Abstract
Disappearance of the fixation spot before the appearance of a peripheral target typically reduces average saccadic reaction times (the gap effect) and may also produce a separate population of early or express saccades. The superior colliculus (SC) is generally believed to be critically involved in generating both effects. As the direct sensory input to the SC does not encode colour information, to determine whether this input was critical in generating the gap effect or express saccades we used coloured targets which this pathway cannot distinguish. Our observers still made early saccades to colour-defined targets, but these were anticipations in response to the offset of the non-coloured fixation target. We also show that a gap effect still occurs when either the fixation target or the peripheral target is colour defined, suggesting that direct sensory input to the SC is not required and that information about the location of colour-defined targets is abstracted prior to processing within the SC.
Keywords: express saccade, gap effect, superior colliculus, S-cone, retinotectal pathway
1. Introduction
Saccadic latency is the delay between the sudden appearance of an object and a saccadic eye movement to look at it. Though latency varies randomly from trial-to-trial, in general its reciprocal follows a Gaussian distribution (Carpenter 1981), although a small subpopulation of saccades with shorter than expected latency may sometimes be found. In extreme cases, this early population may make the distribution bimodal, in which case they are generally called ‘express’ saccades (Fischer & Ramsperger 1984). A gap task (Saslow 1967), wherein a fixation point disappears shortly before a peripheral target appears, primarily acts to speed the main distribution of saccadic latencies (the gap effect) but can also increase the proportion of express saccades (Fischer & Ramsperger 1984). Since it has long been suspected that the superior colliculus (SC) is critically involved in generating express saccades (Fischer et al. 1993) and the gap effect (Munoz & Wurtz 1993a,b), we decided to use targets that do not directly stimulate the SC to investigate what role it might play in generating express saccades and the gap effect.
(a) Visual inputs to the SC
The shortest pathway that directly links the retina to the superficial layers of the SC is the retinotectal pathway. Electrophysiological studies in monkeys suggest that the retinotectal pathway is almost exclusively driven by an achromatic system (Schiller & Malpeli 1977; Schiller et al. 1979) that lacks input from short-wavelength sensitive cone photoreceptors (S-cones; de Monasterio 1978). There are several alternative ways by which sensory information can get to the SC, including projections from the visual cortex, extrastriate areas, parietal and frontal cortices (Krauzlis 2005). In monkeys, the pathways carrying visual information from the cortex to the SC appear to depend primarily upon the magnocellular subdivision of the geniculostriate pathway (Schiller et al. 1979). As the magnocellular pathway is not colour opponent and so does not carry colour information, this renders the SC insensitive to colour (Marrocco & Li 1977). Although colour-opponent saccade-related activity can develop within the SC under appropriate circumstances, the initial sensory response of neurons is non-opponent (Ottes et al. 1987). Therefore, it should be possible to abolish all direct visual input to the SC—whether it be from the retina or from the cortex—by using appropriately selected coloured targets. More specifically, should S-cone-stimulating targets act differently from other coloured targets, this would suggest a specific role for the retinotectal pathway in generating the underlying function. Such arguments presume that human SC cells share similar chromatic properties to those of primates: psychophysical evidence suggests that this is not an unreasonable assumption, however (Sumner et al. 2002).
(b) The role of the retinotectal pathway in express saccades
The idea that the SC might be critical in generating express saccades is based in large part on the observation that they are abolished in monkeys when the SC is ablated (Schiller et al. 1987). Given the short latency with which express saccades occur (approx. 100 ms), it might be thought that the visual signals driving express saccade travel the short retinotectal pathway.
However, a number of observations have led to this idea being largely dismissed. Firstly, lesioning the retinogeniculate pathway in monkeys abolishes all saccades (Schiller et al. 1990), suggesting that activity in the retinotectal pathway is not sufficient on its own to produce saccades. However, the task used in this lesion study (overlap task with randomized target location and interleaved catch trials) is not one in which express saccades would be expected anyway (Fischer et al. 1993; Jüttner & Wolf 1994). Secondly, ablation of monkey visual cortex abolishes visually driven responses in the deep, visuomotor layers of the SC (Schiller et al. 1974). Unfortunately, such lesioning studies do not prove that the retinotectal pathway does not provide visual input to the SC in express saccades, as it might be that the visual cortex serves to control the flow of visual information from the superficial to the deep layers of the SC (Schiller et al. 1974; Isa & Kobayashi 2004) and that cortical ablation disrupts this modulatory effect. Indeed, it has been shown that activity in the superficial layers remains largely unaltered when the cortex is ablated (Schiller et al. 1974). Therefore, despite the commonly held view that the retinotectal pathway is not involved in express saccade generation (Schiller et al. 1987; McPeek & Schiller 1994), this is yet to be explicitly demonstrated.
(c) Saccadic responses to coloured targets
Previous work has shown that express saccades still occur to peripheral coloured targets that are nominally isoluminant with a background (Weber et al. 1991; McPeek & Schiller 1994). While it is tempting to argue that visual input to the SC is therefore not critically involved in express saccade generation, a number of methodological problems prevent this being done with any confidence. Firstly, it is known that activity in the magnocellular pathway in monkeys cannot be completely abolished by isoluminant coloured targets as magnocellular cells do not share a common isoluminant point (Schiller & Colby 1983; Logothetis et al. 1990). In addition, a magnocellular cell can still signal the onset and offset of a colour change even at its particular isoluminant point (Schiller & Colby 1983; Logothetis et al. 1990), and presumably this signal could be used to detect the presence—albeit not the colour—of a coloured target. Isolation of the chromatic visual pathway is further hampered by the potential for stimulus artefacts when abruptly presenting hard-edged coloured targets on video monitors (Vingrys & King-Smith 1986; Regan et al. 1994). For these reasons, the role of direct visual input to the SC in express saccades is not established.
It is also worth considering the role of the fixation target in a gap task in generating both express saccades and the gap effect. The disappearance of a fixation target releases intracollicular inhibition arising from fixation cells (Munoz & Wurtz 1992, 1993a,b), allowing target-related responses to potentially trigger express saccades directly (Dorris et al. 1997). The gap effect may also involve fixation cells, with the offset of the fixation target releasing intracollicular inhibition that would otherwise lengthen saccadic latencies (Dorris & Munoz 1995). However, Kingstone & Klein (1993a) found a gap effect with ‘fixation’ targets located outside the central 2° reported to stimulate fixation cells (Munoz & Wurtz 1993a,b), and have argued that this represents a generalized preparation effect caused by the offset of any target within the visual field. A recent study (Sumner et al. 2006) that appeared while this paper was in preparation has accounted for this preparation effect, and has shown that direct visual input to the SC from the fixation target is not required to generate a gap effect. The study reported mean latencies rather than latency distributions, however, and so it is possible that some of the gap effect found actually resulted from changes in the proportion of express saccades rather than a speeding of the main distribution. In addition, the study did not investigate whether or not direct visual input from the targets themselves—rather than the fixation point—was of importance in a gap task.
