Abstract
An urgent current need in regenerative medicine is that of identifying a plentiful, safe and ethically acceptable stem cell source for the development of therapeutic strategies to restore functionality in damaged or diseased organs and tissues. In this context, human term placenta represents a prime candidate, as it is available in nearly unlimited supply, is ethically problem-free and easily procured. Placental cells display differentiation capacity toward all three germ layers, while also displaying immunomodulatory effects, therefore supporting the possibility that they could be applied in an allogeneic transplantation setting. Although promising data have been reported to date, further study is required to fully characterize the differentiation potential of placenta-derived cells and to identify their possible clinical applications. Here, we provide a snapshot of current knowledge regarding the potential of cells from the amniotic membrane of human term placenta to address current shortcomings in the field of regenerative medicine.
Keywords: Placenta-derived cells, Amnion, Fetal membranes, Cell therapy
Current needs in regenerative medicine
Regenerative medicine is a newly emerging and multidisciplinary field which draws on biology, medicine and genetic manipulation for the development of strategies aimed at maintaining, enhancing or restoring the function of tissues or organs which has been compromised through disease or injury. Due to their ability to differentiate toward multiple cell types, stem cells will undoubtedly play a key role in the development of such strategies. However, although stem cells hold great promise in this context, the stem cell sources which have been most extensively investigated to date have several limitations which would need to be overcome in order to make the development of effective and readily available treatments a clinical reality. For example, embryonic stem cells require destruction of the human embryo for their procurement and are associated with a high rate of tumor induction (teratoma) following transplantation, while mesenchymal stromal cells from bone marrow carry with them a risk of viral infection (Eichna et al. 2008), and the differentiation capacity of these cells has been seen to decrease with donor age (D’Ippolito et al. 1999; Mareschi et al. 2006). The need therefore remains to identify a source of stem cells that is safe, easily accessible, provides a high cell yield and for which cell procurement does not provoke ethical debate.
Why study placenta as a source of stem cells?
In addressing the complex scenario described above, several groups have recently turned their attention to the human term placenta as a possible source of progenitor/stem cells. The fact that placental tissues originate during the first stages of embryological development supports the possibility that these tissues may contain cells which have retained the plasticity of the early embryonic cells from which they derive. Meanwhile, the fact that the placenta is fundamental for maintaining fetomaternal tolerance during pregnancy suggests that cells present in placental tissue may have immunomodulatory characteristics. These two key aspects make cells from placenta good candidates for possible use in cell therapy approaches, with the possibility of providing cells that are capable of differentiating into multiple different cell types, and which also display immunological properties that would allow their use in an allo-transplantation setting. Furthermore, given that the placenta is generally discarded after birth, this tissue is available in large supply, the recovery of cells from this tissue does not involve any invasive procedures for the donor, and their use does not pose any ethical problems.
Embryological origin of human placenta—clues to potentiality
The hypothesis that placental tissues may harbour cells which display plasticity that is characteristic of pre-gastrulation embryonic cells, and which may therefore have the potential to differentiate toward different lineages, is well-supported by the fact that these tissues originate during early embryological development, even before gastrulation occurs.
Four to five days after fertilization, the morula, which results from the stepwise division of the zygote to a cluster of around 16 cells (termed blastomeres), enters the uterine cavity. Within the morula, a cavity known as the blastocoel then forms, giving rise to the blastula. Formation of the blastocyst follows, whereby the inner blastomeres compact to form the inner cell mass, which is positioned to one side of the blastocoel, while the outer, enclosing blastomeres form a layer termed the trophoblast.
After the blastocyst has attached to the endometrium, the proliferating trophoblastic cells differentiate into two layers: the invasive, multinucleated outer layer termed syncytiotrophoblast, which is formed by fusion of neighbouring trophoblast cells, and the inner mononucleated cytotrophoblast layer. This arrangement of an outer trophoblastic layer which is in contact with the endometrium and which surrounds the inner cell mass represents the paradigm which will be maintained for the rest of the gestation period, whereby the trophoblast will continue to differentiate into more specialized cell types that will allow the formation of an interface between maternal and fetal circulation for nutrient and waste exchange, while the fetus itself will derive from cells of the inner cell mass.
As implantation progresses into the second week after fertilization, morphological changes occur in the inner cell mass giving rise to the bilaminar embryonic disc composed of two layers: the epiblast, which is adjacent to the trophoblast, and the hypoblast, which is closest to the blastocoel. At this point, the epiblast separates from the trophoblast, thereby creating a space that will later become the amniotic cavity, and which is lined by cells derived from the epiblast itself, termed amnioblasts, which will later form the amniotic epithelium.
Meanwhile, some cells from the hypoblast migrate along the inner wall of the blastocoel giving rise to the exocoelomic membrane, while the former blastocoel becomes the yolk sac. Cells of the exocoelomic membrane and the adjacent trophoblast form the extraembryonic reticulum. Some hypoblast cells then migrate along the outer edges of extraembryonic reticulum to form a connective tissue known as the extraembryonic mesoderm, which surrounds the yolk sac and amniotic cavity, and later forms the amniotic mesoderm (AM) and chorionic mesoderm (CM).
Therefore, to summarize, the amniotic epithelial region is derived from the epiblast, while the mesodermal regions of both the amnion and chorion are derived from the extraembryonic mesoderm. The chorionic trophoblastic (CT) region, as its name suggests, is derived from the trophoblast.
