Abstract
A bidirectional association between mood disorders and cardiovascular diseases has been described in humans, yet the precise neurobiological mechanisms that underlie this association are not fully understood. This article is focused on neurobiological processes and mediators in mood and cardiovascular disorders, with an emphasis on common mechanisms including stressor reactivity, neuroendocrine and neurohumoral changes, immune alterations, autonomic and cardiovascular dysregulation, and central neurotransmitter and neuropeptide dysfunction. A discussion of the utility of experimental investigations with rodent models, including those in rats and prairie voles (Microtus ochrogaster), is presented. Specific studies using these models are reviewed, focusing on the analysis of behavioral, physiological and neural mechanisms underlying depressive disorders and cardiovascular disease. Considered in combination with studies using human samples, the investigation of mechanisms underlying depressive behaviors and cardiovascular regulation using animal models will enhance our understanding of the association of depression and cardiovascular disease, and will promote the development of improved interventions for individuals with these detrimental disorders.
Keywords: Autonomic nervous system, Behavior, Cardiovascular, Chronic mild stress, Cytokines, Depression, Endocrine system, Heart disease, Hypothalamic, pituitary, adrenal, Immune system, Neurohumoral, Oxytocin, Prairie voles, Rodent models, Social behavior, Social isolation
1. The Importance of Altered Mood and Cardiovascular Dysfunction
Evidence obtained from epidemiological and clinical investigations in humans and a limited number of experimental investigations in non-human animals suggest that there is a bidirectional association between mood and cardiovascular dysfunction. Cardiovascular pathophysiology, such as coronary artery disease, myocardial infarction and congestive heart failure (CHF), is significantly related to altered mood states, and conversely depressive syndromes are considered to be risk factors for cardiac morbidity and mortality (Penninx et al., 2001; Carney and Freedland, 2003; Freedland et al., 2003; Van der Kooy et al., 2007; Glassman, 2007). The association between altered mood and cardiovascular disease is independent of traditional cardiovascular risk factors (e.g., hypertension, high cholesterol, increased body mass index, history of cardiac-related problems, disease severity), and has been demonstrated in individuals both with and without a history of cardiac pathophysiology (Penninx et al., 2001; Carney and Freedland, 2003; Frasure-Smith and Lespérance, 2003; Wulsin and Singal, 2003).
The influence of affective states on cardiovascular dysfunction is well documented. Previous studies suggest that the presence of depression doubles the risk that patients with newly diagnosed cardiovascular disease will experience an adverse cardiovascular event within 12 months (Carney et al., 1988), and individuals with depression are at a greater risk of cardiac-related mortality for up to 10 years following the diagnosis of established cardiovascular disease, relative to non-depressed control subjects (Barefoot et al., 1996). Similarly, major depression is a significant predictor of mortality in patients at both 6 and 18 months following myocardial infarction, independent of other factors such as arrhythmias and history of previous myocardial infarction (Frasure-Smith et al., 1993; Frasure-Smith et al., 1995). Depression also predicts incidence of cardiovascular disease and cardiac-related mortality in initially healthy individuals (i.e., those without a history of cardiovascular pathophysiology). For instance, both major and minor depression are related to an increased risk of cardiac-related mortality in patients without cardiac diseases at baseline (however the excess mortality risk is higher for major versus minor depression) (Penninx et al., 2001). Additional prospective studies, reviewed elsewhere (Wulsin and Singal, 2003), have reported similar effects.
Psychological status influences cardiovascular function, and because the cardiovascular system feeds back to the brain, cardiovascular function in turn can directly or indirectly produce altered mood states. For instance, compared to a prevalence of approximately 2–3% (men) and 5–9% (women) of depression in the general population (American Psychiatric Association, 2000), its prevalence in patients following myocardial infarction may be approximately 45% (Schleifer et al., 1989), and might be even higher in patients with chronic cardiovascular conditions such as CHF (Freedland et al., 2003).
The bidirectional link between mood disorders and cardiovascular dysfunction represents an important worldwide public health concern. Statistics from The Global Burden of Disease (Mathers and Loncar, 2005) describe cardiovascular disease and psychological depression as two of the most detrimental conditions in developed countries, and they are predicted to remain so for several years. Furthermore, it has been estimated that approximately 75,000 deaths each year in the United States among patients discharged following myocardial infarction may be attributable to comorbid depression (Carney et al., 1999). Considered together, these data suggest that understanding the mechanisms responsible for the association of mood and cardiovascular function will have a positive impact on public health and welfare.
Although a large number of studies have indicated the importance of the association between mood disorders and heart disease, the precise pathophysiological mechanisms underlying this association remain unclear. In combination with research involving human populations, experimental approaches that focus on reliable and valid animal disease models will provide translational results and offer insight into causal and common mechanisms underlying the link between mood and cardiovascular regulation. Given these considerations, the purpose of the present article is twofold. First, it will provide a brief summary of common mechanisms in depression and altered cardiovascular regulation. Second, it will discuss the value of investigating potential neural, physiological and behavioral processes involved in this association using model systems in rodents.
2. Potential Common Mechanisms Underlying Mood and Cardiovascular Function
Common mechanisms involved in the link between depression and cardiovascular disease may include reactivity to exogenous stressors, alterations of neurohumoral, immune and autonomic regulation, and dysfunction of neurotransmitter systems. While not an exhaustive list of potential mechanisms, these neurobiological changes will be summarized with a specific focus on clinical and experimental research and the role of integrative processes.
2.1 Behavioral and Physiological Reactivity to Exogenous Stressors
Exposure to environmental stressors has been shown to be responsible for influencing the development of both mood disorders and cardiovascular diseases. The presence of chronic, unpredictable or uncontrollable stressors does not favor behavioral or physiological adaptation, and therefore may play an important role in the development of depressive signs and symptoms (Anisman and Matheson, 2005) and cardiovascular dysregulation (Sgoifo et al., 2001b). The predisposing influence of environmental stressors on depression has been reviewed in detail elsewhere; several lines of evidence indicate that uncontrollable and unpredictable stressors are associated with depressive syndromes in humans and depression-like behaviors in animal models (Swaab et al., 2005; Anisman and Matheson, 2005; Monroe and Harkness, 2005). Exposure to these types of stressors also contributes to cardiovascular diseases and their antecedent risk factors, such as hypertension, changes in vascular resistance, endothelial dysfunction, altered baroreceptor reflex function and ventricular arrhythmias (Johnson and Anderson, 1990; Sanders and Lawler, 1992; Bairey Merz et al., 2002; Schwartz et al., 2003). Exposure to environmental stressors produces alterations in central processes, including changes in norepinephrine, dopamine, serotonin (5-HT) and corticotropin-releasing factor (CRF), as well as activation of neuroendocrine, immune, and autonomic nervous systems (Herman et al., 1982; Joseph and Kennett, 1983; Adell et al., 1988; Vaidya, 2000; Anisman and Matheson, 2005), producing alterations in mood and cardiac dysfunction (see additional discussion of these systems in Sections 2.2 and 2.3).
2.2 The Integration of Neurohumoral, Immune and Autonomic Function
It is possible that reactivity to exogenous stressors leads to altered mood and cardiovascular regulation via neuroendocrine or neuroimmune systems, or through a disruption of autonomic function. For example, the hypothalamic-pituitary-adrenal (HPA) axis is dysregulated in depressed individuals, including: (1) alterations of CRF, (2) increases in circulating adrenocorticotropic hormone (ACTH), cortisol or corticosterone, and (3) impaired feedback regulation of the axis (Carroll et al., 1976; Raadsheer et al., 1995; Maes et al., 1998; Weber et al., 2000); these changes are not dissimilar to those observed following exposure to chronic stressors. Similar changes – such as (1) alterations in corticosterone and ACTH, (2) impaired feedback control of HPA axis functioning, (3) impaired glucocorticoid receptor binding in the hippocampus, cortex and dorsal raphe nucleus, and (4) altered CRF input to the dorsal raphe nucleus – have been observed in several animal models of depression (Froger et al., 2004; Maier and Watkins, 2005; Grippo et al., 2005a; Grippo et al., 2005b). These changes provide evidence for neuroendocrine dysfunction in depression, which may influence cardiovascular regulation via several potential processes, such as activation of the immune system, release of humoral factors, or activation of the sympathetic nervous system.
