Abstract
Adaptive theory predicts that mothers would be advantaged by adjusting the sex ratio of their offspring in relation to their offspring's future reproductive success. Studies investigating sex ratio variation in mammals, including humans, have obtained notoriously inconsistent results, except when maternal condition is measured around conception. Several mechanisms for sex ratio adjustment have been proposed. Here, we test the hypothesis that glucose concentrations around conception influence sex ratios. The change in glucose levels resulted in a change in sex ratios, with more daughters being born to females with experimentally lowered glucose, and with the change in glucose levels being more predictive than the glucose levels per se. We provide evidence for a mechanism, which, in tandem with other mechanisms, could explain observed sex ratio variation in mammals.
Keywords: Trivers–Willard hypothesis, sex allocation, glucose, maternal investment
1. Introduction
Variation in the production of sons and daughters is a key variable in life-history and evolutionary theory, with sex ratio at birth and hatching varying considerably (Clutton-Brock & Iason 1986; Cameron 2004; Rosenfeld & Roberts 2004; Sheldon & West 2004). Adaptive hypotheses predict systematic variation in the sex ratio when the fitness returns of producing sons and daughters vary between individual parents (Trivers & Willard 1973; Clark 1978). The Trivers–Willard hypothesis (TWH; Trivers & Willard 1973) is the most influential of these hypotheses. The TWH posits that, if one sex has more variable reproductive success (males in polygynous species), then (i) mothers in good condition with more resources to allocate would be advantaged by producing sons, as highly competitive sons would out–reproduce highly competitive daughters, who are constrained to a less variable reproductive rate, and (ii) mothers with less resources to allocate would be advantaged by producing a daughter, since even a moderately successful daughter would out-reproduce an unsuccessful son. This hypothesis depends on three key assumptions: the condition of young at the end of maternal investment tends to be correlated with maternal condition; these differences in condition endure into adulthood; and the slight condition advantages will have a greater influence on male reproductive success (Trivers & Willard 1973; Hewison & Gaillard 1999).
This hypothesis is logically appealing and has been extensively tested in a wide variety of mammalian taxa (Cameron 2004; Sheldon & West 2004). In mammals, few studies have produced conclusive results either confirming or refuting this hypothesis, and the inconsistent results have proved difficult to interpret (Festa-Bianchet 1996; Brown 2001; Cameron 2004). However, two recent reviews have suggested that the most consistent support for the TWH in mammals occurs when condition scores are taken around conception, whereas condition scores taken at other times during the reproductive cycle provide less consistent support (Cameron 2004; Sheldon & West 2004). This suggests that sex ratio adjustment is most likely to occur around conception (Cameron 2004). In addition, some of the variation arises owing to inconsistencies in the condition measures themselves (Cameron 2004). Recent studies have also shown consistent support for the TWH where change in condition around conception is used as a variable, rather than absolute condition (Roche et al. 2006; Cameron & Linklater 2007), suggesting that change in condition might be an overlooked variable.
The lack of a known mechanism for sex ratio adjustment hampers our understanding and interpretation of results both in mammals and other taxa (e.g. birds; Pike & Petrie 2003). Several hypotheses in mammals focus on the differences around conception or early development in relation to hormone fluctuations (e.g. Grant 1996, 1998; James 1996, 2004) and asynchrony in early embryo development (e.g. Krackow 1995; Krackow & Burgoyne 1998; Forchhamer 2000). It is probable that more than one process could influence sex ratios (Sheldon & West 2004), and another mechanism by which sex ratios might be adjusted was suggested recently (Cameron 2004). Briefly, male and female conceptuses are sexually dimorphic in their response to glucose (Gutiérrez-Adán et al. 2001) and in their ability to survive in mediums with different glucose concentrations (Larson et al. 2001). Added glucose enhances the development of the male conceptus, but inhibits female conceptus growth and development. This explains a frequently observed phenomenon: the embryos become increasingly male biased as they develop from differentiated cells to expanded blastocyst in vitro but not in vivo (Catt et al. 1997; Pegoraro et al. 1998; Hasler et al. 2002). The glucose added to in vitro cultures enhances male conceptus growth and development, resulting in a difference in sex ratios between in vivo and in vitro raised conceptuses. Other lines of evidence also support the hypothesis (Cameron 2004). For example, studies investigating diabetes that induce an increase in circulating glucose result in male-biased sex ratios (Machado et al. 2001). Furthermore, a recent study showed that a high-fat diet resulted in more sons even when the total caloric value was the same as a low-fat diet (Rosenfeld et al. 2003). The role of glucose levels in offspring sex ratio has yet to be tested in vivo.
