Abstract
To date there is still no proper neuroimaging methods suitable for noninvasively providing both detailed spatial and temporal information of neural interaction across large-scale brain networks. This limitation has impeded the advance of neuroscience research. In an attempt to overcome this challenge, Ogawa et al applied a paired-stimulus paradigm, which is composed of a pair of stimuli separated by a variable inter-stimulus interval (ISI), to decode temporal information of neural interaction from amplitude modulation of the blood-oxygenation-level-dependent (BOLD) responses elicited by the neural interaction pursued (Ogawa et al., 2000). Although application of this paradigm has been demonstrated in a few publications, most of them only focused on investigating cortico-cortical interaction. Considering the vital roles that cortico-thalamic networks play in brain communication and function, extending the applicability of this method to studying cortico-thalamic neural interaction should be significant. In this study, we applied the paired-visual-stimulus paradigm to simultaneously measure the BOLD amplitude modulations as a function of ISI in the lateral geniculate nucleus (LGN) and primary visual cortex (V1) in the cat brain. The results reveal that both V1 and LGN BOLD responses were significantly suppressed when the visual system was within the refractory period at ISI ≤ 1s and the suppression extent gradually recovered when ISI became longer. Both BOLD and electrophysiological measurements show a facilitatory activity in V1 at ISI ≈ 1.5 s, but not in LGN. Furthermore, there was additional and consistent reduction in the LGN BOLD response compared to V1 within the range of ISI below 4s, which is likely controlled by inhibitory effects through the cortico-geniculate feedback. These findings together suggest that the dynamic fMRI approach is sensitive to neuronal inhibitory and facilitatory interactions and it should be useful for noninvasively investigating large-scale cortico-thalamic neural networks.
Keywords: Lateral geniculate nucleus, Primary visual cortex, Cortico-thalamic network, Visual system, fMRI, Neural interaction
Introduction
The normal brain consists of numerous neural networks coordinately working together and accomplishing a specific task. For these neural networks to function properly, many neuronal groups inside and outside of a specific network need to harmonize sequentially or concurrently, as electrophysiological signals propagate along the network. This feature of brain function makes neural interaction a ubiquitous phenomenon at various brain function levels. Consequently, understanding detailed spatiotemporal characteristics of different types of neural interaction is the cornerstone of uncovering the brain network and, ultimately, the brain function. To accomplish this task, a neuroimaging technique with the capability of simultaneously and non-invasively detecting spatial and temporal information of neuronal events and interaction in large-scale brain networks is required. Unfortunately, current neuroimaging techniques with relatively high-spatial resolution including positron emission tomography (PET), single photon emission computed tomography (SPECT), optical imaging, near infrared spectroscopy (NIRS) and functional magnetic resonance imaging (fMRI) all have low temporal resolvability due to the sluggish and lagged vascular responses they measure. On the other hand, neuroimaging techniques such as electroencephalography (EEG) and magnetoencephalography (MEG) have very high temporal resolution that enables them to trace the time evolution of neuronal events, typically on the scale of tens to hundreds of milliseconds. These techniques, however, suffer from the difficulty of precisely defining spatial origins of the brain activity, particularly in the deep brain regions such as thalamus, due to their inherent mathematical difficulties and low spatial resolution (Dale and Halgren, 2001; He and Lian, 2002).
The lack of a neuroimaging modality suitable for investigating neural interaction and brain networks has impeded the advancement of the neuroscience field. In an attempt to overcome this challenge, Ogawa et al combined a paired-stimulus paradigm and fMRI to decode temporal information of neural interaction from the amplitude modulation of the blood-oxygenation-level-dependent (BOLD) signal (Ogawa et al., 2000). The paired-stimulus paradigm is composed of two stimuli separated by an inter-stimulus interval (ISI). With substantial evidence coming from neuronal recording (Nelson, 1991a, 1991b, 1991c), PET (Henson, 2003; Henson and Rugg, 2003) and fMRI (Ogawa et al., 2000; Zhang and Chen, 2006; Zhang et al., 2007; Zhang et al., 2005) studies in the species of cat, monkey and human, it has been well demonstrated that desired neural interaction can be elicited using this stimulation paradigm, resulting in amplitude variations of the elevated brain activity and hemodynamic change sensitive to ISI. Consequently, the spatiotemporal information of neural interaction and brain activation can be obtained from a series of fMRI maps with characteristic BOLD amplitude modulations at different ISIs.
The concept of using BOLD amplitude modulations to extract temporal (or dynamic) information of neural interaction has been demonstrated in several publications (Ogawa et al., 2000; Zhang and Chen, 2006; Zhang et al., 2007; Zhang et al., 2005). However, all of the dynamic fMRI studies only focused on neural interaction at local cortical regions while there is no study attempting to simultaneously map BOLD amplitude modulations induced by neural interactions in a cortico-thalamic neural network covering large-scale brain regions. Considering the vital roles that cortico-thalamic networks play in brain communication and function, extending the applicability of this method to studying cortico-thalamic neural interaction should be significant.