The endocrine system interacts with the immune system both within the brain and in the periphery. Depression is associated with activation of immune system factors and disruption of immune processes; this evidence is reviewed in detail elsewhere (Maes, 1995; Zorrilla et al., 2001; Pollak and Yirmiya, 2002; Dantzer, 2006). However, it is not clear whether this activation is a cause or a consequence of altered mood states. For instance, activation of the HPA axis in depression or under conditions of stress can facilitate activation of the immune system via a network involving catecholamine and glucocorticoid actions (see Hawkley et al., 2007). Alternatively, Smith (1991) initially proposed a macrophage theory of depression, suggesting that excessive secretion of monokines such as interleukin (IL)-1, tumor necrosis factor (TNF)-α and interferon, contribute to the pathophysiology of depression. While the precise causal mechanisms involving depression and immune system changes are not entirely elucidated, activation of the immune system also is associated with specific cardiovascular disorders. For instance, pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 are released into the systemic circulation in CHF (Das, 2000). These have adverse effects on the heart and circulation (Kapadia et al., 1998), and therefore may feed back to the central nervous system to induce directly signs and symptoms of depression.
In addition to the immune system, the renin-angiotensin-aldosterone system (RAAS) is activated in some forms of heart disease, such as CHF, resulting in high circulating levels of angiotensin II and aldosterone (Felder et al., 2001). Several of these factors interact in the central and peripheral nervous systems, which may influence mood and cardiovascular function. For instance, angiotensin and the pro-inflammatory cytokines both activate the HPA axis to increase circulating glucocorticoids and catecholamines (Pollak and Yirmiya, 2002). These systems interact in the brain, where aldosterone stimulates circulating TNF-α levels, and in the periphery, where cytokines prevent the feedback inhibition of renin release by circulating angiotensin II (Felder et al., 2001). Such interactions can create a cycle whereby immune and other humoral changes during states of cardiovascular pathophysiology influence central nervous system processes, affecting mood states as well as endocrine and autonomic outflow.
Neurohumoral activation, manifest in both generalized and specific HPA axis dysfunction, RAAS activation, and altered immune function, interacts with autonomic regulation of the heart (Thayer and Sternberg, 2006). Depression may be characterized by changes in autonomic function, including activation of the sympathetic nervous system, withdrawal of vagal tone to the heart, elevations in heart rate, reductions in heart rate variability and altered baroreceptor reflex function (Carney et al., 1995; Krittayaphong et al., 1997; Watkins and Grossman, 1999; Pitzalis et al., 2001; Barton et al., 2007). Similar autonomic changes are associated with cardiovascular risk factors such as hypertension, increased body mass index and increased blood glucose, and have been observed in both acute and chronic cardiovascular conditions including atherosclerosis, myocardial ischemia, arrhythmias and CHF (Kannel et al., 1987; Carney et al., 1993; Kristal-Boneh et al., 1995; La Rovere et al., 1998; Esler and Kaye, 2000). Depression may influence directly the development and/or progression of cardiovascular disease via effects on autonomic imbalance, cardiac rate or rhythm disturbances, or ventricular instability; via afferent feedback, these changes can influence neurohumoral and neuroimmune activation. However, there are some discrepancies in the literature regarding the precise autonomic and cardiac changes associated with depressive disorders (Watkins et al., 2002; Barton et al., 2007). Additional experimental investigations focused on whole-organism analyses may provide insight into the integration of neurohumoral, immune and autonomic regulation in mood disorders and cardiovascular disease.
2.3 The Role of Central Neurotransmitter and Neuropeptide Systems
Several central nervous system processes are altered in both mood disorders and cardiovascular disease, and can be affected by behavioral, physiological or other neural inputs. Disrupted monoamine function has been implicated in the pathophysiology of depression (Lambert et al., 2000). Evidence from pharmacological studies indicates that monoamine oxidase inhibitors and tricyclic antidepressants have been used previously as effective antidepressants in some patients (see Garlow and Nemeroff, 2004). However, in the context of heart disease, these antidepressants have cardiotoxic effects, including pro-arrhythmic properties (Glassman, 1998). As a result, research has focused more specifically on the role of the serotonergic system in depression. As reviewed elsewhere (Maes and Meltzer, 1995; Lucki, 1998; Berman et al., 1999; Cryan et al., 2005), it has been reported that: (1) 5-HT plays a significant role in behaviors that are disrupted in depression (e.g., mood, sleep, appetite), (2) a decrease in brain 5-HT concentration can precipitate depression in recovering patients, (3) depression is associated with several changes in central 5-HT and 5-HT receptors (e.g., decreased tryptophan concentrations, impaired 5-HT synthesis or release, changes in 5-hydroxyindoleacetic acid, malfunctions at postsynaptic 5-HT receptors, alterations in 5-HT transporter density), and (4) pharmacological agents that alter 5-HT are effective antidepressants. Further, changes in the function of 5-HT type 1A (5-HT1A) receptors may play role in the link between depression and cardiovascular disorders (Nalivaiko, 2006).
Central monoamine systems interact with endocrine and autonomic function to influence cardiovascular regulation. The hypothalamic paraventricular nucleus receives serotonergic innervation and also projects to the intermediolateral cell column of the spinal cord, rostral ventrolateral medulla and dorsal vagal complex, which can influence both sympathetic and parasympathetic outflow in depression or during periods of chronic stress (Swanson and Sawchenko, 1980; Badoer, 2001). Also, 5-HT actions in the hypothalamus may regulate hormonal responses to stressors (Van de Kar and Blair, 1999). In related research, altered 5-HT levels have been found in the central nervous system of rodents with myocardial ischemia, and a specific 5-HT transporter gene polymorphism has been associated with a higher risk of myocardial infarction in males who survived an initial heart attack (Sole et al., 1983; Fumeron et al., 2002).
Pharmacological evidence suggests that treatment with the 5-HT reuptake inhibitor fluoxetine increased 24-hour heart rate variability in depressed individuals (Khaykin et al., 1998). Similarly, the 5-HT2A receptor antagonist nefazadone reduced blood pressure and altered sympathetic tone in patients with major depression (Agelink et al., 2001). However, the treatment of depressed patients with 5-HT-altering drugs does not always result in improved autonomic or cardiovascular function; some studies have found a lack of significant cardiovascular changes in depressed patients treated with 5-HT reuptake inhibitors (Nemeroff et al., 1998; Roose et al., 1998), while others have reported adverse cardiac effects (Dawood et al., 2007). It is possible that central 5-HT may mediate sympathetic outflow to affect heart rate variability through its ultimate effects on discharge of peripheral noradrenergic neurons or by its interactions with other central neurotransmitters such as dopamine.
A neuropeptide that has been receiving increased attention in the context of behavior and autonomic function is oxytocin (OT). In combination with arginine vasopressin (AVP), OT lies at the center of a neuroendocrine network to coordinate social behaviors and responses to various stressors (Carter, 1998; Neumann, 2002). OT may decrease the withdrawal characteristics of fear and anxiety, and increase tolerance for stressful stimuli, protecting the vulnerable mammalian nervous system from regressing into the primitive states of lower brainstem dominance (e.g., freezing, down-regulation of cortical processes) (Porges, 2007).
OT is particularly relevant to the consequences and causes of sociality, and to psychological disorders such as depression (Carter, 1998; Carter and Altemus, 2005; Heinrichs and Domes, 2008). Both OT and AVP have been shown to be altered in depressed patients; while AVP has been consistently increased, it is not clear whether OT’s importance is due to an increase or a decrease of this peptide (Purba et al., 1996; van Londen et al., 2001; Carter and Altemus, 2005). Also, of particular relevance are findings from non-human animals showing that social withdrawal and behavioral immobilization in the face of environmental challenges, which are features associated with depressive disorders, are modulated by OT and AVP (Carter, 1998; Porges, 2001). Similarly, 5-HT reuptake inhibitors can cause an increase in OT and a reduction in AVP secretion (see Carter and Altemus, 2005).