Dexamethasone (DEX) is a steroid that inhibits glucose transport and reduces plasma glucose concentrations (Hahn et al. 1999; Buren et al. 2002) with little impact on follicular development (Maciel et al. 2001). Social stress causes male-biased sex ratios in many species (Krackow & Hoeck 1989; Perret 1990) and DEX has been successfully used to reduce these stress-related litter changes (Pratt & Lisk 1990). While the study by Pratt & Lisk (1990) attributed the differences to a reduction in stress levels caused by DEX, it could be also due to glucose concentrations since stress results in higher levels of circulating glucose (Battilana et al. 2001).
We aimed to test the hypothesis that a change in circulating glucose concentration during early cell division can result in biased sex ratios in mice using DEX to reduce circulating glucose concentrations in the blood during early cell division. Previous studies have argued that the assumptions of the TWH hold in mice (e.g. Meikle & Westberg 2001) and significant sex ratio variation in line with the TWH has been reported (e.g. Rosenfeld et al. 2003).
2. Material and methods
We used 40 mice of the National Medical Research Institute (NMRI) strain from the UK, which were laboratory bred at South African Vaccine Producers, Edenvale, Johannesburg. They were kept under 12 L : 12 D photoperiod in a temperature-controlled room, and provided with food and water ad libitum.
Nulliparous females were housed in pairs from 42 days of age. At 56 days of age, females were weighed and had a small blood sample taken from the ventral tail vein for blood glucose analysis using an Accu-Chek Advantage blood glucose test with Advantage II test strips. Males were now introduced and left in the cage with the two females for 3 days and nights, during which time mating occurred. Only two females failed to conceive, so we had a sample size of 18 DEX-treated females and 20 control females. While the male was present, 1.0 μg ml−1 of DEX was added to the drinking water of the treatment females for 3 days following the introduction of the male, thereby including conception and early conceptus development, but very little gestation period. Although this leads to a variable dosage of DEX, it was preferable to injection, as the stress of handling and injection could result in the elevation of cortisol (Tornello et al. 1982; Pratt & Lisk 1990), which in turn would elevate glucose concentrations, thereby potentially negating the treatment effects. After 3 days, females were once again weighed and blood samples taken for glucose analysis. Males were removed from the cages and all water was replaced with fresh tap water.
Females were maintained individually until parturition 19–20 days later. As soon as possible after birth, we counted the number of pups in case infanticide occurred. No pups were lost and there was no evidence of infanticide. At 21 days after birth, the pups were sexed by anogenital distance and the presence of small scrotal sacs. Pups were sexed blind as to whether they were born to a treatment or control female.
We treated litters as replicates for statistical analysis. Therefore, all results are reported on the litter sex ratio, unless stated otherwise. All tests were two tailed and means reported ±1 s.e. Logistic regression was used to model the effects of G1 (pre-treatment glucose level), G2 (post-treatment glucose level), treatment (treatment group) and change from pre- to post-treatment of circulating blood glucose (ΔG). We used information theoretic approaches (Burnham & Anderson 2002) to assess a priori hypotheses explaining variation in sex ratio. Specifically, we used Akaike information criterion and normalized Akaike weights (Burnham & Anderson 2002) to assess the probability that a specific hypothesis was most likely among the considered. We also used the sum of Akaike weights across models containing a specific variable (e.g. G2) to assess the importance of specific variables in explaining variation in sex ratio. We also report estimates and standard errors of parameters linking explanatory variables to sex ratio in order to evaluate functional linkage between these variables and sex ratio.