In this study, we use colour-defined targets to abolish visual input to the SC and thereby determine whether such targets could elicit express saccades. In addition, these targets can be used to investigate whether there are visual inputs to the collicular fixation cells underlying the gap effect or to the saccade-related cells they inhibit. As our study examines distributions of saccadic latencies, rather than averages, we can also address the following question: is the gap effect noted with coloured targets (Sumner et al. 2006) due to a speeding of the main distribution of saccades, or due to changes in the proportion of early or express saccades? An important feature of our experimental procedure is that our targets are embedded in random luminous noise, ensuring that residual luminous cues—either from stimulus artefacts (Vingrys & King-Smith 1986; Regan et al. 1994), the signalling of onset and offset transients in the magnocellular pathway (Schiller & Colby 1983; Logothetis et al. 1990), or the absence of a common isoluminant point for cells in the magnocellular pathway (Schiller & Colby 1983; Logothetis et al. 1990)—cannot contribute to target detection. We used both an S-cone isolating colour and a non-S-cone isolating colour to see whether any differences existed, and so whether any critical visual input to the SC was being provided by the retinotectal pathway. We compared our results from coloured targets with those from a luminous increment.
2. Material and methods
We presented stimuli on a calibrated video monitor system (ViSaGe graphics card: Cambridge Research Systems Ltd., Kent, UK and GDM-F520 monitor, frame rate 100 Hz; Sony, Tokyo, Japan, or Diamond Pro 2070SB monitor, frame rate 100 Hz; Mitsubishi, Tokyo, Japan) in a dimly illuminated room. The monitor subtended 22° by 17° (H×V) at 1 m viewing distance. We measured the spectral radiance of the phosphors using a Photo Research PR-650 SpectraScan spectrophotometer, and determined the MacLeod–Boynton (1979) chromaticity of each phosphor using the procedure described by Golz & MacLeod (2003). For all experiments, subjects adapted to the equi-energy white (MacLeod–Boynton l=0.6654, s=0.0161; luminance 22.5 cd m−2) stimulus background for at least a minute prior to any testing.
We used three different stimulus types. The first was an S-cone isolating stimulus (+S/(L+M) or simply +S). As it is possible that any effect found with such a stimulus could be due to it being coloured, rather than isolating S-cones per se, we investigated a second colour that nominally increased M-cone excitation while simultaneously decreasing L-cone excitation (−L/(L+M) or simply +M). Our third stimulus was a luminous increment (Lum) 2.5 cd m−2 above the background.
All procedures conformed to the standards set by the Declaration of Helsinki and were approved by our institutional ethic committees. Subjects gave informed consent before participating in our experiments.
(a) Finding an S-cone isolating stimulus
We determined the location of an observer's tritan axis (i.e. that locus of colours that modulate only the S-cones) using the technique described by Smithson et al. (2003). Briefly, this technique determines colour detection thresholds shortly after the offset of a bright yellow background, with thresholds to colours lying along the tritan axis showing a profound elevation, a phenomenon dubbed transient tritanopia (Mollon & Polden 1975). We modified this technique slightly (see electronic supplementary material).
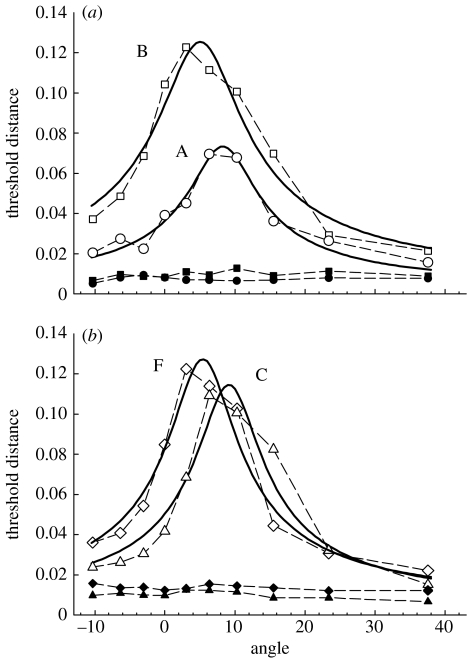
To locate the maximum threshold elevation, and hence the tritan line, we used a modified version of the two-parameter template used previously (Smithson et al. 2003). Our template incorporated an additional parameter to quantify the level of desensitization of the S-opponent channel caused by the yellow mask, rather than assuming the mask completely desensitized the channel. Without the addition of this parameter, we found that our least-squares procedure failed to suitably fit our data. Indeed, there are theoretical reasons why a two-parameter template may fail to find the correct tritan line location: should a datum point lie on the underlying tritan line, a two-parameter template correctly centred on this line will produce an infinite sum of squares, and any fitting procedure will therefore offset the template in order to reduce this sum. Our fitted curves are shown in figure 1, with the chromaticity coordinates of the colours used for each subject given in table 2 (see electronic supplementary material).
Figure 1.
Results of the tritan line location experiment, for (a) subjects A (circles) and B (squares), and (b) subjects C (triangles) and F (diamonds). Unfilled symbols give thresholds following the offset of a bright yellow adaptation background, and filled symbols give thresholds using a constant white background. Thresholds are in a scaled version of MacLeod–Boynton space (S/(L+M)×4.0), with angles giving the clockwise angular rotation of the theoretical tritan line. Solid curves give best fitting (least squares) three parameter templates described in the text.
(b) Determining isoluminance
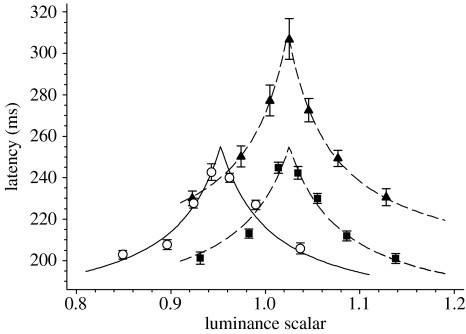
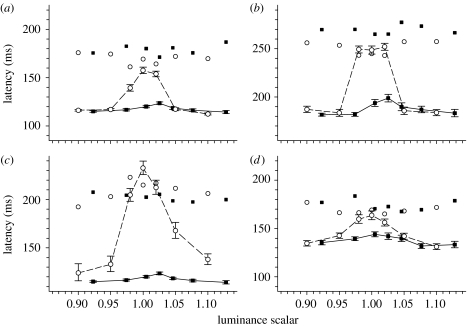
We estimated isoluminance by flickering our coloured targets at 16 Hz and having the subject adjust the luminance in 1% steps until the flicker was a minimum. Targets were Gaussian patches (s.d.=0.3°). We performed this procedure six times for both types of coloured stimuli, centred at both 4° left and 4° right of fixation. Subjects reported that the flicker percept disappeared over a wide range of approximately 10 steps, and so we refined our isoluminance estimates using the following paradigm. Each subject performed a run of 300 saccades with seven interleaved stimuli whose luminance ratios were −10, −5, −2,+0, 2, 5 and 10% relative to the initial estimate of the isoluminant value. The resulting median saccadic latencies formed an inverted V-shape (figure 2) when plotted as a function of luminance, for which the true isoluminant point was assumed to be the peak of this function (McKeefry et al. 2003). To objectively locate this peak, we developed a simple model for saccadic latency λ that assumed
| (2.1) |
where λ0 is a fixed delay; K is a constant; and C is the stimulus contrast (Hildreth 1973). C was assumed to be the linear sum of two components, such that
| (2.2) |
where Ccolour was the chromatic contrast, expressed as an equivalent luminous contrast; L was the luminance of the stimulus; and Lisolum was the isoluminant point. Although our model is undoubtedly an oversimplification, the idea that the sensation of contrast results from a single mechanism that sums appropriately scaled inputs from various chromatic directions (including luminance) is consistent with some psychophysical observations of suprathreshold contrast matching (Switkes & Crognale 1999). In addition, while there are theoretical reasons to think that the relationship between contrast and latency is more complex than modelled above (Taylor et al. 2006), the simple function used is sufficient for the purposes of locating a peak within our data. We assumed that K and λ0 were constant for a given subject and were not influenced by the stimulus colour. The model was fit to the data using a least-squares method.