While the precise relationship between OT and depression requires further investigation, OT regulates several processes that influence both mood disorders and cardiovascular regulation. OT can down-regulate the HPA axis and corresponding affective reactions to stressors in humans (Legros et al., 1987; Heinrichs et al., 2003). In rats, intracerebroventricular OT administration attenuated stressor-induced corticosterone increases and anxiety-like responses (Windle et al., 1997). Neumann et al. (2002) have reviewed evidence for the role of OT in mediating stressor reactivity, suggesting that its involvement is both brain region- and stressor-specific. OT also has several peripheral actions which can influence cardiovascular outcomes. For instance, OT has beneficial effects on blood pressure, and may play a role in regulating autonomic tone (Holst et al., 2002; Michelini et al., 2003). This peptide also has negative inotropic and chronotropic effects in isolated heart preparations, via its actions on cardiac OT receptors (Mukaddam-Daher et al., 2001). Additional studies will elucidate the precise interactions among neuropeptides, alterations in mood and cardiovascular function.
3. The Value of Experimental Investigations Using Animal Models
Difficulties exist in forming comprehensive theories to describe the mechanisms of interaction between mood disorders and cardiovascular disease, in part due to the lack of useful “biological markers” of mood disorders (see Mössner et al., 2007) and the limited experimental research focusing on neural, physiological and genetic processes underlying these conditions. Integrative research using valid and reliable preclinical models of disease, with a focus on whole-organism studies as well as cross-species comparisons, can permit a mechanistic analysis of the factors that influence depression and cardiovascular risk. Research with animal model systems that includes a specific focus on core behavioral and neurobiological features of the conditions in question which can be operationally defined, quantified, observed and systematically investigated will offer the greatest translational potential. For instance, the key feature of anhedonia, which is central to depression (American Psychiatric Association, 2000), is influenced by psychological, neurochemical and physiological factors. This behavioral sign can be effectively mimicked in animal models, and can be combined with the systematic and quantitative evaluation of cardiovascular dysfunction such as autonomic imbalance, vascular changes, arrhythmias or myocardial ischemia.
Several animal models of depression have been developed and reasonably well validated. Non-human primate models involving cynomolgus monkeys (Macaca fascicularis) have focused on vascular responsiveness, neurotransmitter functions and social behaviors, among others, in the context of depression and/or cardiovascular disease (Hamm Jr. et al., 1983; Shively and Bethea, 2004). In rodents, such as rats or mice, validated models such as learned helplessness (Seligman, 1974; Maier and Watkins, 2005), chronic mild stress (CMS) (Katz, 1982; Willner, 2005) and behavioral despair (Porsolt et al., 1977; Cryan et al., 2005) have focused on behavioral and physiological consequences of depression, mechanisms of antidepressant treatments and potential etiological factors in mood disorders. Newer models in rodents have focused on exploring the role of the social environment in mediating affective signs and autonomic function (Sgoifo et al., 2001b; Bartolomucci et al., 2003; Grippo et al., 2007b). To highlight the utility of model systems involving rodents, the following sections will focus on two models that our laboratories have utilized as tools for investigating mechanisms involved in the link between depressive disorders and cardiovascular dysfunction.
3.1 Example 1: Chronic Mild Stress in Rats
The CMS model of depression involves exposing rodents to a chronic period of unpredictable mild stressors such as strobe light, white noise, damp bedding and an empty water bottle; initial studies with this model in rats focused on behaviors related to depression and mechanisms underlying antidepressant treatments (Katz, 1982). The validity, reliability and utility of CMS have been described previously (Willner, 1997; Willner, 2005). Given the need for systematic and experimental investigation of the association between depression and cardiovascular disease, we have used the CMS model (Sprague-Dawley rats) to examine behavioral, physiological, and central nervous system processes that may underlie these conditions.
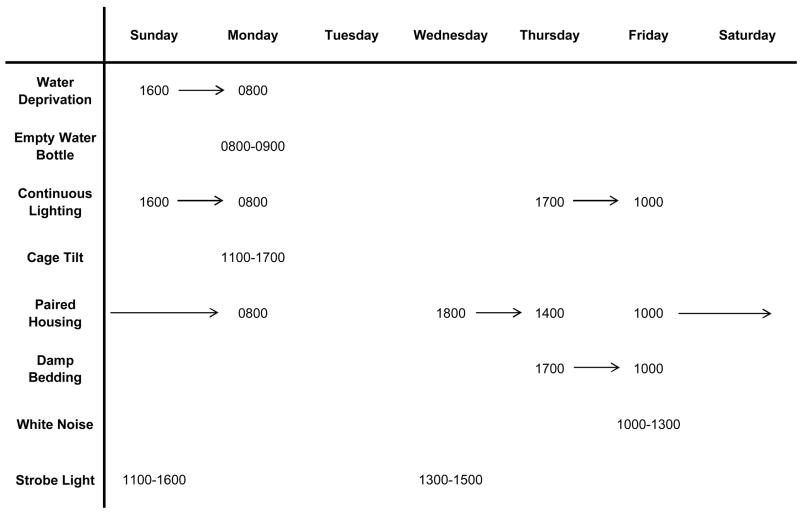
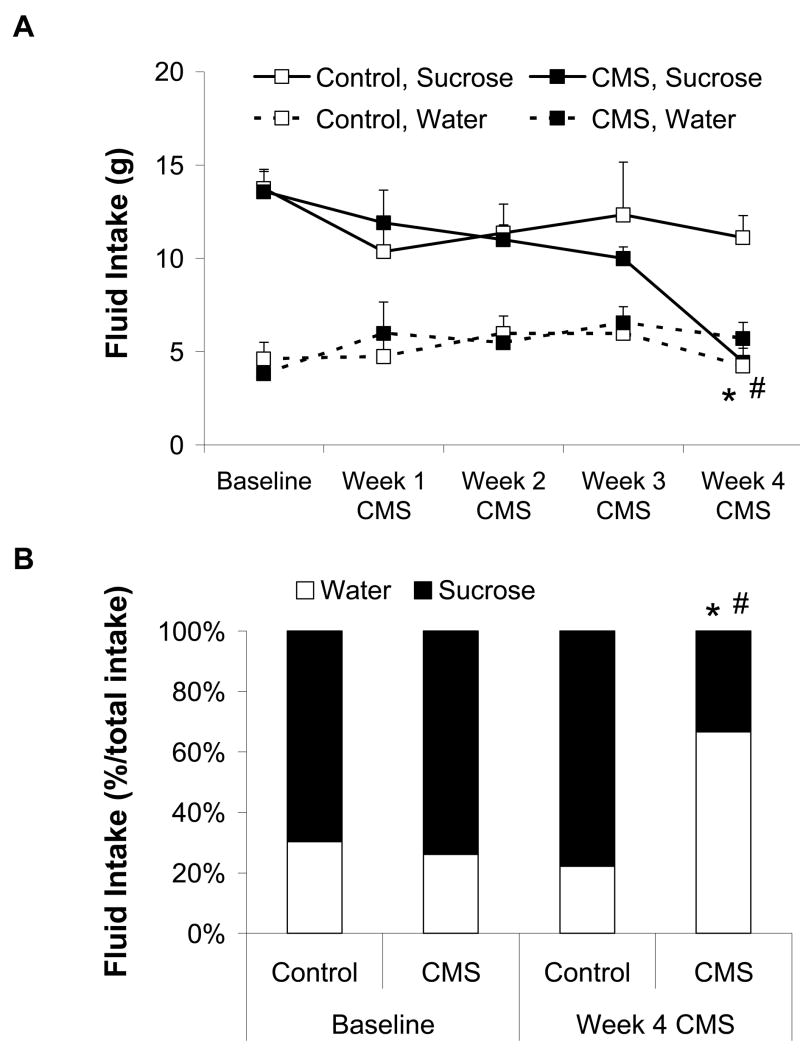
Recent studies with the CMS model have provided insight into potential behavioral and physiological processes that are dysregulated in depression and cardiovascular disease. In a series of studies, adult, male rats were exposed to 4 weeks of CMS (versus undisturbed control conditions; see Figure 1 for a typical CMS paradigm), which produced behavioral changes consistent with depressive syndromes (Grippo et al., 2002; Grippo et al., 2003). These changes include reduced physical activity (reduced spontaneous activity in a running wheel) and anhedonia (reduced responsiveness to a previously-defined rewarding stimulus), shown by a reduction in the consumption of a palatable sucrose solution (Figure 2). CMS also produced several cardiac alterations, including elevated resting heart rate and reduced heart rate variability (time-domain analysis). Furthermore, rats that were previously exposed to CMS (versus control) displayed exaggerated pressor and heart rate reactivity when perturbed with a novel acute stressor (air jet stress). While the behavioral changes associated with CMS recover within a few weeks following cessation of the stressors, these cardiovascular disruptions persist across time, suggesting that simple remediation of the depressive signs is not associated with alleviation of the underlying cardiovascular pathophysiology. Consistent with these findings are those from Carney et al. (2000), suggesting that while heart rate and heart rate variability may improve in treated depressed patients, they may never return to baseline levels.