One particularly interesting cortico-thalamic neural network is related to the visual sensory system. The retinal afferents cross at the optical chiasm and project bilaterally to the lateral geniculate nucleus (LGN) via the optic tracts; the LGN in turn projects to the primary visual cortex (V1) through optic radiation (Hubel and Wiesel, 1968, 1972; Wiesel et al., 1974); visual information is then passed further from V1 to higher-tier visual processing areas (e.g. V2, V3 and MT). In addition, the LGN also receives a large number of backward projection fibers from V1 (Murphy and Sillito, 1996), indicating a possible two-way (bottom-up and top-down) communication between LGN and V1. Neural interactions among different brain regions involved in the visual system are vital to ensure visual information not only be smoothly passed along, but also appropriately processed so as to generate a proper visual perception. Therefore, probing cortico-thalamic neural interactions within each and across multiple mutually interacted visual regions will be of great interest.
Aiming to explore this capability, in this study we employed the dynamic fMRI approach with a pair of visual stimuli separated by a number of ISIs to elicit different levels of inhibition in the cat visual system, and simultaneously measured the BOLD amplitude modulations as a function of ISI at LGN and V1. Both LGN and V1 exhibited similar temporal inhibition dynamics during the refractory period, suggesting a common state elicited by the paradigm in the visual system. At the same time, we observed additional inhibition occurring in the LGN which might be not relevant to refractory inhibition because it occurs in a much longer-lasting temporal window. The possible mechanisms underlying these two types of inhibition were discussed in this article. The results of the present study suggest that it is feasible to investigate neural interaction within cortico-thalamic networks by combining an appropriately designed paired-stimulus paradigm and fMRI.
Materials and Methods
Animal preparation
Six cats (body weight: 1.4±0.2 kg) were used to map cortico-thalamic interaction using the dynamic fMRI approach. Five of them were used to investigate the neuronal response to the same paradigm in the visual cortex. Cats were initially anesthetized with a mixture of ketamine (15.0 mg/kg, i.v.) and xylazine (2.5 mg/kg). After oral intubation and mechanical ventilation (30–33 stokes/min), anesthesia was switched to 0.9–1.2% isoflurane in a N2O/O2 mixture of 70:30 volume ratio throughout the experiment. The pupils of the cat were dilated with a drop of atropine sulfate solution; corrective contact lenses were placed to focus the eyes on the visual stimulus by refracting and locating the fovea of the cat retina with the aid of a fundus camera (Zeiss, Germany). They can also protect the corneas from drying. The animal was placed in a cradle and restrained in normal postural position. The head of the cat was firmly positioned by a home-built head holder with mouth and ear bars to avoid head movement. The rectal temperature of cats was monitored with a temperature sensor (PhysiTemp Instrument, Clifton, NJ) and maintained at 38.3±0.3 °C by using a heated circulating water blanket. The end-tidal CO2 was monitored (Capnomac Ultima; Instrumentarium Corp. Finland) and maintained at normal conditions (between 3.8% and 4.2%) by adjusting respiratory rate and tidal volume. All animal surgical procedures and experimental protocol were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Visual Stimuli
Visual stimulation presented as ultrashort flashing light (10 ms duration per flash; 100% contrast light) was generated by a pair of red LED goggles (Grass Instruments, Quincy, MA) and a home-built amplifier and pulsing device. Full-field visual stimuli were displayed either singly or in pairs separated by an ISI. Fourteen ISI values ranging from 0 ms to 4000 ms were chosen to cover a full range of the refractory period of the cat visual system (Nelson, 1991a, 1991b). During the baseline condition, cats were in uniform darkness.
Experiment procedure and data acquisition
All the fMRI studies were performed on a 9.4T horizontal magnet (Magnex Scientific, UK) interfaced with a Varian INOVA console (Varian Inc., Palo Alto, CA). A half-volume radiofrequency (RF) surface coil was used to ensure sufficient MRI detection sensitivity in the brain volume where cat visual cortex and LGN are located. At first, multi-slice anatomical images were acquired using a conventional T1-weighted TurboFLASH imaging method (Haase, 1990). On the basis of the anatomic images, slices covering the cat LGN and V1 areas were appropriately selected for acquiring fMRI data using multi-slice gradient echo planar images (EPI) with the parameters: repetition time (TR) = 252 ms; echo time (TE) = 14 ms; 5 adjacent axial EPI slices; field of view = 5×5 cm2; 780µm×780µm in-plane spatial resolution; 1 mm slice thickness, 1 mm gap between adjacent image slices.
The fMRI experiment was conducted using an event-related paradigm design. Stimulus onset was time locked to the onset of TR of acquiring each EPI volume. The duration of stimulus and ISI were controlled by a home-built pulsing device. For each single or paired visual-stimulation task, 15 trials were repeated in each fMRI run. Successive trials of single or paired stimuli were separated by an inter-trial interval (ITI) of 20 seconds. A total of 15 fMRI runs corresponding to 15 tasks (1 single and 14 paired visual stimulation tasks) were acquired in a pseudo-randomized order in each experiment.
To confirm that the measured BOLD response indeed reflects the neuronal activity behavior, the visual evoked potential (VEP) response to the same paradigm was acquired to investigate the neuronal activity change as a function of ISI in V1. This experiment was conducted outside of the magnet scanner. The physiologic conditions of the cats were monitored and maintained in the same way as in the fMRI experiment. A small hole was drilled in the skull overlaying the visual cortical area 17, and the dura underneath was carefully removed. Continuous intracranial electroencephalography (EEG) signals were acquired from an EEG electrode inserted into the cortical area 17. The VEP signal was digitized at 1000 Hz with 0.1–30 Hz bandpass filtering. Then the signals were segmented from 100 ms before the onset to 3000 ms after the offset of visual stimulation for each epoch. At least 200 epochs were averaged to obtain the final VEP signal.