Figure 2.
Saccadic latency for coloured stimuli as a function of relative luminance, for a single observer (A). A scalar of 1.0 means that colours had the same luminance (CIE 1931 colour space) as the background. Targets were Gaussian patches (s.d.=0.3°) presented either centrally (upon which the subject looked towards 0.2° black dot continuously present at 4° peripherally) or 4° peripherally (circles, +S (peripheral); squares, +M (peripheral); and triangles, +M (central)). The lines give the best fitting (least squares) model described in the text (equation (2.2)).
It is worth noting that the V-shaped function relating luminance to saccadic (figure 2) and manual latencies (McKeefry et al. 2003) is absent in the results of Weber et al. (1991), where latency at presumed isoluminance is identical to when there is significant luminous contrast between the target and the surround. It is possible that the flicker photometry technique used by Weber et al. (1991) was not sensitive enough to uniquely determine the isoluminant point, consistent with our findings above. In addition, it is unlikely that the addition of a brighter (13%) and dimmer (17%) stimulus in Weber et al. (1991) were sufficiently tightly spaced to guarantee that the isoluminant point was tested.
(c) Experimental protocols
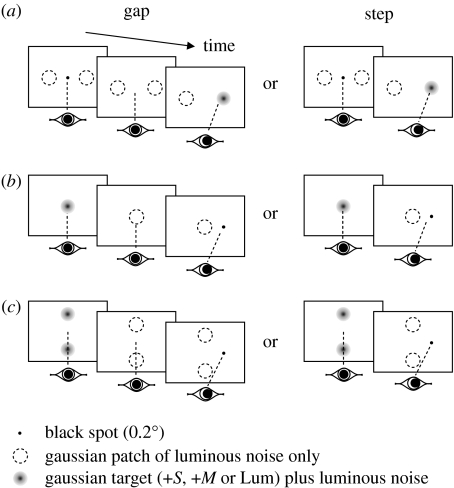
We performed four principal experiments using Gaussian patches (s.d.=0.3°) for both our coloured and luminous increment stimuli to reduce the possibility of detectable artefacts resulting from chromatic aberration in the eye (Regan et al. 1994). Patches were centred 4° from fixation when presented peripherally, an eccentricity at which express saccades have been reported previously (Weber et al. 1992). Unless otherwise noted, we added luminous noise to the patches to eliminate the usefulness of any residual luminous cues from misalignment between phosphor guns (Vingrys & King-Smith 1986) or residual firing in the nominally achromatic pathway at isoluminance (Schiller & Colby 1983; Logothetis et al. 1990). Noise was generated by randomly altering the nominal luminance by either +2 cd m−2 or −2 cd m−2 every frame (100 Hz). When no colour or luminous increment was present, the luminous noise was centred on the background luminance (=noise-only patch). A schematic of the first three protocols, described below, are given in figure 3: for these protocols, step and gap tasks were randomly interleaved, as were the colours or luminous increments to be detected.
Figure 3.
Schematic of the protocols for the first three experiments ((a) experiment 1, (b) experiment 2, and (c) experiment 3). In each case, the final target is shown on the right although in 50% of trials it appeared on the left. Step and gap tasks were randomly interleaved on a trial-by-trial basis.
(i) Experiment 1: can targets providing no visual input to the SC generate early saccades?
Subjects initially stared at a central black fixation dot (0.2° diameter) flanked either side (±4°) by two noise-only patches. The fixation dot disappeared at a random time uniformly distributed between 500 and 1500 ms after the beginning of a trial, after which a single randomly selected patch became coloured or increased in average luminance either instantly (step task) or after a 200 ms delay (gap task). The subject then looked towards the appropriate target. Each subject made approximately 500 saccades for each of the six stimulus conditions.
(ii) Experiment 2: can fixation targets providing no visual input to the SC generate a gap effect?
Subjects initially stared at a centrally located patch that was either coloured or of greater average luminance than the background, but changed to a noise-only patch after a random time between 500 and 1500 ms after the beginning of a trial, after which a black dot (0.2° diameter) appeared either instantly (step task) or after a 200 ms delay (gap task) at 4° either to the left or to the right of fixation. The subject then looked towards the black dot. Subjects made approximately 200 saccades for each of the six stimulus conditions.
As we determined our +S stimuli at 4° eccentricity, it is possible that they did not perfectly isolate S-cones when presented centrally. It is reasonable to expect that any contribution to detection by other chromatic mechanisms would be small, however, and so are unlikely to explain the comparable effect found between +S and other targets in experiment 2 (see §3, below).
(iii) Experiment 3: can peripheral ‘fixation’ targets providing no visual input to the SC generate a gap effect?
Subjects initially stared in between two patches placed above and below (±4°) the centre of the screen. The patches were either coloured or of greater average luminance than the background, but changed to a noise-only patch after a random time between 500 and 1500 ms after the beginning of a trial, after which a black dot (0.2° diameter) appeared either instantly (step task) or after a 200 ms delay (gap task) at either 4° to the left or to the right of fixation. The subject then looked towards the black dot. Subjects made approximately 500 saccades for each of the six stimulus conditions.
(iv) Experiment 4: determining preparation effects in the gap task
This experiment was designed to investigate the role, if any, of a generalized preparation effect in our gap task experiments (see §1). Subjects initially stared at a central, flickering patch in between two flickering patches placed above and below (±4°). In our gap task, either the two peripheral patches (peripheral gap) or the central patch (central gap) changed to a noise-only patch(s) after a random time between 500 and 1500 ms after the beginning of a trial, after which a black dot (0.2° diameter) appeared after a 200 ms delay at either 4° to the left or to the right of fixation. The subject then looked towards the black dot. In our overlap task, the three patches remained unchanged, with the black dot appearing 500 and 1500 ms after the beginning of a trial. Gap (peripheral and central) and overlap tasks were randomly interleaved in a single run, with the data for the various types of patches (+S, +M and Lum) collected in a counterbalanced fashion between runs. Subjects made approximately 200 saccades for each condition.