Figure 1.
Example of a chronic mild stress paradigm. Reprinted from (Grippo et al., 2006); permission requested.
Figure 2.
Mean (+ SEM) absolute water and sucrose consumption (Panel A) and percent preference for sucrose (relative to total fluid intake; Panel B) during a 1-hour fluid intake test in chronic mild stress (CMS) and control groups at baseline and during 4 weeks of CMS. The groups did not differ in the amount of absolute fluid intake or sucrose preference during the baseline period. Following 4 weeks of CMS, the CMS group consumed significantly less sucrose, and showed a significantly reduced sucrose preference, versus the baseline period and the control group (*P < 0.05 vs. control group at the same time point; #P < 0.05 vs. respective baseline value). Modified from (Grippo et al., 2002); permission requested.
The elucidation of specific mechanisms underlying cardiovascular changes in the CMS model will be useful for developing novel and effective treatments for patients with depression and heart disease. To this end, we have investigated potential autonomic and cardiac mechanisms underlying the basal and stressor-reactive changes in the CMS model. Autonomic blockade with the β-adrenergic receptor antagonist, propranolol hydrochloride (2 mg/kg, iv), indicated that sympathetic drive is elevated following exposure to CMS (Grippo et al., 2002; Grippo et al., 2003). Direct lumbar sympathetic nerve measurement suggested also that sympathetic activity is increased in the CMS model (Grippo et al., 2008a). Activation of the sympathetic nervous system mediates directly the elevation in heart rate and decrease in heart rate variability observed in this animal model of depression, and is consistent with autonomic changes in humans with depression and/or heart disease (e.g., Section 2.2).
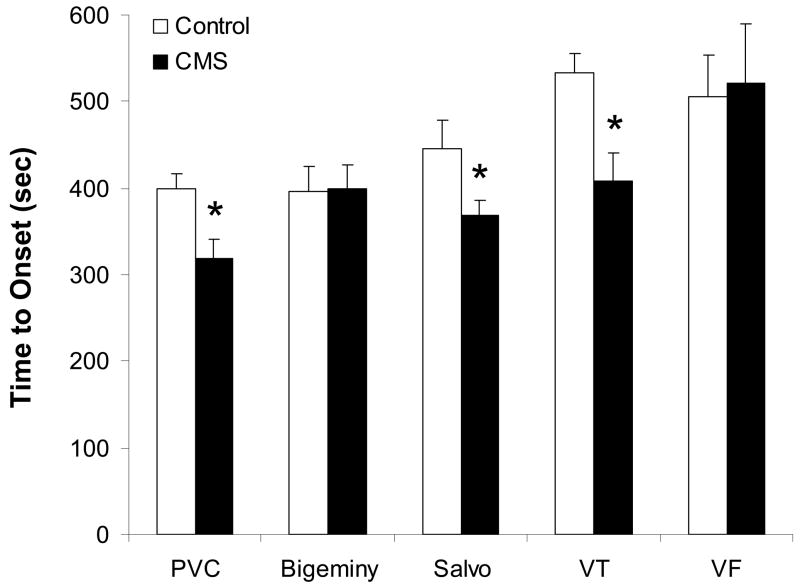
CMS also may be associated with an increased vulnerability to ventricular arrhythmias. Following 4 weeks of CMS in adult, male rats, we examined the types and time course of ventricular arrhythmia development following intravenous administration of the pro-arrhythmic agent, aconitine (Grippo et al., 2004). CMS (versus control) was associated with a decreased threshold for the development of premature ventricular contractions (PVCs), salvos and ventricular tachycardia (Figure 3). Further, an increased incidence of ventricular tachycardia was observed in the CMS group versus the control group, following aconitine administration. These findings suggest that signs of depression may be associated with ventricular electrical instability, which can in turn influence cardiovascular function and disease outcomes. These results are especially significant when considered in the context of findings from human populations. Carney and colleagues (1993) showed that patients with coronary artery disease and depression had a higher prevalence of ventricular tachycardia versus patients without corresponding depression. Similarly, post-myocardial infarct patients are at a greater risk of mortality if they have a combination of PVCs (greater than 10 per hour) and a high score on the Beck Depression Inventory (BDI) relative to patients with fewer PVCs or those with a low BDI score (Frasure-Smith et al., 1995). Further study of ventricular electrical instability in animal models, such as measures of the amount and type of arrhythmias in response to acute and chronic stressors, will provide additional insight into electrical instability as a mechanism underlying signs of depression and cardiovascular disease.
Figure 3.
Mean (+ SEM) time to onset of premature ventricular contractions (PVC), bigeminy, salvos, ventricular tachycardia (VT) and ventricular fibrillation (VF) following intravenous aconitine administration (5 μg/kg/min) following 4 weeks of chronic mild stress (CMS) or undisturbed control conditions. CMS reduced the onset time to PVC, salvo and VT (*P < 0.05 vs. respective control value). Modified from (Grippo et al., 2004), with permission, copyright 2004 American Physiological Society.
In addition to investigations of specific autonomic and cardiac responses, a related study involving the CMS model has experimentally investigated neuroendocrine and immune function related to depression (Grippo et al., 2005a). 4 weeks of CMS exposure produced several neurohumoral and immune changes versus undisturbed control conditions, including: (1) increased circulating aldosterone and plasma renin activity, (2) increased circulating corticosterone, and (3) activation of the pro-inflammatory cytokines, TNF-α and IL-1β, in the plasma and in key central nervous system structures. These changes mirror those observed in human depression, and also are common in individuals with cardiovascular disease (e.g., Section 2.2). The findings from this study suggest that interactions of cytokines and the RAAS with central processes, such as activation of the HPA axis, might influence downstream functions such as the release of corticosterone or catecholamines, regulation of circulating cytokines and alterations in renin and aldosterone secretion, providing a potential physiological link between affective states and cardiovascular dysfunction.
The CMS model has utility for investigating behavioral and physiological changes associated with depressive disorders and the interactions of behaviors with cardiovascular function. In addition, this model may be especially useful for examining central nervous system processes associated with depression, such as the serotonergic system. In an initial study, we investigated the effects of fluoxetine on behavioral and cardiovascular consequences of CMS in rats by administering this 5-HT reuptake inhibitor daily for 4 weeks (10 mg/kg, sc), concurrent with 4 weeks of CMS exposure (Grippo et al., 2006). Versus vehicle, fluoxetine administration in the CMS group prevented anhedonia, but only partially prevented the cardiovascular consequences of the stressors. Specifically, basal heart rate in the CMS group was significantly elevated (relative to control), but administration of fluoxetine only slightly attenuated this increase in CMS-exposed rats. A similar pattern of responses was observed for the elevation of sympathetic tone in the CMS group. These findings have implications for understanding the role of 5-HT in mediating depressive signs and cardiovascular dysfunction, and provide additional evidence for the hypothesis that reduction of depressive signs with traditional therapies does not automatically reduce the underlying cardiovascular pathophysiology associated with this condition. Data regarding fluoxetine administration in humans with depression and heart disease are inconsistent (see Nemeroff et al., 1998; Roose et al., 1998; Khaykin et al., 1998; Dawood et al., 2007); therefore, additional studies with rodent models may provide insight into the role of 5-HT reuptake inhibitor therapy and serotonergic mechanisms underlying mood and cardiovascular dysfunction.
