Abstract
Vasculogenesis, the formation of blood vessels in embryonic or fetal tissue mediated by immature vascular cells (i.e., angioblasts), is poorly understood. Here we report a summary of our recent studies on the identification of a population of vascular progenitor cells (VPCs) in human fetal aorta. These undifferentiated mesenchymal cells co-express endothelial and myogenic markers (CD133+, CD34+, KDR+, desmin+) and are localized in outer layer of the aortic stroma of 11–12 weeks old human fetuses. Under stimulation with VEGF-A or PDGF-BB, VPCs give origin to a mixed population of mature endothelial and mural cells, respectively. When embedded in a three-dimensional collagen gel, VPCs organize into cohesive cellular cords that resembled mature vascular structures. The therapeutic efficacy of a small number of VPCs transplanted into ischemic limb muscle was demonstrated in immunodeficient mice. Investigation of the effect of VPCs on experimental heart ischemia and on diabetic ischemic ulcers in mice is in progress and seems to confirm their efficacy. On the whole, fetal aorta represents an important source for the investigation of phenotypic and functional features of human vascular progenitor cells.
Keywords: Vasculogenesis, Vascular progenitors, Angiogenesis, Endothelium, Ischemic disease
Introduction
Angiogenesis, the sprouting of new vessels from pre-existing vessels, plays an important role in physiologic and pathologic processes (Folkman 1985). Angiogenesis occurs normally during embryonic development and in adult during menstrual cycle and under many pathological conditions, particularly in cancer (Folkman and Shing 1992). In contrast, vasculogenesis, the new formation of blood vessels from undifferentiated vascular precursor cells (angioblasts), occurs mainly during embryonic of fetal development (Risau 1995).
This brief report is aimed to summarize the present knowledge on vascular stem cells. It will be focused particularly on vascular progenitor cells (VPCs) isolated from human fetal aorta (FA) and their potential application in ischemic diseases.
Identification and characterization of VPCs in human fetal aorta
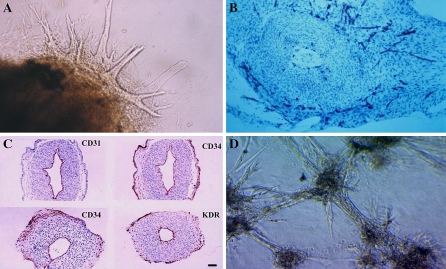
Angioblasts define a population of embryonic mesenchymal cells from which blood cells and blood vessels arise. Hematopoiesis and vasculogeneis begin in the blood islands of the yolk sac in early embryogenesis (Haar and Ackerman 1971; Moore and Metcalf 1970). In addition, endothelial and hematopoietic lineages share a number of phenotypic markers and genes (Asahara et al. 1997; Fina et al. 1990; Kabrun et al. 1997; Kallianpur et al. 1994; Millauer et al. 1993). This led to the hypothesis that both cell types arise from a common precursor named “hemangioblast” (Eichmann et al. 1997; Murray 1932; Sabin 1920). However more recently, this hypothesis has been challenged by kinetics analysis of vascular endothelial and hematopoietic progenitors in early gastrulation of mouse embryos (Furuta et al. 2006). Furuta et al. (2006) propose a distinct pathway of development in which the angioblast lineage directly diverges from mesoderm prior to and independent of hemangioblast development. Therefore the common embryonic origin of blood and vascular cells still remains controversial. Less controversial appear the hypothesis proposed by Yamashita et al. (2000), for a common progenitor cell for endothelial and mural cells (pericytes and vascular smooth muscle cells). By using mouse embryonic stem cells, they found that embryonic stem cells expressing Flk-1/KDR (vascular endothelial growth factor receptor 2) if differentially stimulated with PDGF or VEGF, may give rise respectively, to mural and endothelial cells (ECs). Similarly, Minasi et al. (2002) have demonstrated that rat dorsal embryonic aorta contains multipotent self-renewing cells able to differentiate into most mesodermal tissues, including myogenic and vascular cell lineages. In spite of these findings, the identification of VPCs and their mechanism of differentiation into mature vascular structures, particularly in human, is far from clear (LeDourain 1973; Pardanaud et al. 1987; Risau and Flamme 1995). In an attempt to fill this gap, since 1999 our group has been involved in the identification of human VPCs. We used the rat aortic ring assay developed by Nicosia et al. (1982) and Nicosia and Ottinetti (1990) to