(d) Collecting and fitting latency data
We measured horizontal eye position using an infrared reflection oculometer (Ober Consulting, Poznan, Poland) directed at the medial limbus of each eye (Ober et al. 2003). A computer sampled eye position in 10 ms bins exactly synchronized to individual frames of the display and automatically determined latency (Carpenter 1994). At the conclusion of a run, a human observer reviewed the eye movement traces and deleted those that had been misclassified due to blinks, head movements or other irregularities.
The reciprocal of reaction time (promptness) tends to be normally distributed, and so when promptness is plotted against cumulative frequency on a probit scale (a reciprobit plot), a straight line usually results (Carpenter 1981). We estimated median reaction time and its standard deviation using a normal distribution fitted to the promptness data by minimizing the Kolmogorov–Smirnov (K–S) one-sample statistic. The K–S test is relatively insensitive to deviations in the extreme tails of a cumulative distribution (Press et al. 1992), as may occur when a modest number of express saccades are present. We searched for significant (p<0.05) gap effects by comparing mean promptness values for step and gap tasks for a particular stimulus type using an unpaired t-test. In our gap task in experiment 1, there were clearly two components of the distributions on our reciprobit plots, one consisting of early saccades and one of saccades with more typical latencies. To capture this data form, we fitted our gap experiment results in this experiment with two sequential line elements, using a sum-of-squares derived from each data point. To minimize the influence of any irregularities in the extremes of the tails of our cumulative distributions, we fitted only latencies greater than or equal to 70 ms and within ±3 s.d.s of the mean of the cumulative distribution.
(e) Participants
Four observers (A, B, C and F) had their individual tritan axes determined, with a subset of three of these observers used in experiments involving +S targets. We tested a further two subjects (D and E) to confirm results for the +M and Lum conditions in certain circumstances. Overall, we tested three observers for all experiments, except for the +M and Lum conditions in experiment 2 (five and four observers for the first and second parts of this experiment, respectively).
3. Results
(a) Experiment 1: can targets providing no input to the SC generate early saccades?
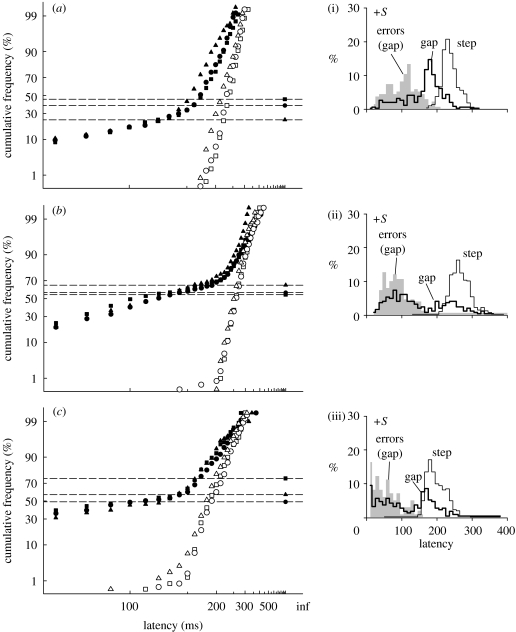
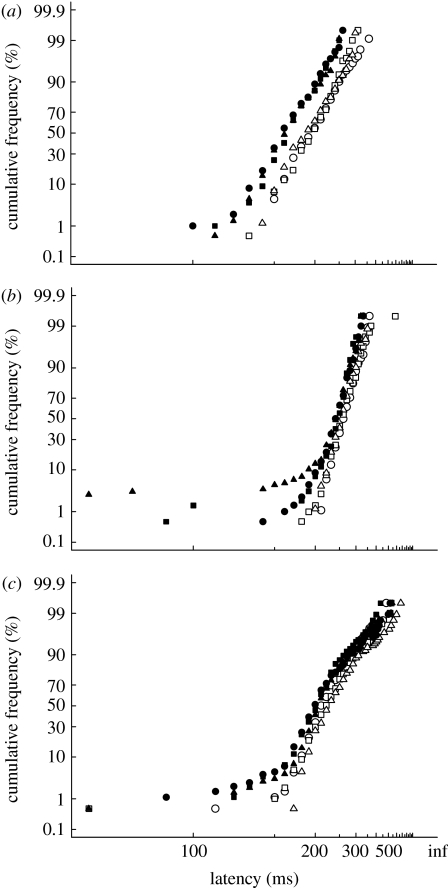
Figure 4 shows the results for the gap and step tasks to coloured or luminous targets in luminous noise for three subjects, plotted as reciprobit plots (left-hand panels: see §2). All subjects show a gap effect for all three stimulus types, as indicated by the speeded latencies relative to the step condition. In addition, the gap task produces a prominent tail of very short latencies extending to below 100 ms. These tails are indistinguishable for all stimulus types, suggesting that the proportion of early saccades is not influenced by stimulus colour or luminance. The tails also create a clearly bimodal distribution of latencies for two of the subjects (figure 4ii and iii, thick lines, subjects B and C).
Figure 4.
Experiment 1: (a–c) reciprobit plots of saccadic latency for saccades in the correct direction, for a gap and step tasks for three observers. Targets were Gaussian patches located 4° either side of a black fixation spot. Isolated data points to the right of each panel give the number of saccades made in the opposite direction to the target (errors), expressed as a frequency (=errors/number of correct saccades): CIs for these frequencies are given in table 1. Median latencies (ms) were: (a) subject A (step: 230, 237, 214; gap: 182, 188, 166); (b) subject B (step: 259, 258, 250; gap: 150, 112, 128); and (c) subject C (step: 190, 186, 180; gap: 139, 103, 133; step task: open circles, open squares and open triangles; gap task: filled circles, filled squares and filled triangles (+S, +M, Lum), respectively). (i–iii) Latency distributions for the +S stimulus for both step (thin lines) and gap (thick lines) tasks and error distributions for the gap task (grey fill).
The horizontal lines in figure 4 give the number of saccades in the incorrect direction for the gap task, expressed as a proportion of the total number of correct saccades for the same task. These proportions are similar to the proportions of saccades making up the early tails of the distributions (table 1), which is precisely the pattern expected if early saccades are not visually driven by the target onset but rather are anticipations in response to the disappearance of the fixation target.
Table 1.