3.2 Example 2: Disruption of Social Bonds in Prairie Voles
The prairie vole (Microtus Ochrogaster) is a highly social rodent species that is particularly dependent on social interactions for the regulation of behavior, endocrine and autonomic function. Socially monogamous species, such as prairie voles, share with humans a cluster of physiological and behavioral characteristics including the capacity to form social bonds and to develop extended families (Carter et al., 1995). Socially monogamous rodents are especially sensitive to their social context, and offer a powerful translational model for understanding the mechanisms through which both negative and positive social experiences influence behavior and cardiovascular function. Thus, the investigation of behavioral, physiological and neural consequences of disrupting established social bonds may be very informative in the context of mechanisms underlying mood and cardiovascular dysfunction.
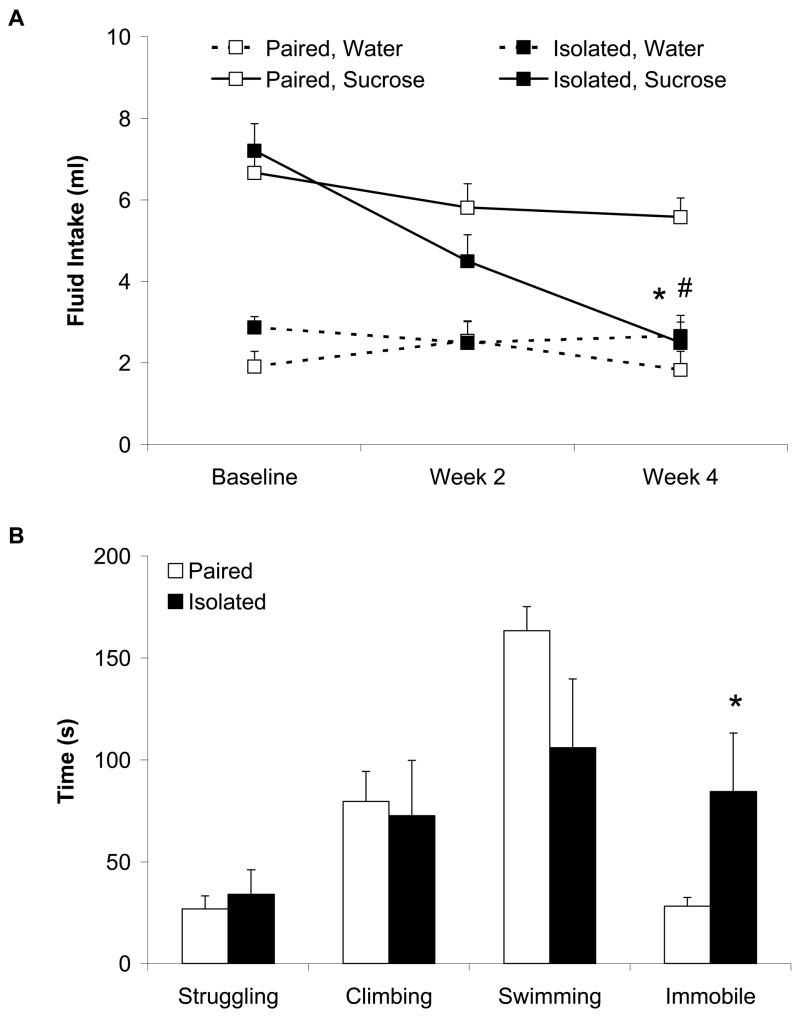
Our recent research has focused on the behavioral and neurobiological consequences of long-term social isolation in adult female and male prairie voles. Our findings have demonstrated that at least 4 weeks of isolation from a same-sex sibling induces behaviors relevant to depression using validated operational measures, including anhedonia (reduced intake of sucrose) and learned helplessness (increased immobility in the forced swim test) (Figure 4) (Grippo et al., 2007b; Grippo et al., 2008b). These behaviors mimic those described in human depression (American Psychiatric Association, 2000), and are consistent with behavioral changes observed in the CMS model of depression (e.g., Section 3.1) and other studies using the prairie vole model (Bosch et al., 2004).
Figure 4.
Mean (+ SEM) water and sucrose intake in female prairie voles during a 1-hour fluid intake test at baseline and following 2 and 4 weeks of isolation or pairing (Panel A), and amount of time spent struggling, climbing, swimming and immobile during a 5-minute forced swim test following 4 weeks of isolation or pairing (Panel B). Social isolation reduced sucrose intake and increased immobility time in the forced swim test (*P < 0.05 vs. respective paired value; #P < 0.05 vs. respective baseline value). Modified from (Grippo et al., 2008b); permission requested.
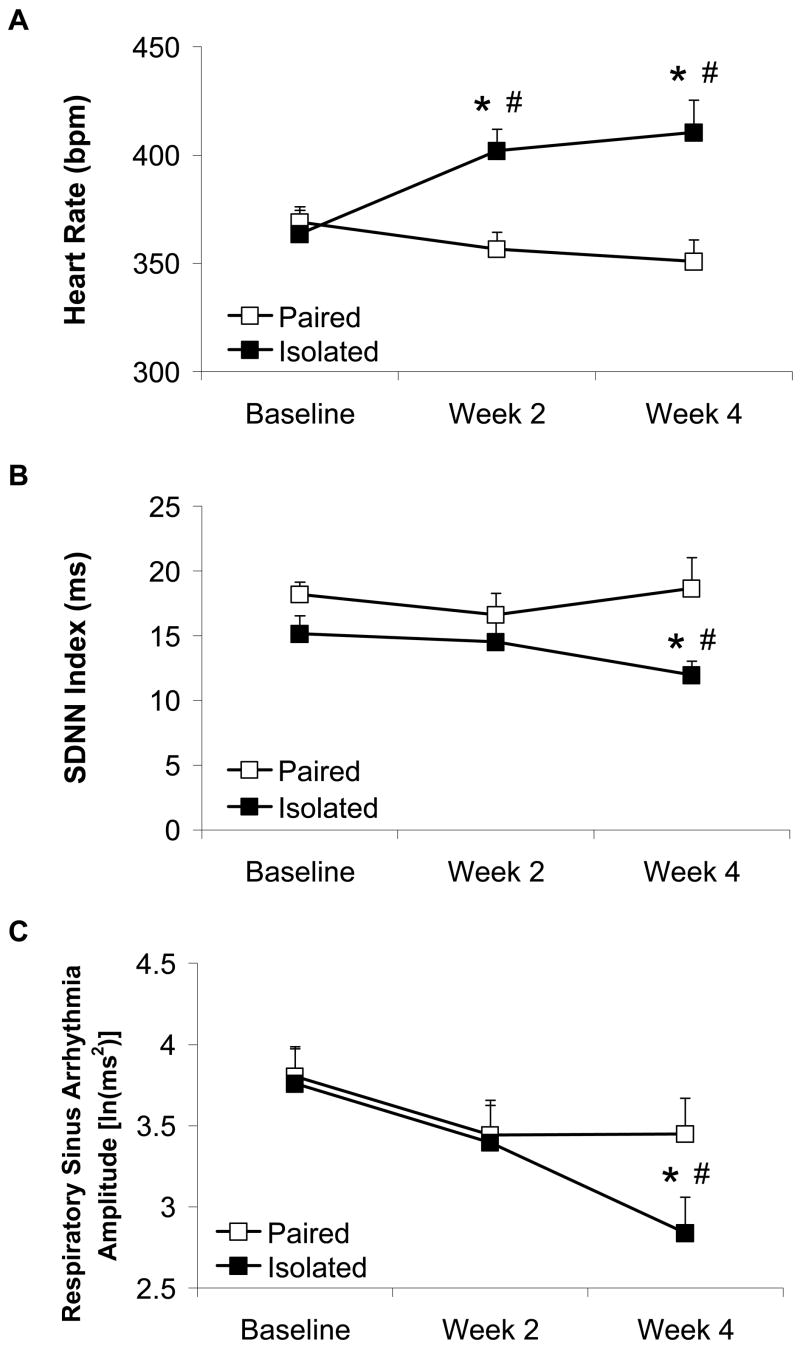
In addition to behavioral changes, we have begun to investigate autonomic and cardiac responses via radiotelemetry in freely behaving, untethered prairie voles as a function of social housing with a sibling versus isolation (Grippo et al., 2007b). 4 weeks of social isolation in adult, female prairie voles induced significant and progressive cardiac disturbances relative to paired conditions, including increased heart rate and reduced heart rate variability (Figure 5); these changes are similar to those described following 4 weeks of CMS. Social isolation (versus paired conditions) also led to exaggerated tachycardic responses to an acute environmental stressor (5-minute resident-intruder paradigm), as well as an increase in cardiac recovery rate following this stressor. These cardiac disturbances appear to be mediated by a significant withdrawal of vagal tone to the heart in isolated prairie voles, evidenced by a reduction in respiratory sinus arrhythmia amplitude (see Figure 5C) and an attenuation of cardiac responsiveness to cholinergic receptor blockade with atropine methyl nitrate (4 mg/kg, ip). Thus, social isolation has potent inhibitory effects on various behavioral and autonomic measures, providing a potential novel model for the investigation of the association between mood and cardiovascular dysfunction. Related findings from rats suggest that social stressors can influence physiological conditions such as cardiovascular disease, as well as affective disorders (Sgoifo et al., 2001a).
