Abstract
Cytokinins are distributed through the vascular system and trigger responses of target cells via receptor-mediated signal transduction. Perception and transduction of the signal can occur at the plasma membrane or in the cytosol. The signal is terminated by the action of extra- or intracellular cytokinin oxidases. While radiotracer studies have been used to study transport and metabolism of cytokinins in plants, little is known about the kinetic properties of cytokinin transport. To provide a reference dataset, radiolabeled trans-zeatin (tZ) was used for uptake studies in Arabidopsis (Arabidopsis thaliana) cell culture. Uptake kinetics of tZ are multiphasic, indicating the presence of both low- and high-affinity transport systems. The protonophore carbonyl cyanide m-chlorophenylhydrazone is an effective inhibitor of cytokinin uptake, consistent with H+-mediated uptake. Other physiological cytokinins, such as isopentenyl adenine and benzylaminopurine, are effective competitors of tZ uptake, whereas allantoin has no inhibitory effect. Adenine competes for zeatin uptake, indicating that the degradation product of cytokinin oxidases is transported by the same systems. Comparison of adenine and tZ uptake in Arabidopsis seedlings reveals similar uptake kinetics. Kinetic properties, as well as substrate specificity determined in cell cultures, are compatible with the hypothesis that members of the plant-specific purine permease family play a role in adenine transport for scavenging extracellular adenine and may, in addition, be involved in low-affinity cytokinin uptake.
Due to their immobile nature, plants require highly efficient mechanisms for acclimation to rapidly changing environmental conditions and for communication between the distal organs of the plant. Besides classical hormones, such as steroids, oligopeptides, and eicosanoid-like compounds, plants have developed a specific set of phytohormones. Most phytohormones are synthesized by few conversions from common metabolic intermediates. The phytohormone cytokinin is mainly generated by isopentylation of AMP, ADP, or ATP by adenosine-phosphate-isopentenyl transferase (IPT), resulting in the production of corresponding isopentenyl adenosine-5′-phosphates. These isopentenyl adenine (iP) nucleotides are converted to trans-zeatin (tZ) derivatives by trans-hydroxylases CYP735A1 and CYP735A2 (Takei et al., 2004b). Further dephosphorylation and deribosylation of iP and tZ nucleotides result in their biological activity. In addition, cytokinin nucleotides can be activated by the phosphoribohydrolase LOG (Kurakawa et al., 2007). In Arabidopsis (Arabidopsis thaliana), seven IPT genes have been identified (AtIPT1, AtIPT3–AtIPT8; Kakimoto, 2001; Takei et al., 2001), which are specifically expressed in a wide range of organs and cell types (Miyawaki et al., 2004; Takei et al., 2004a). Their products, preferentially isopentylate ATP and ADP (Kakimoto, 2001; Sakakibara, 2006), represent the bulk of iP- and tZ-type cytokinins (Miyawaki et al., 2006). In addition, degradation of isopentylated tRNAs has been suggested as a possible source of cytokinins. Two Arabidopsis tRNA-IPTs, AtIPT2 and AtIPT9, catalyze isopentylation of tRNA and are required for cis-zeatin-type cytokinin synthesis (Golovko et al., 2002; Miyawaki et al., 2006).
The level of cytokinins in plants is controlled by de novo biosynthesis, conversion between free bases, nucleosides, and nucleotides, inactivation, degradation, and translocation. The irreversible degradation of cytokinins is catalyzed by cytokinin oxidase/dehydrogenase (CKX). Analysis of the entire Arabidopsis genome identified seven CKX genes (AtCKX1–AtCKX7; Schmülling et al., 2003; Werner et al., 2006); the rice (Oryza sativa) genome contains at least 11 CKX homologs (OsCKX1–OsCKX11; Werner et al., 2006). Interestingly, a rice CKX was identified as a major quantitative trait locus controlling grain number (Ashikari et al., 2005). Ectopic overexpression of cytokinin oxidase genes has dramatic effects on the root-to-shoot ratio and leaf cell number (Werner et al., 2001). The presence of signal sequences, together with heterologous expression data, indicates that some of the enzymes are secreted, whereas others may be localized in the vacuole or in the cytoplasm (Werner et al., 2003). Besides a role in cytokinin degradation, secreted cytokinin oxidases may thus also control cytokinin concentrations available to the plasma membrane-localized cytokinin receptors. In Arabidopsis, three plasma membrane cytokinin receptors have been identified (AHK2, AHK3, and AHK4/CRE1/WOL), belonging to the class of two-component His kinase systems (Higuchi et al., 2004; Müller and Sheen, 2007a). Downstream elements of the cytokinin-mediated signal transduction cascade are His phosphotransfer proteins and nuclear response regulators (ARRs), serving as transcriptional regulators (Müller and Sheen, 2007b). In addition to plasma membrane-associated receptors, the presence of potential intracellular receptors has been shown, suggesting that, besides extracellular signal perception, the uptake of cytokinins may also be important (Kulaeva et al., 1995, 1998; Brault et al., 1997).