3. Results
The overall sex ratio in both groups combined was biased slightly towards females (52% females versus 48% males), but did not differ significantly from the expectation of a 50 : 50 sex ratio (two-tailed binomial test, p=0.57). DEX treatment had a significant effect on plasma glucose levels (control 5.24±0.22, range 4.4–7.8; DEX 6.47±0.19, range 4.9–8.4; t36=4.27, p<0.001). A change in glucose levels was therefore recorded for DEX-treated females, but not for control females (control 0.01±0.25; DEX −0.79±0.16; t36=2.72, p=0.01). There was a correlation between glucose levels and change in glucose levels, but the explanatory power was low (regression F1,36=5.86, r2=0.14). The sex ratio differed significantly between the treatment and control groups (rank-sum test: Z=−2.18, p=0.03), with DEX females giving birth to fewer sons (41.9%) than control females (53.5%). The sex ratio for control females did not differ significantly from the expectation of 50% (54%, two-tailed binomial test, p=0.33), but DEX-treated females gave birth to more females than would be expected (42%, two-tailed binomial test, p=0.04). As a consequence, females treated with a glucose blocker gave birth to fewer sons than control females, as well as less than those predicted from an expectation of parity. However, in our model selection analysis, treatment alone was not a strong predictor of sex ratio (ΔAIC=13.446; table 1). Additionally, there was no significant difference in litter size between the two groups (two-tailed t-test, p=0.13), although DEX females tended to have slightly smaller litters (control 10.45±0.60; DEX 9.17±0.62; t=1.53, p=0.13).
Table 1.
Models used to estimate the effects of blood glucose on litter sex ratio of mice.
| Modela | AICb | ΔAIC | MWc | DEVd | NPe |
|---|---|---|---|---|---|
| ΔG | 503.512 | 0.0 | 0.5446 | 499.512 | 2 |
| ΔG+G2 | 505.237 | 1.725 | 0.2299 | 499.237 | 3 |
| TRT+ΔG | 505.371 | 1.859 | 0.2150 | 499.371 | 3 |
| ΔG×G2 | 507.116 | 3.604 | 0.0824 | 499.116 | 4 |
| G2 | 512.269 | 8.757 | 0.0063 | 08.084 | 2 |
| TRT+G2 | 514.084 | 10.572 | 0.0028 | 509.822 | 3 |
| TRT | 516.958 | 13.446 | 0.0007 | 512.958 | 2 |
| (·) | 520.089 | 16.577 | 0.0001 | 518.089 | 1 |
| G1 | 520.583 | 17.071 | 0.0001 | 516.583 | 2 |
Notation for models follows where letters indicate that the model varies according to the given parameter. The following designation implies: ΔG, delta glucose; G1, glucose level before treatment; G2, glucose level after treatment; TRT, treatment; (·), constant model.
Akaike information criterion.
MW, model weight, probability that the given model is best among the suite of models considered.
DEV, deviance (−2 log L).
NP, number of parameters in the model.
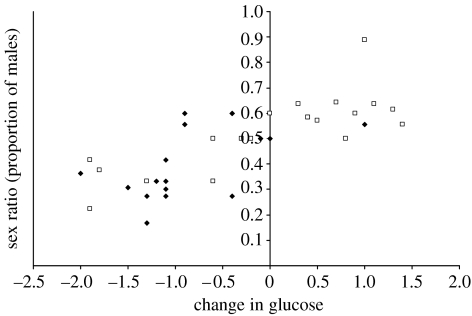
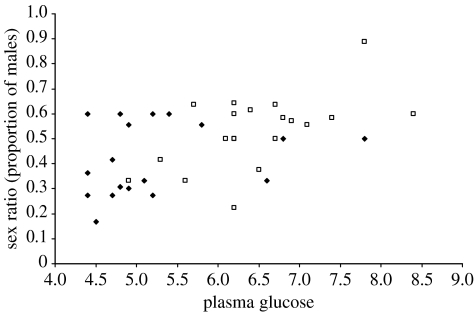
Our model selection analysis revealed that change in circulating blood glucose (ΔG) was the most important predictor of sex ratio (table 1). Given that the top three models fall within 2 ΔAIC, we summed the Akaike weights of the top variables (i.e. treatment, G2, ΔG) in order to quantify the importance of each variable (Burnham & Anderson 2002). The change in circulating blood glucose (ΔG) had overwhelming support with an Akaike weight of 0.9911, while circulating blood glucose post-conception (Akaike weight=0.2395) and treatment (Akaike weight=0.2185) were not well supported (table 1). The change in circulating blood glucose (ΔG) also explained more than half the variation in sex ratio within our dataset (R2=0.5425; figure 1), while circulating blood glucose post-conception (R2=0.2868; figure 2) and treatment (R2=0.1498) explained less of the variation. Consequently, the change in glucose levels was a more important predictor of sex ratio in litters than the concentrations of glucose per se.