Data analysis
All the fMRI data analysis was performed using the STIMULATE software package (Stimulate, Center for Magnetic Resonance Research, University of Minnesota) (Strupp, 1996) and Matlab software (The Mathworks Inc., Natick, MA, USA). The fMRI time series were segmented from 5 s (10 image volumes) before and 20 s (80 image volumes) after the onset of stimulation. Epochs identified were selectively averaged for each task, resulting in a total of 15 averaged time series for the single- and paired-flash tasks.
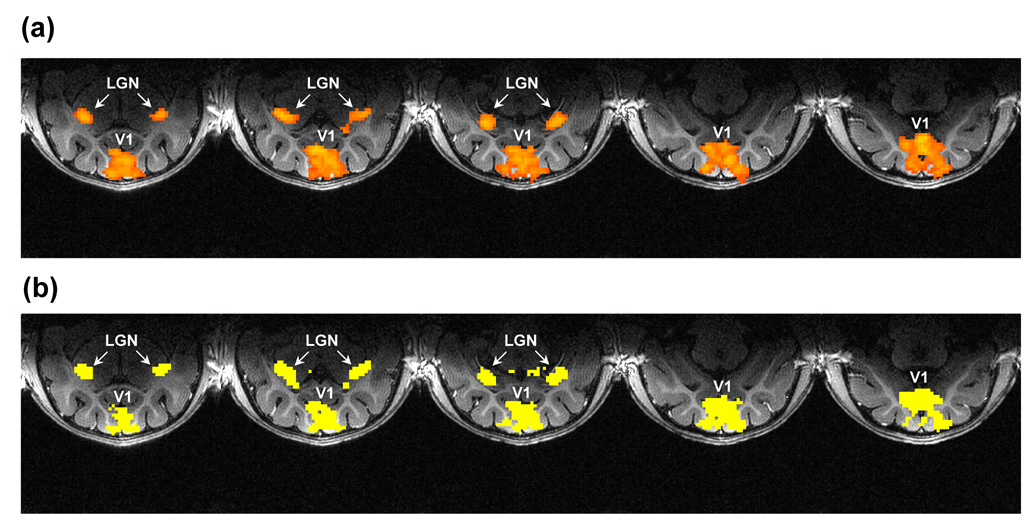
Activation maps were generated using a time-shifted cross-correlation (CC) method (Bandettini et al., 1993; Xiong et al., 1995). The activated pixels for each stimulation task were identified by correlating the averaged fMRI time course with a trapezoidal function (the modified box-car function with the capability of accounting for hemodynamic delays). This procedure generated typical activation maps in LGN and V1 (area 17 and area 18, as shown in Figure 1a), which were consistent across different cats. We chose a statistical threshold of p < 0.01 to generate activation maps for individual tasks. Furthermore, to ensure data reliability in subsequent processing, we created a functional region of interest (ROI) based on the following criterion: a pixel was deemed as activated only if it passed the threshold for at least half of all the tasks: i.e., this pixel was considered activated in at least eight of all the tasks. The created ROIs composed of all activated pixels in either LGNs or in the visual areas 17 and 18 (as shown in Figure 1b) were applied to all the tasks for quantification.
Figure 1.
(a) Activation maps generated using the time shifted cross-correlation method in the task with ISI=1000 ms in one cat. The threshold is chosen at p < 0.01. (b) Common activation map generated based on the following criterion: a pixel is deemed as activated in the common activation map only if it passes the threshold for at least half of all the tasks: i.e., this pixel is considered activated in at least eight of all the tasks. This common activation map composed of all activated pixels in LGN and areas 17 and 18 (i.e., V1) created based on this criterion is used for quantifying BOLD amplitudes for all the tasks.
A ROI-based analysis was used to quantify BOLD amplitudes for all tasks. First, the BOLD time course was averaged across all activated pixels within the ROI for each task. The averaged time course was then calculated as percentage change relative to the baseline condition. Subsequently, the BOLD amplitude was quantified by integrating positive BOLD signal expressed in percentage change for each task. Lastly, BOLD integrals of all paired-stimulus tasks were normalized to the single-stimulus task (i.e., BOLD integral ratio between them). The relative BOLD integral after normalization was used to quantify the modulation of hemodynamic response as a function of ISI in both LGN and V1 (i.e. a value smaller than 2 indicates proportional BOLD suppression owing to neuronal interaction).
Neuronal activity in V1 was quantified by the amplitude of VEP peak, which was defined as the difference between the peak height and peak offset (Arthurs et al., 2000; Brinker et al., 1999; Ogawa et al., 2000; Zhang et al., 2007). All VEP amplitudes were also normalized to the single-flash condition. Relative VEP amplitude after normalization was used to quantify the modulation of neuronal response as a function of ISI in V1 (i.e. a value smaller than 2 indicates proportional suppression of neuronal activity).
All results were presented as mean±standard deviation.