The distinct sites of expression of genes for cytokinin biosynthesis (Miyawaki et al., 2004; Takei et al., 2004a), degradation (Werner et al., 2003, 2006), and signaling (D'Agostino et al., 2000; Kiba et al., 2002; Mason et al., 2004, 2005; Tajima et al., 2004; Ferreira and Kieber, 2005; Yokoyama et al., 2007; Ishida et al., 2008) lead to the hypothesis for local function of cytokinins. On the other hand, the translocation of cytokinins from roots to shoots via the xylem and their reflux occurring in the phloem support the hypothesis that they serve as long-distance signaling molecules (Weiler and Ziegler, 1981; Beveridge et al., 1997; Emery et al., 2000). Thus, multiple cellular importers and exporters are required to allow efficient mobilization and targeted translocation. However, in contrast to the mechanisms of polar transport of the hormone auxin, little is known about the mechanisms of cytokinin transport (Swarup et al., 2000). It has been shown that cell cultures rapidly take up and inactivate free cytokinin bases by glycosylation and storage in the vacuole, indicating the presence of cellular uptake systems (Fußeder et al., 1989). Furthermore, if a significant portion of cytokinin is catabolized extracellularly by CKX, transporters are required for the recycling of the released adenine. Efficient salvage of adenine to AMP and ATP is important for the synthesis of nucleotide cofactors and DNA, and for the supply of the cell with energy carriers (Lee and Moffat, 1994; Moffat and Ashihara, 2002).
Cellular uptake and turnover of cytokinin appear to be highly controlled because a local supply of cytokinins by microorganisms on senescing leaves leads to delay of senescence. Furthermore, grafts of wild-type shoots onto root stocks of transgenic tobacco (Nicotiana tabacum), overproducing cytokinins due to ectopic expression of the Agrobacterium tumefaciens ipt gene, did not lead to release of lateral shoot buds from suppression or to accelerated senescence (Faiss et al., 1997). Localized induction of ipt gene expression also had only local effects, leading to a paracrine hypothesis of cytokinin action. Thus, the major step, which is least understood and molecularly still not defined, is the role of cytokinin and cytokinin catabolite transport.
Recently, low-affinity transporters for cytokinin nucleobases belonging to the purine permease (PUP) family in Arabidopsis (Gillissen et al., 2000; Bürkle et al., 2003) and for cytokinin nucleosides belonging to the equilibrative nucleoside transporter (ENT) family in rice and Arabidopsis (Hirose et al., 2005, 2008) have been identified. The cytokinin transporters of the PUP family have been isolated by an indirect approach based on the structural similarity of cytokinins and purine bases. If metabolism occurs at least in part in the apoplasm, one may expect the coexistence of adenine and cytokinin transport systems in the plasma membrane. A purine transport-deficient yeast mutant fcy2 was used for suppression cloning of the plant transporter gene PUP1, which belongs to a plant-specific gene family encoding a new class of small, integral membrane proteins. PUP1 mediates high-affinity transport of adenine and other nucleobases (Gillissen et al., 2000). Competition studies showed that PUP1 was also able to recognize cytokinins. The ability of PUP1 to transport cytokinins was demonstrated by direct uptake of radiolabeled tZ into yeast expressing this transporter (Bürkle et al., 2003). Analysis of expression using promoter-reporter fusions indicates that PUP1 may play a role in the retrieval of nucleobase derivatives, potentially including xylem-delivered cytokinins, in the epithem of hydathodes and at the stigma surface of siliques (Bürkle et al., 2003). Evidence for this hypothesis is provided by a recent study demonstrating an accumulation of free cytokinins in shoot tissues known for high transpiration, such as hydathodes, guard cells, trichomes, and stigmas in ARR5-GUS plants (Aloni et al., 2005). Two homologs of PUP1 were cloned and expressed in yeast. PUP2 is able to mediate adenine uptake and to recognize tZ and cis-zeatin, iP, kinetin, and benzylaminopurine as substrates; in contrast, PUP3 did not show detectable activity in yeast. The PUP2 promoter drives expression of the GUS reporter gene in the phloem of Arabidopsis leaves. This result, together with recent findings demonstrating the expression of IPT3 (Miyawaki et al., 2004; Takei et al., 2004a) in companion cells of sieve elements and the accumulation of cytokinins in phloem of ARR5-GUS plants (Aloni et al., 2005), may implicate a role of PUP2 in phloem loading and transport of adenine and possibly cytokinins.
This study describes the properties of adenine and tZ transport in cultured Arabidopsis cells. The uptake of tZ is inhibited by other cytokinins, as well as adenine.
RESULTS
Stability of Radiolabeled tZ in Arabidopsis Cell Culture Supernatant
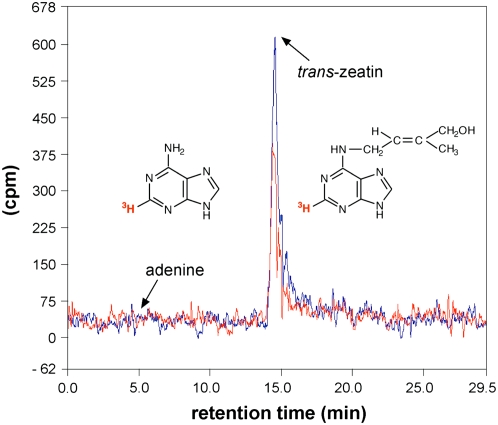
Cytokinins are irreversibly degraded by cytokinin oxidases to produce the structurally related adenine. The presence of signal sequences and the accumulation of extracellular cytokinin oxidase activity in yeast indicate that some of the enzymes may be secreted (Werner et al., 2001). The tritium label of radiolabeled tZ is located on the purine ring (Fig. 1). To verify uptake of intact tZ into the cells, rather than the degradation product 14C-adenine, the stability of the radiolabeled tZ in Arabidopsis cell culture supernatant was tested. HPLC analysis showed that almost all the detected radioactivity had the same retention time as tZ, whereas no radioactivity peak with the retention time corresponding to adenine was detected (Fig. 1). These results indicate that at least within the first 5 min no significant degradation of tZ occurs in the cell supernatant and support the notion that the measured uptake corresponds to import of tZ into Arabidopsis cultured cells.
Figure 1.