Proportion of early saccades and error saccades (=incorrect direction) in the gap task of experiment 1. (The proportion of early saccades was determined from the intersection between two sequential line elements fitted to the gap data in figure 4 (see §2), and binomial CIs calculated using the formula of Clopper & Pearson (1934). The proportion of early saccades and errors were significantly different (Fischer's exact test, p<0.05) for all conditions except for first and last rows of the table.)
| subject | stimulus | early (%) | 95% CI | errors (%) | 95% CI |
|---|---|---|---|---|---|
| A | +S | 38.5 | 33.4–43.7 | 33.6 | 28.9–38.6 |
| +M | 45.7 | 40.3–51.2 | 36.1 | 31.1–41.3 | |
| Lum | 24.0 | 19.8–28.6 | 31.6 | 27.0–36.3 | |
| B | +S | 60.6 | 54.6–66.4 | 70.9 | 65.5–75.8 |
| +M | 54.7 | 48.8–60.4 | 74.9 | 69.7–79.5 | |
| Lum | 65.4 | 59.5–70.9 | 75.1 | 69.8–79.9 | |
| C | +S | 49.1 | 42.3–55.9 | 58.9 | 52.2–65.2 |
| +M | 75.6 | 67.7–80.7 | 57.7 | 50.4–64.5 | |
| Lum | 57.5 | 50.4–64.3 | 56.7 | 50.0–63.4 |
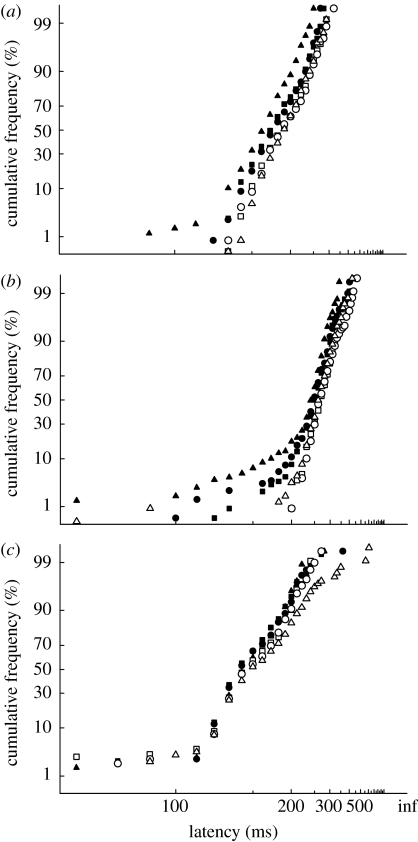
(b) Experiment 2: can fixation targets providing no visual input to the SC generate a gap effect?
In experiment 1, the offset of the black fixation target produced a gap effect for all experimental conditions. In this experiment, we investigated whether fixation targets providing no visual input to the SC could produce similar gap effects. Figure 5 shows the results for the gap and step tasks to a peripheral black spot when the fixation target was either a coloured or luminous increment in luminous noise. For all three subjects and all stimulus conditions, there is a significant gap effect (t-test, p<0.01). The magnitude of the gap effect for the S-cone target is similar to that from our +M target (subject A: 35 and 31 ms, +S and +M, respectively; subject B: 21 and 14 ms; subject F: 14 and 20 ms), suggesting that isolating S-cones does not influence the gap effect in a way that differs from other isoluminant colours. Importantly, the absence of a prominent tail of very short saccades indicates that the gap effect is due to a shift in the main saccadic distribution rather than the presence of sub-population of early saccades. We confirmed our observation that coloured fixation targets (+M) could produce a significant gap effect on two further subjects (subjects D and E, p<0.001: results not shown).
Figure 5.
Experiment 2: reciprobit plots of saccadic latency, for a gap and step task. Fixation targets were Gaussian patches, and the peripheral target a black spot. Median latencies for the curves were: (a) subject A (step: 193, 195, 185 ms; gap: 159, 164, 162 ms); (b) subject B (step: 261, 257, 254 ms, gap: 240, 243, 237 ms); and (c) subject F (step: 213, 221, 229 ms, gap: 199, 201, 207 ms; step task: open circles, open squares and open triangles; gap task: filled circles, filled squares and filled triangles (+S, +M, Lum), respectively). All stimulus conditions gave a significant gap effect for all subjects (subject F, +S, p=0.006: all others p<0.001).
To ensure that our use of luminous noise itself was not influencing the results, we repeated the experiment but without any luminous noise. Instead, on each trial, the luminance of the now non-flickering Gaussian fixation patch was selected from one of seven values ranging from above to below the subject's nominal isoluminant point. The luminous fixation target, being the same colour as the background, disappeared at isoluminance, thereby reducing our gap and step tasks to an appearance task for this condition. Figure 6 shows the results from four observers, who all showed that the gap task using coloured fixation targets (filled squares with errors bars) decreased latencies when compared with an appearance task using the same fixation targets (filled squares without error bars). Systematically altering the luminance of these coloured fixation targets failed to abolish this gap effect. In contrast, a gap effect was only present for the luminous target when there was sufficient luminous contrast between it and the background.
Figure 6.
Mean reaction times for a step (unjoined data points) and gap (joined with lines) task as a function of fixation target luminance, for a luminous increment (circles) and a+M cone stimulus (squares), both presented without luminous noise: (a) subject A; (b) subject B; (c) subject C; and (d) subject D. A luminance scalar of 1.0 means that target had the same luminance (CIE 1931 colour space) as the background. The peripheral target was a black spot. Error bars give ±s.e.m. of the mean for the gap task only. Each datum point represents the mean of approximately 55 latencies.
(c) Experiment 3: can peripheral ‘fixation’ targets providing no visual input to the SC generate a gap effect?
Figure 7 shows the results for the gap and step tasks to a black spot using peripheral ‘fixation’ targets located 4° above and below the centre of the screen. All subjects showed a significant gap effect for all stimulus conditions. As in experiment 2, the gap effect was due to a shifting of the main saccadic distribution, rather than the emergence of a significant population of early saccades.
Figure 7.
Experiment 3: (a–c) reciprobit plots of saccadic latency, for a gap and step task for three observers. The target was a black spot and ‘fixation’ targets were Gaussian patches placed 4° above and below the centre of the screen. Median latencies for the curves were: (a) subject A (step: 188, 186, 189 ms; gap: 175, 172, 160 ms); (b) subject B (step: 266, 266, 261 ms, gap: 246, 249, 236 ms); and (c) subject C (step: 147, 146, 151 ms, gap: 141, 140, 135 ms; step task: open circles, open squares and open triangles; gap task: filled circles, filled squares and filled triangles (+S, +M, Lum), respectively). A significant gap effect was present for all stimulus conditions for all three subjects (subjects A and B, p<0.001; subject C p=0.01, 0.02 and <0.001 for +S, +M and Lum, respectively).
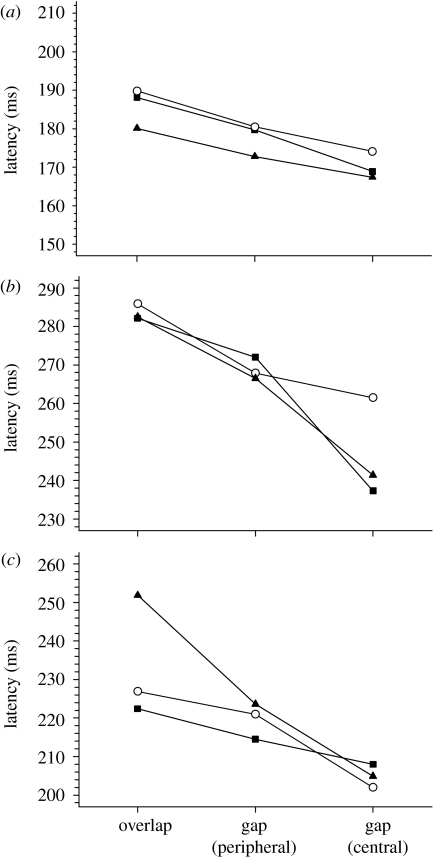
(d) Experiment 4: determining preparation effects in the gap task
Figure 8 shows the median latency for two different gap tasks (disappearance of either a central or two peripheral fixation patches) as well as an overlap task (neither central nor peripheral patches disappearing). For all three observers and for all target types, there is a significantly greater gap effect (=gap−overlap condition) present centrally than peripherally. Assuming that the peripheral fixation patches measure a generalized preparation effect in the gap task, our results suggest that there is a specific fixation-offset effect for central targets that acts in addition to this generalized preparation effect (Kingstone & Klein 1993a).