Gastrulation, the process through which the bilaminar disc differentiates into the three germ layers (ectoderm, mesoderm and endoderm), then proceeds during the third week after fertilization (Benirschke and Kaufmann 2000; Moore and Persaud 1998).
Structure of human term placenta
Before considering whether the human term placenta can provide a source of stem or progenitor cells for research and clinical applications, a detailed understanding of its different regions is necessary to allow undertaking of a precise and systematic analysis of the cell types present in these regions.
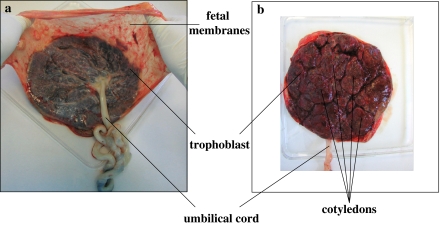
The term placenta is discoid in shape with a diameter of 15–20 cm and a thickness of 2–3 cm (Fig. 1). It is composed of the chorionic plate, from which villi extend and make intimate contact with the uterine decidua during pregnancy, and the fetal membranes (amnion and chorion), which enclose the fetus.
Fig. 1.
Human term placenta. a Fetal surface. It is possible to distinguish the trophoblast, the fetal membranes which continue from the edge of the placenta, and the umbilical cord. b Maternal surface. The placenta is subdivided into irregular lobed structures, termed cotyledons
The multilayered chorionic plate faces the amniotic cavity, and from this extend the chorionic villi, which are composed of a central stromal core containing embedded fetal capillaries as well as fibroblasts and macrophages known as Hofbauer cells, with an outer multinucleated layer of syncytiotrophoblast in direct contact with maternal blood. A basement membrane separates the stromal core from the syncytiotrophoblast. Between the syncytiotrophoblast and its basement membrane are single or aggregated cytotrophoblast cells (also known as Langhans cells).
Most villi terminate in the intervillous space and are bathed in maternal blood, while others invade the decidua basalis, which forms the maternal portion of the placenta, thereby anchoring the placenta to the endometrium. Invasion of the decidua by anchoring villi results in the formation of placental septa that divide the trophoblastic region of the placenta into irregular cotyledons.
The amniotic and chorionic membranes extend from the margins of the chorionic plate, enclosing the fetus in the amniotic cavity.
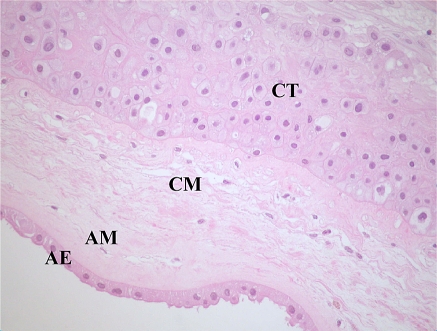
The amnion is a thin, avascular membrane composed of an uninterrupted single layer of cuboidal and columnar epithelial cells (termed the amniotic epithelium, AE) which is bathed in amniotic fluid and is contiguous over the umbilical cord with the fetal skin. The AE is attached to a distinct basement membrane that is, in turn, connected to the AM, an acellular compact layer composed of collagens I, III and fibronectin. Deeper in the AM, a network of dispersed fibroblast-like mesenchymal cells and rare macrophages are observed. A spongy layer of loosely arranged collagen fibers separates the AM and CM, both of which are similar in composition. During pregnancy, the greater part of the chorion is in contact with the decidua capsularis, and over this region, the contained vessels undergo atrophy to give rise to the smooth region of chorion known as the chorion leave, which consists of a mesodermal and chorion trophoblastic region. The region of chorion in contact with the decidua basalis, and from which the chorionic villi extend toward the endometrium, is known as the chorion frondosum. In the term placenta, a basal lamina separates the CM from the extravillous trophoblast cells, which represent the only residue of the former villi of the chorion frondosum (Benirschke and Kaufmann 2000; Cunningham et al. 1997). A cross-sectional representation of the human fetal membranes is shown in Fig. 2.
Fig. 2.
Hematoxylin/eosin staining of fetal membranes of human term placenta. AE amniotic epithelial layer, AM amniotic mesenchymal layer, CM chorionic mesenchymal layer, CT chorionic trophoblastic layer. (magnification ×400)
From the fetal membranes of human term placenta, four principal cell types can be isolated for study, and these have been defined in a recent review as human amniotic epithelial cells (hAEC), human amniotic mesenchymal stromal cells (hAMSC), human chorionic mesenchymal stromal cells (hCMSC) and human chorionic trophoblastic cells (hCTC) (Parolini et al. 2008).
In this review, we will focus on the potentiality and the phenotypic and immunological characteristics of hAEC and hAMSC.
Human amniotic epithelial cells
Out of all of the cell types present in term placenta, human amniotic epithelial cells (hAEC) have been studied most extensively to date. These cells are typically isolated by manual separation of the amniotic membrane from the chorion, followed by digestion with trypsin or dispase to release the epithelial cells, while the mesenchymal layer remains intact and can be removed. Isolated hAEC adopt a cobblestone-shaped morphology in vitro and can be maintained for 2–6 culture passages before proliferation ceases (Parolini et al. 2008). In culture, a high proportion of hAEC express epithelial markers such as cytokeratins, while mesenchymal markers are generally absent. Interestingly, the mesenchymal marker vimentin, although absent on freshly isolated hAEC, has also been shown to appear during culture (Miki and Strom 2006; Toda et al. 2007). The significance of the expression of both epithelial and mesenchymal markers by hAEC remains to be elucidated, although it could be due to the spontaneous commencement of differentiation during culture, or perhaps to the so-called epithelial to mesenchymal transition in the amnion, as also suggested by Sakuragawa et al. (2004).
