Abstract
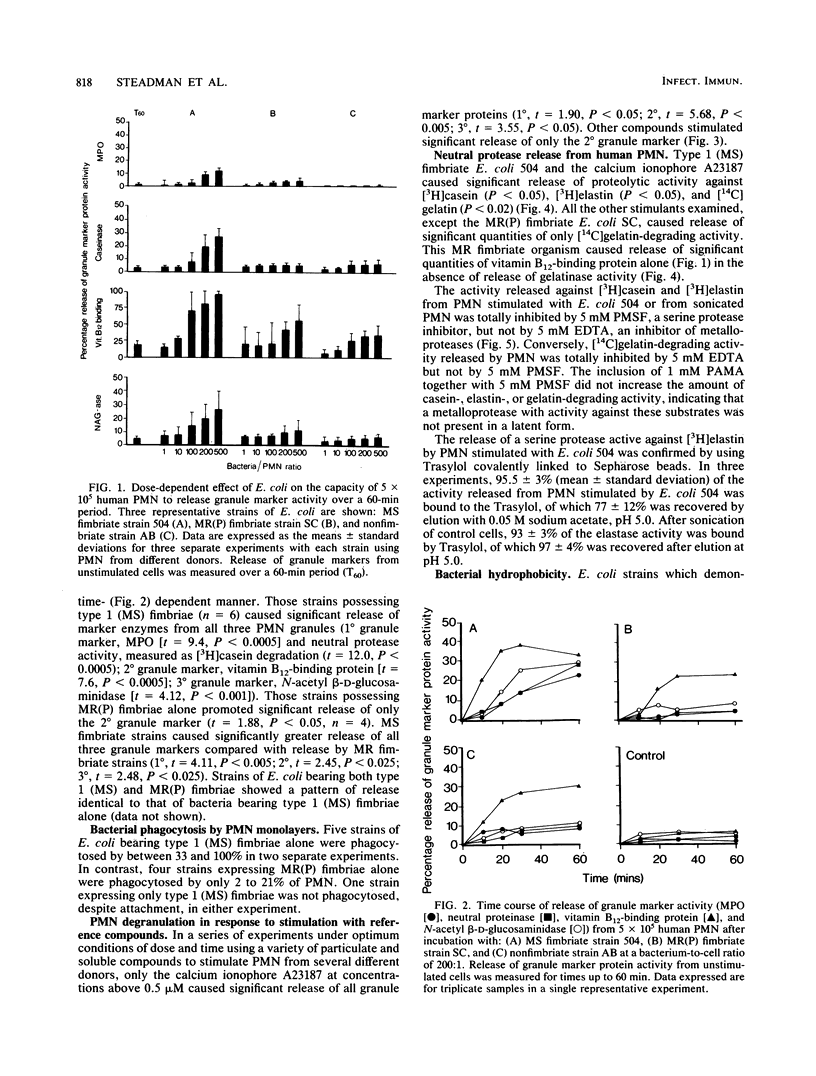
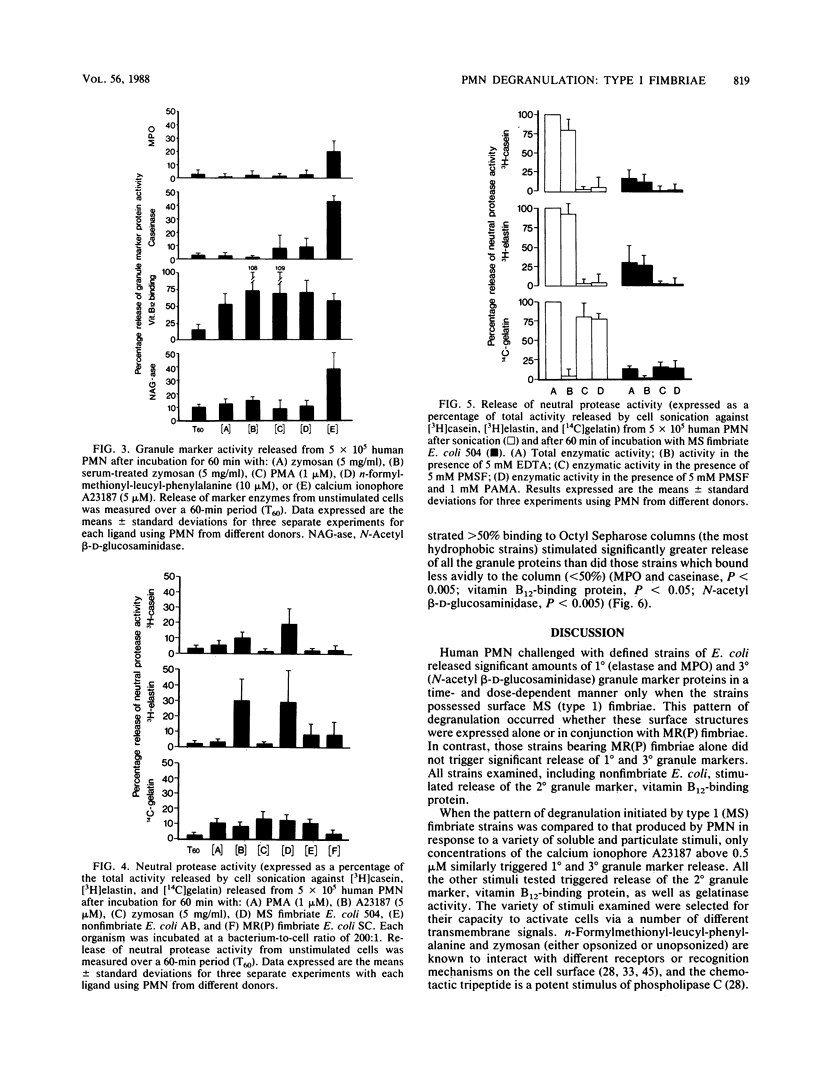
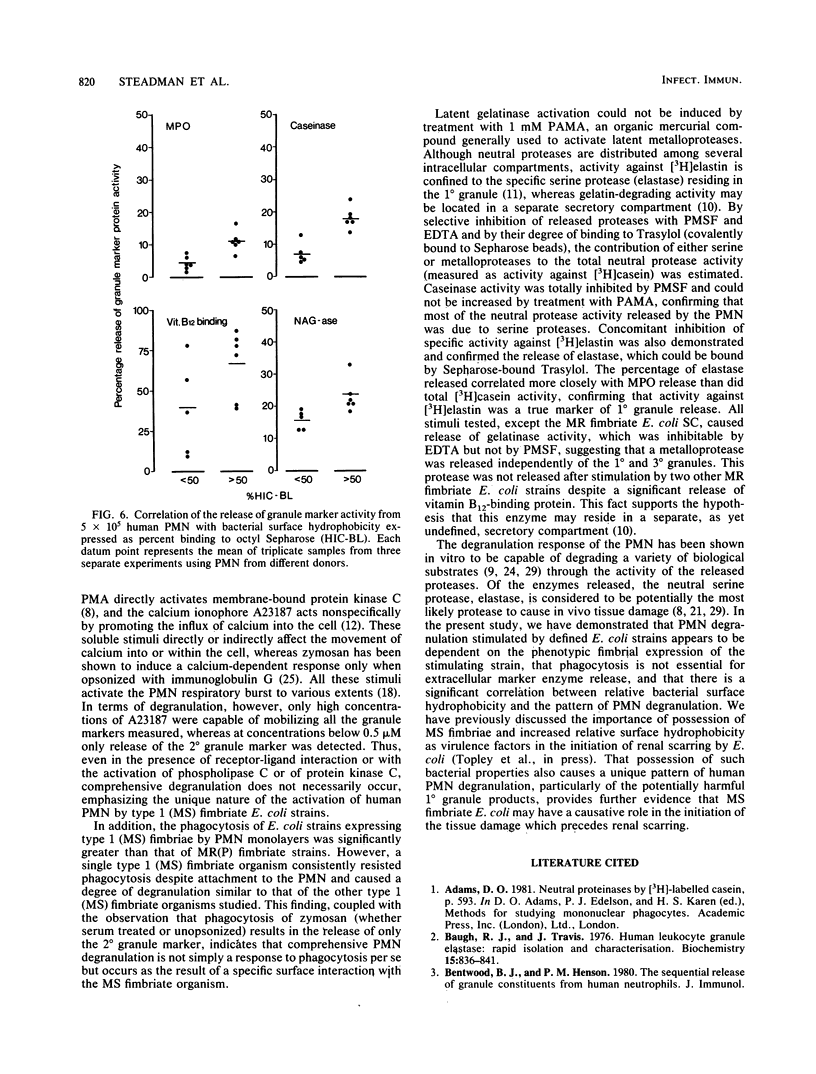
Uropathogenic strains of Escherichia coli bearing mannose-sensitive (type 1) fimbriae promote a unique pattern of degranulation from human polymorphonuclear leukocytes (PMN). Significant quantities of the primary (1 degree) and tertiary (3 degree) granule markers, neutral protease-myeloperoxidase and N-acetyl-beta-D-glucosaminidase, respectively, were released by PMN in a dose- and time-dependent manner when stimulated by these defined bacterial strains. Organisms bearing mannose-resistant (P) fimbriae promoted release of only the secondary (2 degree) granule marker, vitamin B12-binding protein. When this pattern of degranulation was compared to that produced by PMN in response to a variety of soluble and particulate stimuli, only the calcium ionophore A23187 similarly triggered 1 degree and 3 degree granule marker release. All the other stimuli tested--zymosan, serum-treated and unopsonized; n-formylmethionyl-leucyl-phenylalanine; and phorbol myristate acetate--promoted release of only the 2 degree granule marker. These results demonstrate selectivity of PMN degranulation in response to a number of transmembrane signals. In addition, the capacity of E. coli to promote PMN degranulation is dependent on its phenotypic fimbrial expression, a surface characteristic which correlates significantly with its relative surface hydrophobicity as measured by binding to octyl Sepharose. Those bacteria demonstrating the greatest hydrophobicity were capable of triggering discharge of all three granule marker proteins. Thus, the mannose-sensitive fimbriae of uropathogenic E. coli may contribute significantly to their potential pathophysiologic role in renal scarring.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Blumenstock E., Jann K. Adhesion of piliated Escherichia coli strains to phagocytes: differences between bacteria with mannose-sensitive pili and those with mannose-resistant pili. Infect Immun. 