Radiochemical purity and stability of 3H-tZ. Radiolabeled 2-3H-tZ was mixed with Murashige and Skoog medium supplemented with 2% Suc (blue line) or with cultured cells (red line) to a final concentration of 500 nm in the total volume of 900 μL and incubated for 5 min. Samples were centrifuged, filtrated, and the resulting supernatant was analyzed by HPLC using a radioactivity detector. Retention times of adenine (4.63 min) and tZ (13.91 min) are marked by arrows.
Low-Affinity Transport System for tZ in Arabidopsis Cell Culture
The presence of a low-affinity transport system for tZ in Arabidopsis cell culture was analyzed by direct uptake measurements with radiolabeled 3H-tZ. The uptake rate at a substrate concentration of 20 μm was linear for at least 3 min and sensitive to the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP), potentially suggesting that energization is necessary for the transport (Fig. 2, A and B). At 20 μm substrate concentration, the uptake rates were in the range of 0.4 pmol tZ mg−1 min−1 fresh weight (Fig. 2A), or an estimated 0.09 fmol tZ cell−1 min−1 corresponding to 741 fmol tZ cm−2 membrane surface of an average culture cell (diameter 31.05 ± 7.33 μm; n = 36). Uptake rates were concentration dependent; however, they did not show clear Michaelis-Menten saturation (Fig. 2C). Using an Eadie-Hofstee plot of the data, two kinetic components with Km values of 4 μm and approximately 100 μm were determined (Fig. 2C, inset), suggesting the presence of at least two transport systems with different affinity.
Figure 2.
Kinetics of tZ uptake into Arabidopsis suspension cells in the low-affinity range. A and B, Time and energy dependence. Arabidopsis cells were assayed for 2-3H-tZ uptake at 20 μm substrate concentration, pH 5.7, in the presence (○) or absence (□) of 100 μm CCCP. CCCP was added 2 min before (A) and 1.5 min after (B) starting the assay. C, tZ uptake at different substrate concentrations. Inset, Eadie-Hofstee plot of the data; the two fitted curves indicate the presence of at least two transporter activities with Km values of 3.9 and 98.8 μm, respectively. D, Inhibition of tZ uptake by adenine or cytokinins. Uptake of 3H-tZ was determined at 20 μm tZ in the presence of 10-fold excess (200 μm) of unlabeled competitors. BA, Benzylaminopurine; ZR, tZ riboside; WW, wet weight. Values represent the mean of three independent measurements ± sd.
Competition experiments with a 10-fold excess of unlabeled tZ, adenine, iP, and benzylaminopurine reduced the uptake rate, indicating that adenine and cytokinins may be taken up by a common transport system (Fig. 2D). The uptake was significantly inhibited by tZ riboside. Allantoin had no inhibitory effect, properties similar to that of the Arabidopsis transporter PUP1 when expressed heterologously in yeast (Gillissen et al., 2000).
High-Affinity Transport of tZ in Arabidopsis Cell Culture
The presence of nanomolar concentrations of cytokinin in phloem and xylem sap (Beck and Wagner, 1994; Takei et al., 2001) suggests that physiologically relevant high-affinity transport systems may exist in plant cells. Therefore, uptake measurements with nanomolar 3H-tZ concentrations in Arabidopsis cultured cells were performed to test for the presence of such a system. As shown in Figure 3, A and B, Arabidopsis cells take up tZ at a substrate concentration of 90 nm. The uptake rates remain constant for at least 3 min and are completely abolished by the protonophore CCCP, compatible with the involvement of a secondary active transport system. At 90 nm substrate concentration, the uptake rates were in the range of 0.092 pmol tZ mg−1 min−1 fresh weight (Fig. 3A), or an estimated 0.021 fmol tZ cell−1 min−1 corresponding to 173 fmol tZ cm−2 membrane surface of an average cell culture cell (diameter 31.05 ± 7.33 μm; n = 36).
Figure 3.
Kinetics of tZ uptake into Arabidopsis suspension cells in the high-affinity range. A and B, Time and energy dependence. Arabidopsis cells were assayed for 2-3H-tZ uptake at 90 nm substrate concentration, pH 5.7, in the presence (○) or absence (□) of 100 μm CCCP. CCCP was added 2 min before (A) and 1.5 min after (B) starting the assay. C, tZ uptake at different substrate concentrations. Inset, Eadie-Hofstee plot of the data, showing a transport activity with the Km value of 211 nm. D, Inhibition of tZ uptake by adenine or cytokinins. Uptake of 3H-tZ was determined at 200 nm tZ in the presence of 10-fold excess (2 μm) of unlabeled competitors. BA, Benzylaminopurine; ZR, tZ riboside; WW, wet weight. Values represent the mean of three independent measurements ± sd.
The uptake rates were concentration dependent and displayed saturation kinetics (Fig. 3C) with a Km value of 211 nm (Fig. 3C, inset).
Surprisingly also, the high-affinity import of 3H-tZ into Arabidopsis cells was strongly inhibited by an excess of unlabeled adenine. Other cytokinins, such as iP, benzylaminopurine, and tZ riboside, significantly reduced the uptake rates, whereas allantoin did not compete (Fig. 3D). Together, the results indicate that the high-affinity transport system recognizes both cytokinins and adenine as substrates.
Relative Uptake Rates for Adenine and tZ in Arabidopsis Cell Culture
Because transport studies in cultured cells and yeast transformed with PUP1 or PUP2 indicate that adenine and cytokinins use the same low-affinity transport system (this article; Gillissen et al., 2000; Bürkle et al., 2003), the uptake rates for cytokinin and adenine were compared. At a substrate concentration of 50 μm, the uptake of adenine into Arabidopsis cells was about 3-fold higher compared to that of tZ (Fig. 4A). The difference was even greater at a substrate concentration of 200 nm (Fig. 4B). The transport system thus appears to have a higher capacity for adenine; alternatively, additional adenine transporters may contribute to the observed rates.