Figure 1.
The relationship between the change in blood glucose concentrations from pre-treatment to post-treatment and the litter sex ratio of laboratory mice. Filled diamonds, litters from mothers treated with DEX to block glucose uptake; open squares, control mothers.
Figure 2.
The relationship between blood glucose concentrations after conception and the litter sex ratio in laboratory mice. Filled diamonds, litters from mothers treated with DEX to block glucose uptake; open squares, control mothers.
4. Discussion
These results demonstrate a mechanism to explain how sex ratio can be influenced in utero as a result of changing glucose concentration. The level of circulating glucose during early cell division influences the sex ratio of mice litters, consistent with studies in vitro, which show that added glucose differentially influences the survival of male and female conceptuses (e.g. Larson et al. 2001). Further research is needed to confirm if a similar effect is seen in monotocous species. Although it is possible that DEX administration had a direct effect on sex ratio, it is unlikely since the level of glucose was a significant predictor of sex ratio, regardless of treatment group (figure 1). The litter size did not vary significantly, and therefore the treatment seems to influence the ratio of surviving male and female blastocysts rather than the total number, although this may be due to a lack of statistical power owing to the small sample size. No difference in litter size would be intriguing, since the glucose hypothesis would predict differential survival of male and female blastocysts in relation to glucose levels. Although more corpora lutea are typically produced than being developed into blastocysts (Krackow & Burgoyne 1998), this result suggests that other factors are probably operating in tandem with any glucose effect. Nonetheless, variation in glucose levels provides a potential explanation for sex ratio adjustment in line with adaptive hypotheses proposed to take place in mammals. In addition, our results support recent studies that suggest that sex ratio adjustment occurs at or around the time of conception (Cameron 2004; Sheldon & West 2004) and provide a link between maternal condition and diet. Females only had their glucose levels altered during conception and early cell division, thus precluding any influences later in gestation. However, further investigations on the influence of glucose levels in the uterine fluid are required to ensure that variations in plasma glucose are reflected in utero.
If plasma glucose concentrations during the early development of the conceptus mediate sex ratios, either directly or through interaction with other factors (Cameron 2004), then several confusing results from previous studies investigating sex ratio variation could be explained. For example, social stress leads to the production of more sons than daughters (Krackow & Hoeck 1989; Perret 1990), which does not appear to have an adaptive explanation. An interaction between glucose levels and early development may also provide a link to other hypotheses (e.g. Krackow 1995; Forchhamer 2000) that focus on different developmental rates in males and females.
The change in plasma glucose concentration had a markedly greater influence on sex ratio than the level of glucose per se. In evolutionary terms, changing condition would be more likely to predict maternal condition once offspring were born, thereby influencing the mother's longer term ability to invest in offspring, providing a link with the assumptions of the TWH. Studies on sex ratio variation investigate indices of condition, but the rate of change of condition around conception has only rarely been considered as a variable that may be influencing sex ratios (see Roche et al. 2006; Cameron & Linklater 2007). We suggest that this may be a fruitful area for future research into sex ratio adjustment.
Variation in glucose levels might also provide a link with hypotheses related to maternal hormone levels (James 1996, 2004). Glucose levels are important for reproductive functioning through their interaction with luteinizing hormone (LH). Glucose enhances the LH secretion and release from the pituitary, whereas reduced glucose concentrations can inhibit the LH pulse (Murahashi et al. 1996; Nagatani et al. 1996). The LH in turn has an enhanced effect on glycolytic activity, enhancing glucose availability to the oocyte (Zuelke & Brackett 1992). Therefore, there is a positive feedback loop between the glucose levels and the LH; glucose enhances the LH secretion, which in turn enhances the glucose availability to the oocyte. The timing of insemination appears to be an important factor for skewing sex ratio, second only to manipulated food in terms of studies supporting the TWH (Cameron 2004). This could be explained by the close link between glucose and circulating LH. Furthermore, variation in human sex determination with time of ovulation can also be explained by the same relationship. Males are conceived either early or late in the fertile period (James 2000), coinciding with peaks in the LH production, and therefore availability of glucose to the conceptus. Variation in glucose levels, in tandem with other mechanisms, may mediate the sex of an offspring. Consequently, there may just be some truth in the traditional beliefs about certain foods dictating the sex of a conceptus.