investigate the angio-forming properties of aortic explants from human fetus and the role of vasculogenesis in this process. The ring aortic assay consists in culturing fetal aorta (FA) explants obtained from legal abortion of 11–12 week old fetus in a collagen gel (Alessandri et al. 2001). Under these culture conditions FA ring produced outgrowth of vascular-like cords that invade the surrounding collagen matrix (Fig 1a). The cords and the capillary tubes appeared to be formed by mesenchymal cells that stained strongly for endothelial markers such as CD31, CD34, and KDR (Fig 1b) and less intensive for von Willebrand Factor (vWF). It is interesting to observe that most neovascular proliferation occurred primarily in the outer layer of the aortic rings, whereas in a very limited number of cases, it seemed to originate from pre-existing ECs lining the aortic lumen. Since mature endothelial markers like CD31 and vWF were not expressed in the outer layer of aorta parenchyma, but it stained positive for immature endothelial markers CD34 and KDR (Fig 1c), we concluded that most of the neovessels arising from aorta explants originated from immature vascular progenitors (Alessandri et al. 2001). Most recently we confirmed and expanded this conclusion (Invernici et al. 2007). Indeed we found that upon enzymatic digestion, FACS analysis revealed that FA is very rich in CD34+ cells, (around 30% of the total aortic cells), only 1% of the CD34+ cells were also CD31+vWF+; most of the CD34+ cells were KDR+ and also expressing CD133, a marker found on neural stem cells (Uchida et al. 2000) and also on adult immature bone marrow derived EPCs (Reyes et al. 2001). Under culture conditions that induce endothelial differentiation the CD34+KDR+CD133+CD31−vWF− became CD34+KDR+CD31+vWF+CD133−, assuming the typical morphology of ECs in culture, and if embedded in collagen gel, CD34+KDR+CD133+ cells organized cords that resemble vascular capillaries-like structures (Fig. 1d) composed by pericytes/smooth muscle and ECs. In addition we found that around 30% of CD34+KDR+CD133+ cells expressed also desmin, a myogenic marker (Comelison and Wold 1997), and upon stimulation with VEGF or PDGF-BB, CD34+KDR+CD133+ differentiated into endothelial or mural cells. Thus we concluded that human FA is a rich source of immature cells able to differentiate into all cellular components of mature vessels (Invernici et al. 2007).
Fig. 1.
Identification of VPCs in Human FA. a Micrographs of collagen gel culture of human fetal aorta explants: an example of outgrowths of branching capillary-like tubes after 72 h of culture. The cords grow haphazardly and divide into branches to form complex structures. b Immunohistochemistry of capillary-like structures arising from aortic rings paraffine-embedded after 72 h of culture in collagen gel matrix: cross-section of an aortic ring showing many CD31-positive cells organized into capillary-like structures. These outgrowths appear to arise from undifferentiated mesenchymal cells at the periphery of explants. c Immunohistochemistry of untreated fetal aorta. The tissues were immediately fixed after recovery and then embedded in paraffin and cross-sectioned. Left upper panel shows aortic ring stained with CD31, left lower and right upper panel stained with CD34, lower right panel stained with KDR. CD31 (mature endothelial marker) stained only pre-existing endothelial lining the aorta lumen it was absent in the parenchyma or at periphery of aorta. In contrast CD34 and KDR stained both mature ECs and a lot of mesenchymal immature cells present particularly in the outer layer of aorta stroma. d Immature sorted CD34+CD133+KDR+VPCs grow in culture as cells aggregates. When seeded in a collagen gel they start to form vascular-like structures that connect each other
Effect of VPCs on experimental ischemic diseases
Progenitor cells of fetal origin have been successfully used to replace BM in hematological disease (Madeddu et al. 2004). Likewise, naïve VPCs could provide a new way to treat ischemic diseases. Thus, we wished to test in vivo whether the myogenic and vasculogenic properties of VPCs could be clinically exploited to treat ischemic disease. By using a murine model of peripheral ischemia we observed that VPCs transplantation into ischemic muscles significantly ameliorates the clinical outcome of ischemic mice (Invernici et al. 2007).