Figure 4.
Comparison of uptake rates for adenine and tZ in Arabidopsis suspension cells. The uptake of adenine (○) and tZ (□) was determined at 50 μm (A) and 200 nm (B) substrate concentrations. Results represent the mean of three measurements ± sd. WW, Wet weight.
Uptake of Adenine and tZ by Arabidopsis Seedlings
The presence of a transport system for adenine and cytokinins was also demonstrated in 8-d-old Arabidopsis seedlings by direct uptake studies with radiolabeled tZ and adenine. Adenine and tZ are both taken up by Arabidopsis seedlings (Fig. 5A). The uptake is sensitive to the protonophore CCCP, suggesting that transport is energy dependent. Similarly, as in the case of the cell culture, the uptake rates are higher for 14C-adenine compared to 3H-tZ.
Figure 5.
Adenine and cytokinin accumulation in 8-d-old Arabidopsis seedlings. A, Uptake of adenine and tZ into Arabidopsis seedlings in the presence and the absence of CCCP. B, Analysis of PUP1, PUP2, and PUP3 expression in Arabidopsis seedlings by RT-PCR. RNA from 8-d-old Arabidopsis seedlings was converted to cDNA by RT. A 524-bp fragment of PUP1 and a 619-bp fragment of PUP2 were amplified by 32 cycles (+, with template; −, without template; M, molecular mass marker). C, Analysis of PUP1 and PUP2 expression in Arabidopsis seedlings by promoter-GUS fusions. Arabidopsis plants expressing either PUP1- or PUP2-GUS (lines 10 and 50, respectively) were stained with 1 mm X-gluc, cleared in ethanol, and documented (PUP3-GUS did not show staining). D, Tissue-specificity of PUP family members derived from Genevestigator (Zimmermann et al., 2005). Members not present on the ATH1 22K Arabidopsis Affymetrix GeneChip are PUP2 (At2g33750), PUP6, PUP7, and PUP8 (AT4g18190, At4g18197, and At4g18195), PUP12 (At5g41160), PUP16 (At1g09860), and PUP19, PUP20, and PUP21 (At1g47603, At1g47590, and At4g18205).
Arabidopsis PUP1 and PUP2 may contribute to the observed accumulation of adenine and cytokinins because reverse transcription (RT)-PCR analysis with gene-specific primers demonstrated high expression in Arabidopsis seedling tissue (Fig. 5B). As shown in Figure 5C, reporter gene activity was detectable at the tip of cotyledons in PUP1-GUS seedlings at a similar developmental stage as used for the uptake studies, and in the vascular tissue of PUP2-GUS cotyledons. For PUP3, no RT-PCR signal and no GUS activity was obtained in Arabidopsis seedlings, consistent with its pollen-restricted expression pattern described previously (Bürkle et al., 2003). The PUP family of Arabidopsis comprises 21 members, of which 12 are present on the ATH1 chip (22K full-genome Arabidopsis Affymetrix GeneChip). Figure 5D summarizes the organ-specific expression data for the PUP gene family from the Genevestigator database (Zimmermann et al., 2005). Only a subset of 12 PUPs is represented on the chip. Among the ones present on the chip, PUP11, PUP14, and PUP18 show the highest expression levels across all tissues. In roots, PUP4 is expressed to high levels in addition to PUP11, PUP14, and PUP18. PUP4 is relatively closely related to PUP1 and PUP2, whereas the other three PUPs share only a low level of homology (Bürkle et al., 2003). Overall, the majority of PUPs was found to be expressed in callus and cell suspensions (Fig. 5D). For none of these members has a purine transporter function been demonstrated yet.
Sensitivity of Germination to Adenine
Nucleobases deriving from deteriorating biological material are present at low levels in soil. Whereas Arabidopsis germination is unaffected on medium containing up to 2 mm cytosine, thymine, or uracil, seedling development was severely affected on medium supplemented with 2 mm adenine (Fig. 6). Guanine was not tested due to its low solubility in water. Lower adenine concentrations affected seedling growth as well and led to chlorosis. Growth arrest in roots was detectable at concentrations above 0.5 mm adenine (Fig. 5N). As expected, based on the hypothesis that adenine competes for cytokinin, the observed phenotype is apparently different from growth inhibition by iP (Fig. 6, N–P). Lateral root formation was inhibited even at the lowest cytokinin concentrations, whereas it was less affected by adenine.
Figure 6.
Effect of adenine on germination and growth of Arabidopsis seedlings. A to M, Growth of Arabidopsis seedlings on medium containing nucleobases. Arabidopsis seeds were plated on Murashige and Skoog medium supplemented with 2% Suc supplemented with adenine (0.5 mm [A]; 1 mm[B]; 2 mm [C]); cytosine (0.5 mm [D]; 1 mm [E]; 2 mm [F]); uracil (0.5 mm [G]; 1 mm [H]; 2 mm [I]); and thymine (0.5 mm [K]; 1 mm [L]; 2 mm [M]), and grown for 18 d in the light. N and O, Sterilized seeds of Arabidopsis were plated on Murashige and Skoog medium supplemented with 2% Suc supplemented with adenine (N) or iP (O) at the concentrations indicated. P, Root length at different adenine and iP concentrations.