Acknowledgments
We thank S. Alwen for assisting us with the care of the mice, and C.J. Potgieter for logistic support. Funding was provided by the University of Pretoria Research Development Programme to P.W.B. This manuscript has been substantially improved by comments from three anonymous referees, and we appreciate their inputs.
References
- Battilana P, Seematter G, Schneiter P, Jequier E, Tappy L. Effects of free fatty acids on insulin sensitivity and hemodynamics during mental stress. J. Clin. Endocrinol. Metab. 2001;86:124–128. doi: 10.1210/jcem.86.1.7096. doi:10.1210/jc.86.1.124 [DOI] [PubMed] [Google Scholar]
- Brown G.R. Sex-biased investment in nonhuman primates: can Trivers & Willard's theory be tested? Anim. Behav. 2001;61:683–694. doi:10.1006/anbe.2000.1659 [Google Scholar]
- Buren J, Liu H.X, Jensen J, Eriksson J.W. Dexamethasone impairs insulin signalling and glucose transport by depletion of insulin receptor substrate-1, phosphatidylinositol 3-kinase and protein kinase B in primary cultured rat adipocytes. Eur. J. Endocrinol. 2002;146:419–429. doi: 10.1530/eje.0.1460419. doi:10.1530/eje.0.1460419 [DOI] [PubMed] [Google Scholar]
- Burnham K.P, Anderson D.R. 2nd edn. Springer; New York, NY: 2002. Model selection and multimodel inference: a practical information-theoretic approach. [Google Scholar]
- Cameron E.Z. Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc. R. Soc. B. 2004;271:1723–1728. doi: 10.1098/rspb.2004.2773. doi:10.1098/rspb.2004.2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron E.Z, Linklater W.L. Extreme sex ratio variation in relation to change in condition around conception. Biol. Lett. 2007;3:395–397. doi: 10.1098/rsbl.2007.0089. doi:10.1098/rsbl.2007.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt S.L, O'Brien J.K, Maxwell W.M.C, Evans G. Effects of rate of development of in vitro-produced ovine embryos on sex ratio and in vivo survival after embryo transfer. Theriogenology. 1997;48:1369–1378. doi:10.1016/S0093-691X(97)00378-6 [Google Scholar]
- Clark A.B. Sex ratio and local resource competition in a prosimian primate. Science. 1978;201:163–165. doi: 10.1126/science.201.4351.163. doi:10.1126/science.201.4351.163 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Iason G.R. Sex ratio variation in mammals. Q. Rev. Biol. 1986;61:339–374. doi: 10.1086/415033. doi:10.1086/415033 [DOI] [PubMed] [Google Scholar]
- Festa-Bianchet M. Offspring sex ratio studies of mammals: does publication depend upon the quality of the research or the direction of the results? Ecoscience. 1996;3:42–44. [Google Scholar]
- Forchhamer M.C. Timing of foetal growth spurt can explain sex ratio variation in polygynous mammals. Ecol. Lett. 2000;3:1–4. doi:10.1046/j.1461-0248.2000.00101.x [Google Scholar]
- Grant V.J. Sex determination and the maternal dominance hypothesis. Hum. Reprod. 1996;11:2371–2375. doi: 10.1093/oxfordjournals.humrep.a019117. [DOI] [PubMed] [Google Scholar]
- Grant V.J. Routledge; London, UK: 1998. Maternal personality, evolution and the sex ratio: do mothers control the sex of the offspring? [Google Scholar]
- Gutiérrez-Adán A, Granados J, Pintado B, de la Fuente J. Influence of glucose on the sex ratio of bovine IM/IVF embryos cultured in vitro. Reprod. Fert. Dev. 2001;13:361–365. doi: 10.1071/rd00039. doi:10.1071/RD00039. [DOI] [PubMed] [Google Scholar]