Figure 8.
Median latencies for overlap and gap tasks. ‘Fixation’ targets were Gaussian patches placed above and below the centre of the screen, and the target to saccade was a black spot. Each datum point represents the median of approximately 200 saccadic latencies, except for subject F who performed twice this number for the +M condition. For all observers, latencies were significantly faster for the central-gap condition than the peripheral-gap condition ((a) subject A: p=0.02,<0.001 and 0.04 [+S, +M and Lum]; (b) subject B: p=0.006,<0.001 &<0.001; and (c) subject F: p=0.001, 0.05 and <0.001; circles, +S; squares, +M; and triangles, Lum).
4. Discussion
Our results show that peripheral targets defined only by colour can still evoke early saccades in a gap task (figure 4). The number of these early saccades is approximately the same as those made in a direction opposite to the target (table 1), suggesting that they are not driven by the target but are merely anticipatory saccades made in response to the offset of the fixation spot. It is therefore not surprising that changing the target characteristics does not modulate such responses (figure 4). Coloured central fixation targets can also generate a significant gap effect (figure 5) that is not an artefact of an increased proportion of early saccades, and is not an artefact of the luminous noise used in our experiment as it persists when noise is removed (figure 6). Neither is the gap effect seen with coloured fixation targets simply a generalized warning effect, but rather appears to reflect a true fixation-offset effect (Kingstone & Klein 1993a; figure 8).
(a) Express saccades and anticipations
While we found a clear population of early saccades in experiment 1 (figure 4), our analysis showed that these were most likely anticipations driven by the offset of the fixation target. These anticipations may be due to the relatively low visibility of our targets (11% Weber contrast for the luminous condition, with visibility further reduced by the presence of luminous noise) in comparison with the highly visible fixation offset, producing a clearly defined beginning to the gap period yet a less well-defined end. Consistent with this, when highly visible targets are used (experiments 2, 3 and 4) the proportion of early saccades is very low. Target visibility may also explain why these anticipations persist until quite long latencies (150–200 ms), given the relationship between contrast and latency (Taylor et al. 2006): when high-contrast (more than 260% contrast) targets are used, anticipatory errors are confined to much shorter latencies (Wenban-Smith & Findlay 1991).
Previous authors have similarly suggested that express saccades might represent anticipatory eye movements that happen to be in the correct direction (Kalesnykas & Hallett 1987). It is not apparent that express saccades are always anticipatory, however, with a number of experimental findings suggesting that express saccades are visually driven (Fischer & Ramsperger 1984; Fischer et al. 1993; Rohrer & Sparks 1993; McPeek & Schiller 1994), though these findings have failed to convince some authors (Fischer & Weber 1993). Certainly, interpretive difficulties can occur: recently, Schiller et al. (2004) argued that their monkey's express saccades were visually driven as variations in fixation target duration were not fully reflected in the variation of the express-saccade distribution. This argument seems flawed, as it would be expected that variation in the gap duration, rather than that fixation spot, might modulate the latency of anticipatory saccades: in their study, the gap duration was fixed. One difficulty with all express saccade studies is defining what an express saccade actually is, with neither the absolute latency nor the presence of bimodality appearing adequate to define a saccade as being express under all experimental conditions (Kingstone & Klein 1993b). In addition, the proportion of human subjects who demonstrate express saccades is, in many studies, quite low (Kingstone & Klein 1993b), which may explain our inability to show visually driven express saccades in our three subjects tested (figure 4).
(b) S-cone stimuli and the gap effect
Our experiment 2 shows that colour-defined fixation targets can produce a significant gap effect, suggesting that visual input to SC fixation cells was not required for their generation. This result agrees with the recent work of Sumner et al. (2006). Our analysis of the distribution of latencies additionally demonstrates that the gap effect found by Sumner et al. (2006) does not result from the appearance of express saccades that lower the average latency, but rather from a true shift in the median latency of the main saccadic distribution. In addition, the absence of luminous noise does not alter our conclusions (figure 6), and so the use of this technique does not fundamentally change how either luminous or chromatic targets are detected and responded to by the oculomotor system, at least for central fixation targets. Of note also is that a gap effect still manifested when more peripheral (4°) targets were used (experiment 3, figure 7) that were largely outside the central 1–2° that stimulate rostral pole fixation cells in monkeys (Munoz & Wurtz 1993a,b). A gap effect has previously been noted with targets placed in a similar location, and appeared to be independent of whether or not the peripheral target was attended to (Kingstone & Klein 1993a). It has been suggested that a peripheral gap effect can result from the offset of any target within the visual field and is the result of a generalized preparation effect, and that this effect is distinct from the additional oculomotor specific effect found when a fixation point is offset (the fixation-offset effect; Kingstone & Klein 1993a). It is therefore possible that visual input to the SC is necessary for this latter fixation-offset effect, and that the gap effect seen with coloured stimuli in our experiment 2 (figure 5) actually represents a generalized preparation effect only. Our results from experiment 4 (figure 8) show that this is not the case, however, as there is a significantly greater gap effect when coloured targets are used centrally than peripherally: this increased gap effect is due to the addition of a fixation-offset effect for central targets (Kingstone & Klein 1993a). This finding indicates that rostral pole fixation cells are not critically dependent upon visual input, which is consistent with work suggesting that both auditory (Ross & Ross 1981; Taylor et al. 1999) and attentional inputs (Pratt et al. 2006) can modulate the gap effect. As the SC is thought to be critically involved in the gap effect (Munoz & Wurtz 1992, 1993a,b), yet sensory activity in the SC is achromatic (Marrocco & Li 1977), at least initially (Ottes et al. 1987), information about the position of coloured targets must be abstracted elsewhere and then conveyed to the SC in order for a gap effect to be generated (Sumner et al. 2006).
In summary, we can explain our observers' early saccades in terms of anticipatory responses to the offset of the achromatic fixation target, rather than the onset of the peripheral coloured target. Although we cannot show that coloured targets produce visually driven express saccades in our experiment, this probably reflects the fact that such saccades are relatively uncommon in human observers (Kingstone & Klein 1993b) rather than that visual input to the SC is required. We find no evidence that visual input to the SC is required to generate the gap effect, or that the gap effect is the consequence, either fully or in part, of a change in the proportion of early saccades. Despite clear advances in understanding the local neural mechanisms responsible for fixation-related effects such as the gap effect, the exact nature of the inputs into these mechanisms still requires further elaboration.