DISCUSSION
Previous studies had shown that cell suspension cultures of Chenobium rubrum and tobacco are able to take up and metabolize cytokinins (Laloue et al., 1981; Fußeder et al., 1989). However, to the best of our knowledge, no detailed analysis of the characteristics of cytokinin uptake systems has been published to date. Here, a detailed analysis of the kinetics of 3H-labeled tZ uptake in Arabidopsis cell cultures is provided. Cytokinin uptake rates were concentration dependent and showed multiphasic characteristics, indicating the presence of multiple low- and high-affinity transport systems. In the low-affinity range, two activities with apparent Km values for tZ of 4 μm and approximately 100 μm could be identified, respectively. In addition, a high-affinity transport system was identified in the Arabidopsis cell cultures, which is characterized by a Km of approximately 200 nm for tZ. The affinity of the high- and medium-affinity systems is compatible with the observed range for cytokinins in plants (Komor et al., 1993; Weiler and Ziegler, 1981; Beck and Wagner, 1994; Takei et al., 2001). In contrast, it is not clear whether the observed systems with an affinity of 100 μm will be relevant in planta. It should, however, be kept in mind that comparable low affinities have previously been described for enzymes involved in cytokinin biosynthesis and metabolism (Turner et al., 1987; Dixon et al., 1989; Galuszka et al., 1999; Bilyeu et al., 2001), leaving the possibility open that low-affinity cytokinin transport could play a physiological role. The systems appear to be able to transport a variety of cytokinins because tZ uptake was inhibited by other cytokinins, such as iP and benzylaminopurine.
Moreover, cytokinin uptake into cell cultures was competitively inhibited by adenine, lending support to the hypothesis of the existence of a common transport system for adenine and cytokinins. Such systems may either contribute to retrieval of cytokinin oxidase-derived adenine from the apoplasm or they may serve functions in retrieval of adenine derived from other biological processes. As a consequence, one would expect that transport rates of cytokinins will be influenced whenever adenine is present due to the higher capacity for adenine transport. However, in general, adenine levels appear to be very low (Ashihara et al., 1990). Interestingly, adenine uptake rates were significantly higher compared to uptake rates for tZ when analyzed in Arabidopsis cells. Similarly, as in cell cultures, also the uptake of tZ into seedlings was sensitive to competition by adenine. To test for the physiological relevance of the competition of cytokinin and adenine for uptake, the effect of both sets of compounds on root growth was tested. Surprisingly, adenine, but not other nucleobases, inhibited root development. Despite the fact that both adenine and cytokinin affect root growth and development, the phenotypes were clearly different, a finding compatible with a cytokinin-independent mechanism or that could be due to reduced cytokinin flux in the root.
Previous studies had suggested a diffusion mechanism for cytokinin uptake based on the high permeability of cell membranes to free cytokinin bases and ribosides (Laloue et al., 1981). The sensitivity of the cytokinin uptake into Arabidopsis cells and seedlings to CCCP suggests that high-, medium-, and low-affinity systems are mediated by secondary active transport systems. Similarly, uptake into seedlings was protonophore sensitive. Taken together, the data show that Arabidopsis cell cultures have at least three kinetically distinct uptake systems for cytokinins that share similar properties, such as protonophore sensitivity and competition by adenine.
Candidates for Adenine and Cytokinin Transport Function
The molecular nature of the cytokinin transport systems described here is still unknown. Using suppression cloning in yeast, a new family of plant-specific transporters had been identified, members of which mediate protonophore-sensitive uptake of both adenine and cytokinins (Gillissen et al., 2000; Bürkle et al., 2003). When expressed in yeast, the Km values for the Arabidopsis transporters PUP1 and PUP2 for tZ were in the range of 35 to 40 μm. The affinity of PUP1 for adenine was similar to that for tZ in low micromolar range (Bürkle et al., 2003). The affinities of the PUPs for adenine were in a similar range as the value of 370 μm obtained for uptake in Ricinus cotyledons (Kombrink and Beevers, 1983). A variety of PUP members are expressed in Arabidopsis callus and cell cultures; it is thus possible that well-characterized PUPs, or other members of the family that have not been functionally characterized yet, could contribute to the observed cytokinin uptake in the cell cultures. In principle, three hypotheses can be formulated: The affinity of PUPs expressed in yeast does not correspond to their in planta activity due to the lack of additional factors or posttranslational modifications; other members of the PUP family, which contains 21 members in the Arabidopsis genome, are responsible for the high-affinity component (Bürkle et al., 2003); or other transporters unrelated to PUPs may be involved in the cytokinin transport in the nanomolar range. Direct proof for a role of PUPs in cytokinin transport in planta is still outstanding. The large number of paralogs will potentially make analysis of insertional mutants difficult due to overlapping activities and compensation phenomena.
Interestingly, the adenine uptake into Arabidopsis cells was also not competed by tZ riboside, possibly indicating that the transport of this compound occurs via transporters with selectivity for other cytokinins but not to adenine. Potential candidates for such function could be the newly identified and characterized plant ENTs (Möhlmann et al., 2001; Li et al., 2003; Wormit et al., 2004), which transport cytokinin ribosides (Hirose et al., 2005, 2008; Sun et al., 2005). iP riboside inhibited adenosine transport mediated by one of the four rice homologs of the ENT family, OsENT2, indicating that OsENT2 can recognize cytokinin nucleosides. Furthermore, direct measurements with radiolabeled cytokinins showed that OsENT2 transports iP riboside and tZ riboside with Km values in the low-affinity range of 32 and 660 μm, respectively (Hirose et al., 2005). The Arabidopsis genome contains eight ENT genes (AtENT1–AtENT8; Li et al., 2003). In competition experiments, the uptake of adenosine by AtENT6-expressing yeast cells was significantly inhibited by tZ riboside and iP riboside. Direct transport measurements revealed Km values of 17 μm for iP riboside and 630 μm for tZ riboside (Hirose et al., 2008). Interestingly, the adenosine uptake mediated by OsENT2 was also significantly inhibited by tZ, whereas adenine had no inhibitory effect (Hirose et al., 2005). This finding indicates that the substrate specificity OsENT2 is not only restricted to cytokinin nucleosides and that at least OsENT2 can also recognize other cytokinins. Although competition studies with some AtENTs revealed no indication for transport of kinetin or zeatin (Wormit et al., 2004), it cannot be excluded that other members of the Arabidopsis ENT family may be involved in the tZ riboside uptake system described in cultured cells in this study. Because AtENTs function as nucleoside transporters (Möhlmann et al., 2001; Li et al., 2003; Wormit et al., 2004), they may also be responsible for the transport of cytokinin ribosides.