Immediately after isolation, hAEC appear to express very low levels of HLA-A,B,C (Terada et al. 2000), however, by passage 2, significant levels are observed.
Other antigens which are found on the surface of hAEC include the ATP-binding cassette transporter G2 (ABCG2/BCRP), CD9, CD24, E-cadherin, integrins alpha 6 and beta 1, c-met (HGF receptor), stage specific embryonic antigens (SSEA) 3 and 4 and tumor rejection antigens (TRA) 1–60 and 1–81 (Miki et al. 2005; Miki and Strom 2006), while hAEC are thought to be negative for SSEA-1, CD34, and CD133. Other markers such as CD117 (c-kit) and CCR4 (CC chemokine receptor) are either absent or may be expressed on some cells at very low levels. Although initial cell isolates express very low levels of CD90 (Thy-1), the expression of this antigen increases rapidly in culture (Miki et al. 2005; Miki and Strom 2006).
As alluded to previously, the fact that the amniotic epithelium originates before gastrulation suggests that some cells from this region may also display pluripotency. In support of this, hAEC have been demonstrated to express molecular markers of pluripotent stem cells, including octamer-binding-protein-4 (OCT-4), SRY-related HMG-box gene 2 (SOX-2), and Nanog (Ilancheran et al. 2007; Miki et al. 2005).
The suggestion that hAEC may be pluripotent is further supported by the report of Tamagawa et al. (2004), whereby cells isolated from whole amnion were mixed with mouse embryonic stem cells, in order to create a xenogeneic chimera in vitro. This chimeric embryo was maintained until all three germ layers had formed, with demonstration that amnion-derived cells had contributed to all three layers.
Several groups have shown that hAEC are able to differentiate toward cells of the mesodermal lineage in vitro, with directed differentiation toward cells of the osteogenic, chondrogenic and adipogenic lineages reported (Ilancheran et al. 2007; Portmann-Lanz et al. 2006; Wolbank et al. 2007).
Investigation of the neuronal differentiation potential of hAEC has shown that culture of these cells in neuronal differentiation media results in expression neural lineage markers including nestin, MAP2 and the astrocytic protein GFAP, as well as a morphological shift from the fibroblast-like morphology observed under normal culture conditions to an elongated, distinctly neuronal-like morphology (Ilancheran et al. 2007; Miki et al. 2005). Pioneering research by Sakuragawa et al. (1996) demonstrated not only that hAEC express markers of glial and neuronal progenitor cells, but also perform multiple neuronal functions such as synthesis and release of acetylcholine, catecholamines, neurotrophic factors, activin and noggin (Elwan and Sakuragawa 1997; Koyano et al. 2002; Sakuragawa et al. 1997; Uchida et al. 2000). As well as performing functions that are characteristic of neuronal cells, other studies report evidence that cells of the amniotic epithelium also provide support for growth of neuronal cell types in vitro. hAEC conditioned medium has been shown to have neurotrophic effects on rat cortical cells (Uchida et al. 2000) and support the survival of chicken neural retinal cells (Tcheng et al. 1994), while another study has shown that chick dorsal root ganglia are able to survive and grow on human amniotic membrane in the absence of neurotrophic factors, and it appears that these effects may be mediated at least in part by the amniotic epithelial layer (Schroeder et al. 2007).
The in vitro results regarding neuronal differentiation and neurogenic/neuroprotective properties of hAEC are supported by results from pre-clinical studies in animal models, which demonstrate that hAEC may be useful for repairing central nervous system damage by eliciting neuroprotective and neuroregenerative effects during acute phases of injury.
After transplantation into rat models of Parkinson’s disease, hAEC have been shown to produce dopamine and prevent neuron degeneration (Kakishita et al. 2000, 2003). The neuroprotective effect of hAEC in an animal model of Parkinson’s disease is further supported by the recent study of Kong et al. (2008), who show that hAEC display neuroprotective and neurogenic effects when transplanted into the striatum of Parkinson’s disease mice, and result in behavioural improvement in transplanted mice with respect to controls.
After transplantation into brains of rats which had undergone middle cerebral artery occlusion, hAEC have been shown to survive and migrate to the ischemic area and express neuronal markers, while significant amelioration of behavioural dysfunction and reduced infarct volume was also observed in cell-treated animals compared to controls (Liu et al. 2008).
Finally, after transplantation into lesioned areas of a contusion model of spinal cord injury in monkeys without immunosuppression, hAEC survived up to 120 days with no evidence of inflammation or rejection, with improved performance in locomotor tests observed in cell-treated animals compared to lesion control animals (Sankar and Muthusamy 2003), again suggesting that hAEC exert neuroprotective effects which result in functional improvement after injury.