1982 Jan;35(1):264–269. doi: 10.1128/iai.35.1.264-269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Chick S., Harber M. J., Mackenzie R., Asscher A. W. Modified method for studying bacterial adhesion to isolated uroepithelial cells and uromucoid. Infect Immun. 1981 Oct;34(1):256–261. doi: 10.1128/iai.34.1.256-261.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Casnellie M. R., Segel G. B., Lichtman M. A. Concanavalin A and phorbol ester cause opposite subcellular redistribution of protein kinase C. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1139–1144. doi: 10.1016/0006-291x(85)91255-0. [DOI] [PubMed] [Google Scholar]
- Davies M., Barrett A. J., Travis J., Sanders E., Coles G. A. The degradation of human glomerular basement membrane with purified lysosomal proteinases: evidence for the pathogenic role of the polymorphonuclear leucocyte in glomerulonephritis. Clin Sci Mol Med. 1978 Mar;54(3):233–240. doi: 10.1042/cs0540233. [DOI] [PubMed] [Google Scholar]
- Dewald B., Bretz U., Baggiolini M. Release of gelatinase from a novel secretory compartment of human neutrophils. J Clin Invest. 1982 Sep;70(3):518–525. doi: 10.1172/JCI110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., Reusch M. E., Epstein M. L., Hill H. R. Role of Ca2+ and Mg2+ in some human neutrophil functions as indicated by ionophore A23187. Infect Immun. 1976 Jan;13(1):146–151. doi: 10.1128/iai.13.1.146-151.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Glauser M. P., Lyons J. M., Braude A. I. Prevention of chronic experimental pyelonephritis by suppression of acute suppuration. J Clin Invest. 1978 Feb;61(2):403–407. doi: 10.1172/JCI108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L. I., Douglas S. D., Kay N. E., Yamada O., Osserman E. F., Jacob H. S. Modulation of neutrophil function by lysozyme. Potential negative feedback system of inflammation. J Clin Invest. 1979 Jul;64(1):226–232. doi: 10.1172/JCI109443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber M. J., Mackenzie R., Asscher A. W. A rapid bioluminescence method for quantifying bacterial adhesion to polystyrene. J Gen Microbiol. 1983 Mar;129(3):621–632. doi: 10.1099/00221287-129-3-621. [DOI] [PubMed] [Google Scholar]