In summary, this article provides evidence for the presence of proton-coupled high-, medium-, and low-affinity cytokinin transport systems in Arabidopsis culture cells. The transport systems share some properties with PUP1 and PUP2, the so-far only functionally characterized members of this large gene family with 21 members. The characterization of other PUP family members, the characterization of cell cultures from insertional mutants, RNAi-repressed or overexpressor Arabidopsis plants, and cloning of new transporters different from PUPs may help to obtain further information regarding the cytokinin and purine transport and its function in planta.
MATERIALS AND METHODS
Transport Measurements in Arabidopsis Suspension Cells
The suspension cell culture from Arabidopsis (Arabidopsis thaliana) ecotype Landsberg erecta (May et al., 1998) was a gift from Mike Bevan (John Innes Institute). Cells were maintained as described by Fuerst et al. (1996). For the uptake experiments, cells were harvested 5 d after transfer into new medium by centrifugation at 300 rpm for 3 min and resuspended in fresh cytokinin-free medium containing 10 mm potassium phosphate buffer (pH 5.7) at a concentration of 0.25 mL packed cells/1 mL suspension. To start the reaction, 850 μL cells were mixed with 50 μL medium containing 2-3H-tZ at a final concentration of 43.2 Bq/μL (specific activity, 1.2 TBq/mmol; OlchemIm). Adenine was added as 14C-adenine of a final concentration of 12.3 Bq/μL (specific activity, 10.6 GBq/mmol; Amersham-Pharmacia). The unlabeled analogs (Sigma) were added to the final concentration as indicated. Samples of 170 μL were removed after 1, 2, 3, and 4 min, filtered on glass fiber filters, and washed twice with 10 mL medium. Radioactivity on the filters was determined by liquid scintillation spectrometry (Beckman). For the uptake measurements in the nanomolar range, cells were additionally washed with medium and diluted 1:12. The final concentration for 2-3H-tZ was 14.4 Bq/μL. The protonophore CCCP was added 2 min before and 1.5 min after starting the assay. Competition experiments were performed with a 10-fold excess (2 or 200 μm) of the respective unlabeled competitor. Cell diameter was determined as described by Richard et al. (2001).
Analysis of 3H-tZ Stability in Arabidopsis Cell Culture Supernatant
The Arabidopsis suspension cells were harvested by centrifugation, washed, and diluted as described above. After 6-h incubation in fresh medium, an aliquot of 850 μL cells was mixed with 50 μL 3H-tZ-containing medium to the final concentration of 500 nm and incubated for further 5 min. The sample was than centrifuged for 3 min at 500 rpm and filtered. Twenty microliters of the obtained supernatant was analyzed by HPLC using the LB 507B radiodetector (Berthold). Adenine (Sigma) and tZ (Sigma) were used as standards for estimation of the retention time.
Transport Measurements into Arabidopsis Seedlings
Arabidopsis seeds (ecotype Columbia-0) were plated on Murashige and Skoog medium supplemented with 2% Suc. Uptake measurements were performed with 10 8-d-old seedlings in Murashige and Skoog medium supplemented with 2% Suc containing 2-3H-tZ (final concentration 43.2 Bq/μL) or 14C-adenine (final concentration 12.3 Bq/μL) and the unlabeled analog to the final concentration of 5 μm. Seedlings were floated in the medium containing the radiotracer. After 45 min, the seedlings were washed twice with 5 mm adenine solution and incubated overnight in p-diisobutyl-cresoxyethoxyethyl dimethylbenzylammonium hydroxide to solubilize the tissue. Radioactivity was quantified by scintillation counting.
RT-PCR Analysis
RNA for RT-PCR analysis was extracted from 8-d-old Arabidopsis seedlings grown on Murashige and Skoog medium supplemented with 2% Suc according to the SDS-phenol method. Aliquots of 2 μg RNA were used as template for first-strand synthesis using RETROscript kit (Ambion). Two microliters of the first-strand cDNA or 150 ng RNA (as negative control) were used for PCR with gene-specific primers. To avoid amplification of genomic DNA, reverse primers were positioned on intron/exon borders. A 524-bp PUP1 fragment was amplified by 32 PCR cycles using the oligonucleotides 5′-CTAACAACGCGGAAAACAAGC-3′ and 5′-CTCTTGCTATCACCTTAAAATCTC-3′. A 619-bp PUP2 transcript was obtained by 32 PCR cycles with specific primers: 5′-TATCTTGGTACCAAAGGATCTGGTTTCCAAGC-3′ and 5′-TCCTGCTATCACCTTGAAATCG-3′. The primers 5′-ACAATGTGGGTGATAGTACAAG-3′ and 5′-CTTTGGTAAGGCCTTGAAAATC-3′ were used to analyze the expression of PUP3 amplifying a 515-bp region.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At1g28230 (PUP1), At2g33750 (PUP2), and At1g28220 (PUP3).