Figure 5.
Mean (+ SEM) heart rate (Panel A), standard deviation of normal-to-normal intervals (SDNN index; Panel B), and amplitude of respiratory sinus arrhythmia (Panel C) in prairie voles at baseline and following 2 and 4 weeks of social isolation or pairing. Social isolation increased heart rate, and reduced SDNN index and respiratory sinus arrhythmia amplitude (*P < 0.05 vs. respective paired value; #P < 0.05 vs. respective baseline value). Reprinted from (Grippo et al., 2007b); permission requested.
A related series of studies has focused on behavioral and neuroendocrine regulation in socially isolated prairie voles, and may provide insight into central and peripheral changes relevant to both mood disorders and cardiovascular dysfunction. For example, 4 weeks of isolation from a same-sex sibling (versus pairing) was associated with anhedonia (progressive reduction in sucrose intake) in both female and male prairie voles (Grippo et al., 2007a). Additionally, both female and male isolated animals showed increased circulating OT and increased activation of OT neurons in the hypothalamic paraventricular nucleus following an acute stressor (5-minute resident-intruder paradigm). However, isolation alone (without an additional acute stressor) produced an increase in central and circulating OT only in females. It is possible that females are more sensitive than males to the effects of social isolation; females and males may show a different time course of responsiveness to acute and chronic social stressors; or differences in the synthesis and/or effects of central neuropeptides exist between the sexes. Additional studies of social isolation, behavior, and physiological dysfunction are required to determine the precise nature of sex differences in social and neural mechanisms underlying depressive signs and physiological dysregulation. Indeed, as the prevalence of depression is greater in women than in men (American Psychiatric Association, 2000) – which may be a result of sex-based physiological differences, experience-based cultural, behavioral or social differences, or differences in reporting or clinical decision-making practices (among other potential reasons) – it is important to gain a better understanding of sex and gender differences in depression and the association of this condition with cardiovascular dysfunction.
While the precise mechanisms of the changes in OT in isolated female and male prairie voles have not been elucidated, it may be possible that the oxytocinergic system is activated in an attempt to compensate for altered neuroendocrine and autonomic regulation as a result of disrupted established social bonds. This possibility, along with previous findings suggesting that OT may serve to buffer behavioral and physiological responses to stressors (Legros et al., 1987; Windle et al., 1997; Neumann, 2002; Heinrichs et al., 2003; Heinrichs and Domes, 2008), has led us to investigate the effects of exogenously administered OT on the behavioral and cardiovascular consequences of social isolation using the prairie vole model. Our preliminary findings suggest that daily administration of OT (20 μg/vole) during the final 2 weeks of a 4-week isolation period may prevent some of the detrimental behavioral and cardiac effects of isolation in female prairie voles, including anhedonia, increased heart rate and reduced vagal tone (Grippo et al., 2007c). Related findings suggest that OT has antidepressant properties (Arletti and Bertolini, 1987), and may modulate behavioral responses to short-term separation in rats (Insel and Wintink, 1991), however additional analyses are necessary to understand the precise role of the oxytocinergic system in mediating the association between depression and cardiovascular dysregulation.
3.3 Summary of Findings and Limitations from Animal Model Investigations
Studies that focus on animal models can increase our understanding of the mechanisms of stress, autonomic and cardiac dysfunction, and social and neurobiological processes underlying mood and cardiovascular dysfunction. Investigations involving both rats and prairie voles indicate that reactions to the environmental context (e.g., responses to CMS or negative social stressors) can produce important behavioral and physiological changes that mimic the alterations observed in the human conditions. These experimental analyses indicate that sympathovagal imbalance – manifest in the form of altered heart rate and heart rate variability, increased cardiac reactivity both during and following acute stressors, susceptibility to ventricular electrical instability, an increase in sympathetic drive, and a withdrawal of vagal tone to the heart – is an important mechanism for understanding the association of depressive behaviors and cardiovascular dysfunction. Additionally, neuroendocrine and immune dysfunction, including activation of the HPA axis, central and peripheral peptides, and pro-inflammatory cytokines, play an important role in mediating behaviors, cardiovascular dysregulation, and responses to stressors. Finally, administration of pharmacological substances, such as 5-HT reuptake inhibitors or peptides, provide additional insight into potential central processes (e.g., 5-HT or OT) that may underlie the link between depression and cardiovascular disease.
While experimental investigation of animal models can provide useful insight into some of the neurobiological mechanisms underlying the association of depression and cardiovascular disease, inherent limitations exist in animal analog research. Because mental disorders – such as depression –are multifaceted syndromes, including not only physiological and behavioral disturbances but also social and cognitive changes and self-reported symptomatology, it is not possible (or scientifically relevant) to model each aspect of these conditions in non-human animals. To overcome this barrier, it is important for animal model studies to include a focus on species-relevant manipulations (for instance, studies of social isolation in prairie voles because this species, like humans, is socially monogamous), employ validated and translational methodology (such as radiotelemetry for long-term recording of cardiovascular parameters in freely-moving, conscious animals), and use observable, quantifiable, reliable, and operationally-defined dependent measures (such as sucrose intake as an operational definition of anhedonia in rodents). When these practices are employed, the findings from animal model investigations can be interpreted in the context of the empirical data, and the conclusions considered in the larger context of basic science investigations as well as clinical, epidemiological, and experimental studies in human samples. This can lead to a greater understanding of stress, depression, behavior, and cardiovascular regulation.
4. Considerations for Future Research
Depression and cardiovascular disease are highly detrimental conditions; the comorbidity of these conditions is an important public health concern. To adequately address the mechanisms underlying these disorders, it will be beneficial to continue investigations of behavioral and neurobiological processes using valid, reliable and useful preclinical animal models in addition to research with community and clinical samples. Additional research will benefit from focusing on the relevance of the models in question and the validity of the methodological procedures to maximize the translational potential of the findings.
Integrative studies involving multi-system and cross-species analyses will enhance our understanding of the link between mood disorders and cardiovascular disease. This will require increased communication and collaboration among varying scientific fields, including clinical and experimental psychology, physiology and neuroscience. To this end, comparative studies conducted by interdisciplinary teams, such as those described by Willner et al. (1998) and Vaidya et al. (2004), support the utility of parallel analyses in humans and preclinical animal models. The continued focus on animal model systems, considered in combination with findings from human samples, will promote the development of more effective treatments and improve the quality of life for individuals with comorbid mood and cardiovascular disorders.
Acknowledgments
The author is grateful to C. Sue Carter, A. Kim Johnson, Stephen W. Porges, and Louis D. Van de Kar for invaluable support. Financial support provided by United States Public Health Service grants MH65839, MH73233, and MH77581.