Results
Relative BOLD amplitudes at LGN and V1 as a function of ISI
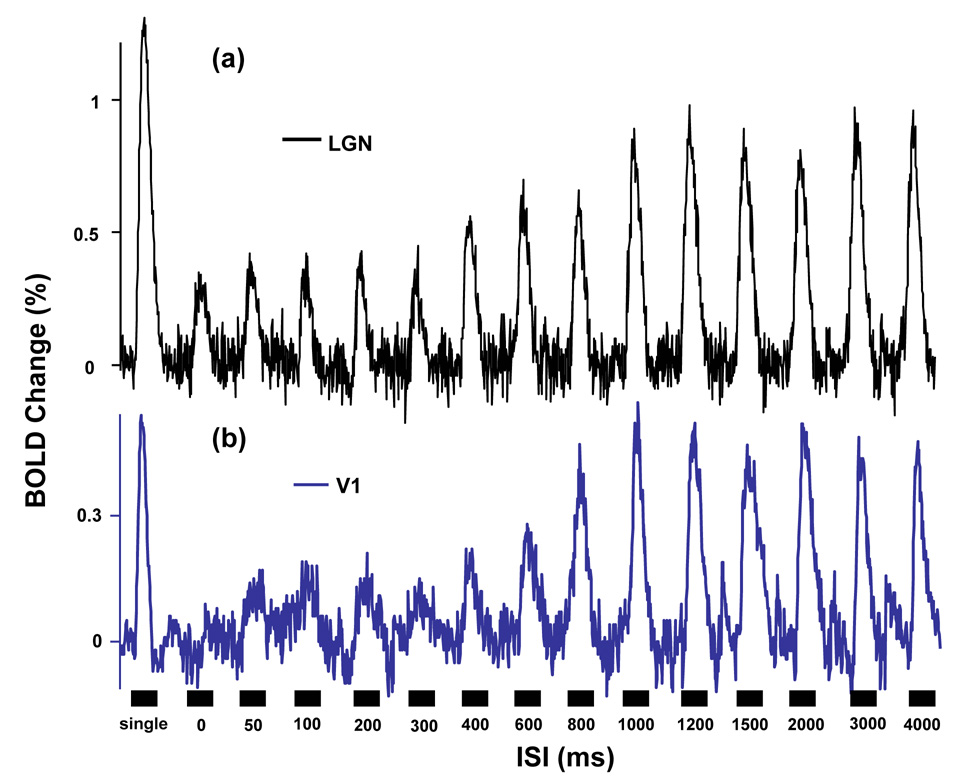
Figures 2a and 2b show the averaged BOLD time courses of all tasks in both ROIs of LGN (Fig. 2a) and V1 (Fig. 2b). We have demonstrated that when ITI is long enough, there is no interference between consecutive stimulation pairs (Ogawa et al., 2000; Zhang et al., 2007). Therefore, BOLD response to the second stimulus at a paired-stimulus condition can be individuated by subtracting the BOLD time course at the single-stimulus condition from that at the paired-stimulus condition. Figure 3 shows the averaged time courses of BOLD responses to the single stimulus (first one on the left side in Fig. 3) and to the second stimulus at all ISIs in LGN (Fig. 3a) and V1 (Fig. 3b). The time courses suggest that within individual ROIs of LGN and V1, BOLD responses were significantly suppressed when the visual system was in a refractory period (ISI < ~1 s), based on the fact that BOLD amplitude in response to the second stimulus was smaller than that in response to the single stimulus when ISI was relatively short. Nevertheless, the suppression of BOLD response to the second stimulus at ISI > 1 s completely disappeared in V1 but was sustained in LGN.
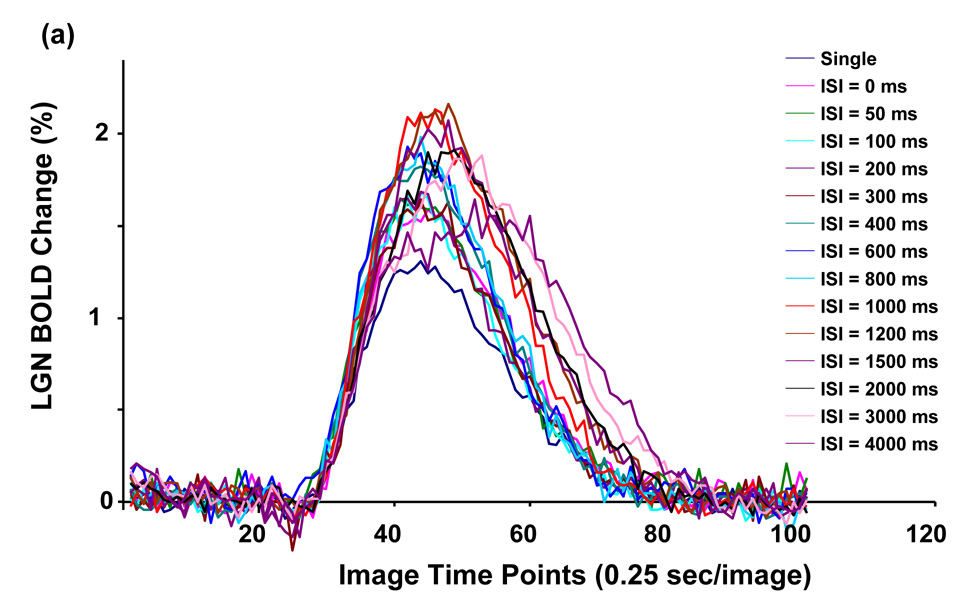
Figure 2.
Averaged BOLD time courses of all tasks from the ROI of (a) LGN and (b) V1. The tasks include a single stimulus task and paired-stimulus tasks with varied ISIs.
Figure 3.