Acknowledgments
All procedures conformed to the standards set by the Declaration of Helsinki and were approved by our institutional ethics committees.
This work was supported by a Wellcome Trust research grant RG 35796 to R.H.S.C. and an Australian Research Council Discovery Project grant DP0663915 to A.J.A. and R.H.S.C.
Footnotes
Electronic supplementary material is available at http://dx.doi.org/10.1098/rspb.2007.1394 or via http://journals.royalsociety.org.
Supplementary Material
References
- Carpenter R.H.S. Oculomotor procrastination. In: Fisher D.F, Monty R.A, Senders J.W, editors. Eye movements: cognition and visual perception. Lawrence Erlbaum Associates; Hillsdale, NJ: 1981. pp. 237–246. [Google Scholar]
- Carpenter R.H.S. SPIC: a PC-based system for rapid measurements of saccadic responses. J. Physiol. (Proc.) 1994;480:4. [Google Scholar]
- Clopper C.J, Pearson E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. doi:10.1093/biomet/26.4.404 [Google Scholar]
- de Monasterio F.M. Properties of concentrically organized X and Y ganglion cells of macaque retina. J. Neurophysiol. 1978;41:1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]
- Dorris M.C, Munoz D.P. A neural correlate for the gap effect on saccadic reaction times in monkey. J. Neurophysiol. 1995;73:2558–2562. doi: 10.1152/jn.1995.73.6.2558. [DOI] [PubMed] [Google Scholar]
- Dorris M.C, Paré M, Munoz D.P. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J. Neurosci. 1997;17:8566–8579. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express saccades: extremely short reaction times of goal directed eye movements. Exp. Brain Res. 1984;57:191–195. doi: 10.1007/BF00231145. doi:10.1007/BF00231145 [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behav. Brain Sci. 1993;16:553–610. [Google Scholar]
- Fischer B, Weber H, Biscaldi M, Aiple F, Otto P, Stuhr V. Separate populations of visually guided saccades in humans: reaction times and amplitudes. Exp. Brain Res. 1993;92:528–541. doi: 10.1007/BF00229043. doi:10.1007/BF00229043 [DOI] [PubMed] [Google Scholar]
- Golz J, MacLeod D.I.A. Colorimetry for CRT displays. J. Opt. Soc. Am. A. 2003;20:769–781. doi: 10.1364/josaa.20.000769. doi:10.1364/JOSAA.20.000769 [DOI] [PubMed] [Google Scholar]
- Hildreth J.D. Bloch's law and a temporal integration model for simple reaction time to light. Percept. Psychophys. 1973;14:421–432. [Google Scholar]
- Isa T, Kobayashi Y. Switching between cortical and subcortical sensorimotor pathways. Prog. Brain Res. 2004;143:299–305. doi: 10.1016/S0079-6123(03)43029-X. [DOI] [PubMed] [Google Scholar]
- Jüttner M, Wolf W. Stimulus sequence effects on human express saccades described by a Markov model. Biol. Cybern. 1994;70:247–253. doi: 10.1007/BF00197605. doi:10.1007/BF00197605 [DOI] [PubMed] [Google Scholar]
- Kalesnykas R.P, Hallett P.E. The differentiation of visually guided and anticipatory saccades in gap and overlap paradigms. Exp. Brain Res. 1987;68:115–121. doi: 10.1007/BF00255238. doi:10.1007/BF00255238 [DOI] [PubMed] [Google Scholar]
- Kingstone A, Klein R.M. Visual offsets facilitate saccadic latency: does predisengagement of visuospatial attention mediate this gap effect? J. Exp. Psychol. Hum. Percept. Perform. 1993a;19:1251–1265. doi: 10.1037//0096-1523.19.6.1251. doi:10.1037/0096-1523.19.6.1251 [DOI] [PubMed] [Google Scholar]
- Kingstone A, Klein R.M. What are human express saccades? Percept. Psychophys. 1993b;54:260–273. doi: 10.3758/bf03211762. [DOI] [PubMed] [Google Scholar]
- Krauzlis R.J. The control of voluntary eye movements: new perspectives. Neuroscientist. 2005;11:124–137. doi: 10.1177/1073858404271196. doi:10.1177/1073858404271196 [DOI] [PubMed] [Google Scholar]
- Logothetis N.K, Schiller P.H, Charles E.R, Hurlbert A.C. Perceptual deficits and the activity of the color-opponent and broad-band pathways at isoluminance. Science. 1990;247:214–217. doi: 10.1126/science.2294602. doi:10.1126/science.2294602 [DOI] [PubMed] [Google Scholar]
- MacLeod D.I.A, Boynton R.M. Chromaticity diagram showing cone excitation by stimuli of equal luminance. J. Opt. Soc. Am. 1979;69:1183–1186. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- Marrocco R.T, Li R.H. Monkey superior colliculus: properties of single cells and their afferent inputs. J. Neurophysiol. 1977;40:844–860. doi: 10.1152/jn.1977.40.4.844. [DOI] [PubMed] [Google Scholar]
- McKeefry D.J, Parry N.R.A, Murray I.J. Simple reaction times in color space: the influence of chromaticity, contrast, and cone opponency. Invest. Ophthalmol. Vis. Sci. 2003;44:2267–2276. doi: 10.1167/iovs.02-0772. doi:10.1167/iovs.02-0772 [DOI] [PubMed] [Google Scholar]
- McPeek R.M, Schiller P.H. The effects of visual scene composition on the latency of saccadic eye movements of the rhesus monkey. Vis. Res. 1994;34:2293–2305. doi: 10.1016/0042-6989(94)90108-2. doi:10.1016/0042-6989(94)90108-2 [DOI] [PubMed] [Google Scholar]
- Mollon J.D, Polden P.G. Colour illusion and evidence for interaction between cone mechanisms. Nature. 1975;258:421–422. doi: 10.1038/258421a0. doi:10.1038/258421a0 [DOI] [PubMed] [Google Scholar]
- Munoz D.P, Wurtz R.H. Role of the rostral superior colliculus in active visual fixation and execution of express saccades. J. Neurophysiol. 1992;67:1000–1002. doi: 10.1152/jn.1992.67.4.1000. [DOI] [PubMed] [Google Scholar]
- Munoz D.P, Wurtz R.H. Fixation cells in monkey superior colliculus I. Characteristics of cell discharge. J. Neurophysiol. 1993a;70:559–575. doi: 10.1152/jn.1993.70.2.559. [DOI] [PubMed] [Google Scholar]
- Munoz D.P, Wurtz R.H. Fixation cells in monkey superior colliculus II. Reversible activation and deactivation. J. Neurophysiol. 1993b;70:576–589. doi: 10.1152/jn.1993.70.2.576. [DOI] [PubMed] [Google Scholar]