References
- Adell A, Garcia-Marquez C, Armario A, Gelpi E. Chronic stress increases serotonin and noradrenaline in rat brain and sensitizes their responses to a further acute stress. J Neurochem. 1988;50:1678–1681. doi: 10.1111/j.1471-4159.1988.tb02462.x. [DOI] [PubMed] [Google Scholar]
- Agelink MW, Majewski T, Wurthmann C, Postert T, Linka T, Rotterdam S, Klieser E. Autonomic neurocardiac function in patients with major depression and effects of antihypertensive treatment with nefazodone. J Affect Disord. 2001;62:187–198. doi: 10.1016/s0165-0327(99)00202-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987;41:1725–1730. doi: 10.1016/0024-3205(87)90600-x. [DOI] [PubMed] [Google Scholar]
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- Bairey Merz CN, Dwyer J, Nordstrom CK, Walton KG, Salerno JW, Schneider RH. Psychosocial stress and cardiovascular disease: pathophysiological links. Behav Med. 2002;27:141–147. doi: 10.1080/08964280209596039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot JC, Helms MJ, Mark DB, Blumenthal JA, Califf RM, Haney TL, O’Connor CM, Siegler IC, Williams RB. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol. 1996;78:613–617. doi: 10.1016/s0002-9149(96)00380-3. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Costoli T, Savani E, Laviola G, Parmigiani S, Sgoifo A. Chronic psychosocial stress persistently alters autonomic function and physical activity in mice. Physiol Behav. 2003;80:57–67. doi: 10.1016/s0031-9384(03)00209-9. [DOI] [PubMed] [Google Scholar]
- Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25:2117–2124. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- Berman RM, Belanoff JK, Charney DS, Schatzberg AF. Principles of the pharmacotherapy of depression. In: Charney DS, Nestler EJ, Bunney BS, editors. Neurobiology of mental illness. Oxford University Press; New York: 1999. pp. 419–432. [Google Scholar]
- Bosch OJ, Nair HP, Neumann ID, Young LJ. 2004 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington DC: 2004. Pair-bonded prairie voles display depression-like behavior after separation. Program No. 762.12 (Online) [Google Scholar]
- Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Rich MW, Smith LJ, Jaffe AS. Ventricular tachycardia and psychiatric depression in patients with coronary artery disease. Am J Med. 1993;95:23–28. doi: 10.1016/0002-9343(93)90228-h. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med. 2000;62:639–647. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Veith RC, Jaffe AS. Can treating depression reduce mortality after an acute myocardial infarction? Psychosom Med. 1999;61:666–675. doi: 10.1097/00006842-199909000-00009. [DOI] [PubMed] [Google Scholar]
- Carney RM, Rich MW, Freedland KE, Saini J, teVelde A, Simeone C, Clark K. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med. 1988;50:627–633. doi: 10.1097/00006842-198811000-00009. [DOI] [PubMed] [Google Scholar]
- Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. 1995;76:562–564. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II. discrimination of depressed from nondepressed patients. Arch Gen Psychiatry. 1976;33:1051–1058. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Oxytocin, vasopressin and depression. In: den Boer JA, George MS, Ter Horst GJ, editors. Current and future developments in psychopharmacology. Benecke N.I.; Amsterdam: 2005. pp. 201–216. [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das UN. Free radicals, cytokines and nitric oxide in cardiac failure and myocardial infarction. Mol Cell Biochem. 2000;215:145–152. doi: 10.1023/a:1026579422132. [DOI] [PubMed] [Google Scholar]
- Dawood T, Lambert EA, Barton DA, Laude D, Elghozi JL, Esler MD, Haikerwal D, Kaye DM, Hotchkin EJ, Lambert GW. Specific serotonin reuptake inhibition in major depressive disorder adversely affects novel markers of cardiac risk. Hypertens Res. 2007;30:285–293. doi: 10.1291/hypres.30.285. [DOI] [PubMed] [Google Scholar]
- Esler M, Kaye D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J Cardiovasc Pharmacol. 2000;35(Suppl 4):S1–S7. doi: 10.1097/00005344-200000004-00001. [DOI] [PubMed] [Google Scholar]
- Felder RB, Francis J, Weiss RM, Zhang ZH, Wei SG, Johnson AK. Neurohumoral regulation in ischemia-induced heart failure. Role of the forebrain Ann NY Acad Sci. 2001;940:444–453. doi: 10.1111/j.1749-6632.2001.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry. 2003;60:627–636. doi: 10.1001/archpsyc.60.6.627. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- Freedland KE, Rich MW, Skala JA, Carney RM, Dávila-Román VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003;65:119–128. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C, Dutriez-Casteloot I, Enache M, Barden N, Cohen-Salmon C, Hamon M, Lanfumey L. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci. 2004;24:2787–2796. doi: 10.1523/JNEUROSCI.4132-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumeron F, Betoulle D, Nicaud V, Evans A, Kee F, Ruidavets JB, Arveiler D, Luc G, Cambien F. Serotonin transporter gene polymorphism and myocardial infarction: Etude Cas-Temoins de l’Infarctus du Myocarde (ECTIM) Circulation. 2002;105:2943–2945. doi: 10.1161/01.cir.0000022603.92986.99. [DOI] [PubMed] [Google Scholar]
- Garlow SJ, Nemeroff CB. The neurochemistry of mood disorders: clinical studies. In: Charney DS, Nestler EJ, editors. Neurobiology of mental illness. Oxford University Press; New York: 2004. pp. 440–460. [Google Scholar]
- Glassman AH. Depression and cardiovascular comorbidity. Dialogues Clin Neurosci. 2007;9:9–17. doi: 10.31887/DCNS.2007.9.1/ahglassman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH. Cardiovascular effects of antidepressant drugs: updated. Int Clin Psychopharmacol. 1998;13(Suppl 5):S25–S30. doi: 10.1097/00004850-199809005-00006. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–316. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005a;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007a;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry. 2007b;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Porges SW, Carter CS. 2007 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington DC: 2007c. Oxytocin prevents detrimental cardiac effects of social isolation in monogamous prairie voles. Program No. 84.9 (Online) [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Evaluation of baroreceptor reflex function in the chronic mild stress rodent model of depression. Psychosom Med. 2008a;70:435–443. doi: 10.1097/PSY.0b013e31816ff7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Santos CM, Johnson RF, Beltz TG, Martins JB, Felder RB, Johnson AK. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. Am J Physiol Heart Circ Physiol. 2004;286:H619–H626. doi: 10.1152/ajpheart.00450.2003. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology. 2005b;179:769–780. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008b doi: 10.1002/da.20375. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm TE, Jr, Kaplan JR, Clarkson TB, Bullock BC. Effects of gender and social behavior on the development of coronary artery atherosclerosis in cynomolgus macaques. Atherosclerosis. 1983;48:221–233. doi: 10.1016/0021-9150(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Bosch JA, Engeland CG, Marucha PT, Cacioppo JT. Loneliness, dysphoria, stress, and immunity: a role for cytokines. In: Plotnikoff NP, Faith RE, Murgo AJ, Good RA, editors. Cytokines: stress and immunity. CRC Press; Boca Raton, FL: 2007. pp. 67–85. [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behavior: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008 doi: 10.1016/S0079-6123(08)00428-7. in press. [DOI] [PubMed] [Google Scholar]
- Herman JP, Guillonneau D, Dantzer R, Scatton B, Semerdjian-Rouquier L, LeMoal M. Differential effects of inescapable footshocks and of stimuli previously paired with inescapable footshocks on dopamine turnover in cortical and limbic areas of the rat. Life Sci. 1982;30:2207–2214. doi: 10.1016/0024-3205(82)90295-8. [DOI] [PubMed] [Google Scholar]
- Holst S, Uvnäs-Moberg K, Petersson M. Postnatal oxytocin treatment and postnatal stroking of rats reduce blood pressure in adulthood. Autonomic Neuroscience: Basic and Clinical. 2002;99:85–90. doi: 10.1016/s1566-0702(02)00134-0. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wintink AJ. Central administration of oxytocin modulates the infant rat’s response to social isolation. Eur J Pharmacol. 1991;203:149–152. doi: 10.1016/0014-2999(91)90806-2. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Anderson EA. Stress and arousal. In: Cacioppo JT, Tassinary LG, editors. Principles of psychophysiology: physical, social, and inferential elements. Cambridge University Press; Cambridge: 1990. pp. 216–252. [Google Scholar]