(a) The averaged time courses of BOLD responses to the first (the leftmost one) and second stimulus at each ISI in LGN. BOLD response to the second stimulus at a paired-stimulus condition is individuated by subtracting the BOLD time course at the single-stimulus conditions from that at the paired-stimulus condition. (b) The averaged time courses of BOLD responses to the first (the leftmost one) and second stimulus at each ISI in V1.
The pattern of BOLD modulation at different ISIs is more evident in Fig. 4, showing the normalized BOLD amplitudes in the ROIs of LGN and V1 at all ISIs. In both ROIs, BOLD responses were significantly suppressed (i.e., below the dotted reference line) at ISIs of < 1 s. When ISI got longer, BOLD suppression at both ROIs gradually became smaller. These results confirm that the cat visual system is in a refractory state if the delay after the initial stimulation is shorter than 1 s. In contrast to this similarity between LGN and V1, a very different behavior between LGN and V1 was also observed. First, for shorter ISIs (< 1 s) when both V1 and LGN are in the refractory state, the relative BOLD integral ratio in V1 was always larger than that in LGN at each corresponding ISI indicating a faster recovery from the suppression in V1. Second, suppression in V1 BOLD amplitude completely disappeared when ISI ≥ 1 s, whereas BOLD suppression in LGN was sustained for even longer ISIs (e.g. 4 s). Third, a facilitatory effect was clearly detected in V1 when ISI was between 1 and 2 seconds, and such phenomenon was not observed in LGN (see Fig. 4). Taken together, the data suggests that although LGN and V1 have similarity in temporal behavior of suppression, reflecting a common state of the visual system, there is still substantial difference in it. Considering the observation that the BOLD response to the second stimulus recovers to the same level of the response to the single stimulus in V1 at ISI > 1 s, we hypothesize that the additional suppression observed in LGN might come from the inhibitory effect of cortico-thalamus feedback. A possible mechanism to explain this hypothesis will be described in more detail in Discussion.
Figure 4.
Normalized BOLD integral as a function of ISI in LGN and V1. At both ROIs, BOLD responses are significantly suppressed (i.e., below the reference line) at ISIs of < 1 second. When ISI gets longer, BOLD suppression at both ROIs gradually becomes smaller. Suppression in V1 BOLD activity completely disappears when ISI ≥ 1 second, whereas BOLD suppression in LGN sustains for even longer ISIs. V1 activity also shows a short period (~ 1 s) of facilitation (i.e., above the reference line) after ISI > 1 second.
Pooling relative BOLD integrals at all ISIs in both V1 and LGN can provide comprehensive information regarding BOLD response to the paired-stimulus paradigm. Statistically, there was a significant reduction in relative BOLD integrals compared to the state of no inhibition in both V1 and LGN (two-tail paired t-test, p < 0.005 for V1 and p < 10−6 for LGN, n = 15). Paired t-tests also revealed a statistically significant difference in relative BOLD integrals between LGN and V1 (two-tail paired t-test, p < 3×10−5, n = 15).
Relative VEP amplitude in V1 as a function of ISI
To further confirm the notion that variation of BOLD integral as a function of ISI results from the corresponding variation of neuronal activity at the same stimulation condition, we measured VEP response to the same paradigm in V1. Figure 5 shows the VEP time courses at two ISIs from one representative cat. Figure 5a indicates that at a short ISI, the neuronal response to the second stimulus was significantly suppressed in V1. However, when ISI became longer (e.g., ISI = 3 seconds) this suppression disappeared as shown in Fig. 5b.
Figure 5.
VEP signals detected from one representative cat visual cortex at (a) ISI = 100 ms and (b) ISI = 3000 ms, respectively.
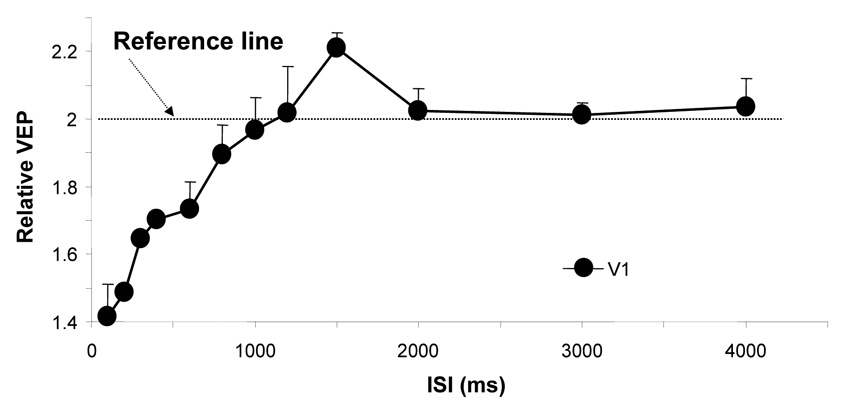
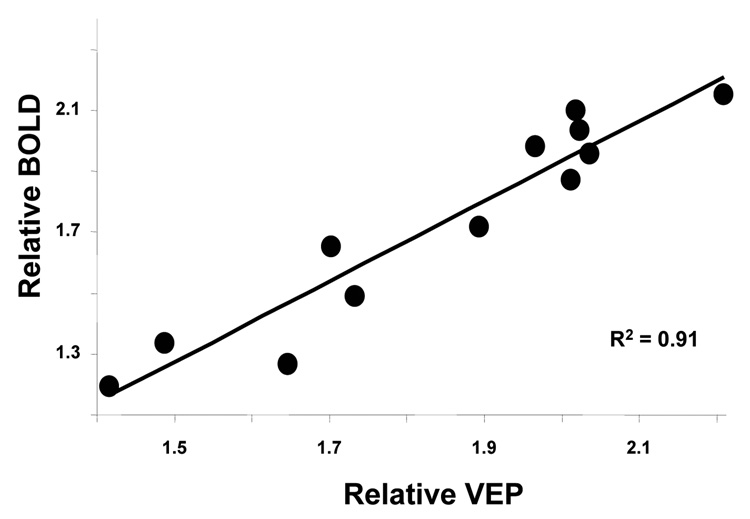