- Hahn T, Barth S, Graf R, Engelmann M, Baslagic D, Reul J.M.H.M, Holsboer F, Dohr G, Doesoye G. Placental glucose transporter expression is regulated by glucocorticoids. J. Clin. Endocrinol. Metab. 1999;84:1445–1452. doi: 10.1210/jcem.84.4.5607. doi:10.1210/jc.84.4.1445 [DOI] [PubMed] [Google Scholar]
- Hasler J.F, Cardey E, Stokes J.E, Bredbacka P. Nonelectrophoretic PCR-sexing of bovine embryos in a commercial environment. Theriogenology. 2002;58:1457–1469. doi: 10.1016/s0093-691x(02)01044-0. doi:10.1016/S0093-691X(02)01044-0 [DOI] [PubMed] [Google Scholar]
- Hewison A.J.M, Gaillard J.M. Successful sons or advantaged daughters? The Trivers–Willard model and sex biased maternal investment in ungulates. Trends Ecol. Evol. 1999;14:229–234. doi: 10.1016/s0169-5347(99)01592-x. doi:10.1016/S0169-5347(99)01592-X [DOI] [PubMed] [Google Scholar]
- James W.H. Evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels at the time of conception. J. Theor. Biol. 1996;180:271–286. doi: 10.1006/jtbi.1996.0102. doi:10.1006/jtbi.1996.0102 [DOI] [PubMed] [Google Scholar]
- James W.H. Analysing data on the sex ratio of human births by cycle day of conception. Hum. Reprod. 2000;15:1206–1207. doi: 10.1093/humrep/15.5.1206. doi:10.1093/humrep/15.5.1206 [DOI] [PubMed] [Google Scholar]
- James W.H. Further evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels at the time of conception. Hum. Reprod. 2004;19:1250–1256. doi: 10.1093/humrep/deh245. doi:10.1093/humrep/deh245 [DOI] [PubMed] [Google Scholar]
- Krackow S. The developmental asynchrony hypothesis for sex ratio manipulation. J. Theor. Biol. 1995;176:273–280. doi: 10.1006/jtbi.1995.0197. doi:10.1006/jtbi.1995.0197 [DOI] [PubMed] [Google Scholar]
- Krackow S, Burgoyne P.S. Timing of mating, developmental asynchrony and the sex ratio in mice. Physiol. Behav. 1998;63:81–84. doi: 10.1016/s0031-9384(97)00393-4. doi:10.1016/S0031-9384(97)00393-4 [DOI] [PubMed] [Google Scholar]
- Krackow S, Hoeck H.N. Sex ratio manipulation, maternal investment and behaviour during concurrent pregnancy and lactation in house mice. Anim. Behav. 1989;37:177–186. doi:10.1016/0003-3472(89)90108-5 [Google Scholar]
- Larson M.A, Kimura K, Kubisch H.M, Roberts R.M. Sexual dimorphism among bovine embryos in their ability to make the transition to expanded blastocyst and in the expression of the signaling molecule IFN-tau. Proc. Natl Acad. Sci. USA. 2001;98:9677–9682. doi: 10.1073/pnas.171305398. doi:10.1073/pnas.171305398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A.F, Zimmerman E.F, Hovland D.N, Weiss R, Collins M.D. Diabetic embryopathy in C57BL/6J mice: altered fetal sex ratio and impact of the splotch gene. Diabetes. 2001;50:1193–1200. doi: 10.2337/diabetes.50.5.1193. doi:10.2337/diabetes.50.5.1193 [DOI] [PubMed] [Google Scholar]
- Maciel S.M, Chamberlain C.S, Wettermann R.P, Spicer L.J. Dexamethasone influences endocrine and ovarian function in dairy cattle. J. Dairy Sci. 2001;84:1998–2009. doi: 10.3168/jds.S0022-0302(01)74643-7. [DOI] [PubMed] [Google Scholar]
- Meikle D, Westberg M. Maternal nutrition and reproduction of daughters in wild house mice (Mus musculus) Reproduction. 2001;122:437–442. doi: 10.1530/rep.0.1220437. doi:10.1530/rep.0.1220437 [DOI] [PubMed] [Google Scholar]
