ (reverse).
qRT-PCR Analysis
RT was performed using TaqMan Reverse Transcription Regents kit (Applied Biosystems). The cDNA samples were diluted to 10, 5, and 1 ng/μL. Triplicate quantitative assays were performed on 1 μL of each cDNA dilution using the SYBR Green Master mix with an ABI 7900 sequence detection system according to the manufacturer's protocol (Applied Biosystems). Gene-specific primers were designed by using PRIMEREXPRESS 1.0 software (Applied Biosystems). The relative quantization method (ΔΔCT) was used to evaluate quantitative variation between the replicates examined. The amplification of 18S rRNA was used as an internal control to normalize all data. Specific primers of DOR used for qRT-PCR were as follows: 5′-CCAGAATTTGTCTCGGCGAT-3′ (forward) and 5′-CCTCTTCCTTGTCTTAGGTTTTGG-3′ (reverse). Specific primers of 18S rRNA used for qRT-PCR were as follows: 5′-CGGCTACCACATCCAAGGAA-3′ (forward) and 5′-TGTCACTACCTCCCCGTGTCA-3′ (reverse).
Plasmid Construction and Arabidopsis Transformation
The DOR∷GUS Construct
Genomic DNA sequence corresponding to 1,858 bp upstream of the predicted ATG codon of the DOR open reading frame was cloned into pCAMBIA1391 binary vector between SmaI and SalI sites. Junction sequences of the resulting construct DOR∷GUS were sequenced for verification. The PCR primers used were as follows: 5′-ATGTCGACGTGTGGCTAACTCTTCTCTGTC-3′ (forward) and 5′-ATCCCGGGGTTGCAGTAATCAAATTCTTTTGGAAC-3′ (reverse). The open reading frame sequence of the DOR gene was introduced into the pC-TAPa vector (see Fig. 1C). The PCR primers used were as follows: 5′-CACCACAAAATGAAATCACGGCGACAGAATGTG-3′ (forward) and 5′-TATAAGCTTCACATCCTCTACATG-3′ (reverse).
The DORp-DOR Construct
Genomic DNA sequence corresponding to 1,858 bp upstream the predicted ATG codon of DOR and the full-length genomic sequence of DOR was cloned into pCAMBIA1300 binary vector between SmaI and SalI sites. The PCR primers used were as follows: 5′-ATGTCGACGTGTGGCTAACTCTTCTCTGTC-3′ (forward) and 5′-TATAAGCTTCACATCCTCTACATG-3′ (reverse). Transgenic Arabidopsis plants were obtained by Agrobacterium-mediated transformation using the floral dip method (Bechtold and Pelletier, 1998). Analyses of transgenic lines were performed on homozygous T3 progeny plants.
ABA and Water Stress Treatments
For ABA treatments, 3-week-old wild-type and abi1-1 seedling were grown in solid Murashige and Skoog medium with 2% (w/v) Suc and transferred to liquid Murashige and Skoog medium supplemented with 100 μm ABA. For drought stress, both the wild-type and dor plants were grown under normal watering conditions for 24 d and then stressed by completely depriving them of irrigation for 11 d. For water loss measurement, rosette leaves of wild type and the dor mutant were detached as described above. Loss in fresh weight (FW) was monitored at indicated time points. RWC was measured after drought stress. The leaves were detached and weighted for the initial FW, then were submerged in water overnight to measure saturated weight (SW), followed by drying in an oven at 80°C for 24 h for dry weight (DW). RWC was expressed as (FW − DW)/(SW − DW) × 100%.
Affymetrix GeneChip Analysis
RNA Isolation and Purification
Arabidopsis rosette leaf samples were homogenized in liquid nitrogen prior to RNA isolation. Total RNA was extracted using Qiagen Rneasy kit (Valencia). For Affymetrix GeneChip ATH1 analysis, 8 μg of total RNA was used for making biotin-labeled cRNA targets. The process for microarray experiment followed the GeneChip Standard Protocol (Eukaryotic Target Preparation), including cDNA and cRNA synthesis, cRNA fragmentation, hybridization, washing and staining, scanning, etc. In this experiment, we applied Poly-A RNA Control kit and the One-Cycle cDNA Synthesis kit. We used Affymetrix developed GCOS software to do data collection and normalization so that signals from different arrays were comparable. The overall intensity of all probe sets of each array was scaled to 500 so hybridization intensity of all arrays was equivalent, and each probe set was assigned P, A, and M and also a P value from algorithm in GCOS. To find differentially expressed genes between wild type and the mutant or between control and drought stress, the log2-transformed signal ratio of each gene was calculated by applying the GCOS baseline tool.