- Harber M. J., Topley N. Factors affecting the measurement of chemiluminescence in stimulated human polymorphonuclear leucocytes. J Biolumin Chemilumin. 1986 Jun;1(1):15–27. doi: 10.1002/bio.1170010105. [DOI] [PubMed] [Google Scholar]
- Hughes C., Phillips R., Roberts A. P. Serum resistance among Escherichia coli strains causing urinary tract infection in relation to O type and the carriage of hemolysin, colicin, and antibiotic resistance determinants. Infect Immun. 1982 Jan;35(1):270–275. doi: 10.1128/iai.35.1.270-275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Lian J., Burleigh M. C. Role of granulocyte collagenase in collagen degradation. Am J Pathol. 1972 Sep;68(3):565–578. [PMC free article] [PubMed] [Google Scholar]
- Lew D. P., Andersson T., Hed J., Di Virgilio F., Pozzan T., Stendahl O. Ca2+-dependent and Ca2+-independent phagocytosis in human neutrophils. Nature. 1985 Jun 6;315(6019):509–511. doi: 10.1038/315509a0. [DOI] [PubMed] [Google Scholar]
- Mantovani B. Different roles of IgG and complement receptors in phagocytosis by polymorphonuclear leukocytes. J Immunol. 1975 Jul;115(1):15–17. [PubMed] [Google Scholar]
- O'Hanley P., Low D., Romero I., Lark D., Vosti K., Falkow S., Schoolnik G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med. 1985 Aug 15;313(7):414–420. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- Ohta H., Okajima F., Ui M. Inhibition by islet-activating protein of a chemotactic peptide-induced early breakdown of inositol phospholipids and Ca2+ mobilization in guinea pig neutrophils. J Biol Chem. 1985 Dec 15;260(29):15771–15780. [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseas R., Yang H. H., Baehner R. L., Boxer L. A. Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness. Blood. 1981 May;57(5):939–945. [PubMed] [Google Scholar]
- Ringel E. W., Soter N. A., Austen K. F. Localization of histaminase to the specific granule of the human neutrophil. Immunology. 1984 Aug;52(4):649–658. [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Slotki I. N., Asscher A. W. Prevention of scarring in experimental pyelonephritis in the rat by early antibiotic therapy. Nephron. 1982;30(3):262–268. doi: 10.1159/000182484. [DOI] [PubMed] [Google Scholar]