Acknowledgments
We would like to thank Bettina Stadelhofer and Gabi Fiene for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. DE–FG02–04ER15542 to W.B.F.) and the U.S. Department of Energy (grant no. DE–FG02–04ER15542 to W.B.F.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Wolf B. Frommer (wfrommer@stanford.edu).
Open Access articles can be viewed online without a subscription.
References
- Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI (2005) Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. J Exp Bot 56 1535–1544 [DOI] [PubMed] [Google Scholar]
- Ashihara H, Yabuki N, Mitsui K (1990) A high-performance liquid-chromatography method for separation of purine-bases, nucleosides and ureides—application to studies on purine catabolism in higher plants. J Biochem Biophys Methods 21 59–63 [DOI] [PubMed] [Google Scholar]
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M (2005) Cytokinin oxidase regulates rice grain production. Science 309 741–745 [DOI] [PubMed] [Google Scholar]
- Beck E, Wagner BM (1994) Quantification of the daily cytokinin transport from the root to the shoot of Urtica dioica L. Bot Acta 107 342–348 [Google Scholar]
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C (1997) The shoot controls zeatin riboside export from pea roots. Evidence from the branching mutant rms4. Plant J 11 339–345 [Google Scholar]
- Bilyeu KD, Cole JL, Laskey JG, Riekhof WR, Esparza TJ, Kramer MD, Morris RO (2001) Molecular and biochemical characterization of a cytokinin oxidase from maize. Plant Physiol 125 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault M, Maldiney R, Miginiac E (1997) Cytokinin-binding proteins. Physiol Plant 100 520–527 [Google Scholar]
- Bürkle L, Cedzich A, Döpke C, Stransky H, Okumoto S, Gillissen B, Kühn K, Frommer WB (2003) Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. Plant J 34 13–26 [DOI] [PubMed] [Google Scholar]
- D'Agostino IB, Deruere J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol 124 1706–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SC, Martin RC, Mok MC, Shaw G, Mok DWS (1989) Zeatin glycosylation enzymes in Phaseolus: isolation of O-glucosyltransferase from P. lunatus and comparison to O-xylosyltransferase from P. vulgaris. Plant Physiol 90 1316–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery RJ, Ma Q, Atkins CA (2000) The forms and sources of cytokinins in developing white lupine seeds and fruits. Plant Physiol 123 1593–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiss M, Zalubilova J, Strnad M, Schmülling T (1997) Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. Plant J 12 401–415 [DOI] [PubMed] [Google Scholar]
- Ferreira FJ, Kieber JJ (2005) Cytokinin signaling. Curr Opin Plant Biol 8 518–525 [DOI] [PubMed] [Google Scholar]
- Fuerst RA, Soni R, Murray JA, Lindsey K (1996) Modulation of cyclin transcript levels in cultured cells of Arabidopsis thaliana. Plant Physiol 112 1023–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fußeder A, Ziegler P, Peters W, Beck E (1989) Turnover of O-glucosides of dihydrozeatin and dihydrozeatin-9-riboside during the cell growth cycle of photoautotrophic cell suspension cultures of Chenopodium rubrum. Bot Acta 102 335–340 [Google Scholar]
- Galuszka P, Frebort I, Sebala M, Strand M, Pec P (1999) Cytokinin oxidase: the key enzyme in the biodegradation of cytokinins. In M Strand, P Pec, E Beck, eds, Advances in Regulation of Plant Growth and Development. PERES Publishers, Prague, p 39
- Gillissen B, Bürkle L, Andre B, Kühn C, Rentsch D, Brandl B, Frommer WB (2000) A new family of high-affinity transporters for adenine, cytosine, and purine derivatives in Arabidopsis. Plant Cell 12 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovko A, Sitbon F, Tillberg E, Nicander B (2002) Identification of a tRNA isopentenyltransferase gene from Arabidopsis thaliana. Plant Mol Biol 49 161–169 [DOI] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mahonen AP, Miyawaki K, Hashimoto Y, Seki M, Kobayashi M, Shinozaki K, Kato T, Tabata S, et al (2004) In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA 101 8821–8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose N, Makita N, Yamaya T, Sakakibara H (2005) Functional characterization and expression analysis of a gene, OsENT2, encoding an equilibrative nucleoside transporter in rice suggest a function in cytokinin transport. Plant Physiol 138 196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H (2008) Regulation of cytokinin biosynthesis, compartmentalization and translocation. J Exp Bot 59 75–83 [DOI] [PubMed] [Google Scholar]
- Ishida K, Yamashino T, Yokoyama A, Mizuno T (2008) Three type-B response regulators, ARR1, ARR10 and ARR12, play essential but redundant roles in cytokinin signal transduction throughout the life cycle of Arabidopsis thaliana. Plant Cell Physiol 49 47–57 [DOI] [PubMed] [Google Scholar]
- Kakimoto T (2001) Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases. Plant Cell Physiol 42 677–685 [DOI] [PubMed] [Google Scholar]
- Kiba T, Yamada H, Mizuno T (2002) Characterization of the ARR15 and ARR16 response regulators with special reference to the cytokinin signaling pathway mediated by the AHK4 histidine kinase in roots of Arabidopsis thaliana. Plant Cell Physiol 43 1059–1066 [DOI] [PubMed] [Google Scholar]
- Kombrink E, Beevers H (1983) Transport of purine and pyrimidine bases and nucleosides from endosperm to cotyledons in germinating castor bean seedlings. Plant Physiol 73 370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E, Liegl I, Schobert C (1993) Loading and translocation of various cytokinins in phloem and xylem of the seedlings of Ricinus communis L. Planta 191 252–255 [Google Scholar]