ATH1 GeneChip was used for gene expression analysis of wild-type and dor mutant plants under drought treatment (both the wild-type and dor plants were grown under normal watering conditions for 24 d and then stressed by completely depriving them of irrigation for 10 d). Two biological repeat experiments were conducted, and the raw data was analyzed by applying Affymetrix GCOS software. A large number of genes showed significant up- and down-regulations in dor-drought versus control without drought treatment (CK), and wild type-drought versus CK, separately. We further did a comparison for difference between dor mutant and wild type under drought conditions. The fold change for dor-drought/CK versus wild type-drought/CK was calculated. First, ABA biosynthesis and signal transduction pathway-related gene expression in the GeneChip were investigated. Second, we found that some of the significantly changed genes were also identified in the guard cells under an ABA treatment experiment (Leonhardt et al., 2004). We listed the top candidate genes in Table I. The ATH1 GeneChip data for the dor mutant analysis has been submitted to the public microarray database under the accession number GSE10643 (http://www.ncbi.nlm.nih.gov/projects/geo/).
Thermal Imaging
Thermal imaging of drought-stressed plantlets was performed as described previously (Merlot et al., 2001). In brief, plantlets were first grown under well-watered conditions (21°C, 60%–70% relative humidity [RH], 16-h photoperiod) for approximately 1 week. Drought stress then was initiated by withholding watering and transferring the pots to a drier atmosphere (24°C, 50% RH, 16-h photoperiod). Thermal images were obtained using a Thermacam PM250 infrared camera (Inframetrics). Images were saved on a Personal Computer Memory Card International Association card and were analyzed subsequently on a Macintosh computer using version 1.56 of the public domain image-analysis program, NIH Image (http://rsb.info.nih.gov/nih-image/).
Stomatal Aperture Bioassays
Stomatal closing assays were conducted as described previously (Pei et al., 1997). Rosette leaves were floated in a solution containing 50 μm CaCl2, 10 mm KCl, 10 mm MES [2-(N-morpholino)ethanesulfonic acid]-Tris, pH 6.15, and exposed to light (150 μmol m−2 s−1) for 2 h. Subsequently, ABA was added to the solution to assay for stomatal closing. After ABA treatment for 2 h, stomatal apertures were measured as described previously (Pei et al., 1997). Values are means ± ses (n = 50).
Histochemical Analysis of GUS Activity
Histochemical location of GUS activities in the transgenic plants was analyzed after incubating tissues in 5-bromo-4-chloro-3-indolyl-β-glucuronic acid buffer (50 mm sodium phosphate buffer, pH 7.0, 10 mm EDTA, 0.1% Triton X-100, 0.5 mm potassium ferrocyanide, and 2 mg/mL 5-bromo-4-chloro-3-indolyl glucuronide) at 37°C for 12 h and subsequently cleared in a transparent liquid medium (85% lactic acid, chloral hydrate, phenol, clove oil, xylene; weight ratio is 2:2:2:2:1). GUS images were taken with an Olympus BX-RFA fluorescence microscope.
ABA Content Analysis
Drought stress was treated by the method of Lee et al. (2006). In brief, plants grown on Murashige and Skoog plates for 21 d and were exposed to 30% RH for 10 h. The leaves of drought stress and unstressed control were frozen in liquid nitrogen and ground into powder. One gram of the tissue was suspended in 10 mL of extraction solution containing 80% methanol, 100 mg/L butylated hydroxytoluene, and 0.5 g/L citric acid monohydrate. The suspension was stirred overnight at 4°C and centrifuged at 1,000g for 20 min. The supernatant was transferred to a new tube and dried under vacuum. The dry residue was dissolved with 500 μL of Tris-buffered saline (50 mm Tris, 0.1 mm MgCl2·6H2O, and 0.15 m NaCl, pH 7.8). ABA concentration in the solution was then determined using the Phytodetek ABA immunoassay kit (Idetek).