The potential of hAEC to differentiate toward the hepatic lineage has also been investigated. In vitro studies have shown that hAEC produce albumin (Alb) and α-fetoprotein (AFP) (Sakuragawa et al. 2000), as well as performing other hepatic functions such as glycogen storage and expression of liver-enriched transcription factors including hepatocyte nuclear factor (HNF) 3γ and HNF4α, CCAAT/enhancer-binding protein (CEBP) α and β and CYP450 enzymes (Davila et al. 2004; Miki et al. 2005; Takashima et al. 2004).
Results showing hepatic differentiation of hAEC in vitro have been extended through studies in animal models. Following transplantation of hAEC into the livers of SCID mice, positivity for both Alb and AFP has been observed (Sakuragawa et al. 2000). Interestingly, these authors also showed that hAEC which had been genetically modified to express the LacZ gene were able to integrate in liver parenchyma, suggesting that hAEC could also be useful as gene carriers for patients with congenital liver disorders.
Further evidence that hAEC are able to perform hepatic functions in vivo comes from the observation that human alpha 1 antitrypsin could be detected by western blot in serum of SCID mice after transplantation of these cells (Miki and Strom 2006), while human albumin was detected in the sera and peritoneal fluid of SCID mice which had received peritoneal implants of human amniotic membrane (Takashima et al. 2004). Although the in vivo data which have been generated to date are promising, there are as yet no reports of the characterization of hepatic gene expression in long-term recipients of AE transplants, or that the transplantation of hAEC can support animals with acute or long-term defects in liver function. These important pre-clinical studies will be needed before the therapeutic potential of hAEC can be fully assessed.
Differentiation of hAEC to another endodermal tissue, pancreas, has also been examined in a study by Wei et al. (2003), who cultured hAEC for 2–4 weeks in the presence of nicotinamide to induce pancreatic differentiation. Subsequent transplantation of the insulin-expressing hAEC corrected hyperglycemia in streptozotocin-induced diabetic mice. Another study in support of the pancreatic differentiation potential of hAEC has shown that these cells adopt morphological and ultrastructural features characteristic of pancreatic cells, and also express the pancreatic marker AMY2B and produce glucagon, when grown in medium which promotes pancreatic differentiation (Ilancheran et al. 2007).
Taken together, the studies discussed above provide strong support to the hypothesis that the amniotic epithelium contains cells with stem characteristics which could be useful for regeneration of tissues derived from all three germ layers. Although these data are very promising, further studies are clearly needed to fully elucidate the conditions under which differentiation of hAEC to functional cell types of different lineages can be achieved.
Human amniotic mesenchymal stromal cells
Human amniotic mesenchymal stromal cells (hAMSC), which are thought to be derived from extraembryonic mesoderm (Moore and Persaud 1998), have been the subject of phenotypic studies by several groups who have shown that, like hAEC, these cells are also capable of multi-lineage differentiation (Bilic et al. 2004; In ‘t Anker et al. 2004; Portmann-Lanz et al. 2006; Sakuragawa et al. 2004; Wolbank et al. 2007).
hAMSC can be isolated from first-, second- and third-trimester mesoderm of amnion (Bilic et al. 2004; In ‘t Anker et al. 2004; Portmann-Lanz et al. 2006; Sakuragawa et al. 2004; Soncini et al. 2007; Wolbank et al. 2007), however the majority of studies on these cells have been performed using term placenta. hAMSC are generally isolated by mechanical separation of the amniotic membrane from the chorion, followed by digestion with collagenase, or collagenase and DNAse, to release hAMSC from amniotic mesodermal layer (Parolini et al. 2008).
The surface marker profile of cultured term hAMSC is reminiscent of that seen on bone marrow-derived MSC, with both cell types expressing typical mesenchymal markers including CD90, CD105 and CD71, while being negative for hematopoietic (CD34 and CD45) and monocytic (CD14) markers (In ‘t Anker et al. 2004; Portmann-Lanz et al. 2006; Soncini et al. 2007; Wolbank et al. 2007). In support of the hypothesis that hAMSC may display some degree of pluripotency, gene expression of stage specific embryonic antigens SSEA-3 and SSEA-4 and RNA for OCT-4 has been reported (Alviano et al. 2007; Wei et al. 2003; Wolbank et al. 2007; Zhao et al. 2005), although immunofluorescence staining of amniotic mesenchymal tissue failed to show positivity for SSEA-3 or SSEA-4 (Miki et al. 2007).
Both first- and third-trimester hAMSC and chorionic mesenchymal stromal cells express low levels of HLA-ABC, but not HLA-DR, (Portmann-Lanz et al. 2006; Wolbank et al. 2007) indicating that these cells are immunoprivileged and may therefore be applicable in a clinical transplantation setting. In support of this hypothesis, human amniotic and chorionic cells have been shown to successfully and persistently engraft in multiple organs and tissues in vivo after xenogeneic transplantation into neonatal swine and rats, with human chimerism detection in the brain, lung, bone marrow, thymus, spleen, kidney and liver of these animals after either intraperitoneal or intravenous transplantation. The migratory ability of placenta-derived cells which was observed in these animals is consistent with the demonstrated expression by hAMSC of adhesion and migration molecules (L-selectin, VLA-5, CD29, P-selectin Ligand 1), as well as cellular matrix proteinases (MMP-2 and MMP-9) (Bailo et al. 2004).