Confocal microscopy pictures and immunohistochemistry analysis of ischemic muscle of mice treated with a small number of VPCs (2 × 104 cells) showed a significant increment of vascularization and myogenesis as well as a reduction of muscular and vascular apoptotic cells. Moreover, the animals treated with VPCs demonstrated a limited but significant incorporation of the transplanted cells into muscular fibers and vessels (Fig. 2). Interesting, an equal number of transplanted human bone marrow derived EPCs, transplanted as reference cell control, was not able to evoke a comparable ameliorative effect. The clinical efficacy of VPCs on peripheral ischemia was also confirmed by the substantial reduction of ischemia-induced necrotic/autoamputated toes in all mice treated with VPCs. We have in progress experiments to test VPCs efficacy on experimental heart ischemia and on a new experimental model of diabetic ischemic ulcers (Barcelos et al. 2008). Similarly to peripheral limb ischemia, VPCs treatment seems to ameliorate the clinical outcome (Barcelos et al. 2008). Interestingly the analysis of VPCs culture conditioned medium demonstrated that these cells secrete significant amount of angiogenic factors, in particular, they produced VEGF-A, Angiopoietins 1 and 2, IL6, IL8 and SDF-1 (Invernici et al. 2007; Barcelos et al. 2008). Because of the small number of injected cells and the limited capacity of the transplanted cells to undergo in vivo differentiation, we conclude that beneficial effect of VPCs on ischemia may be attributed, at least in part, to their capacity to secrete angiogenic factor.
Fig. 2.
Therapeutic efficacy of VPCs treatment on experimental hind limb ischemia. a Immunohistochemical identification of VPCs injected locally in mouse hind limb ischemic muscle. VPCs colored in green appear localized in the vicinity of capillaries and arterioles colored in red (upper panel) and in muscle fibers colored in red (lower panel). b Immunohistochemistry of control muscle treated with control cells or medium. The absence of cells demonstrated the specificity of the antibodies (anti-human nuclei) used for VPCs identification. c Distribution of necrotic toes in the left foot of mice that were exposed to ipsilateral ischemia. Zero means absence of necrotic toes, 5 indicates all toes being necrotic or absent. One point was scored for each necrotic toe. Each circle is representative of a single mouse. Note that EPCs treatment (at the same dose of VPCs) did not significantly ameliorate the number of necrotic toes, whereas VPCs significantly reduced the necrotic toes. d A representative image of hindlimb region submitted to ischemia and treated with VPCs or control medium (CM). Note the absence of necrosis in the foot of mouse treated with VPCs
Conclusions
Our studies demonstrate for the first time that human FA contains, in the outer layer of its parenchyma, immature cells able to form and regenerate vessels. These cells are defined by a precise phenotypic profile which includes immature vascular markers like CD34, CD133, and KDR, and the myogenic marker desmin. These cells are able to differentiate into both mural and endothelial cells, are endowed of angiogenic potential and have strong vascular and muscular regenerative properties in vivo. We propose FA not only as a tool to study human vasculogenesis mechanism, but also as tissue source to isolate and culture VPCs, potentially exploitable for the treatment of human ischemic diseases.
References
- Alessandri G, Girelli M, Taccagni G, Colombo A, Nicosia R, Caruso A, Baronio M, Pagano S, Cova L, Parati E (2001) Human vasculogenesis ex vivo: embryonal aorta as a tool for isolation of endothelial cell progenitors. Lab Invest 81:875–885 [DOI] [PubMed]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee RUT, Witzenbichies B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967. doi:10.1126/science.275.5302.964 [DOI] [PubMed]
- Barcelos L, Duplan C, Graiani G, Invernici G, Alessandri G, Madeddu P et al (2008) Human CD133+Progenitors cells promote the healing of Diabetic ulcers by paracrine stimulation of angiogenesis and activation of wnt signalling. Submitted for publication [DOI] [PMC free article] [PubMed]
- Comelison DD, Wold BJ (1997) Single cell analysis of regulatory gene expression in quite and activated mouse skeletal muscle satellite cells. Dev Biol 191:270–283. doi:10.1006/dbio.1997.8721 [DOI] [PubMed]
- Eichmann A, Corbel C, Nataf V, Valgot R, Breant C, Le Douarin NM (1997) Ligand-dependent development of the endothelial and hematopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor 2. Proc Natl Acad Sci USA 94:5141–5146. doi:10.1073/pnas.94.10.5141 [DOI] [PMC free article] [PubMed]
- Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Geaves MF (1990) Expression of the CD34 gene in vascular endothelial cells. Blood 75:2417–2426 [PubMed]
- Folkman J (1985) Toward an understanding of angiogenesis: search and discovery. Perspect Biol Med 29:10–38 [DOI] [PubMed]
- Folkman J, Shing Y (1992) Angiogenesis. J Biol Chem 267:10931–11093 [PubMed]
- Furuta C, Ema H, Takayanagi S, Ogaeri T, Okamura D, Matsui Y, Nakauchi H (2006) Discordant development waves of angioblasts and hemangioblasts in early grastrulating mouse embryo. Development 133:2771–2779. doi:10.1242/dev.02440 [DOI] [PubMed]
- Haar JL, Ackerman GA (1971) A phase and electron microscopy study on vasculogeneis and erythropoiesis in the yolk sac of the mouse. Anat Rec 170:199–223. doi:10.1002/ar.1091700206 [DOI] [PubMed]
- Invernici G, Emanueli C, Madeddu P, Cristini S, Gadau S, Benetti A, Ciusani E, Stassi G, Siragusa M, Nicosia R, Peschle C, Fascio U, Colombo A, Rizzati T, Parati EA, Alessandri G (2007) Human fetal aorta contains vascular progenitor cells capable of indicing angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol 170:1879–1892. doi:10.2353/ajpath.2007.060646 [DOI] [PMC free article] [PubMed]
- Kabrun N, Buhring HJ, Choi N, Ullrich A, Risaw W, Keller G (1997) Flk-1 expression define a population of early embryonic haematopoietic precursors. Development 124:2039–2048 [DOI] [PubMed]
- Kallianpur AR, Jordan JE, Brandt SJ (1994) The SCL/TAL-1 gene is expressed in progenitors of both haematopoietic and vascular system during embryogenesis. Blood 83:1200–1208 [PubMed]
- LeDourain N (1973) A biological cell labelling technique and its use in experimental embryology. Dev Biol 30:217–222. doi:10.1016/0012-1606(73)90061-4 [DOI] [PubMed]
- Madeddu P, Emanueli C, Pelosi E, Salis MB, Cerio AM, Bonanno G, Patti M, Stassi G, Condorelli G, Peschle C (2004) Transplantation of a low doses of CD34+KDR+cells promote vascular and muscular regeneration in ischemic limbs. FASEB J 18:1737–1739 [DOI] [PubMed]
- Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Moller NP, Risau W, Ullrich A (1993) High affinity VEGF binding and developmetal expression suggest Flk-1 as major regulator of vasculogenesis and angiogenesis. Cell 72:835–846. doi:10.1016/0092-8674(93)90573-9 [DOI] [PubMed]
- Minasi GM, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G (2002) The meso-angioblast: a multipotent self renewing cell that originated from dorsal aorta and differentiated into most mesodermal tissues. Development 129:2773–2783 [DOI] [PubMed]
- Moore M, Metcalf D (1970) Ontogeny of haematopoietic system: yolk sac origin in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol 18:279–296. doi:10.1111/j.1365-2141.1970.tb01443.x [DOI] [PubMed]
- Murray PDF (1932) The development in vitro of the blood of the early chick embryo. Proc R Soc Lond B Sci 11:497–521
- Nicosia RF, Tchao R, Lieghton J (1982) Histotypic angiogenesis in vitro: light microscopy, ultrastructural and radioautographic studies. In Vitro 18:538–549. doi:10.1007/BF02810077 [DOI] [PubMed]
- Nicosia RF, Ottinetti A (1990) Growth of microvessels in serum-free matrix culture of rat aorta: a quantitative assay of angiogenesis in vitro. Lab Invest 63:115–122 [PubMed]
- Pardanaud L, Altmann C, Kitos P, Dieterien-Lièvre F, Buck CA (1987) Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development 100:339–349 [DOI] [PubMed]
- Reyes M, Lund T, Lenvik T, Aguiar D, Koodle L, Verfaillie CM (2001) Purification and ex vivo expression of post-natal human marrow mesodermal progenitor cells. Blood 98:2615–2625. doi:10.1182/blood.V98.9.2615 [DOI] [PubMed]
- Risau W (1995) Differentiation of endothelium. FASEB J 9:926–933 [PubMed]
- Risau W, Flamme I (1995) Vacsulogenesis. Annu Rev Cell Dev Biol 11:73–91. doi:10.1146/annurev.cb.11.110195.000445 [DOI] [PubMed]
- Sabin FR (1920) Study on the origin of blood vessels and or red corpuscles as seen in the living blastoderm of the chick during the second day of incubation. Contrib Embryol 9:213–262
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL (2000) Direct isolation of human nervous system stem cells. Proc Natl Acad Sci USA 97:14720–14725. doi:10.1073/pnas.97.26.14720 [DOI] [PMC free article] [PubMed]
- Yamashita J, Hiroshi I, Masanori H, Minetaro O, Satomi N, Takami Y, Makoto N, Kazuwa N, Nishikawa S (2000) Flk-1 positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408:92–96. doi:10.1038/35040568 [DOI] [PubMed]