- Kulaeva ON, Karavaiko NN, Selivankina S, Zemlyachenko Ya V, Shipilova SV (1995) Receptor of trans-zeatin involved in transcription activation by cytokinin. FEBS Lett 366 26–28 [DOI] [PubMed] [Google Scholar]
- Kulaeva ON, Zagranichnaya TK, Brovko FA, Karavaiko NN, Selivankina SY, Zemlyachenko YV, Hall M, Lipkin VM, Boziev KM (1998) A new family of cytokinin receptors from Cereales. FEBS Lett 423 239–242 [DOI] [PubMed] [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J (2007) Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445 652–655 [DOI] [PubMed] [Google Scholar]
- Laloue M, Pethe-Terrine C, Guern J (1981) Uptake and metabolism of CKs in tobacco cells: studies in relation to the expression of their biological activities. In J Guern, C Peaud-Lenoel, eds, Metabolism and Molecular Activities of CKs. Springer, Berlin, pp 80–96
- Lee D, Moffat BA (1994) Adenine salvage activity during callus induction and plant growth. Physiol Plant 90 739–747 [Google Scholar]
- Li G, Liu K, Baldwin SA, Wang D (2003) Equilibrative nucleoside transporters of Arabidopsis thaliana. cDNA cloning, expression pattern, and analysis of transport activities. J Biol Chem 278 35732–35742 [DOI] [PubMed] [Google Scholar]
- Mason MG, Li J, Mathews DE, Kieber JJ, Schaller GE (2004) Type-B response regulators display overlapping expression patterns in Arabidopsis. Plant Physiol 135 927–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Mathews DE, Argyros DA, Maxwell BB, Kieber JJ, Alonso JM, Ecker JR, Schaller GE (2005) Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 17 3007–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, van Montagu M, Inzé D (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49 649–667 [Google Scholar]
- Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37 128–138 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T (2006) Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc Natl Acad Sci USA 103 16598–16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat BA, Ashihara H (2002) Purine and pyrimidine nucleotide synthesis and metabolism. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/, www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed]
- Möhlmann T, Mezher Z, Schwerdtfeger G, Neuhaus HE (2001) Characterisation of a concentrative type of adenosine transporter from Arabidopsis thaliana (ENT1,At). FEBS Lett 509 370–374 [DOI] [PubMed] [Google Scholar]
- Müller B, Sheen J (2007. a) Advances in cytokinin signaling. Science 318 68–69 [DOI] [PubMed] [Google Scholar]
- Müller B, Sheen J (2007. b) Arabidopsis cytokinin signaling pathway. Sci STKE 2007 cm5. [DOI] [PubMed] [Google Scholar]
- Richard C, Granier C, Inze D, De Veylder L (2001) Analysis of cell division parameters and cell cycle gene expression during the cultivation of Arabidopsis thaliana cell suspensions. J Exp Bot 52 1625–1633 [PubMed] [Google Scholar]
- Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57 431–449 [DOI] [PubMed] [Google Scholar]
- Schmülling T, Werner T, Riefler M, Krupkova E, Bartrina y Manns I (2003) Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J Plant Res 116 241–252 [DOI] [PubMed] [Google Scholar]
- Sun J, Hirose N, Wang X, Wen P, Xue L, Sakakibara H, Zuo J (2005) Arabidopsis SOI33/AtENT8 gene encodes a putative equilibrative nucleoside transporter that is involved in cytokinin transport in planta. J Integr Plant Biol 47 588–603 [Google Scholar]
- Swarup R, Marchant A, Bennett MJ (2000) Auxin transport: providing a sense of direction during plant development. Biochem Soc Trans 28 481–485 [PubMed] [Google Scholar]
- Tajima Y, Imamura A, Kiba T, Amano Y, Yamashino T, Mizuno T (2004) Comparative studies on the type-B response regulators revealing their distinctive properties in the His-to-Asp phosphorelay signal transduction of Arabidopsis thaliana. Plant Cell Physiol 45 28–39 [DOI] [PubMed] [Google Scholar]
- Takei K, Sakakibara H, Sugiyama T (2001) Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J Biol Chem 276 26405–26410 [DOI] [PubMed] [Google Scholar]
- Takei K, Ueda N, Aoki K, Kuromori T, Hirayama T, Shinozaki K, Yamaya T, Sakakibara H (2004. a) AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant Cell Physiol 45 1053–1062 [DOI] [PubMed] [Google Scholar]
- Takei K, Yamaya T, Sakakibara H (2004. b) Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J Biol Chem 279 41866–41872 [DOI] [PubMed] [Google Scholar]
- Turner JE, Mok DWS, Mok MC, Shaw G (1987) Isolation and purification of an enzyme catalyzing the formation of O-xylosylzeatin in Phaseolus vulgaris embryos. Proc Natl Acad Sci USA 84 3714–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler E, Ziegler H (1981) Determination of phytohormones in the phloem exudate from tree species by radioimmunoassay. Planta 152 168–170 [DOI] [PubMed] [Google Scholar]
- Werner T, Kollmer I, Bartrina I, Holst K, Schmülling T (2006) New insights into the biology of cytokinin degradation. Plant Biol 8 371–381 [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T (2001) Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA 98 10487–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormit A, Traub M, Florchinger M, Neuhaus HE, Möhlmann T (2004) Characterization of three novel members of the Arabidopsis thaliana equilibrative nucleoside transporter (ENT) family. Biochem J 383 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Yamashino T, Amano Y, Tajima Y, Imamura A, Sakakibara H, Mizuno T (2007) Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiol 48 84–96 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hennig L, Gruissem W (2005) Gene-expression analysis and network discovery using Genevestigator. Trends Plant Sci 10 407–409 [DOI] [PubMed] [Google Scholar]