- Joseph MH, Kennett GA. Stress-induced release of 5-HT in the hippocampus and its dependence on increased tryptophan availability: an in vivo electrochemical study. Brain Res. 1983;270:251–257. doi: 10.1016/0006-8993(83)90598-x. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- Kapadia S, Dibbs Z, Kurrelmeyer K, Karla D, Seta Y, Wang F, Bozkurt B, Oral H, Sivasubramanian N, Mann DL. The role of cytokines in the failing human heart. Cardiol Clin. 1998;16:645–656. doi: 10.1016/s0733-8651(05)70041-2. [DOI] [PubMed] [Google Scholar]
- Katz R. Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 1982;16:965–968. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- Khaykin Y, Dorian P, Baker B, Shapiro C, Sandor P, Mironov D, Irvine J, Newman D. Autonomic correlates of antidepressant treatment using heart-rate variability analysis. Can J Psychiatry. 1998;43:183–186. doi: 10.1177/070674379804300209. [DOI] [PubMed] [Google Scholar]
- Kristal-Boneh E, Raifel M, Froom P, Ribak J. Heart rate variability in health and disease. Scand J Work Environ Health. 1995;21:85–95. doi: 10.5271/sjweh.15. [DOI] [PubMed] [Google Scholar]
- Krittayaphong R, Cascio WE, Light KC, Sheffield D, Golden RN, Finkel JB, Glekas G, Koch GG, Sheps DS. Heart rate variability in patients with coronary artery disease: differences in patients with higher and lower depression scores. Psychosom Med. 1997;59:231–235. doi: 10.1097/00006842-199705000-00004. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- Lambert G, Johansson M, Ågren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry. 2000;57:787–793. doi: 10.1001/archpsyc.57.8.787. [DOI] [PubMed] [Google Scholar]
- Legros JJ, Chiodera P, Geenen V, von Frenckell R. Confirmation of the inhibitory influence of exogenous oxytocin in cortisol and ACTH in man: evidence of reproducibility. Acta Endocrinol. 1987;114:345–349. doi: 10.1530/acta.0.1140345. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L, Biondi M, Bosmans E, Kenis G, Scharpe S. Increased 24-hour uninary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatr Scand. 1998;98:328–335. doi: 10.1111/j.1600-0447.1998.tb10092.x. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY. The serotonin hypothesis of major depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven Press; New York: 1995. pp. 933–944. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Updated projections of global mortality and burden of disease, 2002–2030: data sources, methods and results. World Health Organization; Geneva: 2005. [Google Scholar]
- Michelini LC, Marcelo MC, Amico J, Morris M. Oxytocinergic regulation of cardiovascular function: studies in oxytocin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:H2269–H2276. doi: 10.1152/ajpheart.00774.2002. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Mössner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Müller N, Fallgatter AJ, Riederer P. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8:141–174. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- Mukaddam-Daher S, Yin YL, Roy J, Gutkowska J, Cardinal R. Negative inotropic and chronotropic effects of oxytocin. Hypertension. 2001;38:292–296. doi: 10.1161/01.hyp.38.2.292. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E. 5-HT1A receptors in stress-induced cardiac changes: a possible link between mental and cardiac disorders. Clin Exp Pharmacol Physiol. 2006;33:1259–1264. doi: 10.1111/j.1440-1681.2006.04521.x. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Musselman DL, Evans DL. Depression and cardiac disease. Depress Anxiety. 1998;8(Suppl 1):71–79. [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Penninx BWJH, Beekman ATF, Honig A, Deeg DJH, Schoevers RA, van Eijk JTM, van Tilburg W. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- Pitzalis MV, Iacoviello M, Todarello O, Fioretti A, Guida P, Massari F, Mastropasqua F, Russo GD, Rizzon P. Depression but not anxiety influences the autonomic control of heart rate after myocardial infarction. Am Heart J. 2001;141:765–771. doi: 10.1067/mhj.2001.114806. [DOI] [PubMed] [Google Scholar]
- Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5:389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJG, Tilders FJH, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Roose SP, Laghrissi-Thode F, Kennedy JS, Nelson JC, Bigger JT, Jr, Pollock BG, Gaffney A, Narayan M, Finkel MS, McCafferty J, Gergel I. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA. 1998;279:287–291. doi: 10.1001/jama.279.4.287. [DOI] [PubMed] [Google Scholar]
- Sanders BJ, Lawler JE. The borderline hypertensive rat (BHR) as a model for environmentally-induced hypertension: a review and update. Neurosci Biobehav Rev. 1992;16:207–217. doi: 10.1016/s0149-7634(05)80181-2. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Macari-Hinson MM, Coyle DA, Slater WR, Kahn M, Gorlin R, Zucker HD. The nature and course of depression following myocardial infarction. Arch Int Med. 1989;149:1785–1789. [PubMed] [Google Scholar]
- Schwartz AR, Gerin W, Davidson KW, Pickering TG, Brosschot JF, Thayer JF, Christenfeld N, Linden W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosom Med. 2003;65:22–35. doi: 10.1097/01.psy.0000046075.79922.61. [DOI] [PubMed] [Google Scholar]
- Seligman MEP. Depression and learned helplessness. In: Friedman RJ, Katz MM, editors. The psychology of depression: contemporary theory and research. V.H. Winston; Washington, D.C.: 1974. pp. 83–125. [Google Scholar]
- Sgoifo A, Koolhaas J, Alleva E, Musso E, Parmigiani S. Social stress: acute and long-term effects on physiology and behavior. Physiol Behav. 2001a;73:253–254. doi: 10.1016/s0031-9384(01)00544-3. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Pozzato C, Costoli T, Manghi M, Stilli D, Ferrari PF, Ceresini G, Musso E. Cardiac autonomic responses to intermittent social conflict in rats. Physiol Behav. 2001b;73:343–349. doi: 10.1016/s0031-9384(01)00455-3. [DOI] [PubMed] [Google Scholar]
- Shively CA, Bethea CL. Cognition, mood disorders, and sex hormones. Ilar J. 2004;45:189–199. doi: 10.1093/ilar.45.2.189. [DOI] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Sole MJ, Versteeg DH, de Kloet ER, Hussain N, Lixfeld W. The identification of specific serotonergic nuclei inhibited by cardiac vagal afferents during acute myocardial ischemia in the rat. Brain Res. 1983;265:55–61. doi: 10.1016/0006-8993(83)91333-1. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann NY Acad Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Grippo AJ, Johnson AK, Watson D. A comparative developmental study of impulsivity in rats and humans: the role of reward sensitivity. Ann NY Acad Sci. 2004;1021:395–398. doi: 10.1196/annals.1308.051. [DOI] [PubMed] [Google Scholar]
- Vaidya VA. Stress, depression, and hippocampal damage. J Biosci. 2000;25:123–124. [PubMed] [Google Scholar]
- Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- van Londen L, Goekoop JG, Kerkof GA, Zwinderman KH, Wiegant VM, De Wied D. Weak 24-h periodicity of body temperature and increased plasma vasopressin in melancholic depression. Eur Neuropsychopharmacol. 2001;11:7–14. doi: 10.1016/s0924-977x(00)00124-3. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Blumenthal JA, Carney RM. Association of anxiety with reduced baroreflex cardiac control in patients after acute myocardial infarction. Am Heart J. 2002;143:460–466. doi: 10.1067/mhj.2002.120404. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Grossman P. Association of depressive symptoms with reduced baroreflex cardiac control in coronary artery disease. Am Heart J. 1999;137:453–457. doi: 10.1016/s0002-8703(99)70491-6. [DOI] [PubMed] [Google Scholar]
- Weber B, Lewicka S, Deuschle M, Colla M, Vecsei P, Heuser I. Increased diurnal plasma concentrations of cortisone in depressed patients. J Clin Endocrinol Metab. 2000;85:1133–1136. doi: 10.1210/jcem.85.3.6469. [DOI] [PubMed] [Google Scholar]
- Willner P. The chronic mild stress procedure as an animal model of depression: valid, reasonably reliable, and useful. Psychopharmacology. 1997;134:371–377. [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, Morgan M. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology. 1998;136:272–283. doi: 10.1007/s002130050566. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]