Isolation of the dor/abi1-1 Double Mutant
We obtained the dor/abi1-1 homozygous mutant lines by crossing abi1-1 into dor. F2 plants were screened by PCR amplification with the DOR-specific primers of 5′-CAAGCAATGCTTCAAAGCAGAGGG ATGG-3′ and 5′-AGGGTTACATATCGCCGAGAC-3′; the segregation ratio was about 9:3:4. The homozygous dor plants, which had been confirmed by DNA blot (Supplemental Fig. S1), were further confirmed by direct sequencing of the ABI1 loci. The primers of abi1-1 were 5′-GGAATCAGCAGCTGCTGATATAGTCGT-3′ and 5′-TCTCCGAGTCAACTCTCAGGAACGAG-3′.
Immunoblot Analysis
Twenty-one-day-old seedlings of wild type and dor (treated with or without 30% polyethylene glycol 6000 for 5 h) were homogenized with extraction buffer (0.2 m Tris-HCl, pH 6.8, 1 mm dithiothreitol, 4% SDS, and 25% glycerol). The samples were boiled and analyzed by immunoblotting with the PLDα1 Antibody (a gift from Wenhua Zhang, Nanjing Agricultural University, People's Republic of China) and a mouse monoclonal anti-tubulin Ab (Sigma) as described previously (Qiao et al., 2004a).
Plasmid Construction and Yeast Transformation Assay
The coding sequence of the DOR gene was introduced to pGBKT7 bait plasmid (CLONTECH) to produce a fusion protein with the GAL4 DNA-binding domain, and 17 ASK cDNA (ASK1, 2, 3, 4, 6, 7, 8, 9, 10, 11, 12, 13, 14, 16, 17, 18, and 19) were inserted into pGADT7 prey plasmid containing the GAL4 activation domain (Wang et al., 2004). Yeast transformation, growth conditions, and assays for β-galactosidase activity were performed according to the manufacturer's instructions (CLONTECH).
TAPa Purification Procedure
DOR-TAPa and TAPa seedlings (5 g FW) grown in agar under a long-day condition (16 h light, 8 h dark) at 22°C were ground in liquid nitrogen, thawed in 2 volumes of extraction buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10% glycerol, 0.1% Tween 20, 1 mm phenylmethylsulfonyl fluoride, and 1× complete protease inhibitor cocktail; Roche), filtered through four layers of cheesecloth, and centrifuged at 12,000g for 10 min at 4°C. The protein concentration in the supernatant was determined by Bradford assay (Bio-Rad). Extracts containing similar amounts of total protein were incubated with 500 μL IgG beads for 6 h at 4°C with gentle rotation. Elution from the IgG beads was performed by the protocol of IgG Sepharose 6 Fast Flow (Amersham Biosciences). All the steps in the purification procedure were carried out at 4°C. Proteins in each fraction were separated on a 12% SDS-PAGE gel. Protein bands were visualized, independently, by immunoblotting using the Arabidopsis CUL1 antibody (Liu et al., 2002).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number NM-128704.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Southern-blot analyses of the dor mutant.
Supplemental Figure S2. Molecular analyses of the DOR rescued lines.
Supplemental Figure S3. The overexpression line Rescued dor-1S enhances transpiration under drought stress.
Supplemental Figure S4. RWC in leaves of dor and wild-type plants was measured 0 and 10 h after drought stress.
Supplemental Figure S5. qRT-PCR analysis of RAB18 expression in response to 100 μm ABA in wild-type (Ler), wild-type (Col), dor, dor/abi1-1, and abi1-1 seedlings.
Supplemental Figure S6. ABA-induced stomatal closure in dor/pldα1.
Supplemental Figure S7. qRT-PCR confirmation of the GeneChip expression data for three selected genes.
Supplemental Figure S8. qRT-PCR analyses of the expression of RAB18, RD29A, and NCED3 at indicated intervals from the detached leaves of dor and wild-type plants.
Supplemental Figure S9. DOR promoter∷GUS expression in pollen.
Supplementary Material
Acknowledgments
We thank the Arabidopsis Biological Resource Center for the seeds of dor (Salk_050074), Xiangdong Fu for the seeds of the abi1-1 mutant, and Wenhua Zhang for the PLDα1 antibody. We would also like to thank Chunpeng Song for his kind help for ABA false-color infrared imaging.