Figure 6 summarizes the averaged normalized VEP amplitude in V1 as a function of ISI, revealing a very similar temporal behavior as the normalized BOLD amplitudes shown in Fig. 4: at short ISIs (< 1 s), the VEP amplitude was significantly suppressed; while when ISI got longer, this suppression gradually became smaller, eventually disappearing; VEP suppression completely disappeared after approximately 1 second of refractory period. There was a strong correlation (R2 = 0.91) between the normalized VEP and normalized BOLD amplitudes as shown in Fig. 7. This result validates the temporal dynamics in response to the paired-flash paradigm measured by the BOLD signal in the cat visual system and suggests a tight coupling between neuronal activity and its accompanied hemodynamic response measured by the BOLD signal. Interestingly, a significant neuronal facilitatory effect was also observed in relative VEP amplitude at ISI of 1500 ms (two-tail paired t-tests, p < 0.05, n = 6). A closer examination of the relative BOLD integral at the same ISI (Fig. 2) also indicates a similar facilitatory effect, though the effect does not reach statistical significance (two-tail paired t-test, p = 0.16, n = 6), possibly due to a relatively large signal variation and a small sample size.
Figure 6.
Normalized VEP amplitudes in V1 as a function of ISI, showing a similar temporal behavior as BOLD activity. The presented VEP amplitudes were averaged from six measurements; and if they are > 2 indicating neuronal facilitation (i.e., above the reference line) and if < 2 indicating neuronal inhibition (below the reference line).
Figure 7.
Correlation between the normalized VEP and normalized BOLD amplitudes measured under varied ISIs (R2 = 0.91).
Discussion and Conclusion
For a long time, the major role of LGN was viewed merely as a relay of transferring visual information from the retina to visual cortex. However, it has also been documented that the number of backward-projection fibers from V1 to LGN is ten times larger than the forward-projection ones in the cat brain (Murphy and Sillito, 1996). This large-scale cortico-thalamic feedback connection makes it intriguing to assume that there must be strong feedback control from V1 to LGN. In the present study, we investigated the transfer function at LGN and V1 using fMRI and a paired-stimulus paradigm. We found strong inhibitory BOLD and VEP activities in LGN and V1 in response to the second stimulus in the paired visual-stimulation paradigm when the visual system is in a refractory period. There is an additional and consistent reduction in LGN BOLD activity compared to V1 when ISI is within a few seconds. Two different mechanisms can be involved in these inhibitory activities. Each mechanism has its characteristic temporal property and distinct functional implication as discussed in the following.
Retino-geniculate neural interaction induces refractory inhibition in LGN and V1
The major driving inputs in the LGN from the retinal afferents make multiple complex contacts with dendrites of geniculate relay cells and inhibitory interneurons, typically forming a characteristic triad (Guillery and Sherman, 2002). Cox and Sherman showed that the retinal glutamatergic innervation of the inhibitory interneuron terminal involves the metabotropic receptor, and activation of this receptor increases GABA release from the interneuronal terminal (Cox and Sherman, 2000). Based on the innervation and receptor patterns, one can predict that retinal activation will produce monosynaptic excitatory postsynaptic potentials (EPSPs) followed by disynaptic inhibitory postsynaptic potentials (IPSPs) in the relay cell, as suggested by Guillery and Sherman (Guillery and Sherman, 2002). Guillery and Sherman also pointed out that it can be predicted that after a period of strong retinal activation, the disynaptic IPSPs would continue to be present hundreds of milliseconds after the monosynaptic EPSPs faded away. This is because metabotropic receptors in interneurons generate long-lasting PSPs, as opposed to brief PSPs via ionotropic receptors. This prediction exactly conforms to what we have observed in the present study. Following the first stimulus, the BOLD response to the second stimulus is significantly smaller if the delay between the two stimuli is shorter than 1 s and gradually recovers when ISI gets longer.