- Ober J.K, Przedpelska-Ober E, Gryncewicz W, Dylak J, Carpenter R.H.S, Ober J.J. Hand-held system for ambulatory measurement of saccadic durations of neurological patients. In: Gajda J, editor. Modelling and measurement in medicine. Komitet Biocybernityki i Inzyneierii Biomedycznej PAN; Warsaw, Poland: 2003. pp. 187–198. [Google Scholar]
- Ottes F.P, Van Gisbergen J.A.M, Eggermont J.J. Collicular involvement in a saccadic colour discrimination task. Exp. Brain Res. 1987;66:465–478. doi: 10.1007/BF00270679. doi:10.1007/BF00270679 [DOI] [PubMed] [Google Scholar]
- Pratt J, Lajonchere C.M, Abrams R.A. Attentional modulation of the gap effect. Vis. Res. 2006;46:2602–2607. doi: 10.1016/j.visres.2006.01.017. doi:10.1016/j.visres.2006.01.017 [DOI] [PubMed] [Google Scholar]
- Press W.H, Teukolsky S.A, Vetterling W.T, Flannery B.P. Cambridge University Press; Cambridge, UK: 1992. Numerical recipes in C. The art of scientific computing. [Google Scholar]
- Regan B.C, Reffin J.P, Mollon J.D. Luminance noise and the rapid determination of discrimination ellipses in colour deficiency. Vis. Res. 1994;34:1279–1299. doi: 10.1016/0042-6989(94)90203-8. doi:10.1016/0042-6989(94)90203-8 [DOI] [PubMed] [Google Scholar]
- Rohrer W.H, Sparks D.L. Express saccades: the effects of spatial and temporal uncertainty. Vis. Res. 1993;33:2447–2460. doi: 10.1016/0042-6989(93)90125-g. doi:10.1016/0042-6989(93)90125-G [DOI] [PubMed] [Google Scholar]
- Ross S.M, Ross L.E. Saccade latency and warning signals: effects of auditory and visual stimulus onset and offset. Percept. Psychophys. 1981;29:429–437. doi: 10.3758/bf03207356. [DOI] [PubMed] [Google Scholar]
- Saslow M.G. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. J. Opt. Soc. Am. 1967;57:1024–1029. doi: 10.1364/josa.57.001024. [DOI] [PubMed] [Google Scholar]
- Schiller P.H, Colby C.L. The responses of single cells in the lateral geniculate nucleus of the rhesus monkey to color and luminance contrast. Vis. Res. 1983;12:1631–1641. doi: 10.1016/0042-6989(83)90177-3. doi:10.1016/0042-6989(83)90177-3 [DOI] [PubMed] [Google Scholar]
- Schiller P.H, Malpeli J.G. Properties and tectal projections of the monkey retinal ganglion cells. J. Neurophysiol. 1977;40:428–445. doi: 10.1152/jn.1977.40.2.428. [DOI] [PubMed] [Google Scholar]
- Schiller P.H, Stryker M, Cynader M, Berman N. Response characteristics of single cells in the monkey superior colliculus following ablation or cooling of visual cortex. J. Neurophysiol. 1974;37:181–194. doi: 10.1152/jn.1974.37.1.181. [DOI] [PubMed] [Google Scholar]
- Schiller P.H, Malpeli J.G, Schein S.J. Composition of geniculostriate input to superior colliculus of the rhesus monkey. J. Neurophysiol. 1979;42:1124–1133. doi: 10.1152/jn.1979.42.4.1124. [DOI] [PubMed] [Google Scholar]
- Schiller P.H, Sandell J.H, Maunsell J.H.R. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J. Neurophysiol. 1987;57:1033–1049. doi: 10.1152/jn.1987.57.4.1033. [DOI] [PubMed] [Google Scholar]
- Schiller P.H, Logothetis N.K, Charles E.R. Role of the color-opponent and broad-band channels in vision. Vis. Neurosci. 1990;5:321–346. doi: 10.1017/s0952523800000420. [DOI] [PubMed] [Google Scholar]
- Schiller P.H, Haushofer J, Kendall G. How do target predictability and precueing affect the production of express saccades in monkeys? Eur. J. Neurosci. 2004;19:1963–1968. doi: 10.1111/j.1460-9568.2004.03299.x. doi:10.1111/j.1460-9568.2004.03299.x [DOI] [PubMed] [Google Scholar]
- Smithson H.E, Sumner P, Mollon J.D. How to find a tritan line. In: Mollon J.D, Pokorny J, Knoblauch K, editors. Normal and defective colour vision. Oxford University Press; Oxford, UK: 2003. pp. 279–287. [Google Scholar]
- Sumner P, Adamjee T, Mollon J.D. Signals invisible to the colliculus and magnocellular pathway can capture visual attention. Curr. Biol. 2002;3:1312–1316. doi: 10.1016/s0960-9822(02)01020-5. doi:10.1016/S0960-9822(02)01020-5 [DOI] [PubMed] [Google Scholar]
- Sumner P, Nachev P, Castor-Perry S, Isenman H, Kennard C. Which visual pathways cause fixation-related inhibition? J. Neurophysiol. 2006;95:1527–1536. doi: 10.1152/jn.00781.2005. doi:10.1152/jn.00781.2005 [DOI] [PubMed] [Google Scholar]
- Switkes E, Crognale M.A. Comparison of color and luminance contrast: apples versus oranges? Vis. Res. 1999;39:1823–1831. doi: 10.1016/s0042-6989(98)00219-3. doi:10.1016/S0042-6989(98)00219-3 [DOI] [PubMed] [Google Scholar]
- Taylor T.L, Klein R.M, Munoz D.P. Saccadic performance as a function of the presence and disappearance of auditory and visual fixation stimuli. J. Cogn. Neurosci. 1999;11:206–213. doi: 10.1162/089892999563337. doi:10.1162/089892999563337 [DOI] [PubMed] [Google Scholar]
- Taylor M.J, Carpenter R.H, Anderson A.J. A noisy transform predicts saccadic and manual reaction times to changes in contrast. J. Physiol. 2006;15:741–751. doi: 10.1113/jphysiol.2006.105387. doi:10.1113/jphysiol.2006.105387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingrys A.J, King-Smith P.E. Factors in using color video monitors for assessment of visual thresholds. Color Res. Appl. 1986;11:57–62. [Google Scholar]
- Weber H, Fischer B, Bach M, Aiple F. Occurrence of express saccades under isoluminance and low contrast luminance conditions. Vis. Neurosci. 1991;7:505–510. doi: 10.1017/s0952523800009792. [DOI] [PubMed] [Google Scholar]
- Weber H, Aiple F, Fischer B, Latanov A. Dead zone for express saccades. Exp. Brain Res. 1992;89:214–222. doi: 10.1007/BF00229018. doi:10.1007/BF00229018 [DOI] [PubMed] [Google Scholar]
- Wenban-Smith M.G, Findlay J.M. Express saccades: is there a separate population in humans? Exp. Brain Res. 1991;87:218–222. doi: 10.1007/BF00228523. doi:10.1007/BF00228523 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.