This work was supported by the Ministry of Science and Technology of China (grant no. 2003CB114306), by the National Natural Science Foundation of China (grant no. 30870216), by the China Postdoctoral Science Foundation (to Y.Z.), and in part by a National Science Foundation 2010 grant (to X.W.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Yongbiao Xue (ybxue@genetics.ac.cn).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Audran C, Borel C, Frey A, Sotta B, Meyer C, Simonneau T, Marion-Poll A (1998) Expression studies of the zeaxanthin epoxidase gene in Nicotiana plumbaginifolia. Plant Physiol 118 1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82 259–266 [DOI] [PubMed] [Google Scholar]
- Burbidge A, Grieve TM, Jackson A, Thompson A, McCarty DR, Taylor IB (1999) Characterization of the ABA-deficient tomato mutant notabilis and its relationship with maize Vp14. Plant J 17 427–431 [DOI] [PubMed] [Google Scholar]
- Blatt MR (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16 221–241 [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Krochko JE (1999) Formation and breakdown of ABA. Trends Plant Sci 4 472–478 [DOI] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P (1996) A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273 1239–1241 [DOI] [PubMed] [Google Scholar]
- Deshaies RJ (1999) SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol 15 435–467 [DOI] [PubMed] [Google Scholar]
- Dong L, Wang L, Zhang Y, Zhang Y, Deng X, Xue Y (2006) An auxin-inducible F-box protein CEGENDUO negatively regulates auxin-mediated lateral root formation in Arabidopsis. Plant Mol Biol 60 599–615 [DOI] [PubMed] [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, Vierstra RD (2004) Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci USA 101 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zeng Q, Guo J, Cheng J, Ellis BE, Chen JG (2007) Genetic characterization reveals no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabidopsis. Plant J 52 1001–1013 [DOI] [PubMed] [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AA, Vartanian N, Giraudat J (1999) ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11 1897–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2003) Plant responses to ethylene gas are mediated by SCF (EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115 667–677 [DOI] [PubMed] [Google Scholar]
- Hugouvieux V, Kwak JM, Schroeder JI (2001) An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell 106 477–487 [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27 325–333 [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2000) A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol 123 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA, Temple BR, Chen JG, Gao Y, Moriyama EN, Jones AM, Siderovski DP, Willard FS (2007) Comment on “A G protein coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid”. Science 318 914. [DOI] [PubMed] [Google Scholar]
- Ko JH, Yang SH, Han KH (2006) Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J 47 343–355 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, Hilhorst HW, Karssen CM (1989) In vivo inhibition of seed development and reserve protein accumulation in recombinants of abscisic acid biosynthesis and responsiveness mutants in Arabidopsis thaliana. Plant Physiol 90 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z, Ma W, Han B, Liang L, Zhang Y, Hong G, Xue Y (2002) An F-box gene linked to the self-incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol Biol 50 29–42 [DOI] [PubMed] [Google Scholar]
- Lang V, Palva ET (1992) The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20 951–962 [DOI] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126 1109–1120 [DOI] [PubMed] [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI (2004) Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16 596–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J (1994) Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science 264 1448–1452 [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Assmann SM, Albert R (2006) Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling. PLoS Biol 4 e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yue Y, Li B, Nie Y, Li W, Wu WH, Ma L (2007) A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science 315 1712–1716 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Serino G, Deng XW, Dinesh-Kumar SP (2002) Role of SCF ubiquitin-ligase and the COP9 signalosome in the N gene-mediated resistance response to Tobacco mosaic virus. Plant Cell 14 1483–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EA (1998) Signal transduction and ion channels in guard cells. Philos Trans R Soc Lond B Biol Sci 353 1475–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun TP, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15 1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J 25 295–303 [DOI] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E (1994) A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264 1452–1455 [DOI] [PubMed] [Google Scholar]
- Mishra G, Zhang W, Deng F, Zhao J, Wang X (2006) A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312 264–266 [DOI] [PubMed] [Google Scholar]