- Murahashi K, Bucholtz D.C, Nagatani S, Tsukahara S, Tsukamura H, Foster D.L, Maeda K.I. Suppression of luteinizing hormone pulses by restriction of glucose availability is mediated by sensors in the brain stem. Endocrinology. 1996;137:1171–1176. doi: 10.1210/endo.137.4.8625886. doi:10.1210/en.137.4.1171 [DOI] [PubMed] [Google Scholar]
- Nagatani S, Bucholtz D.C, Murahashi K, Estacio M.A, Tsukamura H, Foster D.L, Maeda K.I. Reduction of glucose availability suppresses pulsatile luteinizing hormone release in female and male rats. Endocrinology. 1996;137:1166–1170. doi: 10.1210/endo.137.4.8625885. doi:10.1210/en.137.4.1166 [DOI] [PubMed] [Google Scholar]
- Pegoraro L.M.C, Thuard J.M, Delalleau N, Guérin B, Deschamps J.C, Marquant-Le Guienne B, Humblot P. Comparisons of sex ratio and cell number of IVM–IVF bovine blastocysts co-cultured with bovine oviduct epithelial cells or with vero cells. Theriogenology. 1998;49:1579–1590. doi: 10.1016/s0093-691x(98)00103-4. doi:10.1016/S0093-691X(98)00103-4 [DOI] [PubMed] [Google Scholar]
- Perret M. Influence of social factors on sex ratio at birth, maternal investment and young survival in a prosimian primate. Behav. Ecol. Sociobiol. 1990;27:447–454. doi:10.1007/BF00164072 [Google Scholar]
- Pike T.W, Petrie M. Potential mechanisms of avian sex manipulation. Biol. Rev. 2003;78:553–574. doi: 10.1017/s1464793103006146. doi:10.1017/S1464793103006146 [DOI] [PubMed] [Google Scholar]
- Pratt N.C, Lisk R.D. Dexamethasone can prevent stress-related litter deficits in the golden hamster. Behav. Neural Biol. 1990;54:1–12. doi: 10.1016/0163-1047(90)91201-l. doi:10.1016/0163-1047(90)91201-L [DOI] [PubMed] [Google Scholar]
- Roche J.R, Lee J.M, Berry D.P. Pre-conception energy balance and secondary sex ratio—partial support for the Trivers–Willard hypothesis in dairy cows. J. Dairy Sci. 2006;89:2119–2125. doi: 10.3168/jds.S0022-0302(06)72282-2. [DOI] [PubMed] [Google Scholar]
- Rosenfeld C.S, Roberts R.M. Maternal diet and other factors affecting offspring sex ratio: a review. Biol. Reprod. 2004;71:1063–1070. doi: 10.1095/biolreprod.104.030890. doi:10.1095/biolreprod.104.030890 [DOI] [PubMed] [Google Scholar]
- Rosenfeld C.S, Grimm K.M, Livingston K.A, Lamberson W.E, Roberts R.M. Striking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrate. Proc. Natl Acad. Sci. USA. 2003;100:4628–4632. doi: 10.1073/pnas.0330808100. doi:10.1073/pnas.0330808100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon B.C, West S.A. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 2004;163:40–54. doi: 10.1086/381003. doi:10.1086/381003 [DOI] [PubMed] [Google Scholar]
- Tornello S, Orti E, De Nicola A.F, Rainbow T.C, McEwen B.S. Regulation of glucocorticoid receptors in brain by corticosterone treatment of adrenalectomized rats. Neuroendocrinology. 1982;35:411–417. doi: 10.1159/000123429. [DOI] [PubMed] [Google Scholar]
- Trivers R.L, Willard D. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. doi:10.1126/science.179.4068.90 [DOI] [PubMed] [Google Scholar]
- Zuelke K.A, Brackett B.G. Effects of luteinizing hormone on glucose metabolism in cumulus-enclosed bovine oocytes matured in vitro. Endocrinology. 1992;131:2690–2696. doi: 10.1210/endo.131.6.1446610. doi:10.1210/en.131.6.2690 [DOI] [PubMed] [Google Scholar]