As for hAEC, support for the hypothesis that some hAMSC may still display pluripotency that is characteristic of the pre-gastrulation embryonic cells from which they are derived has come from several recent studies which show that these cells are able to differentiate toward “classic” mesodermal lineages (osteogenic, chondrogenic and adipogenic) (Alviano et al. 2007; In ‘t Anker et al. 2004; Portmann-Lanz et al. 2006; Soncini et al. 2007; Wolbank et al. 2007), as well as toward cell types of all three germ layers—ectoderm (neural) (Portmann-Lanz et al. 2006; Sakuragawa et al. 2004), mesoderm (skeletal muscles, cardiomyocytes and endothelial) (Alviano et al. 2007; Ilancheran et al. 2007; Ventura et al. 2007; Zhao et al. 2005) and endoderm (Wei et al. 2003).
Neuronal and glial marker expression by hAMSC, as well as in vitro differentiation of these cells toward a neuroglial phenotype has been demonstrated (Sakuragawa et al. 2004), although in vivo studies to assess the possible neurogenic/neuroprotective effects of hAMSC have not yet been reported.
In vitro and in vivo studies have provided promising evidence that hAMSC may be useful for the regeneration of cardiac tissue after infarction. Co-culture experiments with neonatal rat heart explants has shown that hAMSC are able to integrate into cardiac tissue and differentiate into cardiomyocyte-like cells, while hAMSC transplantation into myocardial infarcts in rat hearts showed that these cells are able to survive for at least two months and differentiate into cardiomyocyte-like cells (Zhao et al. 2005). In attempting to enhance the cardiac differentiation of placental cells, Ventura and co-workers found that a mixed ester of hyaluronan and butyric and retinoic acids (HBR) promoted cardiogenic/vasculogenic differentiation of human amniochorionic (AC)-derived cells and enhanced the expression of cardiomyogenic genes (GATA4, NKX 2.5) and proteins (sarcomeric myosin heavy chain and alpha sarcomeric actin). Furthermore, cells treated with HBR were also seen to express both cardiac and endothelial markers such as von Willebrand factor (vWF), and enhance cardiac repair in infarcted rat hearts (Alviano et al. 2007; Ventura et al. 2007), thereby providing valuable indication as to the conditions which may prove most useful in directing cardiac differentiation of hAMSC for clinical application.
Immunological features of placenta-derived cells
Immunologically, pregnancy is an exceptional phenomenon whereby the fully competent immune system of the mother is rendered tolerant to the immunologically distinct fetal allograft, thereby creating a situation in which rejection, which would normally ensue in an allogeneic transplantation setting in the absence of immunosuppression, is avoided. Given that the placenta forms the interface between the immunologically distinct mother and fetus, it can therefore be hypothesized that cells from this tissue are likely to have immunoregulatory properties which contribute to fetomaternal tolerance.
Evidence which provides support to the hypothesis that fetal membranes are non-immunogenic comes from clinical studies in which amniotic membrane has been used for treatment of skin wounds, burn injuries, chronic leg ulcers, and prevention of tissue adhesion in surgical procedures (Colocho et al. 1974; Faulk et al. 1980; Gruss and Jirsch 1978; Subrahmanyam 1995; Trelford and Trelford-Sauder 1979; Ward and Bennett 1984; Ward et al. 1989) and, more recently, in ocular surface reconstruction, where amniotic membrane has been used for substrate transplantation to promote the development of normal corneal or conjuntival epithelium (Gomes et al. 2005) without acute rejection in absence of immunosuppressive treatment. Moreover, several clinical trials in humans have proven that allogeneic transplantation of amniotic membrane (Sakuragawa et al. 1992; Scaggiante et al. 1987; Tylki-Szymanska et al. 1985) or hAEC (Akle et al. 1981; Yeager et al. 1985) does not induce acute immune rejection in the absence of immunosuppressive treatment.
Intriguingly, various groups have demonstrated prolonged survival of human amniotic membrane or hAEC after xenogeneic transplantation into various immuno-competent animals, including rabbits (Avila et al. 2001), rats (Kubo et al. 2001), guinea pigs (Yuge et al. 2004) and bonnet monkeys (Sankar and Muthusamy 2003). Additionally, long-term engraftment was observed after intravenous injection of heterogeneous human amniotic and chorionic cells into newborn swine or rats, with human microchimerism detected in several organs (Bailo et al. 2004), suggesting active migration and integration into specific organs, and indicating active tolerance of the xenogeneic cells.
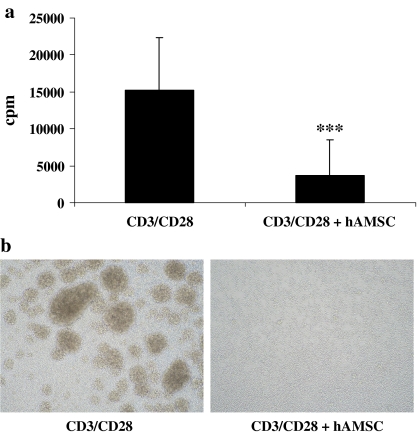
In vitro studies aimed at understanding the mechanisms underlying the immunomodulatory effects observed after allo- or xenogeneic transplantation of placenta-derived cells show that cells isolated from amniotic and chorionic membranes do not induce an allogeneic or xenogeneic immune response in MLR, and are able to actively suppress proliferation of lymphocytes which has been induced either by an allogeneic stimulus in a mixed lymphocyte reaction or by stimulation via the TCR receptor (Fig. 3) (Bailo et al. 2004; Li et al. 2005; Wolbank et al. 2007). The immunomodulatory effects of hAMSC on MLR are observed both in a direct contact setting, or when the hAMSC are separated from lymphocyte responders via a transwell, therefore suggesting that hAMSC secrete soluble immunomodulatory factors. Very recently, the presence of two subpopulations within the amniotic mesenchymal layer, which display either T-cell stimulatory or suppressive effects, has been described (Magatti et al. 2008). Further characterization of the different cell subpopulations present in placental tissues will be required in order to understand how opposing functions (stimulatory and suppressive) may be integrated in order to contribute to fetal tolerance. Such studies are also likely to provide general mechanistic insight into the processes of tolerance versus rejection, as well as indications of better cell sources for the development of novel cell therapy procedures with reduced rates of rejection.