Additional inhibition in LGN might be attributed to the Cortico-thalamic feedback
In addition to driving inputs from the retina, all geniculate relay cells in the LGN receive the modulatory inputs from the layer 6 of visual cortex (Guillery and Sherman, 2002). It has been suggested that the key function of these modulatory inputs to LGN is to control the response mode, either burst or tonic, of relay cells: when geniculate cells are in the tonic mode, retino-geniculate transmission is linear (i.e. the LGN can faithfully transmit the visual information from the retina to the visual cortex), whereas when geniculate cells are in the burst mode, the transmission of visual information is not as effective (Sherman and Guillery, 1996, Sherman, 2002 #25). The key factor in controlling LGN function between the two distinct response modes is a low-threshold Ca2+ conductance, which is in turn determined by membrane potential of the relay cell, with hyperpolarization shifting the response mode from tonic to burst and depolarization shifting the response mode from burst to tonic (Sherman, 2001, Sherman, 1996 #24, Sherman, 2002 #25). Nelson found a long-lasting suppression of spontaneous activity accompanied with a reduction of the antidromic excitability in the cat LGN following the offset of an excitatory visual stimulus (Nelson, 1991b); and this study suggested that in LGN neurons, the offset of an excitatory visual stimulus produces a hyperpolarization that can last 600–800 ms. Hyperpolarization following the stimulus has also been noted in intracellular recording studies of LGN neurons in vivo (Singer and Creutzfeldt, 1970). Collectively, all these results indicate that after a visual stimulus, there is a hyperpolarization in LGN neurons, possibly modulated by cortico-thalamic feedbacks mediated via thalamic reticular nucleus, interneurons and/or cortical modulators, which is long enough to lead to a change of membrane potential and in turn modulates the response mode of LGN to the burst mode. As a result, the LGN response to the second stimulus would remain reduced at the burst mode for up to a few seconds even though the V1 activities have recovered from the refractory period. This notion is further supported by a recent work to record neuronal activity in dorsal LGN when V1 is inhibited by local transcranial magnetic stimulation (de Labra et al., 2007). This study shows that the inhibitory effect on V1 could selectively suppress the sustained component of LGN responses to visual stimulation; and this LGN suppression is the result of a loss of spikes fired in tonic mode. All of these findings reported in the literature suggest that the long-lasting suppression in the LGN activity as observed in our fMRI study is possibly associated with the suppression of the tonic-mode neuronal activity in LGN controlled by the cortico-geniculate feedback pathway.
Functional implications
It has been reported from visual perception studies that one visual stimulus can alter the visibility of the second stimulus. The effects of the forward masking stimulus are commonly suppressive (Breitmeyer, 1980), but facilitatory effects have also been found (Bachmann, 1988). The major function of masking, as suggested by Noda (Noda, 1975), Judge et al (Judge et al., 1980) and others, is to suppress visual information during saccade eye movements. It has been shown in the cat (Noda, 1975) and monkey (Louie et al., 1976) that following saccade, the transfer of visual information through LGN is impaired. In our study, we demonstrated that activities in the LGN and V1 are not as vigorous within a delay of 1 second following a brief visual stimulus. We also observed facilitatory VEP and BOLD activities in V1 during the post-inhibitory period. Like masking, if the reduction in V1 and LGN activities during the refractory period contributes to the function of suppressing visual information during saccadic eye movements, the facilitatory effect observed in V1 during the post-inhibition period could represent the post saccadic enhancement reported in some studies (Bartlett et al., 1976; Noda, 1975).
A significant suppression in the LGN observed at large ISIs in this study could be related to controlling the gain of V1 activity level during the post-inhibitory period. The post-inhibition facilitatory effect in V1 observed in both VEP and BOLD data makes it intriguing to postulate that enhanced V1 activity during the post-inhibitory period sends a stronger inhibitory signal to the LGN through the feedback pathway so as to maintain it in the burst response mode. Sustained suppression in the LGN after the refractory period can in turn prevent the V1 activity from going even higher. As a result, with this negative feedback mechanism V1 activity can be maintained at a steady level under neither inhibitory nor facilitatory states.
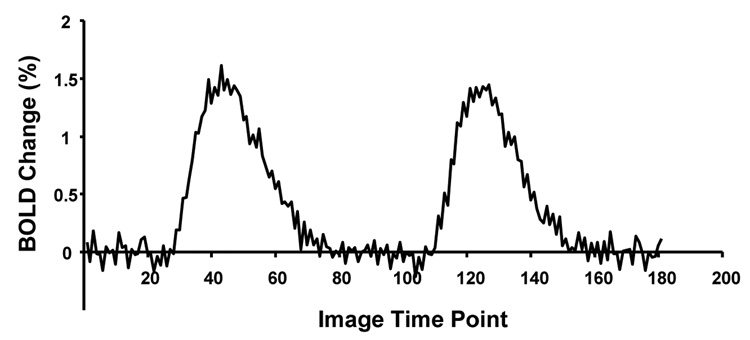
The suppression in the LGN eventually disappeared at a long enough ISI. Figure 8 shows the averaged BOLD time course in response to the paired-flash stimulation at ISI = 20 s. The BOLD responses to the first and second flashing stimulus are very close to each other and also similar to the BOLD response to the single stimulus, indicating that there is no suppression of the LGN activity when ISI is long enough.
Figure 8.
Averaged BOLD time course in response to the paired-flash stimulation at ISI = 20 s.
One limitation in this study is that we did not measure the electrophysiological signal in LGN in response to the same paradigm due to technique challenges. Lack of such information renders us unable to explicitly exclude the possibility that a prolonged inhibition period for LGN (e.g. 4 s) could be due to different vasculature and hemodynamics in the LGN. Nevertheless, the electrophysiological signal measured at V1 suggests a tight coupling between the neuronal activity and its accompanied hemodynamic response.
Finally, the present study demonstrates that fMRI combined with a paired stimulus paradigm is sensitive to the dynamics of neuronal inhibitory and facilitatory interactions during brain activation. In addition, the fMRI approach overcomes the major hurdles of invasiveness and limited spatial information faced by conventional electrophysiological recording approaches; it should provide a robust and completely noninvasive neuroimaging tool to study large-scale neural networks across the entire brain.