- Smith G. P., Peters T. J. The release of granule components from human polymorphonuclear leukocytes in response to both phagocytic and chemical stimuli. Biochim Biophys Acta. 1982 Nov 24;719(2):304–308. doi: 10.1016/0304-4165(82)90103-9. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Jr, Roberts J. A. Chronic pyelonephritis. An electron microscopic study in nonhuman primates. Invest Urol. 1978 Sep;16(2):148–153. [PubMed] [Google Scholar]
- Svanborg Edén C., Bjursten L. M., Hull R., Hull S., Magnusson K. E., Moldovano Z., Leffler H. Influence of adhesins on the interaction of Escherichia coli with human phagocytes. Infect Immun. 1984 Jun;44(3):672–680. doi: 10.1128/iai.44.3.672-680.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Källenius G., Möllby R., Hultberg H., Winberg J. Rapid identification of P-fimbriated Escherichia coli by a receptor-specific particle agglutination test. Infection. 1982;10(4):209–214. doi: 10.1007/BF01666912. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Seifter S., Yang F. C. A new radioactive assay for enzymes with elastolytic activity using reduced tritiated elastin. The effect of sodium dodecyl sulfate on elastolysis. Biochim Biophys Acta. 1973 Nov 15;327(1):138–145. doi: 10.1016/0005-2744(73)90111-3. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Invest. 1984 May;73(5):1297–1303. doi: 10.1172/JCI111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Lee T. H., Lewis R. A., Austen F. Intracellular retention of the 5-lipoxygenase pathway product, leukotriene B4, by human neutrophils activated with unopsonized zymosan. J Immunol. 1985 Apr;134(4):2624–2630. [PubMed] [Google Scholar]
- Williams J. D., Topley N., Alobaidi H. M., Harber M. J. Activation of human polymorphonuclear leucocytes by particulate zymosan is related to both its major carbohydrate components: glucan and mannan. Immunology. 1986 May;58(1):117–124. [PMC free article] [PubMed] [Google Scholar]