Fig. 3.
Human amniotic mesenchymal stromal cells are able to suppress proliferation of T cells stimulated by anti-CD3 and anti-CD28. a T-cell proliferation assessed by [3H]-thymidine incorporation after stimulating T-cells with anti-CD3 plus anti-CD28 antibody in the absence or presence of hAMSC. Proliferation is expressed in counts per minutes (cpm). Data are the mean of 10 independent experiments. ***p < 0.001 b Presence and absence of activation clusters in CD3/CD28-stimulated T-cell cultures in the absence or presence of hAMSC
Summary
The early embryological origin of placental tissues, which begin to develop even before gastrulation occurs, combined with the fact that these tissues play a fundamental role in maintaining fetomaternal tolerance, suggests that the term placenta may harbour cells which have retained the plasticity that is characteristic of the cells from which it is derived, and which may also be able to prevent immune rejection. These features represent the fundamental aspects of regenerative medicine. Starting from these considerations, and in addressing the current need to identify new, ethically acceptable, readily available sources of stem cells for regenerative medicine which can overcome the shortcomings associated with other stem cell sources, several groups have turned to the term placenta and have shown that cells from this tissue do indeed display plasticity and immunomodulatory effects both in vitro and in vivo. Although showing great promise, the study of placenta-derived stem cells still represents a field in its infancy, and further studies are needed to provide a complete understanding of the mechanisms underlying the differentiation potential and immunomodulatory effects of placental cells, which will ultimately allow the development of new and more effective strategies for regenerative medicine and transplantation.
Acknowledgements
We sincerely thank Fondazione Cariplo for providing continuous and generous financial support for research into placenta-derived stem cells at Centro di Ricerca E. Menni, Brescia, Italy.
References
- Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I (1981) Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 2:1003–1005. doi:10.1016/S0140-6736(81)91212-5 [DOI] [PubMed]
- Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, Lanzoni G, Cantoni S, Cavallini C, Bianchi F, Tazzari PL, Pasquinelli G, Foroni L, Ventura C, Grossi A, Bagnara GP (2007) Term Amniotic membrane is a high throughput source for multipotent Mesenchymal Stem Cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol 7:11. doi:10.1186/1471-213X-7-11 [DOI] [PMC free article] [PubMed]
- Avila M, Espana M, Moreno C, Pena C (2001) Reconstruction of ocular surface with heterologous limbal epithelium and amniotic membrane in a rabbit model. Cornea 20:414–420. doi:10.1097/00003226-200105000-00016 [DOI] [PubMed]
- Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P, Albertini A, Zorzi F, Cavagnini A, Candotti F, Wengler GS, Parolini O (2004) Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation 78:1439–1448. doi:10.1097/01.TP.0000144606.84234.49 [DOI] [PubMed]
- Benirschke K, Kaufmann P (2000) Pathology of the human placenta. Springer-Verlag
- Bilic G, Ochsenbein-Kolble N, Hall H, Huch R, Zimmermann R (2004) In vitro lesion repair by human amnion epithelial and mesenchymal cells. Am J Obstet Gynecol 190:87–92. doi:10.1016/j.ajog.2003.07.011 [DOI] [PubMed]
- Colocho G, Graham WP 3rd, Greene AE, Matheson DW, Lynch D (1974) Human amniotic membrane as a physiologic wound dressing. Arch Surg 109:370–373 [DOI] [PubMed]
- Cunningham FG, Hankins GDF, Leveno KJ, Gilstrap LC, Gant NF, Mac Donald PC, Clark SL (1997) Williams obstetrics. McGraw-Hill Medical
- Davila JC, Cezar GG, Thiede M, Strom S, Miki T, Trosko J (2004) Use and application of stem cells in toxicology. Toxicol Sci 79:214–223. doi:10.1093/toxsci/kfh100 [DOI] [PubMed]
- D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA (1999) Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 14:1115–1122. doi:10.1359/jbmr.1999.14.7.1115 [DOI] [PubMed]
- Eichna DM, Brown KS, Breen A, Dean RB (2008) Mucormycosis: a rare but serious infection. Clin J Oncol Nurs 12:108–112. doi:10.1188/08.CJON.108-112 [DOI] [PubMed]
- Elwan MA, Sakuragawa N (1997) Evidence for synthesis and release of catecholamines by human amniotic epithelial cells. Neuroreport 8:3435–3438. doi:10.1097/00001756-199711100-00004 [DOI] [PubMed]
- Faulk WP, Matthews R, Stevens PJ, Bennett JP, Burgos H, Hsi BL (1980) Human amnion as an adjunct in wound healing. Lancet 1:1156–1158. doi:10.1016/S0140-6736(80)91617-7 [DOI] [PubMed]