Acknowledgements
This work was supported in part by NIH grants: NS41262, EB00329, EB00513, P41 RR08079 and P30NS057091; the Keck Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arthurs OJ, Williams EJ, Carpenter TA, Pickard JD, Boniface SJ. Linear coupling between functional magnetic resonance imaging and evoked potential amplitude in human somatosensory cortex. Neuroscience. 2000;101:803–806. doi: 10.1016/s0306-4522(00)00511-x. [DOI] [PubMed] [Google Scholar]
- Bachmann T. Time course of the subjective contrast enhancement for a second stimulus in successively paired above-threshold transient forms: perceptual retouch instead of forward masking. Vision Res. 1988;28:1255–1261. doi: 10.1016/0042-6989(88)90041-7. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bartlett JR, Doty RW, Lee BB, Sr, Sakakura H. Influence of saccadic eye movements on geniculostriate excitability in normal monkeys. Exp Brain Res. 1976;25:487–509. doi: 10.1007/BF00239783. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG. Unmasking visual masking: a look at the "why" behind the veil of the "how". Psychol Rev. 1980;87:52–69. [PubMed] [Google Scholar]
- Brinker G, Bock C, Busch E, Krep H, Hossmann KA, Hoehn-Berlage M. Simultaneous recording of evoked potentials and T2*-weighted MR images during somatosensory stimulation of rat. Magn Reson Med. 1999;41:469–473. doi: 10.1002/(sici)1522-2594(199903)41:3<469::aid-mrm7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Cox CL, Sherman SM. Control of dendritic outputs of inhibitory interneurons in the lateral geniculate nucleus. Neuron. 2000;27:597–610. doi: 10.1016/s0896-6273(00)00069-6. [DOI] [PubMed] [Google Scholar]
- Dale AM, Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Curr Opin Neurobiol. 2001;11:202–208. doi: 10.1016/s0959-4388(00)00197-5. [DOI] [PubMed] [Google Scholar]
- de Labra C, Rivadulla C, Grieve K, Marino J, Espinosa N, Cudeiro J. Changes in visual responses in the feline dLGN: selective thalamic suppression induced by transcranial magnetic stimulation of V1. Cereb Cortex. 2007;17:1376–1385. doi: 10.1093/cercor/bhl048. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Haase A. Snapshot FLASH MRI. Applications to T1, T2, and chemical-shift imaging. Magn Reson Med. 1990;13:77–89. doi: 10.1002/mrm.1910130109. [DOI] [PubMed] [Google Scholar]
- He B, Lian J. High-resolution spatio-temporal functional neuroimaging of brain activity. Crit Rev Biomed Eng. 2002;30:283–306. doi: 10.1615/critrevbiomedeng.v30.i456.30. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972;146:421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Wurtz RH, Richmond BJ. Vision during saccadic eye movements. I. Visual interactions in striate cortex. J Neurophysiol. 1980;43:1133–1155. doi: 10.1152/jn.1980.43.4.1133. [DOI] [PubMed] [Google Scholar]
- Louie TJ, Tally FP, Bartlett JG, Gorbach SL. Rapid microbiological assay for chloramphenicol and tetracyclines. Antimicrob Agents Chemother. 1976;9:874–878. doi: 10.1128/aac.9.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. Functional morphology of the feedback pathway from area 17 of the cat visual cortex to the lateral geniculate nucleus. J Neurosci. 1996;16:1180–1192. doi: 10.1523/JNEUROSCI.16-03-01180.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB. Temporal interactions in the cat visual system. I. Orientation-selective suppression in the visual cortex. J Neurosci. 1991a;11:344–356. doi: 10.1523/JNEUROSCI.11-02-00344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB. Temporal interactions in the cat visual system. II. Suppressive and facilitatory effects in the lateral geniculate nucleus. J Neurosci. 1991b;11:357–368. doi: 10.1523/JNEUROSCI.11-02-00357.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB. Temporal interactions in the cat visual system. III. Pharmacological studies of cortical suppression suggest a presynaptic mechanism. J Neurosci. 1991c;11:369–380. doi: 10.1523/JNEUROSCI.11-02-00369.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H. Depression in the excitability of relay cells of lateral geniculate nucleus following saccadic eye movements in the cat. J Physiol. 1975;249:87–102. doi: 10.1113/jphysiol.1975.sp011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee T-M, Stepnoski R, Chen W, Zhu XH, Ugurbil K. An approach to probe neural systems interaction by functional MRI at neural time scale down to milliseconds. Proc Natl Acad Sci U S A. 2000;97:11026–11031. doi: 10.1073/pnas.97.20.11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–126. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Singer W, Creutzfeldt OD. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10:311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Strupp JP. Stimulate: A GUI based fMRI analysis software package. Neuroimage. 1996;3:S607. [Google Scholar]
- Wiesel TN, Hubel DH, Lam DM. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974;79:273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PH. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995;3:287–301. [Google Scholar]
- Zhang N, Chen W. A dynamic fMRI study of illusory double-flash effect on human visual cortex. Exp Brain Res. 2006;172:57–66. doi: 10.1007/s00221-005-0304-7. [DOI] [PubMed] [Google Scholar]
- Zhang N, Liu Z, He B, Chen W. Noninvasive study of neurovascular coupling during graded neuronal suppression. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600531. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhu XH, Chen W. Influence of gradient acoustic noise on fMRI response in the human visual cortex. Magn Reson Med. 2005;54:258–263. doi: 10.1002/mrm.20512. [DOI] [PubMed] [Google Scholar]