- Moon J, Parry G, Estelle M (2004) The ubiquitin-proteasome pathway and plant development. Plant Cell 16 3181–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56 165–185 [DOI] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452 483–486 [DOI] [PubMed] [Google Scholar]
- Nilson SE, Assmann SM (2007) The control of transpiration. Insights from Arabidopsis. Plant Physiol 143 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI (1998) Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282 287–290 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734 [DOI] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115 679–689 [DOI] [PubMed] [Google Scholar]
- Qiao H, Wang F, Zhao L, Zhou J, Lai Z, Zhang Y, Robbins TP, Xue Y (2004. a) The F-box protein AhSLF-S2 controls the pollen function of S-RNase-based self-incompatibility. Plant Cell 16 2307–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Wang H, Zhao L, Zhou J, Huang J, Zhang Y, Xue Y (2004. b) The F-box protein AhSLF-S2 physically interacts with S-RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in Antirrhinum. Plant Cell 16 582–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Zeevaart JA (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci USA 96 15354–15361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razem FA, El-Kereamy A, Abrams SR, Hill RD (2006) The RNA-binding protein FCA is an abscisic acid receptor. Nature 439 290–294 [DOI] [PubMed] [Google Scholar]
- Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, Hellmann H, Estelle M, Somers DE, Crosby WL (2003) Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J 34 753–767 [DOI] [PubMed] [Google Scholar]
- Rubio V, Shen Y, Saijo Y, Liu Y, Gusmaroli G, Dinesh-Kumar SP, Deng XW (2005) An alternative tandem affinity purification strategy applied to Arabidopsis protein complex isolation. Plant J 41 767–778 [DOI] [PubMed] [Google Scholar]
- Saez A, Apostolova N, Gonzalez-Guzman M, Gonzalez-Garcia MP, Nicolas C, Lorenzo O, Rodriguez PL (2004) Gain-of-function and loss-of-function phenotypes of the protein phosphatase 2C HAB1 reveal its role as a negative regulator of abscisic acid signalling. Plant J 37 354–369 [DOI] [PubMed] [Google Scholar]
- Saez A, Robert N, Maktabi MH, Schroeder JI, Serrano R, Rodriguez PL (2006) Enhancement of abscisic acid sensitivity and reduction of water consumption in Arabidopsis by combined inactivation of the protein phosphatases type 2C ABI1 and HAB1. Plant Physiol 141 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M (2004) Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol 134 1439–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, et al (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299 1896–1898 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52 627–658 [DOI] [PubMed] [Google Scholar]
- Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7 41–48 [DOI] [PubMed] [Google Scholar]
- Shen YY, Wang XF, Wu FQ, Du SY, Cao Z, Shang Y, Wang XL, Peng CC, Yu XC, Zhu SY, et al (2006) The Mg-chelatase H subunit is an abscisic acid receptor. Nature 443 823–826 [DOI] [PubMed] [Google Scholar]
- Smalle J, Vierstra RD (2004) The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol 55 555–590 [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmaki A, Brosche M, Moldau H, Desikan R, et al (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD (2003) The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins. Trends Plant Sci 8 135–142 [DOI] [PubMed] [Google Scholar]
- Wang L, Dong L, Zhang Y, Zhang Y, Wu W, Deng X, Xue Y (2004) Genome-wide analysis of S-Locus F-box-like genes in Arabidopsis thaliana. Plant Mol Biol 56 929–945 [DOI] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assmann SM (2001) G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292 2070–2072 [DOI] [PubMed] [Google Scholar]
- Wang Y, Holroyd G, Hetherington AM, Ng CK (2004) Seeing ‘cool’ and ‘hot’—infrared thermography as a tool for non-invasive, high-throughput screening of Arabidopsis guard cell signalling mutants. J Exp Bot 55 1187–1193 [DOI] [PubMed] [Google Scholar]
- Welin BV, Olson A, Nylander M, Palva ET (1994) Characterization and differential expression of dhn/lea/rab-like genes during cold acclimation and drought stress in Arabidopsis thaliana. Plant Mol Biol 26 131–144 [DOI] [PubMed] [Google Scholar]
- Xiong L, Gong Z, Rock CD, Subramanian S, Guo Y, Xu W, Galbraith D, Zhu JK (2001) Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev Cell 1 771–781 [DOI] [PubMed] [Google Scholar]
- Xiong L, Lee H, Ishitani M, Zhu JK (2002) Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem 277 8588–8596 [DOI] [PubMed] [Google Scholar]
- Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, Peng W, Huang D, Xie D (2002) The SCF(COI1) ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14 1919–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K (1993) Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet 236 331–340 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T, Shinozaki K, Hirayama T (2006) ABA-hypersensitive germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol 140 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Qin C, Zhao J, Wang X (2004) Phospholipase D alpha 1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA 101 9508–9513 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.