- Gomes JA, Romano A, Santos MS, Dua HS (2005) Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol 16:233–240. doi:10.1097/01.icu.0000172827.31985.3a [DOI] [PubMed]
- Gruss JS, Jirsch DW (1978) Human amniotic membrane: a versatile wound dressing. Can Med Assoc J 118:1237–1246 [PMC free article] [PubMed]
- Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U (2007) Stem cells derived from human fetal membranes display multi-lineage differentiation potential. Biol Reprod 77:577–588. doi:10.1095/biolreprod.106.055244 [DOI] [PubMed]
- In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH (2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22:1338–1345 [DOI] [PubMed]
- Kakishita K, Elwan MA, Nakao N, Itakura T, Sakuragawa N (2000) Human amniotic epithelial cells produce dopamine and survive after implantation into the striatum of a rat model of Parkinson’s disease: a potential source of donor for transplantation therapy. Exp Neurol 165:27–34. doi:10.1006/exnr.2000.7449 [DOI] [PubMed]
- Kakishita K, Nakao N, Sakuragawa N, Itakura T (2003) Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions. Brain Res 980:48–56. doi:10.1016/S0006-8993(03)02875-0 [DOI] [PubMed]
- Kong XY, Cai Z, Pan L, Zhang L, Shu J, Dong YL, Yang N, Li Q, Huang XJ, Zuo PP (2008) Transplantation of human amniotic cells exerts neuroprotection in MPTP-induced Parkinson disease mice. Brain Res 1205:108–115. doi:10.1016/j.brainres.2008.02.040 [DOI] [PubMed]
- Koyano S, Fukui A, Uchida S, Yamada K, Asashima M, Sakuragawa N (2002) Synthesis and release of activin and noggin by cultured human amniotic epithelial cells. Dev Growth Differ 44:103–112. doi:10.1046/j.1440-169x.2002.00626.x [DOI] [PubMed]
- Kubo M, Sonoda Y, Muramatsu R, Usui M (2001) Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 42:1539–1546 [PubMed]
- Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, Alizadeh H (2005) Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci 46:900–907. doi:10.1167/iovs.04-0495 [DOI] [PubMed]
- Liu T, Wu J, Huang Q, Hou Y, Jiang Z, Zang S, Guo L (2008) Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model. Shock 29:603–611. doi:10.1097/SHK.0b013e318157e845 [DOI] [PubMed]
- Magatti M, De Munari S, Vertua E, Gibelli L, Wengler GS, Parolini O (2008) Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells 26:182–192. doi:10.1634/stemcells.2007-0491 [DOI] [PubMed]
- Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M, Madon E, Fagioli F (2006) Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem 97:744–754. doi:10.1002/jcb.20681 [DOI] [PubMed]
- Miki T, Strom SC (2006) Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev 2:133–142 [DOI] [PubMed]
- Miki T, Lehmann T, Cai H, Stolz DB, Strom SC (2005) Stem cell characteristics of amniotic epithelial cells. Stem Cells 23:1549–1559. doi:10.1634/stemcells.2004-0357 [DOI] [PubMed]
- Miki T, Mitamura K, Ross MA, Stolz DB, Strom SC (2007) Identification of stem cell marker-positive cells by immunofluorescence in term human amnion. J Reprod Immunol 75:91–96. doi:10.1016/j.jri.2007.03.017 [DOI] [PubMed]
- Moore KL, Persaud TVN (1998) The developing human: clinically oriented embryology. Sanders
- Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells 26:300–311 [DOI] [PubMed]
- Portmann-Lanz CB, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, Surbek DV (2006) Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol 194:664–673. doi:10.1016/j.ajog.2006.01.101 [DOI] [PubMed]
- Sakuragawa N, Yoshikawa H, Sasaki M (1992) Amniotic tissue transplantation: clinical and biochemical evaluations for some lysosomal storage diseases. Brain Dev 14:7–11 [DOI] [PubMed]
- Sakuragawa N, Thangavel R, Mizuguchi M, Hirasawa M, Kamo I (1996) Expression of markers for both neuronal and glial cells in human amniotic epithelial cells. Neurosci Lett 209:9–12. doi:10.1016/0304-3940(96)12599-4 [DOI] [PubMed]
- Sakuragawa N, Misawa H, Ohsugi K, Kakishita K, Ishii T, Thangavel R, Tohyama J, Elwan M, Yokoyama Y, Okuda O, Arai H, Ogino I, Sato K (1997) Evidence for active acetylcholine metabolism in human amniotic epithelial cells: applicable to intracerebral allografting for neurologic disease. Neurosci Lett 232:53–56. doi:10.1016/S0304-3940(97)00570-3 [DOI] [PubMed]
- Sakuragawa N, Enosawa S, Ishii T, Thangavel R, Tashiro T, Okuyama T, Suzuki S (2000) Human amniotic epithelial cells are promising transgene carriers for allogeneic cell transplantation into liver. J Hum Genet 45:171–176. doi:10.1007/s100380050205 [DOI] [PubMed]
- Sakuragawa N, Kakinuma K, Kikuchi A, Okano H, Uchida S, Kamo I, Kobayashi M, Yokoyama Y (2004) Human amnion mesenchyme cells express phenotypes of neuroglial progenitor cells. J Neurosci Res 78:208–214. doi:10.1002/jnr.20257 [DOI] [PubMed]
- Sankar V, Muthusamy R (2003) Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience 118:11–17. doi:10.1016/S0306-4522(02)00929-6 [DOI] [PubMed]
- Scaggiante B, Pineschi A, Sustersich M, Andolina M, Agosti E, Romeo D (1987) Successful therapy of Niemann-Pick disease by implantation of human amniotic membrane. Transplantation 44:59–61. doi:10.1097/00007890-198707000-00014 [DOI] [PubMed]
- Schroeder A, Theiss C, Steuhl KP, Meller K, Meller D (2007) Effects of the human amniotic membrane on axonal outgrowth of dorsal root ganglia neurons in culture. Curr Eye Res 32:731–738. doi:10.1080/02713680701530605 [DOI] [PubMed]
- Soncini M, Vertua E, Gibelli L, Zorzi F, Denegri M, Albertini A, Wengler GS, Parolini O (2007) Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med 1:296–305. doi:10.1002/term.40 [DOI] [PubMed]
- Subrahmanyam M (1995) Amniotic membrane as a cover for microskin grafts. Br J Plast Surg 48:477–478. doi:10.1016/0007-1226(95)90123-X [DOI] [PubMed]
- Takashima S, Ise H, Zhao P, Akaike T, Nikaido T (2004) Human amniotic epithelial cells possess hepatocyte-like characteristics and functions. Cell Struct Funct 29:73–84. doi:10.1247/csf.29.73 [DOI] [PubMed]
- Tamagawa T, Ishiwata I, Saito S (2004) Establishment and characterization of a pluripotent stem cell line derived from human amniotic membranes and initiation of germ layers in vitro. Hum Cell 17:125–130 [DOI] [PubMed]
- Tcheng M, Oliver L, Courtois Y, Jeanny JC (1994) Effects of exogenous FGFs on growth, differentiation, and survival of chick neural retina cells. Exp Cell Res 212:30–35. doi:10.1006/excr.1994.1114 [DOI] [PubMed]
- Terada S, Matsuura K, Enosawa S, Miki M, Hoshika A, Suzuki S, Sakuragawa N (2000) Inducing proliferation of human amniotic epithelial (HAE) cells for cell therapy. Cell Transpl 9:701–704 [DOI] [PubMed]
- Toda A, Okabe M, Yoshida T, Nikaido T (2007) The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci 105:215–228. doi:10.1254/jphs.CR0070034 [DOI] [PubMed]
- Trelford JD, Trelford-Sauder M (1979) The amnion in surgery, past and present. Am J Obstet Gynecol 134:833–845 [DOI] [PubMed]
- Tylki-Szymanska A, Maciejko D, Kidawa M, Jablonska-Budaj U, Czartoryska B (1985) Amniotic tissue transplantation as a trial of treatment in some lysosomal storage diseases. J Inherit Metab Dis 8:101–104. doi:10.1007/BF01819289 [DOI] [PubMed]
- Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N (2000) Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res 62:585–590. doi:10.1002/1097-4547(20001115)62:4<585::AID-JNR13>3.0.CO;2-U [DOI] [PubMed]
- Ventura C, Cantoni S, Bianchi F, Lionetti V, Cavallini C, Scarlata I, Foroni L, Maioli M, Bonsi L, Alviano F, Fossati V, Bagnara GP, Pasquinelli G, Recchia FA, Perbellini A (2007) Hyaluronan mixed esters of butyric and retinoic acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. J Biol Chem 282:14243–14252. doi:10.1074/jbc.M609350200 [DOI] [PubMed]
- Ward DJ, Bennett JP (1984) The long-term results of the use of human amnion in the treatment of leg ulcers. Br J Plast Surg 37:191–193. doi:10.1016/0007-1226(84)90009-2 [DOI] [PubMed]
- Ward DJ, Bennett JP, Burgos H, Fabre J (1989) The healing of chronic venous leg ulcers with prepared human amnion. Br J Plast Surg 42:463–467. doi:10.1016/0007-1226(89)90015-5 [DOI] [PubMed]
- Wei JP, Zhang TS, Kawa S, Aizawa T, Ota M, Akaike T, Kato K, Konishi I, Nikaido T (2003) Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transpl 12:545–552 [DOI] [PubMed]
- Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van Griensven M, Stadler G, Redl H, Gabriel C (2007) Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng 13:1173–1183. doi:10.1089/ten.2006.0313 [DOI] [PubMed]
- Yeager AM, Singer HS, Buck JR, Matalon R, Brennan S, O’Toole SO, Moser HW (1985) A therapeutic trial of amniotic epithelial cell implantation in patients with lysosomal storage diseases. Am J Med Genet 22:347–355. doi:10.1002/ajmg.1320220219 [DOI] [PubMed]
- Yuge I, Takumi Y, Koyabu K, Hashimoto S, Takashima S, Fukuyama T, Nikaido T, Usami S (2004) Transplanted human amniotic epithelial cells express connexin 26 and Na-K-adenosine triphosphatase in the inner ear. Transplantation 77:1452–1454. doi:10.1097/00007890-200405150-00023 [DOI] [PubMed]
- Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T (2005) Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation 79:528–535. doi:10.1097/01.TP.0000149503.92433.39 [DOI] [PubMed]